Abstract
Objective
To assess cerclage benefit in women with short cervix also receiving 17-alpha-hydroxyprogesterone caproate (17P) to prevent recurrent preterm birth (PTB).
Methods
Secondary analysis of a multicenter trial of ultrasound-indicated cerclage for shortened cervical length (CL). Women with prior spontaneous PTB at 16-33 6/7 weeks, singleton gestation and CL<25mm between 16-22 6/7 weeks were counseled on use of 17P and randomized to cerclage or no cerclage. Outcomes of women who received 17P were analyzed by randomization group. Primary outcome was PTB<35 weeks.
Results
99 women received 17P: 47 cerclage; 52 no cerclage. Rates of PTB<35 weeks were similar, 30% for cerclage and 38% for no cerclage (aOR 0.64 (0.27 – 1.52)). In women with CL<15mm, PTB<35 weeks was reduced for the cerclage group (17% versus 75%, p=0.02). However, this difference was nullified after controlling for total progesterone doses received (p=0.40).
Conclusions
Cerclage was shown not to offer additional benefit for the prevention of recurrent PTB in women with short CL<25mm receiving 17P, but the sample size is insufficient for a definite conclusion given the 36% non-significant decrease in the odds of PTB<35 weeks. Cerclage may further offer substantial benefit to women with very short CL<15mm and further study is needed.
Keywords: cerclage, progesterone, cerclage, preterm birth, short cervical length
Introduction
Preterm birth (PTB) is a leading cause of perinatal morbidity and mortality [1] and is a common problem in the United States with annual rates over 12% in 2009 [2]. Women with prior spontaneous PTB(s) have one of the strongest risk factors for recurrent PTB [3,4,5]. Shortened cervical length (CL) on transvaginal ultrasound prior to 24 weeks gestation is currently the best method to predict spontaneous PTB [6,7].
Both cerclage [8-12] and progesterone [13,14] have been investigated as interventions to prevent PTB in women with a prior PTB. Following the detection of a shortened cervical length on transvaginal ultrasound, cerclage has been shown to reduce recurrent PTB <37 weeks, PTB <24 weeks, and perinatal mortality in a large randomized trial [8] and PTB <35 weeks and perinatal morbidity and mortality in a meta-analysis of the prior trials [15]. Treatment with 17-alpha-hydroxyprogesterone (17P) has also been shown to reduce recurrent PTB <37 weeks in a large multicenter trial [13] and this finding was further supported in subsequent meta-analysis [14]. Prior investigation [16] of participants in the trial by Owen and colleagues focused on the effects of progesterone administration for women receiving and not receiving cerclage. However, the efficacy of cerclage in women who develop a short CL<25mm in the second trimester and who receive the recommended 17P for a prior PTB has not been well studied. Furthermore, the additive benefit of cerclage in higher-risk women with very short CL<15mm receiving 17P requires further investigation.
The aim of this study is to quantify and evaluate the effect of cerclage on pregnancy outcomes in high-risk women receiving 17P with a history of PTB, singleton gestation, and shortened CL<25mm (or alternatively CL<15mm).
Methods
This is a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development-sponsored trial to investigate ultrasound-indicated cerclage for the prevention of PTB in high-risk women. The trial is summarized elsewhere [8]. Briefly, characteristics of eligible women were singleton gestation, prior spontaneous PTB <34 weeks, and shortened CL<25mm observed during serial ultrasound screening between 16 and 22 6/7 weeks. From January 2003 to February 2007, 302 women at 15 U.S. clinical sites were randomized to receive McDonald cerclage (n=149) or no cerclage (n=153).
Results from a randomized trial of 17P [13] became available early in the trial, and recommendations that women be counseled regarding the use of progesterone for the prevention of PTB were made by the independent data and safety monitoring board. Subsequently, patients were counseled regarding the availability and potential benefit of 17P, and randomization was stratified by women's intent to use 17P, with a suggested weekly intramuscular dose of 250 mg. Participants were contacted by study nurses to determine reported use after randomization. As reported previously [16], study nurses at the center with the greatest enrollment total reviewed medical records for documented evidence of 17P injections. The agreement between reported and actual administration was acceptably high, 85% (kappa = 0.85), and thus reported use of 17P was deemed a sufficient surrogate for actual 17P usage across all participating centers.
Analyses in this study considered only women who received at least one injection of 17P for the history of PTB after randomization, and we compared those women randomized to cerclage vs those randomized to no cerclage. Similar to the primary study analysis [8], separate analyses were planned for women with CL<25 mm and also the subset of women with very short CL<15mm. Study outcomes included rates of PTB at <37, <35, <32, <28, and <24 weeks, perinatal death, and time to delivery. Characteristics of the comparison groups for each analysis were evaluated. Differences in categorical characteristics and outcomes were analyzed with chi-square tests of association and Fisher's exact test, as appropriate. Differences in quantitative characteristics were assessed with Student t-tests and mean ± one standard deviation are presented. Logistic regression was used to estimate odds ratios for study outcomes. Adjusted odds ratios were obtained using multivariable logistic regression analyses controlling for imbalanced patient characteristics potentially operating as masking effects. Time to delivery was estimated with Kaplan-Meier analysis with differences evaluated using the log-rank test. Analyses of the subset of women with CL<15mm at randomization included Fisher's exact test and the Wilcoxon rank-sum test. To address the small sample size, exact logistic regression was used to obtain adjusted odds ratios controlling for imbalanced patient characteristics. All tests of significance were two-sided and evaluated at a 0.05 level of significance. SAS version 9.2 (SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses.
Results
Ninety-nine women of the 302 randomized received at least one 17P injection and 94 (95%) stated intent to use progesterone at the time of randomization. Of these 99, 47 were randomized to receive cerclage (52 to no cerclage). Women randomized to cerclage were administered their first dose of progesterone less than one week earlier in gestation than those randomized to no cerclage (p=0.048). Other characteristics of the patients did not differ between randomization groups (Table I).
Table I.
Baseline characteristics for women receiving 17P by cerclage randomization assignment.
| Cerclage (n = 47) |
No Cerclage (n = 52) |
P | |
|---|---|---|---|
|
| |||
| Race/ethnicity | |||
| Black (non-Hispanic) | 26 (55) | 32 (62) | 0.57 |
| White (non-Hispanic) | 13 (28) | 13 (25) | |
| Hispanic | 2 (4) | 4 (8) | |
| Other | 6 (13) | 3 (6) | |
|
| |||
| Cigarette use | 12 (26) | 12 (23) | 0.78 |
|
| |||
| Any drug abuse | 3 (6) | 6 (12) | 0.49 |
|
| |||
| One or more prior induced abortion | 7 (15) | 15 (29) | 0.10 |
|
| |||
| Years of Age | 26.9 ± 6.3 | 26.3 ± 4.5 | 0.55 |
|
| |||
| Years of education | 12.5 ± 2.1 | 12.8 ± 1.8 | 0.48 |
|
| |||
| Gestational age (wks) of qualifying birth | 23.2 ± 4.8 | 24.0 ± 5.0 | 0.41 |
|
| |||
| Gestational age (wks) at randomization | 18.9 ± 1.9 | 19.6 ± 2.0 | 0.06 |
|
| |||
| Cervical length (mm) at randomization | 19.0 ± 5.5 | 19.5 ± 5.0 | 0.60 |
|
| |||
| Total reported progesterone doses | 12.4 ± 5.1 | 11.0 ± 4.7 | 0.14 |
|
| |||
| Gestational age (wks) at 1st reported progesterone dose | 19.8 ± 2.3 | 20.7 ± 2.2 | 0.048 |
Data presented as n (%) and mean ± one standard deviation
No significant difference in any PTB outcome was observed between cerclage randomization groups (Table II). Multivariable logistic regression models were used to evaluate group differences in the presence of factors potentially masking an effect: gestational age (GA) at randomization and GA at first 17P dose. All study outcomes remained statistically non-significant, with the adjusted odds ratios for PTB <37, <35, <32, and <28 weeks varying between 0.46 and 0.64 (Table II). The mean GA at delivery was slightly greater for those randomized to cerclage (35.5 ± 4.6 weeks) versus those randomized to no cerclage (34.7 ± 4.8 weeks) though not statistically significant (p=0.39). This null relationship is further illustrated by the Kaplan-Meier plot available online as supplemental Figure S1 (log-rank p=0.42). We conducted the same series of analyses to compare women who actually received cerclage versus those who did not with similar results (data not shown).
Table II.
Perinatal outcomes for women receiving 17P, presented by randomization group. Adjusted odds ratios (aOR) are obtained from logistic regression models adjusting for GA at randomization and GA at first recorded 17P dose.
| Outcome | Cerclage (n = 47) |
No Cerclage (n = 52) |
OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| PTB < 37 weeks | 23 (49) | 31 (60) | 0.65 (0.29 – 1.44) | 0.62 (0.27 – 1.41) |
| PTB < 35 weeks | 14 (30) | 20 (38) | 0.68 (0.29 – 1.57) | 0.64 (0.27 – 1.52) |
| PTB < 32 weeks | 8 (17) | 11 (21) | 0.77 (0.28 – 2.10) | 0.63 (0.22 – 1.81) |
| PTB < 28 weeks | 4 (9) | 8 (15) | 0.51 (0.14 – 1.83) | 0.46 (0.12 – 1.70) |
| Previable birth < 24 weeks | 2 (4) | 1 (2) | 2.27 (0.20 – 25.8) | 1.92 (0.15 – 25.3) |
| Perinatal death | 3 (6) | 2 (4) | 1.67 (0.27 – 10.4) | 1.44 (0.22 – 9.67) |
Data presented as n (%)
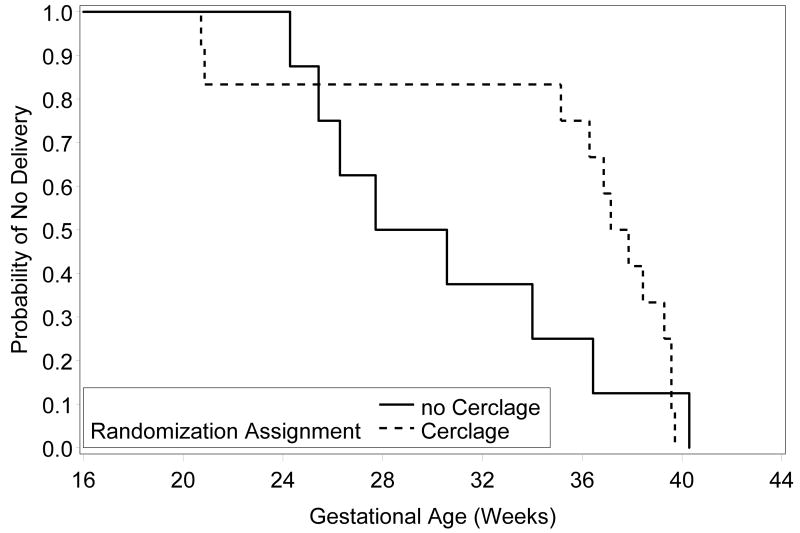
Twenty women received 17P and also had a CL<15mm at randomization: 12 were randomized to receive cerclage (8 no cerclage). Women randomized to cerclage recorded more doses of 17P (median; interquartile range [IQR]: 15.0; 14.0 – 16.0) than those randomized to no cerclage, (8.0; 2.5 – 13.0) p=0.04, and no other differences were found in the patient characteristics (data not shown). PTB was less common in women randomized to receive cerclage at <37 (42% vs 88%), <35 (17% vs 75%), <32 (17% vs 63%), and <28 (17% vs 50%) weeks (supplemental Table S1). This difference was statistically significant for PTB <35 weeks (p=0.02). However, this significant difference was nullified in an exact logistic regression analysis controlling for the number of progesterone doses received (p=0.40). The GA at delivery was greater in the cerclage group with median (IQR) of 37.5 (35.7 – 39.5) weeks compared to 29.2 (25.9 – 35.2) weeks, though this difference was not statistically significant, (p=0.15). The Kaplan-Meier plot in Figure 1 further illustrates this relationship in the very high-risk subgroup (log-rank p=0.33).
Figure 1.
Kaplan-Meier plot, by randomization assignment, for women receiving ≥1 dose of 17P and very short CL<15mm (log-rank p=0.33).
Discussion
In the US, it has been recommended that women with a singleton gestation who have had a prior spontaneous PTB receive weekly 17P beginning at 16-20 weeks [13,17]. Recently, in this same population of singleton gestations with prior PTB, screening of cervical length has been recommended between 16 and 23 weeks [18]. This is based on the fact that, if the cervical length shortens to <25 mm, cerclage has been shown to prevent recurrent PTB by about 30%, and perinatal morbidity and mortality by about 36% (8,15). It remains unclear whether the effects of 17P and cerclage are cumulative in these women with singleton gestations and a prior spontaneous PTB, who then develop a shortened cervical length.
Our study aimed to assess this important clinical question: in a woman with a singleton gestation who is taking 17P because of a prior PTB, is cerclage for short cervical length associated with further benefits beyond those already provided by 17P? Although we observed no differences in PTB and perinatal outcomes between cerclage treatment groups in women who received 17P, our analysis has limitations.
First, we have limited knowledge of 17P administration during the serial sonographic screening during which these women had not yet achieved CL<25mm. Administration of 17P was recorded for all patients following randomization and monitored throughout the remainder of pregnancy. Thus, the data presented here reflect therapy documented only after establishing eligibility for trial participation and any 17P these women may have received prior to randomization cannot be factored into this analysis.
Further, a single dose of progesterone may be insufficient to reduce the risk of preterm birth and defining the administration of 17P as receiving at least one dose during ultrasonographic screening may be a suboptimal evaluation of 17P exposure. We alternatively defined 17P administration as those women receiving at least half of the maximum possible doses between first study ultrasound and delivery (85 women). Even with this definition of exposure, however, we found no statistically significant differences in any of the study outcomes. Similarly, no differences were found for any outcome in exposed women with CL<15mm. Similar analyses considering women with CL<25mm who received >=75% (47 women) of the maximum possible doses receivable found no differences in the study outcomes; however, in women with CL<15mm, we once again saw a difference in the rate of PTB<35 weeks with 3 (100%) of those in the no cerclage group and 1 (11%) in the cerclage group (p=0.02).
Finally, this was a secondary analysis and may have been underpowered to detect a significant difference in any of the primary and secondary study outcomes. For the primary outcome of PTB <35 weeks, an impressive 36% decrease associated with cerclage in the multivariable analysis was seen, but this was not a statistically significant difference in this sample (Table II). To detect a one-third reduction in the rate of PTB <35 weeks (based on the observed rate of 38% in women receiving 17P, but not cerclage) in this high risk population, 400 women would be required to achieve 80% power. Decreases in odds of PTB cutoffs of <37, <32, and <28 weeks of about 36% to 54% were also seen, but were not significant, again, likely due to our sample size (Table II). As PTB <24 weeks and perinatal mortality were exceptionally rare in each of these two groups, these data should be interpreted cautiously.
In the small subset of women with CL<15mm receiving at least one dose of 17P, PTB <35 weeks was significantly less frequent for women randomized to cerclage. However, this effect was nullified when we also considered the number of 17P doses documented. Given the limited sample size for this analysis, and given the great reduction in PTB <35 weeks in women who received ≥75% of the possible doses, these analyses should not be treated as a definitive answer to the question of whether cerclage is beneficial in women with CL<15mm and who are receiving 17P.
Advantages of this study include a regionally diverse US population and rigorous identification of high risk women with a prior PTB, singleton gestation, and CL <25 mm identified through serial sonographic screening. A larger study in this high risk population, particularly those with CL<15mm, is required to better investigate the utility of ultrasound-indicated cerclage in women receiving 17P.
Supplementary Material
Figure S1. Kaplan-Meier plot, by randomization assignment, for women receiving ≥1 dose of 17P (log-rank p=0.42).
Acknowledgments
We wish to acknowledge other members of the Vaginal Ultrasound Trial Consortium: Susan Ramin, MD, Mark Tomlinson, MD, Eric Knudtson, MD, Robert Egerman, MD, Richard Silver, MD, Helen How, MD, Mike Gordon, MD.
Source of funding. The Eunice Kennedy Shriver National Institute of Child Health and Development provided funding via grant U01 HD039939; from the same agency, Dr. Owen also received support via grant 5K24 HD43314-5.
Footnotes
Disclosure of interest. The authors report no declarations of interest.
Contribution to authorship. All authors were the principal investigators in this NIH-sponsored prospective trial, with this secondary analysis proposed by Dr. Berghella before the primary analysis of the primary trial was performed, and approved by Dr. Owen as the Primary PI of the trial. Each author contributed to approval of the protocol, data collection and primary analysis. Dr. Szychowski wrote the manuscript and performed the statistical analyses, with substantial input from Drs. Berghella and Owen, and all authors edited and contributed to the final version of the manuscript.
Ethics approval. Each of the centers obtained Institutional Review Board approval, and each woman signed informed consent at entry into the randomized study.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Mathews TJ, Wilson EC. National vital statistics reports. No. 1. Vol. 60. Hyattsville, MD: national Center for health Statistics; 2011. Births: Final data for 2009. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339:313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 4.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 5.McManemy J, Cooke E, Amon E, Leet T. Recurrence risk for preterm delivery. Am J Obstet Gynecol. 2007;196:576.e1–576.e7. doi: 10.1016/j.ajog.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: A study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1106. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 7.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, III, Miodovnik M, LangerO Sabai B, McNellis D National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–88. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 8.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, Egerman RS, Wing DA, Tomlinson M, Silver R, Ramin SM, Guzman ER, Gordon M, How HY, Knudtson EJ, Szychowski JM, Cliver S, Hauth JC. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix on transvaginal ultrasound: A randomized trial. Am J Obstet Gynecol. 2004;191:1311–1317. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 10.To MS, Alfirevic Z, Heath VCF, Cicero S, Cacho AM, Williamson PR, Nicolaides KH. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomized controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 11.Rust OA, Atlas RO, Reed J, van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: Why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 12.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the cervical incompetence prevention randomized cerclage trial (CIPRACT): Therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–1112. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 13.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 14.MacKenzie R, Walker M, Armson A, Hannah ME. Progesterone for the prevention of preterm birth among women at increase risk: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2006;194:1234–42. doi: 10.1016/j.ajog.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 15.Berghella V, Rafael TJ, Szychowski JM, Orion AR, Owen J. Cerclage for Short Cervix on Ultrasound in Singleton Gestations with Prior Preterm Birth: Meta-analysis of Trials. Obstet Gynecol. 2011;117:663–71. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 16.Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, Sheffield JS, Perez-Delboy A, Wing DA, Guzman ER Vaginal Ultrasound Trial Consortium. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202:351.e1–6. doi: 10.1016/j.ajog.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ACOG Committee Opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115–6. doi: 10.1016/j.obstetgynecol.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 18.De Franco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, Soma-Pillay P, Porter K, How H, Schakis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, CAlda P, Bsharat M, Creasy GW. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan-Meier plot, by randomization assignment, for women receiving ≥1 dose of 17P (log-rank p=0.42).