Abstract
The recent ability to create detailed 3D models of the atrial and ventricular chambers using CT, MRI and rapid prototyping offers unique opportunities to study the size and shape of the different cardiac chambers both before and following operation for complex cardiac anomalies. We here describe the techniques for creating detailed 3D models of the heart and demonstrate the utility of these techniques in a patient studied after the Mustard operation. This can give important insights into the changes in size and shape of the different chambers and the patterns of blood flow from the pulmonary and systemic veins to the ‘appropriate’ ventricle. This information in turn could be extremely helpful in understanding and optimizing the overall hemodynamic function after the Mustard operation.
Introduction
Currently the preferred surgical option for ‘correction’ transposition of the great arteries TGA is the arterial switch operation.1–4 In spite of that, a significant number of patients with TGA and intact inter-ventricular septum present ‘late’ after the small window of time following birth, when the left ventricle is capable of supporting the systemic circulation. In these patients the two-stage anatomical correction can be considered up to the age of 18 months.5 After that point, the preferred option for these patients is atrial ‘correction’ by the Mustard or Senning operation. Although these operations have been well described,6–8 the exact tailoring of the shape and size of the different components of the neo-atria needs to be refined further to generate the optimal post-operative hemodynamics.
Methodology
CT scans were obtained via a Siemens Definition Flash with a slice thickness of 0.6 mm and a slice increment of 0.3 mm. DICOM were imported into Mimics (Materialise, Leuven, Belgium) for 3D reconstruction of the blood volumes in left and right sides of the heart. The processed files were exported as STL files into 3-matic (Materialise, Leuven, Belgium) to create various images showing the cross sections of interest. The STL files were also used for printing on a 3D printer (Fortus 400mc, Stratasys) to produce a model of the heart post surgery.
Case report
A two-year old male patient presented with history of cyanosis since birth. He was diagnosed as D-TGA with ASD and small PDA. He had balloon atrial septosomy on the 8th day of life. On presentation the oxygen saturation was 76%. Echo showed transposition of the great arteries with the aorta anterior and to the right, good sized inter-atrial communication and deconditioned left ventricle.
A Mustard (atrial switch) operation was performed where the superior and inferior vena cavae where directed to the mitral valve using a trousers-shaped patch8 of autologous pericardium, while the pulmonary veins drained to the tricuspid valve.
The patient had a smooth post-operative course and was discharged from hospital six days after operation. His pre-discharge echo showed good ventricular function with no systemic or pulmonary venous obstruction.
The patient was investigated by MRI and CT, 3 months after operation, and 3D images were developed, with rapid prototyping produced by the methods described above. The resulting 3D physical models are described in the following paragraphs.
Three-dimensional models of the cardiac chambers after Mustard operation
1 – Systemic venous phase
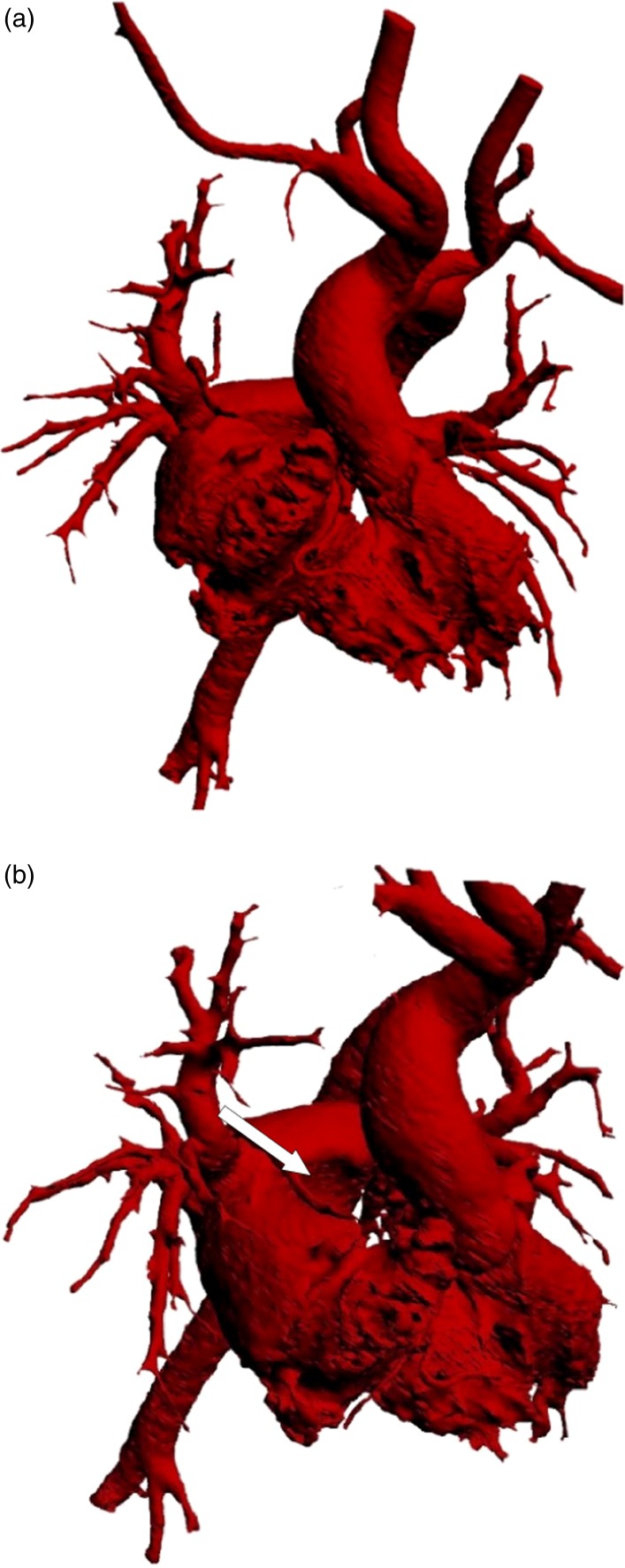
Figure 1 shows a postero-anterior view of the 3D cast showing the systemic venous channels diverting the SVC and IVC to a small supra-mitral chamber, leading to the mitral valve and left ventricle. The size of both channels appears to be adequate, with measurements comparable to the SVC and IVC respectively. In contrast, the supra-mitral chamber is small, and almost certainly inadequate as a reservoir. The SVC channel appears to follow a course almost at a right angle to the SVC, but has adequate dimensions throughout with no evidence of obstruction.
Figure 1.
3-dimensional cast of the systemic venous channels and the left ventricle following the Mustard operation.
Figure 2 is an oblique view showing the asymmetry of the junctions of SVC, situated anteriorly on the roof of the atrium, and the IVC, which is situated on a more posterior plane. This asymmetry has important implications in the normal heart9, as shown in Figure 3.
Figure 2.
Oblique view of the cast of the systemic venous channel, showing spatial asymmetrical connection of the SVC and the IVC with the atrium, which is not maintained by the channels.
Figure 3.
Coloured streamlines computed from magnetic resonance velocity acquisitions in a normal atrium showing the importance of asymmetry of the entrance of the SVC and the IVC in producing a smooth “blending” of the streamlines9.
Unfortunately this asymmetry is not maintained for the entrance of the two channels into the supra-mitral chamber (Figure 2). The hemodynamic effects of the angulation, and more importantly the small supra-mitral chamber, needs to be evaluated further.
2 – Pulmonary venous phase
The un-impeded entrance of the right and left pulmonary veins into the posterior part of the atrial cavity is seen in Figures 4a and 4b. The groove produced by the SVC systemic channel is clearly seen in Figure 4b. The wide communication between the posterior chamber receiving the veins and the anteriorly placed right atrium, with its trabecular part, is visible in Figure 4b.
Figure 4.
(a) Postero-anterior view of the pulmonary venous cast showing the entrance of the right pulmonary veins and the wide communication between the right and left components of the atria. (b) An oblique view showing the groove produced by the SVC channel (white arrow).
3 – Ventricular shapes
The left and right ventricular sizes and shapes are shown in Figure 5, which illustrate the “banana” shaped left ventricle, and the “cup” shaped interventricular septum, which is compressed by the “pear” shaped right ventricle.
Figure 5.
Right and left ventricular shapes (see text).
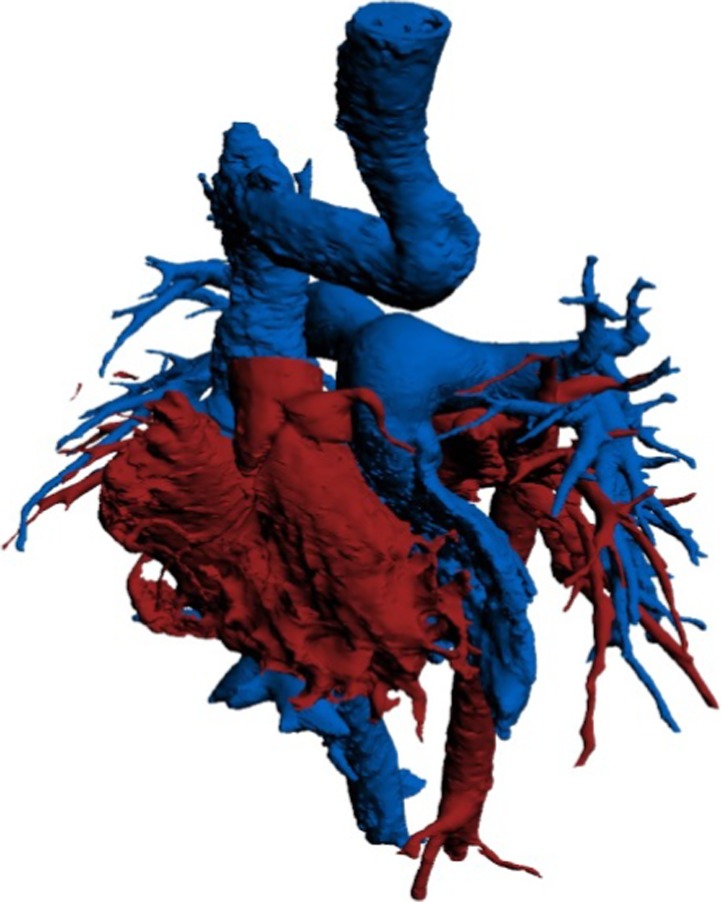
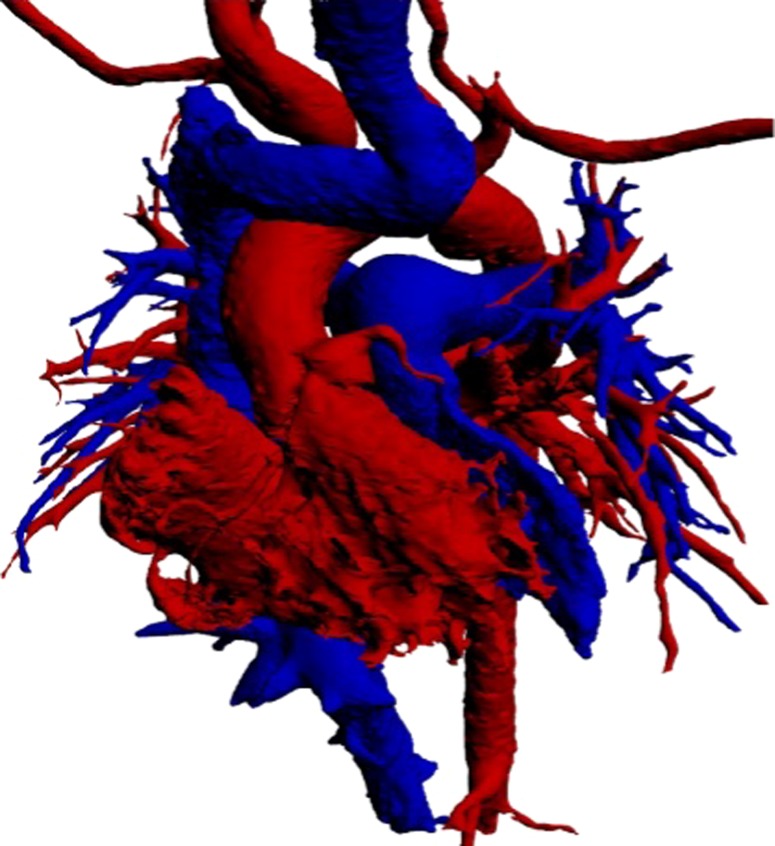
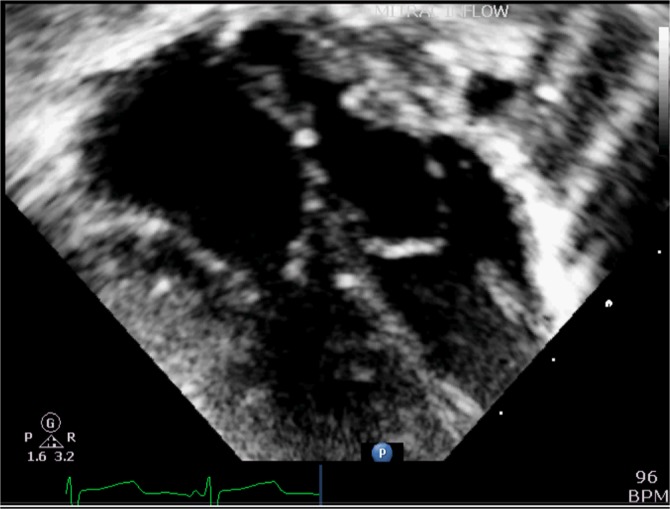
Figure 6 shows the overall 3-dimensional cast, which can be handled and examined in detail, to study the finer features of the morphology and interaction of all the component parts of the atrial and ventricular chambers. The small size of the supra-mitral chamber in contrast to the supra-tricuspid chamber is seen in 2-dimensional echocardiography (Figure 7).
Figure 6.
The whole 3-dimensional cast of the cardiac chambers following the Mustard operation.
Figure 7.
2-dimensional echocradiography showing the small supra-mitral left atrial chamber in comparison to the large supra-tricuspid chamber.
Conclusions and future direction
The techniques described in this paper allow individualized examinations of pre and post operative changes in shape and function of the ventricular chambers, and their potential influence on the dynamics and haemodynamics of the heart.10 Apart from its value in follow up and detecting complications, these techniques could allow the introduction of innovative refinements in technique and management with the aim of optimizing results.
Footnotes
** These two authors have contributed equally.
References
- 1.Kempny A, Wustmann K, Borgia F, Dimopoulos K, Uebing A, Li W, Chen SS, Piorkowski A, Radley-Smith R, Yacoub MH, Gatzoulis MA, Shore DF, Swan L, Diller GP. Outcome in adult patients after arterial switch operation for transposition of the great arteries. Int J Cardiol. 2013 Sep 10;167(6):2588–2593. doi: 10.1016/j.ijcard.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 2.Guilhot M, Godart F, Foucher C, Francart C, Libersa C, Pladys A, Vaksmann G, Brevière GM, Rey C. Long-term results of anatomic repair of transposition of great vessels. Arch Mal Coeur Vaiss. 2000 May;93(5):511–517. [PubMed] [Google Scholar]
- 3.Borow KM, Arensman FW, Webb C, Radley-Smith R, Yacoub MH. Assessment of left ventricular contractile state after anatomic correction of transposition of the great arteries. Circulation. 1984 Jan;69(1):106–112. doi: 10.1161/01.cir.69.1.106. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub MH, Radley-Smith R. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax. 1978 Aug;33(4):418–424. doi: 10.1136/thx.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yacoub MH, Radley-Smith R, Maclaurin R. Two-stage operation for anatomical correction of transposition of the great arteries with intact interventricular septum. Lancet. 1977 Jun 18;1(8025):1275–1278. doi: 10.1016/s0140-6736(77)91317-4. [DOI] [PubMed] [Google Scholar]
- 6.Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959 Jun;45(6):966–980. [PubMed] [Google Scholar]
- 7.Mustard WT. Successful two-stage correction of transposition of the great vessels. Surgery. 1964 Mar;55:469–472. [PubMed] [Google Scholar]
- 8.Quaegebeur JM, Brom AG. The trousers-shaped baffle for use in the Mustard operation. Ann Thorac Surg. 1978 Mar;25(3):240–242. doi: 10.1016/s0003-4975(10)63531-x. [DOI] [PubMed] [Google Scholar]
- 9.Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000 Apr 13;404(6779):759–761. doi: 10.1038/35008075. [DOI] [PubMed] [Google Scholar]
- 10.Mihalef V, Ionasec R, Wang Y, Zheng Y, Georgescu B, Comaniciu D. Patient-specific modeling of left heart anatomy, dynamics and hemodynamics from high resolution 4D CT. 2010. pp. 504–507. IEEE Int. Symp. Biomed. Imaging From Nano to Macro IEEE: 2010.