Abstract
Despite apolipoprotein E’s important role in cholesterol transport and metabolism in the brain as well as its influence on Alzheimer’s disease, the impact of the human APOE genotype on cholesterol metabolism in brain has not been fully examined. This study was carried out to investigate APOE genotype effects on oxysterols measured. In this study the measurement of cholesterol and several oxysterols in the brains of human APOE ε2, ε3 and ε4 knock-in mice at 8 weeks and 1 year of age using gas chromatography mass spectrometry (GC-MS) demonstrated no APOE genotype or age effect on total brain cholesterol and the oxysterol 24-hydroxycholesterol. The level of 27-hydroxycholesterol was elevated in 1 year old animals for all APOE genotypes. Interestingly, lathosterol an indicator of cholesterol synthesis was significantly reduced in the 1 year old animals for all APOE genotypes. APOE ε4 expressing mice exhibited statistically lower levels of lathosterol compared to APOE ε2 in both the young and old mice. Oxidized cholesterol metabolites were significantly lower in APOE ε2 mice compared to other genotypes at 8 weeks old. Although minimal differences were observed between APOE E3 and E4 KI mice, these findings indicate that there are some clear APOE genotype specific effects on brain cholesterol synthesis and associated metabolic pathways, particularly in APOE ε2 KI mice.
Keywords: Oxysterols, APOE KI mice, Alzheimer’s disease, 24-hydroxycholesterol, 27-hydroxycholesterol
Apolipoprotein E (apoE) functions as an important carrier protein and plays a prominent role in the transport and metabolism of cholesterol, triacylglycerols (TAG), and phospholipids among various cells of the body (Lin et al., 1986, Mahley, 1988). It exists in three major isoforms: apoE2, apoE3 and apoE4, with frequencies of 7-8%, 77-78% and 14-15% respectively in the general population (Cedazo-Minguez and Cowburn, 2001), which result from the expression of the ε2, ε3 and ε4 alleles of the APOE gene (Zannis et al., 1981). APOE has been shown to be the major apolipoprotein in the central nervous system, where it plays a key role in neurobiology, and is also an important mediator of cholesterol and lipid transport in the brain (Adibhatla and Hatcher, 2008).The ε4 allele of APOE is recognised universally as the major genetic risk factor for Alzheimer’s disease (AD). APOE ε4 in particular is strongly associated with familial and sporadic late-onset AD (LOAD) (Strittmatter and Roses, 1996).
The brain is the most cholesterol-rich organ in the body (~25% of total amount) and must synthesize its own cholesterol de novo, since cholesterol cannot pass the blood brain barrier (BBB) (Björkhem et al., 1998, Björkhem et al., 2006). Consequently, regulation of cholesterol synthesis and metabolism in brain is largely independent of changes in the periphery (Björkhem et al., 2006). However, there is increasing evidence that changes in cholesterol metabolism plays a role in the pathogenesis of many neurodegenerative disorders, including AD and that brain cholesterol metabolites may act as clinically relevant biomarkers (Björkhem et al., 2006).
Although cholesterol itself is unable to enter the brain from the circulation in significant amounts, side-chain oxidised cholesterol metabolites also known as oxysterols are able to freely pass the BBB (Björkhem et al., 2006, Vaja and Schipper, 2007). Oxysterols including, 7β-hydroxycholesterol and 7-ketocholesterol, are formed as a result of oxidative damage to cholesterol (Figure 2). They can be measured in trace amounts in human tissue (Iuliano et al., 2003, Lee et al., 2008) and are elevated in diseases that involve oxidative stress including AD. Since there is considerable evidence for oxidative damage during neurodegeneration and cholesterol comprises a major lipid target in brain (~2% w/w) these oxysterols have the potential of being important biomarkers of oxidative damage in this tissue.
Figure 2.
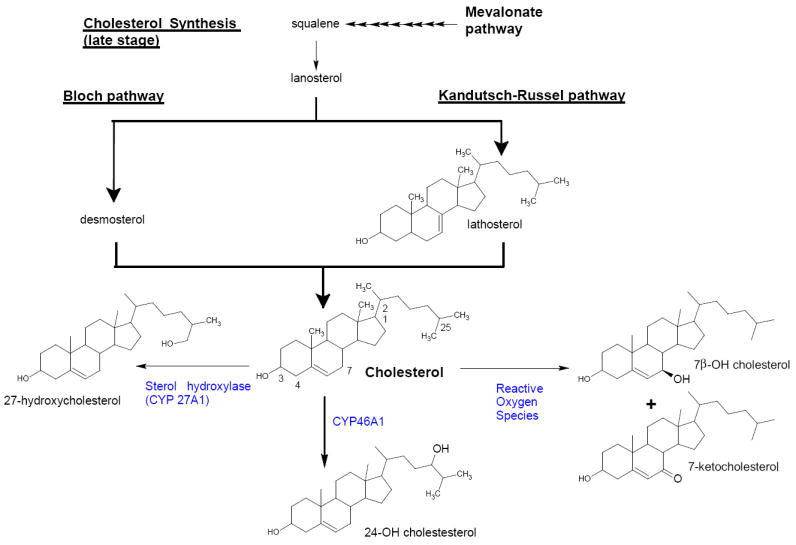
Oxysterol Synthesis Pathway
In spite of apoE’s important role in cholesterol transport and the growing evidence that altered cholesterol metabolism in the brain contributes to the pathogenesis of AD, the impact of different APOE genotype on brain cholesterol metabolism is poorly understood. To date, investigations performed on oxysterols mainly concentrated on 24-OH Chol and 27-OH Chol. It is also interesting to note that despite the critical role APOE plays in the pathogenesis of AD, no oxysterols have yet been studied in APOE knock-in mice. In this study, we have used gas chromatography mass spectrometry (GC-MS) with heavy isotope dilution to reliably and sensitively measure the level of cholesterol precursors, metabolites, plus oxidative damage products in brains from APOE knock-in mice expressing different human apoE isoforms.
Experimental Procedures
Animals
Male APOE knock-in (KI) mice, homozygous for human APOE ε2, ε3 and ε4 were originally obtained from Taconic (Germantown, NY, USA) and a colony derived and maintained at the Animal Resources Centre (ARC, Perth, Western Australia). These mice have been described previously (Piedrahita et al., 1992, Sullivan et al., 1997, Sullivan et al., 1998, Knouff et al., 1999). The mice were maintained on standard rodent chow (rat and mouse cubes, Specialty Feeds, Glen Forrest, WA, Australia) and kept until either 8 weeks or 1 year old. Food and water were accessible ad libitum. This study was conducted in accordance with the Australian code of practice for the care and use of animals for scientific purposes [National Health and medical research Council (NHMRC) 2004], and the experimental protocols were approved by the University of Western Australia Animal Ethics Committee.
Chemicals
Oxysterol standards 7β– hydrocholesterol, and 7-keto cholesterol were obtained from Sigma (St. Louis, MO, USA), lathosterol, 27-OH Chol and β-sitosterol were from Steraloids, (Newport, RI, USA ; 7β– hydroxycholesterol-d7, β-sitosterol-d7, lathosterol-d4 and 7-ketocholesterol-d7 were purchased from CDN Isotopes (Quebec, Canada); 27-OH Chol-d5, 24-OH Chol and 24-OH Chol-d7 were from Medical Isotopes, Inc (Pelham, NH, USA). All standards obtained were of the highest purity (>95%).
Sample preparation
Total brain homogenate (approximately 30mg of brain tissue in 0.5 ml PBS at 4°C) obtained from respective APOE ε2/ε3/ε4 KI mice was added to 2.5ml Folch solution (chloroform:methanol 1:3 containing 0.005% BHT) (4°C) in a 10ml centrifuge tube and incubated for 15minutes with gentle mixing, before centrifugation at 1,000g for 10minutes. The lower organic layer was collected in a clean glass container and after addition of internal standards (80ng of 7β– hydrocholesterol-d7, 120ng of 27-OH-Chol-d5, 400ng of lathosterol-d4, 80ng of 7-ketocholesterol-d7, 400ng of 24-OH-Chol-d7 and 400ng of β-sitosterol-d7) was evaporated under a stream of nitrogen. Hydrolysis was performed to measure the total (free & esterified) forms of oxidized lipids. 1ml of PBS and 1ml of 1M potassium hydroxide (prepared in 100% methanol) was added to the dried lipid extract. Hydrolysis was performed at 23°C for 15 h with gentle agitation. 1.35ml of 80mM formic acid (pH 4.5) was added to the tube and then neutralized with 0.25ml of 2.5M HCl. The mixture was filtered with 25mm nylon disc (60 μm pore size).
Extraction and derivatization of oxysterols
Mixed anion-exchange SPE columns were preconditioned with 2ml methanol; followed by 2ml of 20mM formic acid pH 4.5. The hydrolyzed samples were loaded onto the columns. These columns were washed with 2ml of 2% ammonium hydroxide and then with 2ml of 40% methanol/formic acid pH 4.5. After the wash, 2ml of hexane and then 2ml of ethyl acetate/hexane (30:70) was added to elute cholesterol and oxysterols. The eluted samples were evaporated under a stream of nitrogen. The aliquots were derivatised with 25μl acetonitrile and 25μl BSTFA and 1% TMCS for an hour at room temperature. The samples were evaporated under a stream of nitrogen and later reconstituted in 35μl of undecane prior to injection into the GC-MS.
Analysis of oxysterols by gas chromatography-mass spectrometry
For oxysterols measurement, the derivatized samples were analysed using an Agilent 5975 inert XL mass selective detector. Helium was used as the carrier gas at a flow rate of 0.8ml/min, derivatised samples (1μl) were injected splitless into the GC injection port (280°C). Column temperature was increased from 160°C to 300°C at 40°C/min after 1 min at 160°C then held at 300°C for 6 mins. Selective ion monitoring was performed using electron ionization mode at 70eV (with ion source maintained at 230°C and the quadrupole at 150°C) to monitor one target ion and 2 qualifier ions selected from each compound’s mass spectrum to optimize sensitivity and specificity. Quantitation of oxysterols was achieved by relating its peak area of target ion to its corresponding internal standard peak. Careful minimisations of artifactual oxidation to cholesterol during the analytical procedure, anaerobic and lower temperature hydrolysis conditions that have been reported to minimize oxidation were used (Iuliano et al., 2003).
Statistical analyses
Means and standard deviations were calculated for all variables using conventional methods. A Kruskal-Wallis ANOVA by ranks for nonparametric data was used to evaluate significant differences between the groups of animals. All data were analyzed using Matlab version 6.5, Massachusetts, United States of America.
Results
All sterols except 25- OH Chol were significantly detected in all brain samples. Significant age-associated increases in 27-OH Chol levels were observed for all three APOE genotypes. It was noted that the increase in APOE ε2 KI mice was significantly higher (P<0.01) in 1 year old APOE ε2 KI mice when compared to 8 weeks old APOE ε2 KI mice (Figure 1a). Significant increases in 27-OH Chol were also observed in APOE ε3 (P<0.05) and APOE ε4 (P<0.05) KI mice when compared to their respective 8 weeks old APOE KI matched mice (Figure 1a). There was a stronger statistical age-related difference in APOE ε2 KI mice as compared to the other genotypes but this is predominantly due to the lower variance in the APOE ε2 KI mice. No significant differences were noted in the level of 24-OH Chol across the various APOE KI mice (Figure 1b). There was also no significant difference with age in the respective APOE KI mice for 24-OH Chol levels.
Figure 1.
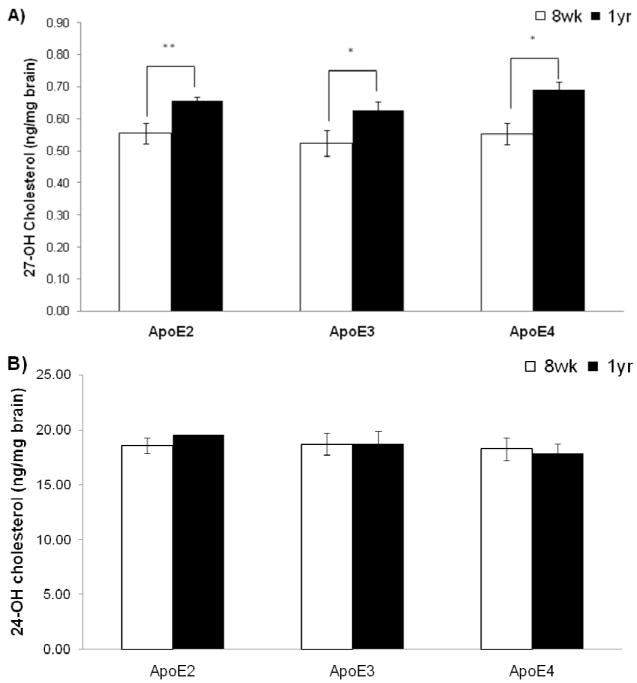
Comparison of A) 27-OH cholesterol levels, and B) 24-OH cholesterol levels in APOE ε2, ε3 and ε4 KI mice aged 8 weeks old and 1 year old. Values are calculated as means ± SEM of 5 animals. * P<0.05 8weeks old vs. 1 year old, ** P<0.01 8 weeks old vs. 1 year old.
Significant increases in 7-β hydroxycholesterol, β-sitosterol and 7-keto cholesterol (P<0.01) were observed in 1year old APOE ε2 KI mice as compared to 8 weeks old APOE ε2 KI mice. However, the level of lathosterol (P<0.01) significantly decreased with age in APOE ε2 KI mice. A significant age-dependent increase in β-sitosterol (P<0.05) level was observed in APOE ε3 KI mice. In aged APOE ε4 KI mice, significant increases in 27-OH Chol (P<0.05) levels were observed. Lathosterol level (P<0.01) significantly declined with age in APOE ε4 KI mice.
There were significant differences in 7-β hydroxycholesterol (P<0.01) and 7-keto cholesterol (P<0.01) when 8 weeks old APOE ε2 KI mice were compared to other age-matched APOE KI mice (Table 1A). However, lathosterol level was only significantly higher in 8 weeks old APOE ε2 KI mice (P<0.05) compared to age-matched APOE ε4 KI mice (Table 1A). The level of lathosterol was significantly decreased in 1 year old APOE ε4 KI mice as compared to age-matched APOE ε2 KI mice (Table 1B). Although significant age dependent increases in 27-OH Chol levels were observed in mice expressing either of the three apoE isoforms (Figure 1), no significant difference was observed between APOE genotypes in these aged matched APOE KI mice (Table 1A & B). The level of lathosterol declined with age in mice expressing either, apoE2, apoE3 or apoE4 isoforms. Levels of lathosterol were highest in APOE ε2 KI mice at both ages. No differences in the lathosterol levels were observed between the APOE ε3 KI and APOE ε4 KI mice. There were no significant differences between APOE ε3 and APOE ε4 KI mice under the current experimental conditions used in this study.
Table 1.
Comparison of oxysterol levels in A) 8 week old and B) 1 year old APOE ε2, ε3 and ε4 KI mice. Values are calculated as means ± SEM of 5 animals.
| 8 weeks (A) | 1 year (B) | |||||
|---|---|---|---|---|---|---|
| APOE ε2 | APOE ε3 | APOE ε4 | APOE ε2 | APOE ε3 | APOE ε4 | |
| lathosterol | 21.82 ± 1.39 o | 18.12 ± 1.66 | 17.19 ± 0.77 | 15.66 ± 0.26 oo | 13.80 ± 0.99 | 12.60 ± 0.92 |
| 7-b OH-cholesterol | 0.67 ± 0.10 ** oo | 2.61 ± 0.35 | 2.67 ± 0.32 | 2.71 ± 0.18 | 2.87 ± 0.37 | 3.58 ± 0.51 |
| b-sitosterol | 3.64 ± 0.29 | 3.57 ±0.40 | 4.14 ± 0.34 | 5.56 ±0.13 | 5.24 ± 0.31 | 5.57 ± 0.60 |
| 24-OH cholesterol | 18.53 ±0.71 | 18.70 ± 0.97 | 18.24 ± 1.03 | 19.50 ± 1.37 | 18.74 ± 1.11 | 17.86 ± 0.84 |
| 7-keto cholesterol | 2.60 ±0.51 ** oo | 8.33 ±1.01 | 7.34 ± 0.58 | 8.70 ±1.29 | 9.20 ± 1.21 | 11.56 ± 1.83 |
| 27-OH cholesterol | 0.56 ± 0.03 | 0.52 ± 0.04 | 0.55 ± 0.03 | 0.66 ± 0.01 | 0.63 ±0.03 | 0.69 ± 0.03 |
P<0.05 APOE ε2 KI mice vs. APOE ε3 KI mice,
P<0.01 APOE ε2 KI mice vs. APOE ε3KI mice,
P<0.05 APOE ε2 KI mice vs. APOE ε4 KI mice,
P<0.01 APOE ε2 KI mice vs. APOE ε4KI mice. No significant differences were detected in APOE ε3 KI mice vs. APOE ε4 KI mice at 8 weeks of age.
Discussion
This is the first study looking at the influence of APOE genotypes on oxysterol levels in the brains of APOE KI mice. Oxysterols, in particular 24-OH Chol, 25-OH Chol and 27-OH Chol are able to freely pass through the BBB. 24-OH Chol is the major neuronal cholesterol metabolite catalysed by CYP46A1 (mainly expressed in brain) (Björkhem et al., 1998, Björkhem et al., 2006) (Figure 2). The conversion of cholesterol into 24-OH Chol in the brain is responsible for maintaining cholesterol homeostasis and the removal of excess cholesterol from the brain.
No significant differences were noted in this study for 24-OH Chol, comparing either age-effect or APOE genotype. This may be due to the lack of any AD-like pathology in these APOE KI mice under the experimental conditions employed in this study. Levels of 24-OH Chol measured in AD patients are correlated to brain atrophy as measured by magnetic resonance imaging (Leoni, 2005) and this cholesterol metabolite has been reported to be elevated in the cerebrospinal fluid (CSF) and serum of AD patients (Lütjohann et al., 2000, Papassotiropoulos et al., 2002). The lack of significant differences in total brain 24-OH Chol between the different animal groups in our study may indicate that there is no APOE genotype specific effects in KI mice on this cholesterol metabolic pathway, including any effect on CYP46A1 activity where there is an absence of environmental challenges such as a high-fat/high-cholesterol diet. Thus, the levels of 24-OH Chol in our study were independent of APOE genotype under the conditions employed. It is possible that potential changes in some specific brain regions were masked by total brain homogenization. Others have previously reported changes of 24-OH Chol in brain regions (Heverin et al., 2004), plasma (Lütjohann et al., 2000), and CSF (Papassotiropoulos et al., 2002). However, there was a lack of consistent findings between reports. Lutjohann et al., 2000 concluded in their study that elevated plasma 24-OH Chol levels in AD patients were not significantly influenced by APOE genotype, whilst Papassotiropoulos et al., (Papassotiropoulos et al., 2002) reported a positive correlation between 24-OH Chol CSF levels and APOE ε4 alleles in 32 AD patients. However, it could be argued that these different findings may be explained by 24-OH Chol levels varying depending on the stage of AD progression. The levels of 24-OH Chol increase in the initial stages of AD as excess cholesterol is released from cholesterol-rich cell membranes that are destroyed during the process of neurodegeneration (Lütjohann et al., 2000, Papassotiropoulos et al., 2002), but as more neurons expressing 24S-hydroxylase die i.e. in advanced AD, the conversion to 24-OH Chol and efflux from the brain is less efficient (Papassotiropoulos et al., 2002). While the effects of APOE genotype are unclear, Abildayeva et al., 2006 demonstrated that 24-OH Chol induces APOE gene expression and increases apoE protein levels in primary rat and mouse astrocytes in a dose dependent manner. Significant correlation between apoE levels and 24-OH Chol levels were observed in the cerebrospinal fluid (CSF) of mild cognitive impairment (MCI) and AD patients (Shafaati et al., 2007). Thus apoE expression is clearly influenced by 24-OH Chol levels irrespective of APOE genotype.
27-OH Chol is formed in multiple organs by sterol hydroxylase (CYP27A1) and can influx across the BBB into the brain from peripheral circulation (Figure 2). We observed significant age associated increases in 27-OH Chol levels in all three APOE genoytpes, which may reflect an increased uptake of 27-OH Chol into the brain of 1 yr old mice, or a reduction in its metabolism. Leoni et al., (2002) demonstrated significant accumulation of 27-OH Chol in the brain of AD patients. The brain is known to have low levels of CYP27A1 consistent with negligible endogenous 27-OH Chol production as opposed to high levels of 24-OH Chol in this organ (Lütjohann et al., 1996). Consistent with our study, significant influx of 27-OH Chol from peripheral circulation into the brain had also been observed in humans (Heverin et al., 2005) and mice (Heverin et al., 2004). In the brain, 27-OH Chol has been demonstrated to be metabolized by a combination of 3 different enzymes in a novel metabolic route forming 7α-hydroxy-3-oxo-4-cholestenoic acid and subsequently efficiently eliminated from the human brain (Meaney et al., 2007). This metabolic route likely explains why low 27-OH Chol levels in brain were detected in our study, in common with previous analytical studies of both mouse and human brain (Heverin et al., 2004, Heverin et al., 2005). Consequently this oxysterol has been hypothesized to provide a link between peripheral hypercholesterolemia and AD, since there is good correlation between plasma levels of cholesterol and 27-OH Chol (Björkhem et al., 2006). There was no association between APOE genotype and brain cholesterol levels in this study.
Lathosterol, a precursor of cholesterol (Figure 2) and established biomarker of de novo synthesis of cholesterol (Heverin et al., 2004, Lütjohann et al., 2004) was significantly lower in APOE ε4 KI mice brain, compared to APOE ε2 mice at both 8 wk and 1 yr old. Such an effect of APOE allele on brain lathosterol has not been reported previously and it is not known by what mechanisms apoE may exert this influence. APOE ε4 may down-regulate or APOE ε2 up-regulate the lathosterol pathway in various cell types, possibly by differences in the type and quantity of oxysterols that are transported by these different apoE isoforms. Different oxysterols are known to have a variety of influences on cholesterol synthesis that is usually dependent on their specific binding to receptors that regulate the transcription of cholesterol synthetic enzymes (Vaja and Schipper, 2007).
Since the level of brain cholesterol was not significantly different between the APOE KI mice, we can conclude that the effect on the lathosterol pathway does not limit the rate of cholesterol synthesis and/or that alternative synthetic pathways leading to cholesterol synthesis are able to compensate, for example the parallel synthetic pathway via desmosterol. In mice Lutojohann et al., (2002) reported that brain lathosterol after 3 months of age increase whilst desmosterol decreases, but another study in mice found an age dependent decline between 2 and 10 months of age in brain desmosterol and lathosterol (Valenza et al., 2007). Similar to the latter study, the levels of lathosterol in our APOE KI study exhibited a decrease with age across all three APOE genotypes, although, this decline was only significant in APOE ε2 and APOE ε4 KI mice.
Examination of cholesterol synthesis in human hippocampi revealed an age dependent decrease in lathosterol, but levels of desmosterol and cholesterol were unaffected (Thelen et al., 2006). Further analytical investigations are required to confirm the potential of lathosterol as a possible differentiation biomarker between apoE2 and apoE4 isoforms during aging in animals and humans. Based on our data that APOE ε4 KI mice continued to have significantly lower brain lathosterol at older ages, coupled with strong evidence for an age dependent decline in brain lathosterol. We therefore speculate that the association of AD with aging and apoE4 may be related to earlier critical decreases in lathosterol levels in AD brains. Further studies will determine whether this effect on lathosterol is selectively increased in APOE ε4 KI mice in response to AD-associated environmental risk factors such as a high fat diet.
Significant age related increases in brain levels of β-sitosterol were also detected in the APOE KI mice in this study. This dietary derived phytosterol is not synthesized in brain but transported in plasma and may pass the BBB more readily during aging in APOE KI mice. In particular APOE ε2 KI mice had larger age dependent increases, although this was predominantly due to lower levels of sterols at a younger age compared to APOE ε3 and APOE ε4 KI mice. Further investigation is required to determine if β-sitosterol may contribute to the protective role apoE2 is thought to play in the pathogenesis of AD. Previous studies also detected a significant increase in β-sitosterol in APP23 transgenic mice (β-amyloid precursor protein carrying the Swedish mutation), but not wild-type mice (Lütjohann et al., 2002).
Due to the fact that APOE ε4 is a major risk factor as well as overwhelming evidence supporting the role of oxidative stress and accumulation of damage in AD amongst the APOE KI mice, one specific aim of our analysis was to measure biomarkers of oxidative damage to cholesterol (7-ketocholesterol and 7-β hydroxycholesterol). Basal levels of each oxysterols in brain measured in this study, representing approximately 2-5/104 molecules of cholesterol have not been reported previously and were higher than our previous measurements in plasma or other tissues of mice (unreported data not shown). This is likely due to the fact that compared to other tissues the brain has the highest oxygen consumption and level of mitochondrial and oxidative metabolism that generates increased levels of reactive oxygen species (ROS). Also, higher cholesterol levels in brain represent an increased target for attack by reactive oxygen species. Our study shows that oxysterol biomarkers at 8 weeks old were reduced in apoE2 KI mice compared to apoE4 KI mice and apoE3 KI mice. A greater significant age dependent increase in cholesterol oxidative damage was observed in apoE2 KI mice than apoE3 KI mice and apoE4 KI mice. Age dependent increases in oxidative stress and damage to brain have been frequently reported in animals and humans (Barnham et al., 2004) and have contributed to the oxidative damage hypothesis of ageing, particularly age dependent neurodegeneration. APOE isoforms have also been demonstrated to have effects on periphery and vasculature. The low density lipoproteins (LDL) concentration and mass are highest in the APOE ε4 KI mice homozygotes compared to the other genotypes, while their concentration is lowest in APOE ε2 KI mice (Murdoch et al., 2007). Similarly, the presence of APOE ε2 allele has a lower incidence of vasoconstriction compared to that of APOE ε4 allele (Paris et al., 1998). However, it is unclear exactly how such mechanisms may influence brain cholesterol and oxysterols since evidence illustrates that blood lipoproteins are unable to cross the blood brain barrier.
To summarise, our data illustrates that APOE genotype, particularly APOE ε2, does have significant effects on cholesterol synthesis and metabolism as well as cholesterol oxidative damage, which is also influenced by ageing. In our study, APOE genotype does not influence total brain cholesterol levels. In addition we observed minimal differences between APOE ε3 and ε4, in contrast to APOE ε4 known elevated risk in AD. Our findings are similar to previous work (Bandaru et al. 2007) suggesting that APOE ε4 is not associated with disturbances in brain lipids without an underlying neurological disorder, such as Alzheimer’s disease. Although the APOE gene is the strongest genetic factor associated with AD, APOE KI mice are not exactly an AD model as they do not accumulate beta-amyloid plaques. However we believe that other physiological stress factors such as a high fat/high cholesterol diet and/or hormonal depletion may also be required (in addition to age) in order to observe significant differences in cholesterol and oxysterols between APOE ε3 and ε4 that influence AD development. The mechanisms regulating the brain cholesterol metabolome and their influence on development of AD have yet to be fully elucidated. Interestingly, the accumulation of 27-OH Chol in the brain of ageing mice from this study was also found to be the most significant change in the oxysterol profile in the brains of AD patients (Leoni et al., 2002). In this study, no significant differences were observed in 24-OH Chol. However, there are contradicting reports in the literature of 24-OH Chol in humans. Lutjohann et al., (2000) concluded in their study that elevated plasma 24-OH Chol levels in AD patients were not significantly influenced by APOE genotype, whilst Papassotiropoulos et al., (2002) reported a positive correlation between 24-OH Chol CSF levels and APOE ε4 alleles in 32 AD patients. The effect of ageing and apoE on cholesterol synthesis and metabolism in the brain, reinforce previous studies highlighting that disturbance of cholesterol turnover is associated with increased risk of AD. Changes in cholesterol intermediate levels may be clinically relevant and potentially serve as diagnostic markers. Further studies are necessary to decipher the exact importance and functional contribution of apoE and cholesterol intermediates/metabolites that may lead to the development of neurodegeneration.
Acknowledgments
Wei Ling Florence Lim is a recipient of a Scholarship for International Research Fees and University International Stipend from University of Western Australia. This work was supported by a program grant from the NIH (2 P01 AG010491-10A2) to RNM and SEG. Andrew Jenner is grateful to the NMRC Singapore for support through the grant (R-183-000-155-214/133). MRW, RNM and MJS were funded by the National Medical Research Council (R-183-000-224-213). MRW was also funded by the Singapore National Research Foundation under CRP Award No. 2007-04, the Biomedical Research Council of Singapore (R-183-000-211-305).
Abbreviations
- 24-OH Chol
24S-hydroxycholesterol
- 27-OH Chol
27-hydroxycholesterol
- 25-OH Chol
25-hydroxycholesterol
- APOE
apolipoprotein E
- AD
Alzheimer’s Disease
- HMG-CoAR
3-hydroxy-3-methyl-CoA reductase
- BBB
blood-brain barrier
- Aβ
amyloid beta
- GC-MS
gas chromatography mass spectrometry
- BHT
butylated hydrotoluene
- SPE
solid phase extraction
- BSTFA
N,O-Bis(trimethylsilyl)trifluoroacetamide
- TMCS
trimethylchlorosilane
- LOAD
Late-onset Alzheimer’s disease
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, Haughey NJ. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiology of Aging. 2009;4:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and Alzheimer’s disease. Acta Neurologica Scandinavica. 2006;114:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Lütjohann D, Diczfalusy U, Ståhle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. Journal of Lipid Research. 1998;39:6. [PubMed] [Google Scholar]
- Cedazo-Minguez A, Cowburn RF. Apolipoprotein E: a major piece in the Alzheimer’s disease puzzle. J Cell Mol Med. 2001;5:254–266. doi: 10.1111/j.1582-4934.2001.tb00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heverin M, Bogdanovic N, Lütjohann D, Bayer T, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Björkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. Journal of Lipid Research. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- Heverin M, Meaney S, Lütjohann D, Diczfalusy U, Wahren J, Björkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. Journal of Lipid Research. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- Iuliano L, Micheletta F, Natoli S, Corradini SG, Iappelli M, Elisei W, Giovannelli L, Violi F, Diczfalusy U. Measurement of oxysterols and α-tocopherol in plasma and tissue samples as indices of oxidant stress status. Analytical Biochemistry. 2003;312:7. doi: 10.1016/s0003-2697(02)00467-0. [DOI] [PubMed] [Google Scholar]
- Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. The Journal of Clinical Investigation. 1999;103:8. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-YJ, Huang SH, Jenner AM, Halliwell B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radical Biology & Medicine. 2008;44:9. doi: 10.1016/j.freeradbiomed.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Leoni V. Department of Laboratory Medicine, Division of Clinical Chemistry. Stockholm, Sweden: Karolinska University Hopital Huddinge; 2005. On the possible use of oxysterols for the diagnosis and evaluation of patients with neurological and neurodegenerative diseases; p. 89. [Google Scholar]
- Leoni V, Masterman T, Diczfalusy U, De Luca G, Hillert J, Bjorkhem I. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci Lett. 2002;331:163–166. doi: 10.1016/s0304-3940(02)00887-x. [DOI] [PubMed] [Google Scholar]
- Lin CT, Xu YF, Wu JY, Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. Journal of Clinical Investigation. 1986;78:947–958. doi: 10.1172/JCI112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütjohann D, Breuer O, Ahlborg G, Nennesmo I, Sidén Å, Diczfalusy U, Björkhem I. Cholesterol homeostasis in human brain: Evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci USA. 1996;93:6. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütjohann D, Brzezinka A, Barth E, Abramowski D, Staufenbiel M, Bergmann Kv, Beyreuther K, Multhaup G, Bayer TA. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. Journal of Lipid Research. 2002;43:8. doi: 10.1194/jlr.m200071-jlr200. [DOI] [PubMed] [Google Scholar]
- Lütjohann D, Papassotiropoulos A, Björkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, Bergmann Kv, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. Journal of Lipid Research. 2000;41:195–198. [PubMed] [Google Scholar]
- Lütjohann D, Stroick M, Bertsch T, Kühl S, Lindenthal B, Karin T, Andersson U, Björkhem I, Bergmann Kv, Fassbender K. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 2004;69:8. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, Brzezinka A, Barth E, Abramowski D, Staufenbiel M, von Bergmann K, Beyreuther K, Multhaup G, Bayer TA. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J Lipid Res. 2002;43:1078–1085. doi: 10.1194/jlr.m200071-jlr200. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, Papassotiropoulos A, Bjorkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von Bergmann K, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Meaney S, Heverin M, Panzenboeck U, Ekström L, Axelsson M, Andersson U, Diczfalusy U, Pikuleva I, Wahren J, Sattler W, Björkhem I. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7α-hydroxy-3-oxo-4-cholestenoic acid. Journal of Lipid Research. 2007;48:8. doi: 10.1194/jlr.M600529-JLR200. [DOI] [PubMed] [Google Scholar]
- Murdoch SJ, Boright AP, Paterson AD, Zinman B, Steffes M, Cleary P, Edwards K, Marcovina SS, Purnell JQ, Brunzell JD. LDL composition in E2/2 subjects and LDL distribution by Apo E genotype in type 1 diabetes. Atherosclerosis. 2007;192:138–147. doi: 10.1016/j.atherosclerosis.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, Ptok U, Bjorkhem I, von Bergmann K, Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res. 2002;36:27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- Paris D, Town T, Parker TA, Humphrey J, Mullan M. Isoform-specific vasoconstriction induced by Apolipoprotein E and modulation of this effect by Alzheimer’s β-amyloid peptide. Neuroscience Letters. 1998;256:73–76. doi: 10.1016/s0304-3940(98)00764-2. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:5. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaati M, Solomon A, Kivipelto M, Björkhem I, Leoni V. Levels of ApoE in cerebrospinal fluid are correlated with Tau and 24S-hydroxycholesterol in patients with cognitive disorders. Neuroscience Letters. 2007;425:5. doi: 10.1016/j.neulet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annual Review of Neuroscience. 1996:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allelle enhances dist-induced hypercholesterolemia and atherosclerosis. Journal of Biological Chemistry. 1997;272:9. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeba N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting fron gene replacement of mouse ApoE with human Apoe*2. The Journal of Clinical Investigation. 1998;102:6. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen KM, Falkai P, Bayer TA, Lütjohann D. Cholesterol synthesis rate in human hippocampus declines with aging. Neuroscience Letters. 2006:15–19. doi: 10.1016/j.neulet.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Vaja J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer’s disease. Journal of Neurochemistry. 2007;102:11. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- Valenza M, Carroll JB, Leoni V, Bertram LN, Björkhem I, Singaraja RR, Di Donato S, Lutjohann D, Hayden MR, Cattaneo E. Cholesterol biosynthesis pathway is disturbed in YAC 128 mice and is modulated by huntingtin mutation. Human Molecular Genetics. 2007;16:12. doi: 10.1093/hmg/ddm170. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Just PW, Breslow JL. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981;33:11–24. [PMC free article] [PubMed] [Google Scholar]


