Abstract
Objective
This study compares the population and repair ability of bone marrow hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) in experimental colitis (EC) rat model after allogeneic stem-cell transplantation (SCT).
Methods
EC was induced by 2, 4, 6-trinitrobenzenesulfonic acid (TNBS). The HSCs, MSCs, HSCs+MSCs, derived from male Sprague-Dawley rats, were cultured and labeled with bromodeoxyuridine and then transplanted into the EC rat. The colon samples were collected for histologic evaluation at days 7, 14, and 21 posttransplantation. Immunohistochemical staining, polymerase chain reaction, and fluorescence in situ hybridization were used to detect donor stem cells population.
Results
EC induced by TNBS had characteristics similar to those of Crohn's disease. A large number of bromodeoxyuridine-labeled HSCs or MSCs were detected on days 7, 14, and 21 posttransplantation. Sex-determining region of Y chromosomes (sry) was found in all EC regions, but not in control and normal tissues. A clear localization of Y chromosomes in the colons of EC rat was detected by fluorescence in situ hybridization. Immunohistochemical staining revealed that HSCs or MSCs had similar population ability. When HSCs and MSCs were combined, gross morphologic scores significantly improved 21 days post-SCT compared with the control without SCT, but only slightly better than that of HSCs or MSCs alone.
Conclusions
Allogeneic transplantation of HSCs or MSCs alone could populate in the injured regions of the colons, both showed similar population ability in the colons of the TNBS-induced EC model rats. Combination transplantation of HSCs with MSCs could improve the gross morphologic scores of EC.
Keywords: Hematopoietic stem cell, Mesenchymal stem cells, Transplantation, Experimental colitis
Inflammatory bowel disease (IBD), both ulcerative colitis and Crohn's disease (CD), is common in the western world. The estimated incidences range from 3.1 to 14.6 and 5.6 per 100,000 persons per year in North America (1) and Europe (2), respectively. Recently, the disease is reported more frequently in Asia-Pacific regions (3, 4). The causes of IBD remain obscure. Severe and continuous inflammation can disrupt the intrinsic repair system, which results in refractory ulcers in the intestine. Most patients with IBD suffer a low quality of life.
There is no known cure for IBD. Present therapies aim to suppress inflammation and relieve symptoms. The goals of treatment are to induce and maintain remission, thus healing of the intestinal mucosa can occur to reconstitute the normal function. Immunosuppressants including aminosalicylates, corticosteroids, and 6-mercaptopurin are commonly used, but none of them have a curative effect.
One possible treatment of IBD is bone marrow transplantation (BMT) or hematopoietic stem-cell transplantation (HSCT), which is an established technique for hematological disorders. Most clinical reports are from patients with hematological disorders who also have IBD. In 1998, Kashyap and Forman (5) first reported a case of non-Hodgkin's lymphoma with CD that had CD remission and sustained symptom free for 7 years after receiving autologous BMT. In a case of six patients with CD undergoing allogeneic BMT for myeloid leukemia, five patients were free of CD-related symptoms for longer than 1 year (6). The followed-up cases of allogeneic and autologous HSCT showed an average 7-year and 20-month remission (7). The data from 11 patients with IBD, who received allogeneic stem-cell transplantation (SCT) for treatment of myeloid leukemia and myelodysplastic syn dromes, showed that 9 of 10 patients were free from IBD, after an average follow-up of 34 months post-SCT (8). In another report of allogeneic HSCT for refractory CD, 11 of 12 patients maintained a sustained remission defined as a CD activity index less than or equal to 150 (9). In a review article, Brittan et al. (10) summarized the literature until 2007. Overall, of 33 cases of CD undergoing autologous or allogeneic BMT 29 remained in remission with transplantation-related mortality in two cases. No deaths occurred in the 14 cases transplanted primarily for the treatment of CD. These studies suggested the efficacy and safety of both allogeneic and autologous BMT in the treatment of IBD (7–11). On the basis of these results, a clinical trial to determine the value of SCT in IBD has been carried out under the supervision of the European Group for Blood and Marrow Transplantation (12).
Although clinical studies showed promising results of SCT for IBD treatment, questions remain concerning the mechanism of SCT. It remains to be determined whether the colon was repaired by grafted stem cells or by colon epithelium cell. In addition, the relative importance of hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) in contribution to colon repair has not been fully addressed. In animal models, bone marrow-derived cells, especially MSCs, were reported to be involved in the healing process after intestinal injury and contribute to various components of the mucosa (13–15). A few histologic analyses revealed the re-population of the epithelial cells with MSCs in human gastrointestinal (GI) tract (6). However, in clinical transplantation treatment, HSCs, not MSCs, are the main source of grafted cells, the allogeneic HSCs can be collected from donor's bone marrow or peripheral blood. Collection of peripheral blood stem cell is a less invasive procedure; therefore, allogeneic peripheral blood stem cell transplantation is being used in more transplant units instead of BMT. Thus, the role of HSCs in the repair ability in IBD needs to be surveyed and defined. In this study, we transplanted HSCs or MSCs and HSCs+MSCs into experimental colitis (EC) rat model to compare their ability to populate and repair the intestinal mucosa of EC after SCT.
METHODS
Experimental Animals
Two male pathogen-free Sprague-Dawley rats, aged 4 weeks, weighing 40 to 50 g and 108 female Sprague-Dawley rats, aged 4 weeks, weighing 180 to 200 g were purchased from Animal Experimental Center of Guangdong Province. Experiments and animal care were carried out in compliance with the guidance suggestion of caring laboratory animals of the Ministry of Science and Technology of the People's Republic of China.
Induction of EC
All rats were allowed free access to standard pellet chow and water ad libitum. They were anesthetized with 40 mg/kg pentobarbital sodium by injections into the abdominal cavities, then subjected to the induction of EC. A polyethylene catheter (OD 1.7 mm) was inserted anorectally into the lumen of the colon with the tip at 8 cm proximal to the anus, approximately at the splenic flexure. EC was induced by instilling 100 mg/kg of 2, 4, 6-trinitrobenzenesulfonic acid (TNBS; Sigma Co., Missouri) dissolved in a 50% ethanol solution into the colonic lumen. In control groups, 50% alcohol or saline was injected (16, 17).
Study Setting
One hundred eight female rats were randomly divided into six groups with each group comprised of 18 rats. EC was induced in four experimental groups followed by transplantation of HSCs (group A), MSCs (group B), and HSCs+MSCs (group C). The transplant control (group F) did not receive any stem cells. Two additional model controls (groups D and E) were induced, they received 50% ethanol or saline injection following HSCs+MSCs transplantation. EC colon regions were examined by bromodeoxyuridine (BrdU)+sex-determining region of Y chromosomes (sry) or BrdU+sry+fluorescence in situ hybridization (FISH).
On days 7, 14, and 21 after the transplantation, rats were killed by cervical dislocation under anesthesia. The entire colons were removed for pathologic and molecular examinations.
HSCs and MSCs Culture and Expansion
SCT was performed using female rats as recipients and male rats as donors. Bone marrow cells were collected by flushing the femur and tibia shafts of two male rats with l-Dulbecco's minimum essential medium, then cultured at 37°C in 5% CO2 concentration and supplemented with fetal calf serum (10%) plus antibiotics (0.1% penicillin and 0.1% streptomycin). The suspension and adhesion cells were separated into two flasks. Stem-cell factor (100 ng/mL) and interleukin-3 (10 ng/mL) were added into the suspension flasks for expanding HSCs, whereas the adhesion cells were expanded and purified for MSCs. When MSCs reached approximately 80% confluence (3–4 days), the culture medium was replaced. The cells were passaged with trypsin-EDTA (Gibco BRL, California) at a dilution of 1:3. After 2 to 3 passages, cells were used for transplantation. CD90+ antigens were analyzed by flowcytometry for identification of the specific surface antigens of HSCs and CD54+ and CD44+ antigens for MSCs (the third passages).
BrdU Labeling of Male Rat Stem Cells
Bone marrow-derived HSCs and MSCs of Sprague-Dawley male rats were cultured in L-Dulbecco's minimum essential medium under the above conditions. Before transplantation, 1, 5-bromo-2′-deoxyuridine (BrdU; 10 μM) was added into the medium and cultured for 24 hr. HSCs and MSCs were collected separately. Cells were washed twice with PBS. BrdU-labeled cells were examined with flowcytometry and counted by microscope.
Stem Cells Transplantation
Twenty-four hours after the establishment of the EC models, a total number of 2×106 HSCs and MSCs suspended in 1-mL saline were injected into the tail veins of female recipient rats of transplant group A and B, respectively. A mixture of HSCs and MSCs (1:1) were engrafted into rats in transplant group C and EC model control groups D and E. Saline (1 mL) in the place of the stem-cell suspension was used in transplant control group F.
Histologic Study
The removed colons were opened by longitudinal incision and immediately examined under a stereomicroscope for gross morphologic damage. A Gross Morphologic Score System was used to quantify the damages as follows: 0, no damage; 1, localized hyperemia, but no ulcers; 2, linear ulcers with no significant inflammation; 3, linear ulcer with inflammation at one site; 4, two or more sites of ulceration and inflammation; and 5, two or more major sites of inflammation and ulceration, or one major site of inflammation and ulceration extending 1 cm along the length of the colon (16). Colon specimens (10 mm) from damaged and nondamaged regions were harvested from each animal, fixed in 1.33 M/L formaldehyde saline, embedded in paraffin, and stained with hematoxylin-eosin after sectioning for histologic analysis. The microscopic alterations were assessed according to the Score Microscopic System: 0, infiltrated normocellular or normal hypercellular lamina, polymorph nuclear cells (PMNs) absent; 1, diffused PMNs at the lamina propria, occasional cryptitis but few cryptic abscesses, minimal glandular destruction, or ulceration; 2, moderate number of PMNs at the lamina propria, cryptitis, and prominent cryptic abscesses, some glandular destruction; and 3, numerous PMNs with abundant cryptitis, cryptic abscesses, extensive cellular destruction, prominent ulceration (18). The results were based mainly on the scores of histologic damages referred to the scores of gross morphologic changes. All assessments were performed by two independent observers unaware of the study.
Immunohistochemistry Study
Tissue samples were cut into 5-μm thick sections and mounted on aminopropyl triethoxysilane coated glass slides, deparaffinized in xylene, and dehydrated in a gradient of ethanol solutions. The samples were stained with immunohisto-chemical S-P assay to detect BrdU-labeled cells. Endogenous peroxidase in the samples was blocked by using 3% hydrogen peroxide for 10 min at 37°C. The sections were treated with 2 M/L HCl for 30 min and 0.125% trypsin for 20 min at 37°C, then goat serum for 15 min at 37°C. Then, the sections were incubated with anti-BrdU antibody in a moist chamber for 16 hr at 4°C followed by incubation with biotin-labeled secondary antibodies for 15 min at 37°C and then with diaminobenzidine for 10 min. Hematoxylin was used as a counter stain. The number of BrdU positive cells was quantified as follows: 1, negative (<1%); 2, weak positive (1%–25%); 3, positive (26%–50%); and 4, strong positive (>50% cells were BrdU positive).
Detection of the Sex-Determining Region of Y Chromosome Gene by Polymerase Chain Reaction
The presence of the sry, derived from the donor, was detected by polymerase chain reaction (PCR). The forward primer sequence was 5′-catctctgacttcctggttgcaa-3′ and the reverse primer sequence was 5′-atgctgggattctgttgagcc-3′ (Synthesized by Bioasia Co. Ltd., Shanghai, China). The amplification cycle was 94°C for 5 min; 35 cycles at 95°C for 50 sec, 56°C for 50 sec, 72°C for 60 sec; and one cycle at 72°C for 10 min. The PCR products were subjected to electrophoresis. The size of the amplified gene products was 241 bp.
Localization of Y Chromosome by FISH
FISH was conducted to detect Y chromosome as a marker of donor cells. Specimens were pretreated using the Pretreatment Reagent Kit (Vysis, Downers Grove, IL) according to the instructions supplied by the manufacturer and hybridized with a rat fluorescein isothiocyanate-labeled Cambio Y-paint probe (Cambio, Cambridge, UK). The hybridized slides were observed under fluorescent microscopy.
Statistical Analysis
All data were expressed as mean±SD. The software of SPSS 11.0 for Windows (SPSS Inc., IL) was used for analysis. Student's two-tailed t test and the chi-square test were used for the evaluations of continuous and categorical variables, respectively. A P value less than 0.05 was considered statistically significant.
RESULTS
Establishment of EC Models
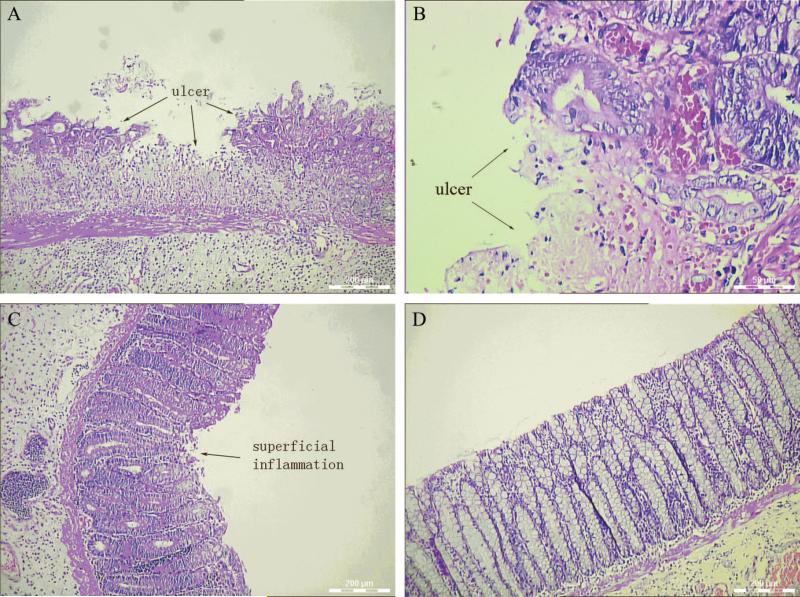
Diarrhea was observed in all EC models rats after the intraluminal administration of 100 mg/kg TNBS. All the rats survived the study until they were killed. Seven days after the administration, all rats developed grossly visible inflammation or ulcers in the mucosa, and thickening of the bowel walls, suggesting the inflammation extended from the mucosal to the submucosal areas (Fig. 1). By light microscopy, all the TNBS-treated rats had mucosal and submucosal damages characterized by edema, hemorrhage, epithelial exfoliation, PMNs infiltration, cryptic abscesses, minimal glandular destruction, and ulceration. In contrast, intracolonic administration of 50% ethanol (in model control D) did not produce apparent histologic changes, only a few areas showed light superficial inflammation, whereas saline alone (model control E) did not produce any detectable damage in the colon (Fig. 2).
FIGURE 1.
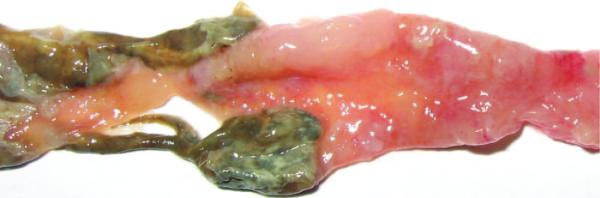
Macroscopic manifestations of the colons 7 days after 2, 4, 6-trinitrobenzenesulfonic acid administration: congestion, edema, erosion, necrosis, and multiple ulcers in the colon were present.
FIGURE 2.
Histological changes of the distal colons induced by 2, 4, 6-trinitrobenzenesulfonic acid 50% ethanol solution on day 7. (A and B) Presence of experimental colitis with ulcers after 2, 4, 6-trinitrobenzenesulfonic acid administration; (C) superficial inflammation of the colon after ethanol administration; (D) no histological changes in control after saline administration.
Culture and Expansion of HSCs and MSCs
Bone marrow cells were divided into suspension (HSCs) and adhesion cells (MSCs) by the adhesion characteristics mentioned above. The HSCs were expanded for 2 weeks in the medium containing SCF and interleukin-3. The positive rate of CD90+ cells, which represented HSCs, ranged 13.6%±4.9%. Adherent MSCs were visible within 24 hr. On day 3 or 4, approximately 80% MSCs reached confluence. Flowcytometric analysis showed that MSCs expressed CD44+ and CD54+ antigens. The CD44+ positive cells were 89.6%±6.0%, and CD54+ were 95.5%±3.9% when analyzed for three passages on day 14.
SCT, Histologic, and Gross Morphologic Evaluation
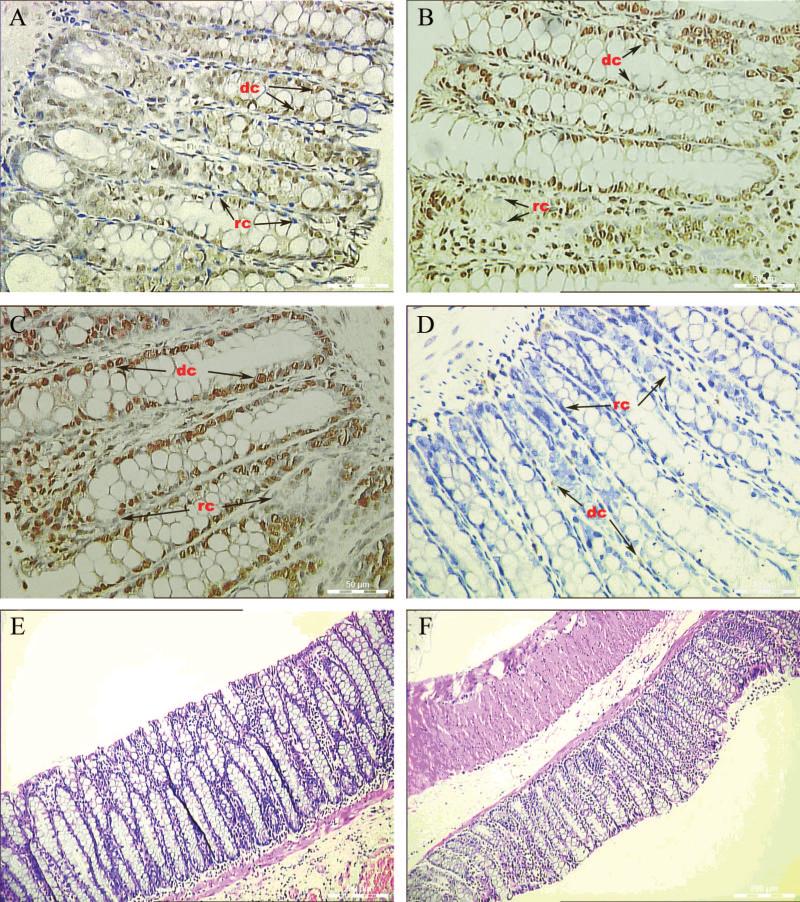
On days 7, 14, and 21 after the transplantation, the BrdU positive cells were exclusively detected in the EC regions, but not in the normal regions, of the recipient rats. The donor's cells replaced most of the recipient's cells in heavily ulcerated regions. By quantification of BrdU positive cells, HSCs or MSCs had strong positive cells and showed similar population ability, whereas the HSCs+MSCs was slightly better than that of HSCs or MSCs alone, but did not reach statistical significant difference. The number of BrdU positive cells was not significantly different among the saline-treated control group E (06), sham transplant group F (06), and ethanol treated group D (less than 12) (Fig. 3). All stem cells were presented as single cells and interspersed in the mucosa of the colons. The density of grafted stem-cell population increased with the severity of inflammation of EC. A few BrdU-labeled stem cells were also detected in the liver tissue of three transplanted groups that developed EC (data were not shown).
FIGURE 3.
BrdU positive cells were found in ulcerated experimental colitis region 21 days after transplantation with hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), or HSCs+MSCs (group A, B, and C). Few BrdU positive donor cells were found in ethanol-treated group (group D). No BrdU positive donor cells were found in saline model (group E) or sham transplantation (group F). (A) 2, 4, 6-trinitrobenzenesulfonic acid (TNBS)+HSCs; (B) TNBS+MSCs; (C) TNBS+HSCs+MSCs (1:1); (D) ethanol+ HSCs+MSCs; (E) saline+HSCs+MSCs; (F) TNBS+saline. dc, donor's cell (brown-yellow-stained nucleus); rc, recipient cell (blue-stained nucleus).
The repair of damaged colons was evaluated by Gross Morphologic Score System. The results of the colon in different groups after SCT or sham SCT were shown in Table 1. Although HSCs or MSCs migrated and populated in the damaged colons, there was no significant difference of gross morphologic score compared with control group F without transplantation or those of each other (P>0.05). However, when HSCs+MSCs were used in the transplantation, a significant improvement in gross morphologic score was noted 21 days after the transplantation (P<0.05). The morphologic score of the combination treatment was also better than that of single treatment, but did not achieve statistical significance (P>0.05).
TABLE 1.
Gross morphological score of damaged colon after stem-cell transplantation at different time points
| Number of the rats receiving gross morphologic score |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day7 (n=6) |
Day 14 (n=6) |
Day 21 (n=6) |
|||||||||||||||||||
| Groups | 0 | 1 | 2 | 3 | 4 | 5 | Total | 0 | 1 | 2 | 3 | 4 | 5 | Total | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| TNBS + HSC | 0 | 0 | 0 | 2 | 4 | 0 | 22 | 0 | 0 | 3 | 2 | 1 | 0 | 16 | 1 | 3 | 2 | 0 | 0 | 0 | 7 |
| TNBS + MSC | 0 | 0 | 0 | 2 | 4 | 0 | 22 | 0 | 0 | 2 | 3 | 1 | 0 | 17 | 1 | 2 | 3 | 0 | 0 | 0 | 8 |
| TNBS + HSC/MSC | 0 | 0 | 0 | 3 | 3 | 0 | 21 | 0 | 1 | 3 | 2 | 0 | 0 | 13 | 2 | 4 | 0 | 0 | 0 | 0 | 4* |
| Ethanol + HSC/MSC | 0 | 5 | 1 | 0 | 0 | 0 | 7 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saline + HSC/MSC | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| TNBS + Saline | 0 | 0 | 0 | 1 | 5 | 0 | 23 | 0 | 0 | 1 | 4 | 1 | 0 | 18 | 0 | 2 | 3 | 1 | 0 | 0 | 11 |
P<0.05 versus TNBS + Saline group.
TNBS, 2, 4, 6-trinitrobenzenesulfonic acid; HSC, hematopoietic stem cell; MSC, mesenchymal stem cell.
Detection of the Sry Gene and Y Chromosome
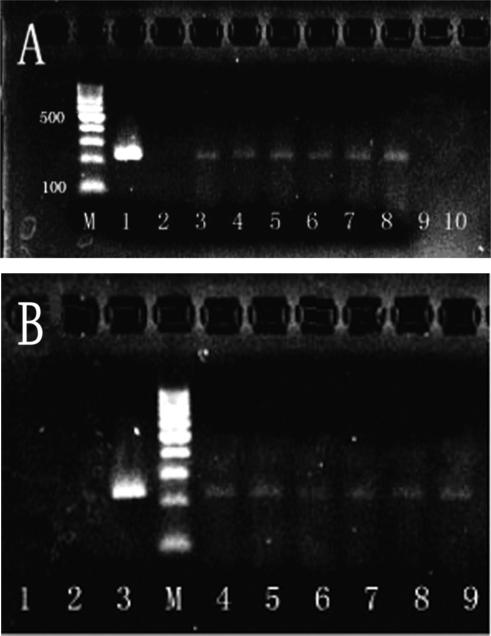
To confirm that the BrdU positive cells were derived from the male donors, the presence of the sry gene and Y chromosome was detected by PCR and FISH, respectively. All the EC regions of the colons in transplanted groups A, B, and C had positive detection of the sry gene on days 7, 14, and 21 posttransplantation. In contrast to the transplanted EC rats, the specimens derived from the control rats, ethanol model control (group D) and saline model control (group E), both received mixture of HSCs and MSCs transplantation, and sham transplant control (group F) were all sry gene negative (Fig. 4).
FIGURE 4.
(A) The detection of the sex-determining region of Y chromosomes in the colons of all experimental colitis transplantation groups on day 21. (lane 1) Male rat colon; (lane 2) 2, 4, 6-trinitrobenzenesulfonic acid (TNBS)+saline (group F); (lanes 3 and 4) TNBS+hematopoietic stem cells (HSCs; group A); (lanes 5 and 6) TNBS+mesenchymal stem cells (MSCs; group B); (lanes 7 and 8) TNBS+HSCs+MSCs (1:1) (group C); (lane 9) ethanol+HSCs+MSCs (group D); (lane 10) saline+HSCs+ MSCs (group E). (B) The detection of the sex-determining region of Y chromosomes in the colons of group C after transplantation at different time points. (lanes 1 and 2) Day 1; (lane 3) male rat colon; (lanes 4 and 5) day 7; (lanes 6 and 7) day 14; (lanes 8 and 9) day 21. (lane M) DNA size marker.
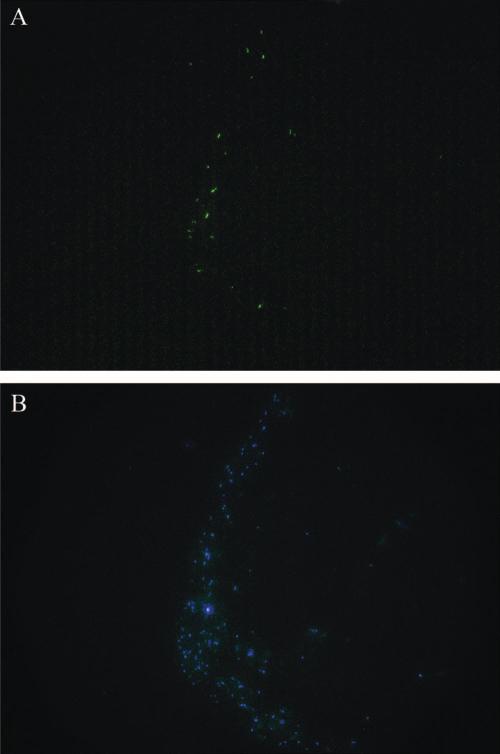
Detection of Y chromosome by FISH determines the location of donor cells in the colon after the transplantation. The fluorescein isothiocyanate-labeled Y chromosomes were found in the EC regions of the colons at 7, 14, and 21 days posttransplantation. The nuclei of the cells were stained blue fluorescence with DAPI (Fig. 5). The detection of Y chromo-some in situ provided direct evidence that the population of transplanted stem cells occurred in the EC regions, and the data were consistent with the BrdU and sry results.
FIGURE 5.
(A) The detection of Y chromosomes (green fluorescence) using FISH indicates the presence of male donor cells in the colon of female recipients 21 days after transplantation in 2, 4, 6-trinitrobenzenesulfonic acid+hematopoietic stem cells+mesenchymal stem cells group (group C). (B) DAPI (blue fluorescence) stain indicates all the cells in the same view.
DISCUSSION
The rapid renewal of the epithelia in the GI tract continues throughout life. When damages occur, the epithelia usually repair by the restitution, proliferation, and differentiation of the cells. In IBD patients, the damage is severe and inflammatory factors in the microenvironment prevent the epithelial renewal system from repairing itself. Bone marrow cell or peripheral blood stem cells are suggested to be the only cells outside GI origins that can contribute to the reparation of the epithelia of the GI tract (19).
The bone marrow contains HSCs and nonhematopoietic MSCs, both of which are derived from the same precursor cells, the vascular and endothelial progenitor cells. Both cell types have the potential to transdifferentiate into a variety of cells under certain microenvironments. These versatile cells possess differentiation potential, expansion capacity, and nurturing and immunomodulatory proficiency and have widespread clinical applications. Komori et al. (13) successfully engrafted green fluorescent protein (GFP)-transgenic bone marrow-derived cells into TNBS-colitis model rats. The results showed a significant amount of donor-derived mucosal and submucosal interstitial cells. MSC is a main stem-cell source in regenerative medicine because of its multipotentiality. When MSCs were transplanted to the gastric tissue surrounding the ulcer, accelerated gastric ulcer healing was noted. These beneficial effects might be mediated by their differentiation into gastric interstitial cells (14, 15). In SCT clinical trials, HSCs are more frequently being collected through peripheral blood due to the less invasive nature it poses to donors. In our study, we identified that HSCs or MSCs alone could migrate and populate in the injured regions of the colons, both cell types showed similar capability in population. When HSCs and MSCs were combined, gross morphologic score was improved. This result is consistent with the report of Kim et al. (20) that MSCs could enhance engraftment of HSCs in humans. Our results and others’ (21, 22) suggested that HSCs or MSCs could populate among the pillar cells of colon epithelia and reconstitute the damaged defense walls. In the colons of control rats, which did not receive TNBS and in the normal regions in the transplanted groups, no donor cells were found. This finding suggested that allogeneic stem cells could not populate in the colon region without damage because neither the microenvironment nor available space could support the population.
This study showed the migration and replacement of the recipient's colon epithelia by donor stem cells in SCT EC model rats. In the EC group that did not receive transplantation, we also found the self-renewed epithelia in the EC regions. However, this automatic epithelium reparation was slower in comparison with the SCT groups. It has been suggested that self-renewal stem cells exist in all tissues including the colons. When injury occurs in the EC models, the self-renewal stem cells are activated to repair the damaged tissues and thus the epithelia can regenerate. Regenerating cells in the transplanted models might come from donor stem cells or self-renewal stem cells, or a combination of the two. In our experimental model, we believed the donor cells are the major source for colon epithelia renewal if it occurred.
Although bone marrow-derived MSCs have been reported to populate and transdifferentiate into GI epithelium cells in EC rat models (13–15, 23), HSC, including peripheral blood stem cell, is the main stem-cell source of SCT in clinical practice. Based on our findings, HSCs are proved to substantially repopulate in the EC colon and have a similar capability of MSCs. Utilization of HSCT, combined with MSCs, may lead to a novel approach for IBD.
ACKNOWLEDGMENT
The authors thank Barbara Brede for editing this manuscript.
This work was supported by the Guangdong Science and Technology Grant (2004-139-50) and Health Bureau Grant of Guangzhou (004-D01).
REFERENCES
- 1.Loftus EV, Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: A systematic review. Aliment Pharmacol Ther. 2002;16:51. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology. 2004;126:1504. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang Q, Tandon R, Goh KL, et al. The emergence of inflammatory bowel disease in the Asian-Pacific region. Curr Opin Gastroenterol. 2005;21:408. [PubMed] [Google Scholar]
- 5.Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin's lymphoma resulting in long-term remission of co-incidental Crohn's disease. Br J Haematol. 1998;103:651. doi: 10.1046/j.1365-2141.1998.01059.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn's disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433. doi: 10.1016/s0016-5085(98)70525-6. [DOI] [PubMed] [Google Scholar]
- 7.Leung Y, Geddes M, Storek J, et al. Hematopoietic cell transplantation for Crohn's disease; is it time? World J Gastroenterol. 2006;12:6665. doi: 10.3748/wjg.v12.i41.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ditschkowski M, Einsele H, Schwerdtfeger R, et al. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75:1745. doi: 10.1097/01.TP.0000062540.29757.E9. [DOI] [PubMed] [Google Scholar]
- 9.Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Brittan M, Alison MR, Schier S, et al. Bone marrow stem cell-mediated regeneration in IBD: Where do we go from here? Gastroenterology. 2007;132:1171. doi: 10.1053/j.gastro.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34+ selection in refractory Crohn's disease. Gut. 2008;57:211. doi: 10.1136/gut.2007.128694. [DOI] [PubMed] [Google Scholar]
- 12.Hawkey CJ, Snowden JA, Lobo A, et al. Stem cell transplantation for inflammatory bowel disease: Practical and ethical issues. Gut. 2000;46:869. doi: 10.1136/gut.46.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Tsuji S, Tsujii M, et al. Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G778. doi: 10.1152/ajpgi.00468.2007. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, Tsuji S, Tsujii M, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 16.Morris GP, Beck PL, Herridge MS, et al. Hapten induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795. [PubMed] [Google Scholar]
- 17.Sandborn WJ, Tremaine WJ, Offord KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis—A randomized, double blind, placebo-controlled trial. Ann Intern Med. 1997;126:361. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Hollenbach E, Vieth M, Roessner A, et al. Inhibition of RICK/nuclear factor-kappa B and p38 signaling attenuates the inflammatory response in a murine model of Crohn's disease. J Biol Chem. 2005;280:14981. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50(suppl 1):S34. doi: 10.1007/s10620-005-2804-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Yoo KH, Yim YS, et al. Co-transplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci. 2006;21:1000. doi: 10.3346/jkms.2006.21.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi Y, Tsuji S, Tsujii M, et al. The transdiffrentiation of bone marrow derived cells in colonic mucosal regeneration after dextran sulfate sodium induced colitis in mice. Pharmacology. 2007;80:193. doi: 10.1159/000104148. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto R, Watanabe M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J Gastroenterol. 2004;39:1. doi: 10.1007/s00535-003-1259-8. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]