Abstract
Background
Essential tremor is the most common type of tremor, with a prevalence of 0.4% in the overall population and 4–7% in persons over age 65. In general, tremor is so common that patients with tremor are frequently treated not only by neurologists, but also by physicians from other specialties.
Method
This review is based on publications retrieved by a selective PubMed search and on guidelines from Germany and abroad.
Results
Particular tremor syndromes are usually diagnosed on the basis of their typical clinical presentation and whatever accompanying manifestations may be present. Ancillary tests are usually unnecessary. Unilateral rest tremor accompanied by rigidity and bradykinesia is typical of Parkinson’s disease. Essential tremor is a bilateral postural tremor. The most common cause of intention tremor is multiple sclerosis. Mild tremor syndromes can often be treated satisfactorily with drugs. In case of severe tremor, which is rarer, a stereotactic operation can be considered. The usual outcome of such procedures is the complete suppression of tremor.
Conclusions
Most patients with tremor can be given a precise diagnosis and offered specific treatment. It is important for the physician to inform the patient about the expected course of tremor over time, its possible genetic causes, and the various available treatments.
Tremor is the most common movement disorder. Essential tremor, with an overall prevalence of 0.4%, affects up to 5% of persons over age 65, and up to 21% of persons over age 95 (e1). Parkinsonian tremor is much rarer, as the prevalence of Parkinson’s disease in persons over age 65 is about 2%, and only about half of patients with the disease have tremor (e2). In most cases, a specific tremor syndrome can be diagnosed on the basis of the clinical presentation; an important step in diagnosis is the assignment of the patient’s tremor to one of the the main clinical types—rest tremor, position tremor, action tremor, and intention tremor. Moreover, any accompanying manifestations should be taken into account as well, e.g., generalized slowness of movement (bradykinesia) in Parkinson’s disease. This review is principally devoted to the differential diagnosis of the common tremor syndromes and the treatment strategies that are available for them. It is based on publications retrieved by a selective PubMed search and on relevant textbooks and guidelines. Studies providing a high level of evidence were preferentially considered.
Learning objectives
After reading this article, readers should
know the diseases that cause tremor,
be acquainted with its possible genetic causes,
and be able to assess the role of the various available treatments in particular situations.
Clinical diagnostic evaluation.
Tremor can affect any or all of the limbs, the trunk, the head, and the voice, individually or in varying combinations.
Clinical evaluation
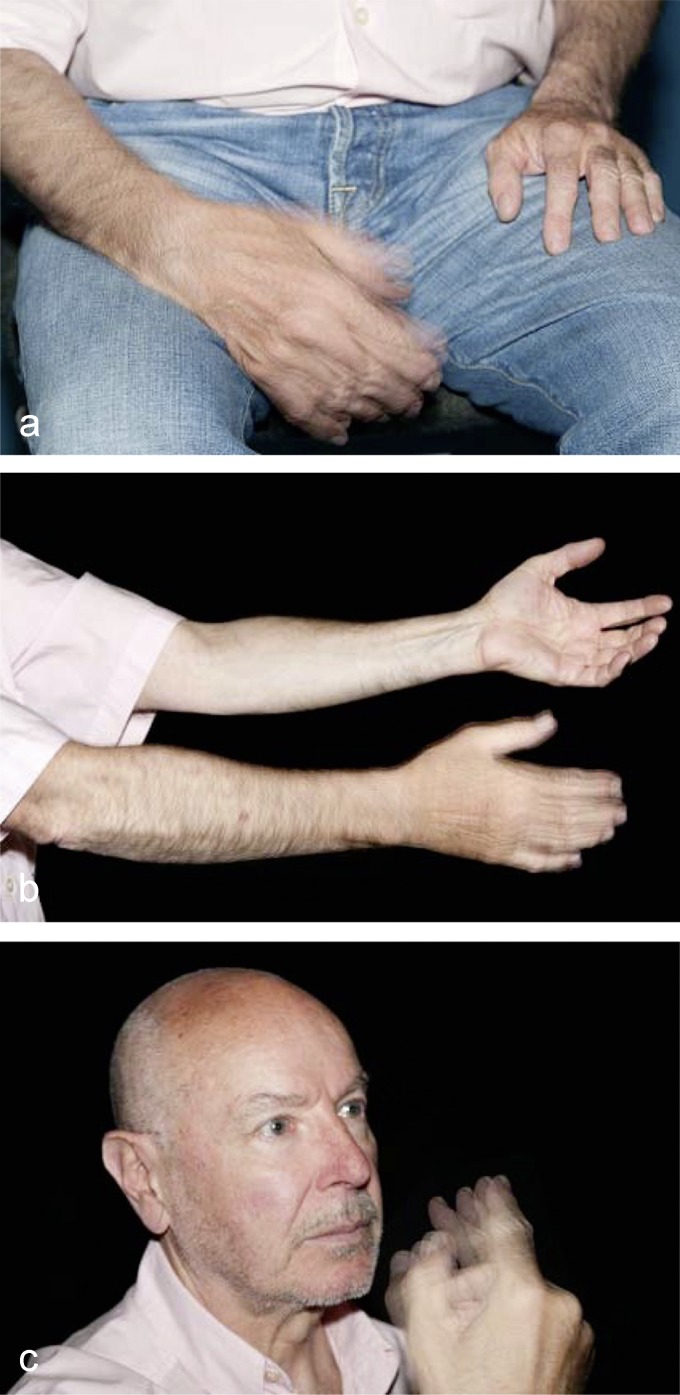
Tremor can affect any or all of the limbs, the trunk, the head, and the voice, individually or in varying combinations. By definition, tremor is a regularly oscillating involuntary movement. Myoclonus can be mistaken for tremor until further assessment reveals it to be non-rhythmic. A prerequisite to the diagnosis of the underlying disease in a patient with tremor is its clinical classification as rest tremor, postural tremor, or intention tremor (Figure 1). Each of these types of tremor is caused by a different set of underlying diseases.
Figure 1.
a) Rest tremor arises when the arm is laid down (at rest). This type of tremor is typical of Parkinson’s disease, in which tremor is usually unilateral at first. Rest tremor can be seen in one or more fingers, the hand, the foot, or the chin. b) Postural tremor regularly arises when the patient holds the arms outstretched. A postural tremor of very low amplitude may be a drug-induced tremor (e.g., due to lithium) or an intensified physiologic tremor in the setting or hyperthyroidism or alcohol withdrawal. Postural tremor with an amplitude greater than 1 cm is usually essential tremor. This entity is always bilateral. c) The finger-to-nose test reveals intention tremor, which arises as the finger approaches the target (nose). Intention tremor is due to cerebellar dysfunction and can be either uni- or bilateral.
A key element of diagnostic evaluation.
A prerequisite to diagnosis in a patient with tremor is its clinical classification as rest tremor, postural tremor, or intention tremor.
Unilateral rest tremor
Unilateral rest tremor should arouse suspicion of Parkinson’s disease and prompt a search for its other manifestations, including rigor and bradykinesia. The latter manifests itself in early Parkinson’s disease mainly as reduced arm-swing on the affected side when the patient walks. Stooped posture and a relatively expressionless face are often also seen early on in the course of the disease. The first signs of the disease may well be noticed by the patient’s family before the patient realizes anything is wrong.
Essential tremor
Unlike rest tremor, essential tremor is a bilateral, usually symmetrical postural tremor. When the patient tries to hold the arms outstretched in front, tremor can be seen, with an amplitude of several centimeters at the fingertips. The patient reports that it has become difficult or impossible to eat with a knife and fork or to drink from a cup or glass. The tremor worsens with excitement (family celebrations, public speaking).
Intention tremor
The third major type of tremor that can be distinguished by physical examination is intention tremor. In this condition, the amplitude of tremor is seen to increase as the fingertips approach a target. In the finger-to-nose test, the index finger trembles only in the vicinity of the nose. Likewise, the hand trembles as it approaches a target, such as a cup. Intention tremor is due to cerebellar dysfunction. The affected patients usually manifest further signs of cerebellar disease, such as dysarthrophonia and a swaying, broad-based gait.
Ancillary tests.
Patients with the new onset of tremor should undergo magnetic resonance imaging (MRI) of the brain.
Ancillary tests
Patients with the new onset of tremor should undergo magnetic resonance imaging (MRI) of the brain. If tremor is accompanied by other signs of parkinsonism, the MRI scan should be inspected for the white-matter changes associated with certain manifestations (such as a gait disturbance) that are easily mistaken for Parkinson’s disease. Intention tremor should arouse suspicion of cerebellar disease and prompt a search for evidence of multiple sclerosis, the most common cause. Clinical criteria alone sometimes do not suffice to tell Parkinson’s disease apart from essential tremor; in such cases, methods of nuclear medicine (above all, dopamine-transporter SPECT [single photon emission computed tomography]) can be used to differentiate these conditions with high specificity and sensitivity (97% and 100%) (e3).
Accompanying manifestations.
Accompanying manifestations (aside from tremor) are generally present in patients with either parkinsonian tremor or cerebellar tremor.
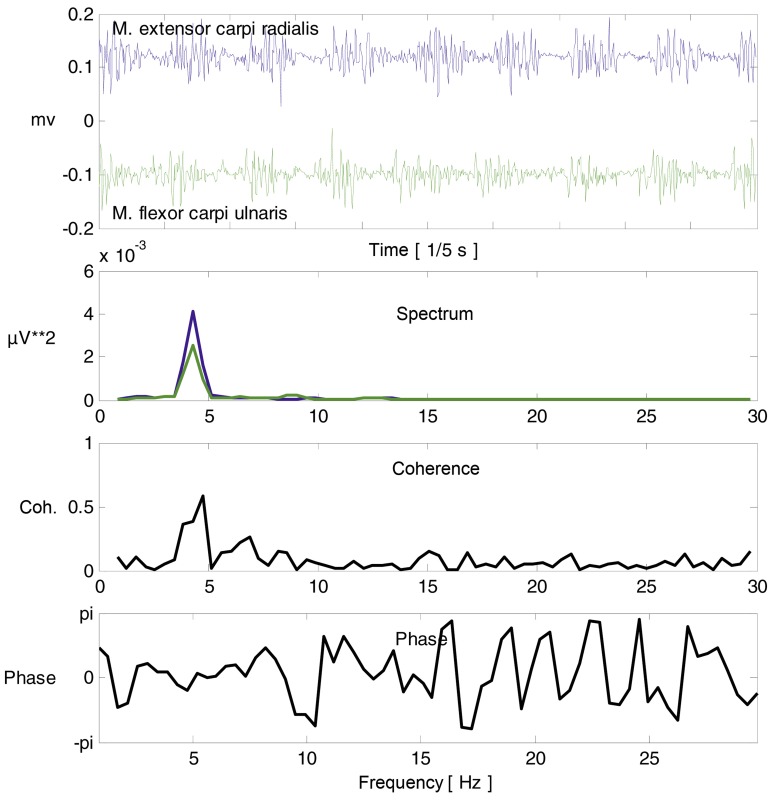
Tremor can be non-invasively recorded with small accelerometers or surface electrodes that detect muscle activity (Figure 2). Such studies help document the tremor frequency, which can be useful in the differential diagnosis of tremor syndromes even thought their typical frequency ranges overlap (1). Cerebellar tremor usually has a frequency of about 4 Hz, parkinsonian tremor about 5 Hz, and essential tremor 4–8 Hz (1). The degree of regularity of a tremor can be more reliably determined by objective tremor analysis (in particular, EMG [electromyography] recording) than by clinical examination alone. Regularity, though not a scientifically validated criterion, can nonetheless be of use in distinguishing a classic tremor syndrome (in which the oscillation is generally regular) from a myoclonus syndrome or psychogenic syndrome (in which it is often irregular).
Figure 2.
Tremor analysis with an outstretched left arm. Activity in the muscles participating in the tremor can be recorded and analyzed. The upper tracing reveals synchronous, regular activity of the hand extensors and flexors. The spectrum has a peak at 4.3 Hz; the movement is thus rhythmic, satisfying the definition of tremor. The coherence curve shows that the activity of each of the two muscles at 4.3 Hz is correlated with the other. The phase information at 4.3 Hz in this case indicates that the muscles are active without any temporal delay between them (i.e., the phase difference here is zero). The finding of activity at 4.3 Hz with simultaneous innervation of agonist and antagonist muscles is typical of essential tremor.
The diagnosis of Parkinson’s disease.
The diagnosis of Parkinson’s disease is based on the clinical observation of its cardinal manifestations—tremor, rigor, and bradykinesia.
Differential diagnosis and treatment
Parkinson’s disease
The diagnosis of Parkinson’s disease is based on the clinical observation of its cardinal manifestations—tremor, rigor, and bradykinesia—and the exclusion of other manifestations that would indicate an atypical parkinsonian syndrome (2). For example, dementia and frequent falls are never seen in the early stage of classic Parkinson’s disease. Although rest tremor is the hallmark of the disease, a moderately severe postural tremor is seen as well in half of all patients (e4). Rarely, a patient may have postural tremor without rest tremor, along with otherwise typical manifestations of Parkinson’s disease. Parkinsonian tremor fluctuates in intensity and increases with mental effort. The tremor usually becomes bilateral several years of after the onset of the disease; the limbs do not tremble in synchrony and are thus assumed to be under the influence of independent tremor generators (e5). Pharmacological replacement of the deficient neurotransmitter dopamine alleviates tremor effectively, even if postural tremor is present in addition (3). Clinical experience indicates that the tremor can actually increase in amplitude when treatment is begun, because rigidity responds first to dopaminergic treatment and tremor only improves at higher doses.
Dopamine replacement in Parkinson’s disease.
Pharmacological replacement of the deficient neurotransmitter dopamine alleviates tremor effectively, even if postural tremor is present in addition.
Bilateral manifestations.
The tremor usually becomes bilateral several years of after the onset of the disease; the limbs do not tremble in synchrony and are thus assumed to be under the influence of independent tremor generators.
The classic dopaminergic drugs include levodopa and a variety of dopamine agonists. If tremor persists when these drugs are given, anticholinergic drugs (bornaprine, biperidene) can be used, as was more commonly done in the past, as long as the major contraindications to their use are absent (dementia, cardiac arrhythmia, prostatic hypertrophy). The efficacy of anticholinergic drugs has not been documented in modern clinical trials, but it was concluded in a systematic review that they work better than placebo (4). Budipine is an effective drug that can be held in reserve (5) (level Ia evidence); it can cause cardiac arrhythmia, however, and patients taking it should have strict treatment monitoring with electrocardiography at regular intervals. Budipine abolishes tremor in about half of all cases and generally lowers its intensity by 15% (e6). Drug-resistant parkinsonian tremor can be treated with deep brain stimulation.
Essential tremor
Essential tremor is a bilateral postural tremor that usually affects the hands. Its amplitude with arms outstretched varies from a few millimeters to 10–15 cm. Head tremor can be present alone or in addition to hand tremor. A vocal tremor is often present as well (1). Writing and other fine motor tasks are particularly impaired, but so are coarse tasks such as drinking from a cup or glass, leading to marked difficulties in everyday life. Patients with advanced essential tremor may become unable to feed themselves. Tremor is the single determining manifestation of this disease. According to the classic definition, essential tremor consists purely of a postural tremor without any other manifestations; more recent studies have shown, however, that patients can also have mild cerebellar ataxia with unsteadiness in tandem gait (e7) and intention tremor on the finger-to-nose test (e8).
The differential diagnosis of essential tremor includes intensified physiological tremor that may arise as a side effect of various drugs or in the setting of a metabolic disorder. The incidence of essential tremor has two peaks: juvenile essential tremor generally arises between the ages of 10 and 20, senile essential tremor between 50 and 60 (e9). It is unclear whether these two entities are really variants of a single disease. The course is slowly progressive. In the juvenile form, the tremor worsens slowly over decades and usually does not cause any major functional impairment until the patient is over 50 years old. Affected young adults should, however, bear in mind when choosing an occupation that their tremor will probably worsen inexorably over the years; they may one day be unable to perform the fine motor tasks that certain jobs demand (e.g., dentistry). Senile essential tremor seems to progress somewhat more rapidly (e8). Essential tremor often improves when the patient drinks alcohol, and this can be used as a diagnostic criterion (e10). In rare cases, this phenomenon leads to chronic alcoholism as a consequence of self-therapy.
Essential tremor.
Essential tremor is a bilateral postural tremor that usually affects the hands.
Twin studies have documented a strong genetic element in the pathogenesis of essential tremor: the concordance rate for essential tremor among monozygotic twins is over 90%. Many large pedigrees have been described in which the disease is evidently transmitted in autosomal dominant fashion. Remarkably, however, no causative gene has been identified to date. Recently, genetic variants in the gene for LINGO1 have been identified as risk factors for essential tremor and confirmed as such in multiple replication studies (6– 8). LINGO1 plays a role in axonal regeneration and oligodendrocyte maturation in the central nervous system (6)
Genetic factors.
Twin studies have documented a strong genetic element in the pathogenesis of essential tremor.
The treatment consists of propranolol at doses of up to 240 mg per day; contraindications to this drug include AV block, depression, and asthma (class I evidence) (9, 10). The tremor amplitude is lowered by 32–75% under treatment (e11). In men, propranolol can make penile erection difficult or abolish it entirely. Another drug with a comparable prospect of success against tremor is primidone, an antiepileptic drug (11). Multiple studies have shown that primidone reduces the tremor amplitude by 42% to 76% (e11). The dose must be ramped up very slowly at first (by 1/4 of a tablet per week), as otherwise the drug would cause such severe fatigue that treatment with it would probably be abandoned. It can also impair the patient’s ability to drive a car. Yet another drug used to treat essential tremor is topiramate (class I evidence): in a placebo-controlled clinical trial, tremor improved by 29% (on a clinical tremor scale) after 24 weeks of treatment with topiramate, compared to 16% with placebo (12). Sensory deficits, nausea, impaired concentration, and somnolence each affected about 3% of patients. Topiramate had to be discontinued in 32% of patients because of adverse side effects. Patients with very severe essential tremor often cannot be adequately treated with drugs alone. Such patients often undergo deep brain stimulation, with good results.
Intention tremor
Intention tremor is due to cerebellar dysfunction. The most common cause is multiple sclerosis, but idiopathic cerebellar degeneration can also present with intention tremor as a leading manifestation. There is no known, effective drug treatment for tremor resulting from a cerebellar lesion, but benzodiazepines, propranolol and antiepileptic drugs are worth trying, as they may benefit individual patients. Recent single case reports have indicated that 4-aminopyridine may be useful in the treatment of cerebellar downbeat nystagmus, tremor, and gait ataxia (13). Deep brain stimulation can ameliorate the tremor component of a cerebellar movement disorder, but it has no effect on dysmetria, which manifests itself in typical cerebellar dysarthria and swaying gait (14, 15).
Psychogenic tremor.
Diagnostic clues include sudden onset, spontaneous remission, distractability, and a peculiar type of tremor that does not seem to fit into any of the known tremor syndromes.
Psychogenic tremor
It is estimated that 2–3% of all neurological patients have psychogenic symptoms (16), with the majority of these (55%) accounted for by psychogenic tremor (17). Diagnostic clues include sudden onset, spontaneous remission, distractability, and a peculiar type of tremor that does not seem to fit into any of the known tremor syndromes. Moreover, psychogenic tremor is often not rhythmic and tends to assume the frequency of voluntary tapping movements performed simultaneously with another limb (18). In bilateral psychogenic tremor, the limbs often tremble in synchrony; this does not occur in Parkinson’s disease or essential tremor (e12). Patients with suspected psychogenic tremor must undergo an extensive diagnostic evaluation to rule out other possible somatic causes, including Wilson’s disease, dystonia, and epileptic myoclonus. Tremor analysis can provide valuable clues but cannot single-handedly establish the diagnosis of psychogenic tremor. There is no evidence-based treatment. Once the diagnosis of psychogenic tremor has been established, a compassionate approach to the patient’s problem should be undertaken, in collaboration with a psychiatrist. Drastic “patient education” with the message that no organic disease is present generally serves no therapeutic purpose. Placebo measures may bring about short-term improvement and also help confirm the diagnosis. Some authors also consider that magnetic stimulation of the cerebral cortex may have a role to play in the treatment of this condition (19). Patients with psychiatric comorbidity should generally be treated with psychiatric medication. Three years after the diagnosis of psychogenic tremor, 64% of patients still report having a moderate or severe tremor, while 15% report spontaneous improvement (17). The odds that treatment will succeed are better if the problem has only been present for a short time.
Rare types of tremor
Physiological tremor—Physiological tremor is normally only demonstrable with ancillary testing, as its amplitude is too small to be seen with the naked eye. It reflects the intrinsic frequency of muscle stretch reflexes. Various metabolic disorders, including hypoglycemia, hyperthyroidism, and ethanol withdrawal syndrome, can accentuate physiological tremor to the point of obvious visibility. It is a bilateral, low-amplitude postural tremor of relatively high frequency (about 7 Hz). Drug-induced tremor, which is also generally of very low amplitude, probably has a similar mechanism; it is most commonly induced by antiepileptic drugs, lithium, valproic acid, and cyclosporine A (1).
Physiological tremor.
Drug-induced tremor is generally of very low amplitude and is most commonly induced by antiepileptic drugs, lithium, valproic acid, and cyclosporine A.
Orthostatic tremor—The main manifestation of orthostatic tremor is an unsteady stance. The patient reports a feeling of uneasiness while standing but is generally unaware of the tremor in the lower limb muscles, which is easily demonstrated by electromyography. The latter reveals rhythmic muscular activity at 13–18 Hz, which is the highest frequency of all known tremor syndromes (1). The phenomenon is rare, and the studies that have been published on this subject are few and easily summarized. The sole randomized, controlled trial of treatment that has been performed to date indicates that gabapentin can lessen subjective symptoms by 50–75% while lowering the tremor amplitude by 79% (e13). The effect was still demonstrable 19 months after the initiation of treatment. Clonazepam (0.5–6.0 mg/day) is recommended as an alternative (e14), but there has been no systematic study of this form of treatment.
Dystonic tremor—Tremor of the limbs or head often arises in the setting of dystonia. Its frequency and amplitude are often irregular; thus, this form of tremor is not really a tremor according to the strict definition of the term. Patients with dystonic head tremor typically have an abnormal head posture (torticollis) or other dystonic manifestations, e.g., writer’s cramp, that can serve as a clue to the diagnosis. Dystonic tremor can also closely resemble unilateral rest tremor of a hand and thus be mistaken for early Parkinson’s disease. The differentiation of these two entities was recently discussed in the literature (e15, e16).
Dystonic tremor.
Tremor of the limbs or head often arises in the setting of dystonia. Its frequency and amplitude are often irregular.
Holmes tremor—This type of tremor is due to midbrain damage and consists of both rest and postural tremor, at a low frequency, generally arising a few months after the acute event. It generally has a wide amplitude and makes the affected limb(s) practically unusable. Because Holmes tremor is very rare, the current recommendation for treatment with high-dose levodopa (up to 750 mg per day) is based on single case reports, as higher-level evidence is unavailable (e17).
Surgical treatment.
Prerequisites for the surgical treatment of the tremor are that the tremor cannot be ameliorated by drugs, that the patient feels substantially impaired by the tremor, and that there are no contraindications to a surgery.
The surgical treatment of tremor
Prerequisites for the surgical treatment of tremor are that the tremor cannot be ameliorated by drugs, that the patient feels substantially impaired by tremor, and that there are no contraindications to surgery, e.g., a coagulopathy or dementia with the risk of postoperative delirium (Table 1). Neurosurgical procedures for the abolition of tremor involve either the creation of a small lesion at a target site deep within the brain (usually by thermocoagulation) (Figure 3) or else the implantation of a system for chronic continuous electrical stimulation to modulate the activity of neurons at and around a target site deep in the brain (deep brain stimulation [DBS], neuro-modulation). The stimulating electrodes for DBS are implanted at sites that are considered to be nodes in the brain’s tremor network. They remain in the brain permanently and are connected to a pulse generator that is implanted subcutaneously (usually just below the clavicle). Such systems have now been in use for two decades and have reached a high degree of technical sophistication; in most cases, they can suppress tremor completely for many years. Pulse generators must be replaced every few years when they run out of charge. Recently, rechargeable pulse generators have come into use that can remain in place for about nine years before they, too, need to be replaced. The complication rate of DBS procedures is 1–2% (20). The major surgical risk is that of brain hemorrhage. Voges et al., reporting on an overall surgical cohort of more than 1000 patients (20), documented asymptomatic brain hemorrhages in 1.6% and symptomatic ones in 1.3%. In most cases of symptomatic brain hemorrhage, the clinical manifestations regressed fully within 30 days. Infection is also a potential complication. Multiple cerebral target structures are available for DBS against tremor; the preferred target structure to be aimed at in each individual case depends on the type of tremor that is present (Table 2).
Table 1. The treatment of tremor (evidence classes in parentheses).
| 1st choice | Additional (optional) | 2nd choice | 3rd choice | Surgical treatment in case of inadequate improvement from drug treatment alone | |
|---|---|---|---|---|---|
| Tremor in Parkinson’s disease | dopamine agonists (Ia) | levodopa (Ia), anticholinergic drugs (III) (biperidene, bornaprine), budipine (II) | clozapine, propranolol | deep brain stimulation (preferably in the subthalamic nucleus; in exceptional cases, nucleus ventrointermedius [VIM] of the thalamus) | |
| Essential tremor | propranolol (Ia), primidone (Ia), these two combined | gabapentin (I), topiramate (I), atenolol, sotalol | clonazepam, clozapine | deep brain stimulation in the VIM nucleus of the thalamus | |
| Cerebellar tremor | low chance of success with carbamazepine, ondansetron | experimental: 4-aminopyridine (approved under the name Fampyra for gait ataxia in MS) | of possible benefit for selected patients: deep brain stimulation in the VIM nucleus of the thalamus | ||
| Dystonic tremor | local injection ofbotulinum toxin | as in the treatment of dystonia: trihexyphenidyl, biperidene, benzodiazepines | |||
| Intensifiedphysiological tremor | diagnosis and treatment of cause, e.g., hyperthyroidism, vitamin B12 deficiency, drugs | ||||
| Orthostatic tremor | gabapentin (Ib) | clonazepam, primidone (II) |
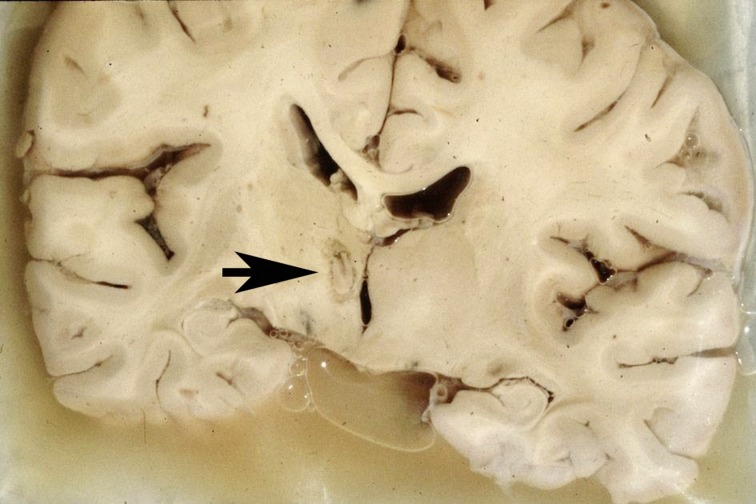
Figure 3.
An anatomical specimen in which the left thalamus bears a lesion in the nucleus ventrointermedius (VIM), created by thermocoagulation (thalamotomy).
Table 2. Indications and targets for the stereotactic treatment of tremor.
| Cause of tremor | Target structure | Lesion-making surgery | Deep brain stimulation |
|---|---|---|---|
| Parkinson’s disease (tremor-dominant) |
VIM/STN | thalamotomy radiosurgery |
VIM stimulation STN stimulation |
| Parkinson’s disease (with both tremor and rigidity/akinesia) |
STN pallidum |
subthalamotomy pallidotomy radiosurgery |
STN stimulation GPI stimulation |
| Essential tremor | VIM zona incerta STN |
thalamotomy subthalamotomy radiosurgery |
VIM stimulation STN stimulation |
| Tremor in multiple sclerosis | VIM | thalamotomy radiosurgery |
VIM stimulation |
| Holmes tremor | VIM | – | VIM stimulation |
| Orthostatic tremor | VIM | – | VIM stimulation |
| Dystonic tremor | VIM, pallidum | thalamotomy | GPI stimulation VIM stimulation possibly, combination of targets |
STN, subthalamic nucleus; VIM, nucleus ventrointermedius of the thalamus; GPI, globus pallidus, pars interna
Thalamotomy
Thalamotomy (local cerebral tissue destruction by high-frequency thermocoagulation at a target site within the thalamus) for the treatment of Parkinson’s disease, and of parkinsonian tremor in particular, was first advocated by Hassler and Riechert in Germany (21). Thousands of thalamotomies were performed in the 1960s, with small anatomic variations among the target sites used (Figure 3). Interestingly, nearly all types of tremor, regardless of their cause and regardless of the type of brain damage underlying them (Table 2), were found to respond favorably to thalamic lesion-making. The same target region was chosen later on for the implantation of DBS electrodes, which is, today, a much more common procedure than thalamotomy. Rest tremor in Parkinson’s disease is completely suppressed by thalamotomy in 70–80% of patients so treated (22), and the same is true of thalamotomy for essential tremor (23, 24). Cerebellar tremor due to multiple sclerosis responds well to thalamotomy for 2 to 3 years after the procedure but then, unfortunately, tends to recur, so that the tremor is still suppressed in only one-third of patients. This fact is explained by the progression of the underlying disease and by the limb dysmetria that often accompanies tremor. A therapeutic lesion in the nucleus ventrointermedius of the thalamus (VIM) for the treatment of parkinsonian tremor can also be created by radiosurgery, i.e., single-shot, stereotactically focused gamma-irradiation (25– 27).
Thalamotomy.
Rest tremor in Parkinson’s disease is completely suppressed by thalamotomy in 70–80% of patients so treated, and the same is true of thalamotomy for essential tremor.
Thalamic stimulation (VIM stimulation)
Deep brain stimulation (DBS) of the nucleus ventrointermedius (VIM) of the thalamus at a frequency of 100–180 Hz is now a well-established treatment for essential tremor (28– 30) and for selected cases of parkinsonian tremor (class I evidence).
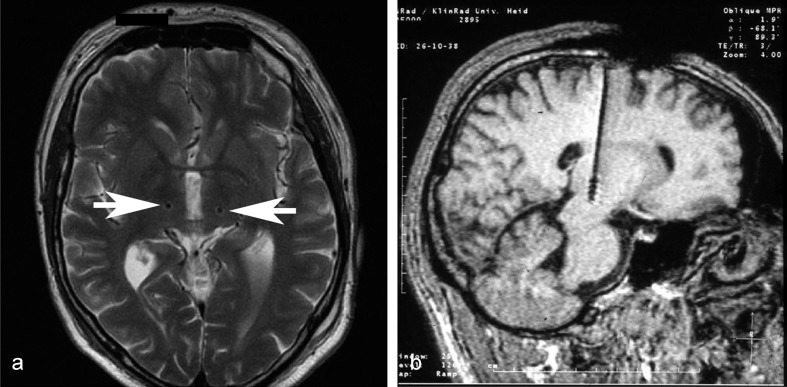
The operation has the same initial steps as a thalamotomy. The stereotactic targets are localized anatomically on the basis of preoperatively obtained neuroimaging studies and then verified intraoperatively with microelectrode recording of intrinsic neuronal activity (optional) and with test stimulation in the awake patient before implantation of the permanent, quadripolar electrode (Figure 4). VIM stimulation primarily improves tremor in the contralateral limbs but has been found to have a less marked effect on the ipsilateral ones as well. In essential tremor, the tremor in the limbs responds well to stimulation, while head tremor and vocal tremor respond less well, and only to bilateral stimulation (31). Moreover, as essential tremor progresses, some patients can develop cerebellar manifestations that cannot be ameliorated by stimulation. It remains less than fully clear whether the deterioration of clinical efficacy experienced by many patients after years of stimulation is due to progression of the underlying disease or to the development of tolerance (32). Patients who do not undergo DBS seem to experience disease progression to the same extent as DBS patients, and this fact makes tolerance an unlikely explanation (33). Some patients in whom tremor becomes more intense after initially efficacious treatment have electrodes that were implanted at suboptimal locations (34). Patients with Parkinson’s disease should only undergo VIM stimulation if their disease manifestations are longstanding and strongly tremor-dominant. Those who have had Parkinson’s disease for a relatively short period of time can be expected to experience progression of the disease to include rigidity and bradykinesia and should therefore undergo deep brain stimulation in the subthalamic nucleus (STN), rather than the VIM. STN stimulation has an excellent effect against tremor (35) while also improving rigidity and bradykinesia.
Figure 4.
a) Axial MRI of the brain showing a DBS electrode. b) MRI with electrodes. Under certain conditions, MRI may be performed despite the presence of implanted DBS electrodes. This enables precise documentation of the position of the electrodes. The images show two stimulating electrodes in the VIM nucleus of the left thalamus and a sagittal view of an electrode in the right thalamus. The true diameter of each electrode is 1.3 mm; the electrodes seem much larger because of the metal-induced MRI artefact.
Thalamic stimulation.
Deep brain stimulation (DBS) of the nucleus ventrointermedius (VIM) of the thalamus at a frequency of 100–180 Hz is now a well-established treatment for essential tremor and for selected cases of parkinsonian tremor.
Tremor in multiple sclerosis is often improved only to a small extent, and/or for a short time, by VIM stimulation, because of progression of the disease. In patients with multiple sclerosis who are being considered for a DBS procedure, postural and intention tremor must be distinguished from cerebellar ataxia (dysmetria), which will be unaffected by stimulation. Thalamotomy remains a viable alternative to thalamic DBS for tremor in multiple sclerosis, because thalamotomy is capable of inactivating larger regions than electrical stimulation.
Only single case studies or small case series are available for DBS in the treatment of other, rarer types of tremor (Holmes tremor, dystonic tremor, orthostatic tremor), and no conclusive judgments can be made at present (36– 39). The opportunity that DBS affords for test stimulation before the definitive implantation of a permanent system makes it possible to employ DBS for a wider spectrum of indications than lesion-making.
Tremor due to multiple sclerosis.
Thalamotomy remains a viable alternative to thalamic DBS for tremor in multiple sclerosis, because thalamotomy is capable of inactivating larger regions than electrical stimulation.
Thalamotomy compared to deep brain stimulation
Schuurman et al. conducted a randomized trial of thalamotomy versus deep brain stimulation in the VIM nucleus in 86 patients suffering from tremor of various causes (Parkinson’s disease, essential tremor, multiple sclerosis) and determined that the two methods suppressed tremor to a comparable extent (30). Thalamotomy suppressed tremor completely or nearly completely in 27 of 34 patients, while DBS did so in 30 of 33 patients. However, the patients who had undergone DBS had a significantly better functional status with respect to activities of daily living (p = 0.01). This was largely due to the significantly higher frequency of side effects in the thalamotomy group, including dysarthria, gait disturbances, and cognitive deficits.
For these reasons, deep brain stimulation is now usually preferred to thalamotomy, particularly when a bilateral procedure is indicated. The authors have published their own patients’ long-term results (40): DBS and thermocoagulation suppressed tremor to a comparable extent. In patients with tremor due to Parkinson’s disease, tremor suppression persisted over the years. In those with essential tremor and multiple sclerosis, there was a significant reduction of the therapeutic effect after five years of follow-up.
Thalamotomy versus deep brain stimulation.
The patients who had undergone DBS had a significantly better functional status with respect to activities of daily living. This was due to the significantly higher frequency of side effects in the thalamotomy group.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 22 June 2014.
The CME unit “Structured Management of Otitis Media” (Issue 9/2014) can be accessed until 25 May 2014.
The CME unit “The Treatment of Type 2 Diabetes” (Issue 5/2014) can be accessed until 27 April 2014.
The CME unit “Rhegmatogenous Retinal Detachment” (Issue 1-2/2014) can be accessed until 30 March 2014.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following constellations of clinical findings is typical of tremor in patients with Parkinson’s disease?
bilateral postural tremor
unilateral rest tremor and diminished ipsilateral arm swing while walking
severe unilateral tremor while holding a cup or glass
tremor that only appears when the patient writes
postural tremor of both hands and ataxic gait
Question 2
A 25-year-old man has a mild postural tremor of both hands that improves when he drinks alcohol. His mother had the same condition. What can you advise him?
He should definitely be evaluated for possible early Parkinson’s disease.
He must get treatment now, as otherwise the condition could worsen.
If treatment is indicated, propranolol or primidone could be given.
Relaxation exercises and physiotherapy are effective treatment options.
Genetic testing is needed to confirm the diagnosis of essential tremor.
Question 3
A man who received the diagnosis of multiple sclerosis two years ago presents to you with the new onset of tremor. What constellation of clinical findings is typical of tremor due to multiple sclerosis?
rest tremor, only occasionally observable when the patient is excited
a tremor that appears sometimes on the left side, sometimes on the right
a tremor that is only present in the morning
a swaying, broad-based gait and an intention tremor
a postural tremor that is easily suppressed by voluntary effort
Question 4
A 55-year-old man with essential tremor says that he can no longer feed himself because of tremor, can dress himself only with great difficulty, and has not had legible handwriting for many years. Drug treatment as recommended in the relevant clinical guidelines brings only slight improvement. What can you advise the patient about the option of surgical treatment?
Deep brain stimulation (DBS) might help but is not available in Germany.
DBS is an experimental technique that is only performed in clinical trials.
DBS is indicated only to treat Parkinson’s disease and plays no role in the treatment of essential tremor.
DBS has a high chance of success in this situation; it is now established as a standard treatment for essential tremor.
DBS is no more effective than pharmacotherapy for this indication.
Question 5
What information is most important for the diagnostic classification of a tremor syndrome?
the clinical findings
brain magnetic resonance imaging (MRI) with fine cerebellar sections
nuclear-medical visualization of brain perfusion
ultrasonography of the basal ganglia
measurement of serum drug levels
Question 6
What findings indicate that tremor may be psychogenic?
no evidence of essential tremor or Parkinson’s disease on brain MRI
a longstanding marital conflict
a tremor of inconstant location that diminishes on distraction and is found to be irregular on tremor analysis
a clearly identifiable underlying psychological conflict
remission after psychotherapy
Question 7
When can tremor be treated surgically?
When the patient is unwilling to take drugs to treat tremor.
When the patient is under 50 years old.
When the tremor cannot be adequately suppressed by drugs and there is no contraindication to surgery.
When the patient is willing to see a neurosurgeon once a week so that brain stimulation can be performed.
When the patient is willing to assume the cost of weekly battery changes.
Question 8
What must be borne in mind with respect to drug treatment for various tremor syndromes?
That the treatment is based on the clinical findings and not on the underlying disease causing tremor.
That causally directed treatment is generally possible only for drug-induced tremor or tremor due to a metabolic disturbance.
That parkinsonian tremor responds best to anticholinergic drugs and does not respond at all to the classic dopamine preparations.
That the cerebellar tremor of multiple sclerosis is treated in exactly the same way as essential tremor.
That essential tremor is usually medically intractable.
Question 9
What drugs can induce tremor?
lithium, valproic acid, cyclosporine A
carbamazepine, propranolol, seroxate
aspirin, diclofenac, paracetamol
penicillin, erythromycin, cephalosporin
antilipid drugs, antidiabetic drugs
Question 10
What is the drug, or drug class, of first choice for the treatment of parkinsonian tremor?
dopaminergic drugs
propanolol
primidone
gabapentin
ondansetron
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Bötzel has received reimbursement of travel and meeting participation expenses, honoraria for the preparation of scientific presentations, and financial support for a research project that he initiated from Medtronic.
Prof. Tronnier has received honoraria for consulting activities, reimbursement of meeting participation fees and of travel and accommodation expenses, and payment for preparing scientific presentations at meetings from Medtronic. Medtronic and St. Jude Medical made funds available to him for a research project that he initiated.
Prof. Gasser states that he has no conflict of interest.
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3 M):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 2.Schulz JB, Gasser T. Parkinson-Syndrome. In: Brandt T, Diener HC, Gerloff C, editors. Therapie und Verlauf neurologischer Erkrankungen. Stuttgart: Kohlhammer; 2012. pp. p. 943–988. [Google Scholar]
- 3.Ebersbach G, Stoeck M, Müller J, Wissel J, Poewe W. Dopa-responsiver Haltetremor bei idiopathischer Parkinson Erkrankung. Aktuelle Neurologie. 2000;27:327–331. [Google Scholar]
- 4.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003735. CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichmann H. Budipine in Parkinson’s tremor. J Neurol Sci. 2006;248:53–55. doi: 10.1016/j.jns.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Stefansson H, Steinberg S, Petursson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet. 2009;41:277–279. doi: 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thier S, Lorenz D, Nothnagel M, et al. LINGO1 polymorphisms are associated with essential tremor in Europeans. Mov Disord. 2010;25:717–723. doi: 10.1002/mds.22887. [DOI] [PubMed] [Google Scholar]
- 8.Tan EK, Teo YY, Prakash KM, et al. LINGO1 variant increases risk of familial essential tremor. Neurology. 2009;73:1161–1162. doi: 10.1212/WNL.0b013e3181bacfc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweet RD, Blumberg J, Lee JE, Mc Dowell FH. Propranolol treatment of essential tremor. Neurology. 1974;24:64–67. doi: 10.1212/wnl.24.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Winkler GF, Young RR. Efficacy of chronic propranolol therapy in action tremors of the familial, senile or essential varieties. N Engl J Med. 1974;290:984–988. doi: 10.1056/NEJM197405022901802. [DOI] [PubMed] [Google Scholar]
- 11.Findley LJ, Cleeves L, Calzetti S. Primidone in essential tremor of the hands and head: a double blind controlled clinical study. J Neurol Neurosurg Psychiatry. 1985;48:911–915. doi: 10.1136/jnnp.48.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66:672–677. doi: 10.1212/01.wnl.0000200779.03748.0f. [DOI] [PubMed] [Google Scholar]
- 13.Schniepp R, Jakl V, Wuehr M, et al. Treatment with 4-aminopyridine improves upper limb tremor of a patient with multiple sclerosis: a video case report. Mult Scler. 2012 doi: 10.1177/1352458512461394. [DOI] [PubMed] [Google Scholar]
- 14.Bötzel K, Steude U. Indikationen der operativen Behandlung des Tremors. Dtsch Med Wochenschr. 1999;124:287–290. doi: 10.1055/s-2007-1024297. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery EB, Jr., Baker KB, Kinkel RP, Barnett G. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625–628. doi: 10.1212/wnl.53.3.625. [DOI] [PubMed] [Google Scholar]
- 16.Nowak DA, Fink GR. Psychogenic movement disorders: aetiology, phenomenology, neuroanatomical correlates and therapeutic approaches. Neuroimage. 2009;47:1015–1025. doi: 10.1016/j.neuroimage.2009.04.082. [DOI] [PubMed] [Google Scholar]
- 17.McKeon A, Ahlskog JE, Bower JH, Josephs KA, Matsumoto JY. Psychogenic tremor: long-term prognosis in patients with electrophysiologically confirmed disease. Mov Disord. 2009;24:72–76. doi: 10.1002/mds.22301. [DOI] [PubMed] [Google Scholar]
- 18.McAuley J, Rothwell J. Identification of psychogenic, dystonic, and other organic tremors by a coherence entrainment test. Mov Disord. 2004;19:253–267. doi: 10.1002/mds.10707. [DOI] [PubMed] [Google Scholar]
- 19.Dafotakis M, Ameli M, Vitinius F, et al. Der Einsatz der transkraniellen Magnetstimulation beim psychogenen Tremor - eine Pilotstudie. Fortschr Neurol Psychiatr. 2011;79:226–233. doi: 10.1055/s-0029-1246094. [DOI] [PubMed] [Google Scholar]
- 20.Voges J, Hilker R, Bötzel K, et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord. 2007;22:1486–1489. doi: 10.1002/mds.21481. [DOI] [PubMed] [Google Scholar]
- 21.Hassler T, Riechert T. Indikationen und Lokalisationsmethode der gezielten Hirnoperationen. Der Nervenarzt. 1954;25:441–447. [PubMed] [Google Scholar]
- 22.Jankovic J, Cardoso F, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for parkinsonian, essential, and other types of tremor. Neurosurgery. 1995;37:263–270. doi: 10.1227/00006123-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg. 1986;65:296–302. doi: 10.3171/jns.1986.65.3.0296. [DOI] [PubMed] [Google Scholar]
- 24.Shahzadi S, Tasker RR, Lozano A. Thalamotomy for essential and cerebellar tremor. Stereotact Funct Neurosurg. 1995;65:11–17. doi: 10.1159/000098890. [DOI] [PubMed] [Google Scholar]
- 25.Duma CM, Jacques DB, Kopyov OV, Mark RJ, Copcutt B, Farokhi HK. Gamma knife radiosurgery for thalamotomy in parkinsonian tremor: a five-year experience. J Neurosurg. 1998;88:1044–1049. doi: 10.3171/jns.1998.88.6.1044. [DOI] [PubMed] [Google Scholar]
- 26.Young RF, Jacques S, Mark R, et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg. 2000;93(Suppl 3):128–135. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 27.Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery. 2012;70:526–535. doi: 10.1227/NEU.0b013e3182350893. discussion 35-6. [DOI] [PubMed] [Google Scholar]
- 28.Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 29.Bittar RG, Hyam J, Nandi D, et al. Thalamotomy versus thalamic stimulation for multiple sclerosis tremor. J Clin Neurosci. 2005;12:638–642. doi: 10.1016/j.jocn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 31.Sixel-Döring F, Benecke R, Fogel W, et al. Tiefe Hirnstimulation bei essenziellem Tremor. Empfehlungen der Deutschen Arbeitsgemeinschaft Tiefe Hirnstimulation. Nervenarzt. 2009;80:662–665. doi: 10.1007/s00115-009-2703-7. [DOI] [PubMed] [Google Scholar]
- 32.Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol. 2011;258:434–439. doi: 10.1007/s00415-010-5773-3. [DOI] [PubMed] [Google Scholar]
- 33.Favilla CG, Ullman D, Wagle Shukla A, et al. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain. 2012;135:1455–1462. doi: 10.1093/brain/aws026. [DOI] [PubMed] [Google Scholar]
- 34.Pilitsis JG, Metman LV, Toleikis JR, Hughes LE, Sani SB, Bakay RA. Factors involved in long-term efficacy of deep brain stimulation of the thalamus for essential tremor. J Neurosurg. 2008;109:640–646. doi: 10.3171/JNS/2008/109/10/0640. [DOI] [PubMed] [Google Scholar]
- 35.Krack P, Benazzouz A, Pollak P, et al. Treatment of tremor in Parkinson’s disease by subthalamic nucleus stimulation. Mov Disord. 1998;13:907–914. doi: 10.1002/mds.870130608. [DOI] [PubMed] [Google Scholar]
- 36.Guridi J, Rodriguez-Oroz MC, Arbizu J, et al. Successful thalamic deep brain stimulation for orthostatic tremor. Mov Disord. 2008;23:1808–1811. doi: 10.1002/mds.22001. [DOI] [PubMed] [Google Scholar]
- 37.Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23:2357–2362. doi: 10.1002/mds.22271. [DOI] [PubMed] [Google Scholar]
- 38.Diederich NJ, Verhagen Metman L, Bakay RA, Alesch F. Ventral intermediate thalamic stimulation in complex tremor syndromes. Stereotact Funct Neurosurg. 2008;86:167–172. doi: 10.1159/000120429. [DOI] [PubMed] [Google Scholar]
- 39.Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson CE, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88:98–104. doi: 10.1159/000289354. [DOI] [PubMed] [Google Scholar]
- 40.Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23:1146–1153. doi: 10.1002/mds.22059. [DOI] [PubMed] [Google Scholar]
- e1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- e2.de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- e3.Tatsch K, Poepperl G. Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: an update. J Nucl Med. 2013;54:1331–1338. doi: 10.2967/jnumed.112.105379. [DOI] [PubMed] [Google Scholar]
- e4.Louis ED, Levy G, Cote LJ, Mejia H, Fahn S, Marder K. Clinical correlates of action tremor in Parkinson disease. Arch Neurol. 2001;58:1630–1634. doi: 10.1001/archneur.58.10.1630. [DOI] [PubMed] [Google Scholar]
- e5.Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord. 2000;15:84–94. doi: 10.1002/1531-8257(200001)15:1<84::aid-mds1014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- e6.Spieker S, Breit S, Klockgether T, Dichgans J. Tremorlytic activity of budipine in Parkinson’s disease. J Neural Transm Suppl. 1999;56:165–172. doi: 10.1007/978-3-7091-6360-3_10. [DOI] [PubMed] [Google Scholar]
- e7.Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture. 2011;34:65–70. doi: 10.1016/j.gaitpost.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123(Pt 8):1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- e9.Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. 2007;29:208–212. doi: 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Voller B, Lines E, McCrossin G, et al. Alcohol challenge and sensitivity to change of the essential tremor rating assessment scale. Mov Disord. 2013 doi: 10.1002/mds.25667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Schneider SA, Deuschl G. The Treatment of Tremor. Neurotherapeutics. 2014;11:128–138. doi: 10.1007/s13311-013-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Raethjen J, Kopper F, Govindan RB, Volkmann J, Deuschl G. Two different pathogenetic mechanisms in psychogenic tremor. Neurology. 2004;63:812–815. doi: 10.1212/01.wnl.0000137012.35029.6b. [DOI] [PubMed] [Google Scholar]
- e13.Rodrigues JP, Edwards DJ, Walters SE, et al. Blinded placebo crossover study ofgabapentin in primary orthostatic tremor. Mov Disord. 2006;21:900–905. doi: 10.1002/mds.20830. [DOI] [PubMed] [Google Scholar]
- e14.Jones L, Bain PG. Orthostatic tremor. Pract Neurol. 2011;11:240–243. doi: 10.1136/practneurol-2011-000022. [DOI] [PubMed] [Google Scholar]
- e15.Erro R, Quinn NP, Schneider SA, Bhatia KP. Does rest tremor exclude the diagnosis of adult-onset primary dystonia? J Neurol Neurosurg Psychiatry. 2013;84:708. doi: 10.1136/jnnp-2012-304779. [DOI] [PubMed] [Google Scholar]
- e16.Schneider SA, Edwards MJ, Mir P, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007;22:2210–2215. doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
- e17.Velez M, Cosentino C, Torres L. Levodopa-responsive rubral (Holmes’) tremor. Mov Disord. 2002;17:741–742. doi: 10.1002/mds.10224. [DOI] [PubMed] [Google Scholar]