Creativity is defined as producing ostensibly out of nothing, something of Beauty, Order or Significance, Peter Medawar (1915–1987)
Creativity is just connecting the dots, Steve Jobs (1955–2011)
Atherosclerosis, obesity, and metabolic syndrome are closely linked and constitute, arguably, the most menacing three conditions to modern society. Thus urgent, concerted efforts at several levels, utilising modern tools, are required to tackle them. Translational research is a rapidly expanding branch of science, dedicated to rapid delivery of discoveries from the bench to the bedside. The growing importance of this specialty in our field is evidenced by the establishment of the International Society of Cardiovascular Translational Research and its dedicated Journal by Nabil Dib and colleagues.1
The fundamental discovery of the relationship between, lipids, cholesterol and atherosclerosis was made in the 1960s.2–4 This led to the discovery of lipid lowering drugs, including statins, which constitute one of the blockbuster drugs of modern times. In spite of that, the toll of the three conditions mentioned above continued almost unabated, particularly in patients with familial hypercholesterolemia (FH) in whom LDL apheresis and/or liver transplantation are needed in many instances to achieve adequate reduction of LDL-cholesterol levels.5,6 The landmark discovery of the role of the LDL receptors in lipid clearance by Goldstein and Brown (Figures 1 and 2)7,8 earned them the Nobel Prize, and opened the door to further translational research which led to several further discoveries, including that of Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9). In addition to establishing the role of the LDL receptors in clearing LDL-C from the circulation, and that FH is due to a mutation in the LDL receptor gene, Brown and Goldstein described three new cellular processes, including receptor-mediated endocytosis (paving the way for further research into the mechanisms of vesicle transport and cellular trafficking; work for which Rothman, Schekman and Sudhof have recently been awarded the Nobel Prize in Medicine), receptor recycling, and feedback regulation of receptors, which led to discovery of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase and statins.
Figure 1.
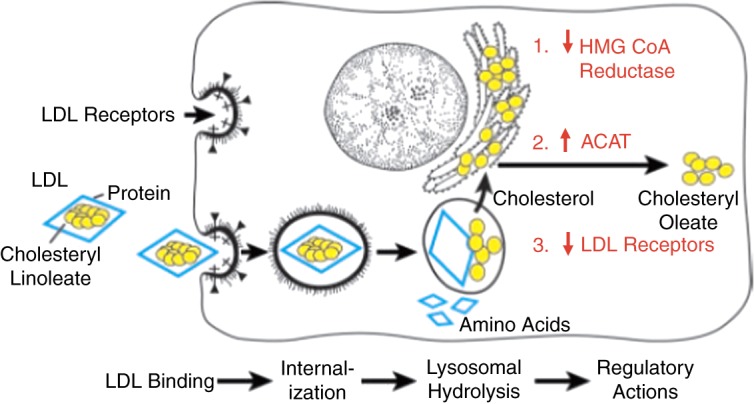
LDL receptor pathway of mammalian cells (from Goldstein J and Brown S. The LDL Receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438).
Figure 2.

Joseph L. Goldstein and Michael S. Brown.
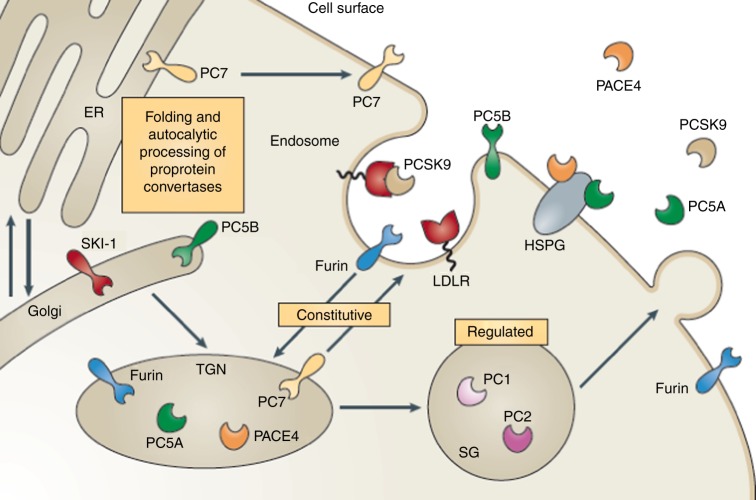
Several years later, the discovery of a major regulatory pathway controlling the number and function of the LDL receptors was made by a large number of researchers working in different fields of translational research. This was started by the massive contribution of Nabil Seidah's group from Montreal (Figure 3). While working on the chemistry and functions of a family of proteins termed proprotein convertases, they identified PCSK9 encoded by a gene on chromosome 1 (Figure 4). Collaborative work with a French group showed that a gain of function mutation in that gene was responsible for FH in a French family.9 This was followed by similar findings from Oslo.10 In contrast, findings from the Dallas Heart Study, showed that loss of function mutations in PCSK9 gene in a subset of Afro-Americans were associated with very low cholesterol levels and markedly reduced incidence of cardiovascular disease.11 The mechanisms involved are schematically shown in Figure 5.
Figure 3.

Nabil Seidah.
Figure 4.
Subcellular localization of proprotein convertases. Upon exiting the endoplasmic reticulum (ER), most of the basic amino acid-specific proprotein convertases traverse the Golgi apparatus towards the trans-Golgi network (TGN). The activated membrane-bound subtilisin kexin isozyme 1 (SKI1) is mostly concentrated in the cis- and medial-Golgi, from where it is then sent to lysosomes for degradation, and does not normally reach the cell surface. Proprotein convertase subtilisin kexin 9 (PCSK9) is secreted from the TGN directly into the medium as an enzymatically inactive non-covalent complex of the protease and its prosegment. Upon binding to the low-density lipoprotein receptor (LDLR) at the cell surface, the PCSK9–LDLR complex is internalized into endosomes and then sent to lysosomes for degradation. (from Seidah N and Prat A. The biology and therapeutic targeting of proprotein convertases. Nat Rev Drug Discov. 2012 May;11(5):367–83).
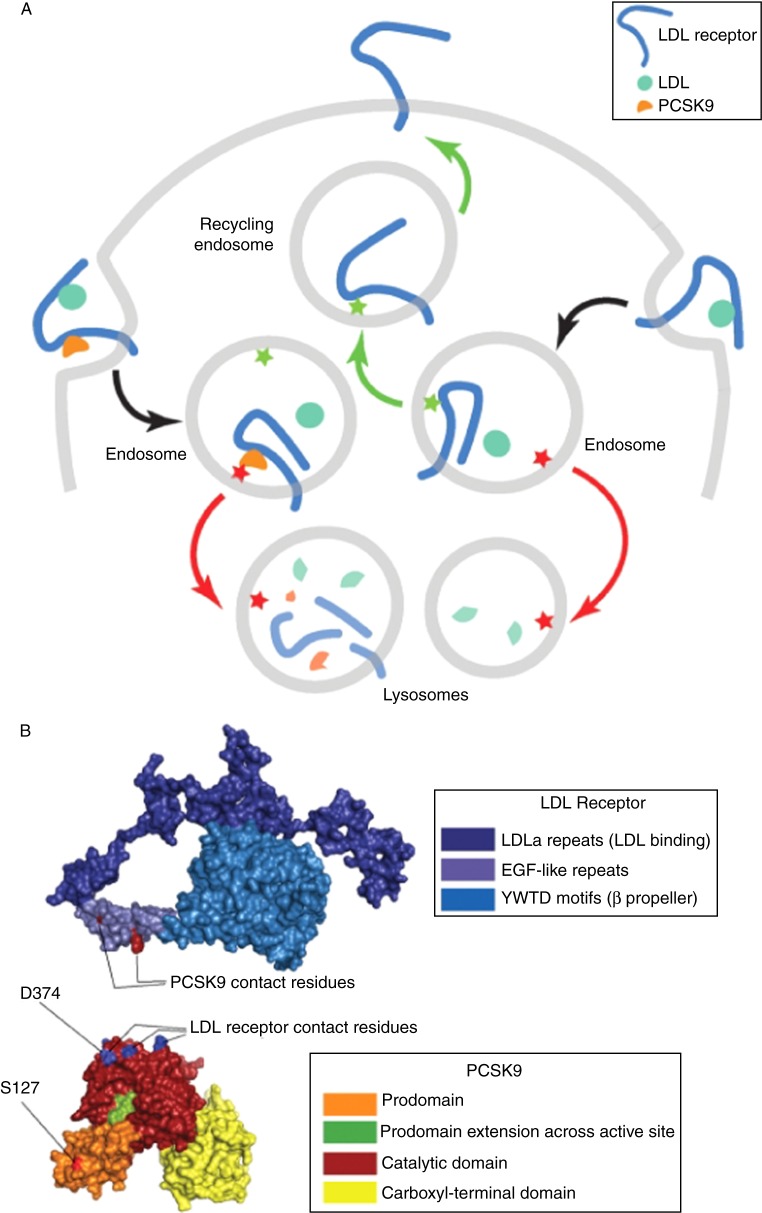
Figure 5.
A: Model of PCSK9-mediated sorting of LDL receptors to lysosomes. The EGFa domain of the LDL receptor is required for proper sorting of the LDL receptor back to the cell surface. The EGFa domain may contain a sorting signal that interacts with an endosomal protein (green star), directing the LDL receptor back to the cell surface on recycling endosomes (green arrows). Binding of PCSK9 might interfere with that signal, preventing the LDL receptor from returning to the cell surface. Alternatively, PCSK9 could contain a distinct sorting signal (red star) that results in the sorting of the PCSK9-LDL receptor complex (red arrows) to lysosomes. The gain-of-function mutation involving S127 of PCSK9 may enhance the sorting of the PCSK9-LDL receptor complex to lysosomes. B: The structure of the LDL receptor and PCSK9 at endosomal pH. The LDL receptor is folded back upon itself at low pH; however, the face of the EGFa domain that binds PCSK9 is exposed. The LDL receptor-binding site on PCSK9 is at the apex of a roughly triangular structure formed by the tripartite domain structure of PCSK9. The D374 residue that is altered in gain-of-function PCSK mutants is located within the apical LDL receptor-binding site, whereas the S127 residue is quite distant from the binding interface. S127 mutations do not affect binding of PCSK9 to the LDL receptor. Gain-of-function mutations affecting residue 127 may reduce LDL receptors by enhancing the sorting of LDL receptors to lysosomes, rather than by affecting the strength of PCSK9-LDL receptor interactions. (figure and accompanying legend from Peterson A, Fong L and Young S. PCSK9 function and Physiology. J Lipid Res. 2008 June; 49(6): 1152–1156).
The rush to the clinic
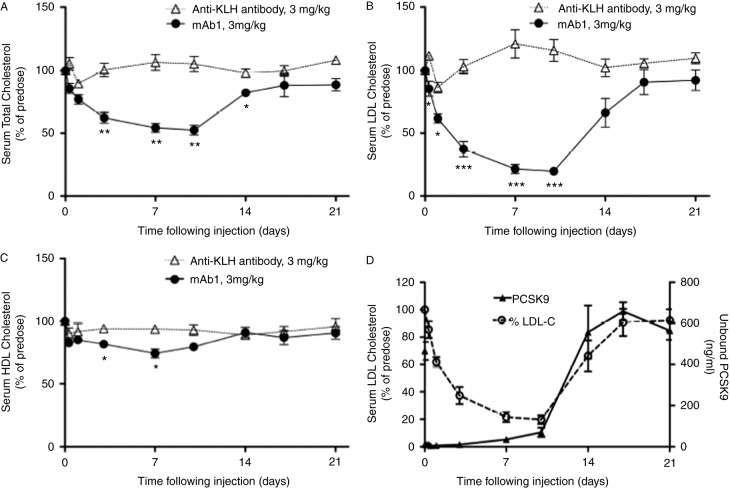
Not surprisingly, the above mentioned exciting findings was followed by the development of several strategies to inhibit the protein or RNA using monoclonal antibodies (Figure 6) or RNA interference drugs respectively,12–19 with extremely promising results in Phase 1 and 2 trials. Inhibition of PCSK9-mediated degradation of LDL receptors by an epidermal growth factor-like repeat A (EGF-A) peptide has also been demonstrated in a mouse model.20
Figure 6.
Total cholesterol, LDL-C and HDL-C levels after injection of a neutralizing monoclonal antibody against PCSK9 compared to a control antibody against keyhole limpet hemocyanin (KLH). Results are expressed as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 vs. anti-KLH control antibody at the same time point, n = 4 per group. (from Chan J. et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9820–5).
The future
The search for other means of inhibiting PCSK9 continues. Increased expression of PCSK9 has recently been shown to be a key mechanism by which human resistin – an adipose tissue-derived adipokine – downregulates hepatocyte LDL receptor expression,21 hence increasing the risk of atherosclerotic cardiovascular disease.22,23 This interesting link opens the door for future research into resistin inhibition as another strategy for inhibiting PCSK9; possibly with added pleotropic effects.24 Such drugs are expected to have a major effect on cardiovascular health, and represent a triumph for translational research. The story stands as a prime example of creativity as defined by the late Sir Peter Medawar, with a methodology that follows Steve Jobs' approach.25
References
- 1.Hall J, Dib N. The path from pre-clinical research to FDA approval. J Cardiovasc Transl Res. 2008;1(2):93–94. doi: 10.1007/s12265-008-9033-1. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J. The problem of elevated blood cholesterol. Am J Public Health Nations Health. 1960;50(3 Pt 2):14–19. doi: 10.2105/ajph.50.3_pt_2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Dawber TR, Friedman GD, Glennon WE, McNamara PM. Risk factors in coronary heart disease. An evaluation of several serum lipids as predictors of coronary heart disease; the framingham study. Ann Intern Med. 1964;61:888–899. doi: 10.7326/0003-4819-61-5-888. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Castelli WP, McNamara PM. Serum lipid fractions and risk of coronary heart disease. The Framingham study. Minn Med. 1969;52:1225–1230. [PubMed] [Google Scholar]
- 5.Hovingh GK, Davidson MH, Kastelein JJP, O'Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34(13):962–971. doi: 10.1093/eurheartj/eht015. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim M, El-Hamamsy I, Barbir M, Yacoub MH. Translational lessons from a case of combined heart and liver transplantation for familial hypercholesterolemia 20 years post-operatively. J Cardiovasc Transl Res. 2012;5(3):351–358. doi: 10.1007/s12265-011-9311-1. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249:5153–5162. [PubMed] [Google Scholar]
- 9.Abifadel M, Varret M, Rabés JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 10.Leren TP. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet. 2004;65:419–422. doi: 10.1111/j.0009-9163.2004.0238.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 12.Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, Tang J, Liu Q, Higbee J, Xia Z, Di Y, Shetterly S, Arimura Z, Salomonis H, Romanow WG, Thibault ST, Zhang R, Cao P, Yang XP, Yu T, Lu M, Retter MW, Kwon G, Henne K, Pan O, Tsai MM, Fuchslocher B, Yang E, Zhou L, Lee KJ, Daris M, Sheng J, Wang Y, Shen WD, Yeh WC, Emery M, Walker NP, Shan B, Schwarz M, Jackson SM. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2009;106:9820–9825. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni YG, Di Marco S, Condra JH, Peterson LB, Wang W, Wang F, Pandit S, Hammond HA, Rosa R, Cummings RT, Wood DD, Liu X, Bottomley MJ, Shen X, Cubbon RM, Wang SP, Johns DG, Volpari C, Hamuro L, Chin J, Huang L, Zhao JZ, Vitelli S, Haytko P, Wisniewski D, Mitnaul LJ, Sparrow CP, Hubbard B, Carfí A, Sitlani A. A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J Lipid Res. 2011;52:78–86. doi: 10.1194/jlr.M011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang H, Chaparro-Riggers J, Strop P, Geng T, Sutton JE, Tsai D, Bai L, Abdiche Y, Dilley J, Yu J, Wu S, Chin SM, Lee NA, Rossi A, Lin JC, Rajpal A, Pons J, Shelton DL. Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates. J Pharmacol Exp Ther. 2012;340:228–236. doi: 10.1124/jpet.111.187419. [DOI] [PubMed] [Google Scholar]
- 15.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Röhl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, Hutabarat RM, Clausen VA, Karsten V, Cehelsky J, Nochur SV, Kotelianski V, Vaishnaw AK, Gollob JA, Simon A. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. doi:10.1016/S0140-6736(13)61914-5 Published Online: 03 October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 18.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 19.Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, Scott R, Wasserman SM, Sabatine MS, for the OSLER Investigators Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-Week results from the open-label study of long-term evaluation against LDL-C (OSLER) Randomized Trial. Circulation. doi: 10.1161/CIRCULATIONAHA.113.007012. doi:10.1161/CIRCULATIONAHA.113.007012 CIRCULATIONAHA.113.007012–(2013) [DOI] [PubMed] [Google Scholar]
- 20.Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Commun. 2008;375:69–73. doi: 10.1016/j.bbrc.2008.07.106. [DOI] [PubMed] [Google Scholar]
- 21.Melone M, Wilsie L, Palyha O, Strack A, Rashid S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J Am Coll Cardiol. 2012;59(19):1697–1705. doi: 10.1016/j.jacc.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 22.Weikert C, Westphal S, Berger K, Dierkes J, Möhlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93(7):2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 23.Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689–702. doi: 10.1038/nrcardio.2012.148. [DOI] [PubMed] [Google Scholar]
- 24.Sahebkar A. Beyond anti-PCSK9 therapies: the potential role of resistin inhibitors. Nat Rev Cardiol. 2014;11(1):12. doi: 10.1038/nrcardio.2013.139-c1. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JL. Juxtapositions in trafalgar square: tip-offs to creativity in art and science. Nat Med. 2013;19:1222–1226. doi: 10.1038/nm.3329. [DOI] [PubMed] [Google Scholar]