Abstract
Energy homeostasis- ensuring that energy availability matches energy requirements- is essential for survival. One way that energy balance is achieved is through coordinated action of neural and neuroendocrine feeding circuits, which promote energy intake when energy supply is limited. Feeding behavior engages multiple somatic and visceral tissues distributed throughout the body – contraction of skeletal and smooth muscles in the head and along the upper digestive tract required to consume and digest food, as well as stimulation of endocrine and exocrine secretions from a wide range of organs. Accordingly, neurons that contribute to feeding behaviors are localized to central, peripheral and enteric nervous systems. To promote energy balance, feeding circuits must be able to identify and respond to energy requirements, as well as the amount of energy available from internal and external sources, and then direct appropriate coordinated responses throughout the body.
Keywords: food intake, CNS circuits, energy balance, obesity
INTRODUCTION
This review will outline a conceptual framework for understanding the ways in which environmental and physiological stimuli impinge upon neural circuits to determine food intake. More comprehensive descriptions of the myriad of circuit components and types of input/output signals characteristic of feeding circuits have been provided elsewhere. This paper will identify points of convergence among outputs and inputs within the context of a “core feeding neuraxis”, and illustrate how this core conceptualization fosters the development of theoretical and experimental approaches to understand feeding behavior regulation under different internal and external contexts.
The framework we present is based on the appreciation that feeding behavior, like all motivated behaviors, arises from the coordinated activity of multiple central neuronal populations. Hence, experimental studies aimed at understanding the neurobiology of feeding must encompass neuroanatomical, neurophysiological, and molecular genetic approaches that contribute to behavioral expression. These approaches commonly involve manipulations of experimentally accessible variables, such as the quantity and composition of the food provided, pharmacological stimulation and inhibition, or constitutive, chronic genetic modifications. Following the rapid progress in identifying neuropeptide and hormonal signals of energy status in the late 20th century, an array of genetic and pharmacological tools were developed to modulate circuits regulating homeostatic aspects of feeding. While it is clear that cognitive and reward circuits significantly contribute to regulate feeding behavior, the tools to precisely interrogate their roles in feeding were not available. However, recent technological advances in the temporal and neuroanatomical precision of neurobiological interventions, discussed in the context of experimental data in this review, provide new opportunities to significantly reduce gaps in our understanding of how neural activity in different brain circuits initiates or terminates feeding.
Food intake is essential to survival, and accordingly, multiple sensorimotor, neuroendocrine and cognitive processes participate in its control. These systems rely on the evaluation of relationships between external environmental stimuli and internal neurohumoral signals related to nutrient availability. Ingestive behavior occurs in two phases: appetitive and consummatory. The appetitive phase of ingestion comprises somatomotor acts and tasks involved in the approach to food, food seeking, and activity required to initiate direct contact with food The consummatory phase of ingestion comprises somatomotor acts that occur during direct contact with food that make it possible to derive the nutrient value of food, such as licking, biting, chewing, and swallowing.
For mammals, including man, the meal is the functional behavioral unit of food intake. Total daily energy intake is determined as the product of the number of meals consumed and the energetic value of each meal. Each meal is an episode of food consumption preceded by food seeking, and ingestion during a meal is maintained by intermittent oral contact with food followed by chewing and swallowing. Meal duration, and the amount of food consumed during an individual meal, is determined by excitatory oral or post-oral signals arising from nutrient contact with the multiple regions spanning the alimentary tract. For example, gustatory sensations arising from oral contact with palatable food stimuli can increase the rate and amount of food consumption during a single feeding episode.
Meals are terminated as a result of the central nervous system processing of neurohumoral signals arising from the sensory consequences of ingestion. These typically include post-oral neural signals and blood borne signals arising from gut peptide factors released during ingestion of a meal. Signals that are important in meal termination, such as gastric distension, intestinal nutrient exposure, and gut satiety peptide release are well characterized. In contrast, the signals important in initiating appetitive responding and meal initiation during normal meal taking are not well understood, yet several “orexigenic”, or feeding-stimulatory, peptides have been proposed to be involved. Agouti related peptide (AgRP) is one such candidate, as it rapidly initiates food seeking and operant responding for food, as do Neuropeptide Y (NPY) and ghrelin (2; 124).
Smith (112) has proposed a conceptual framework of direct and indirect controls of an individual meal, to aid in the identification and characterization of neurobiological substrates and processes important in determining food intake. According to this framework, direct controls are those events arising from direct contact with food during the consummatory phase of ingestion. Examples of direct controls include the gustatory neural signals promoted by oral contact with palatable food, as well as neural gastric distension signals elicited by the accumulation of food in the stomach after a meal. In contrast, indirect control signals are those factors that modulate the ability of direct controls to determine meal size. These indirect controls may include stimuli arising from the external environment, such as those that predict the availability of food or light in the environment, as well as biological processes that modulate the need for nutrients required to maintain metabolic homeostasis, such as the endocrine and energetic correlates of growth and reproduction, or the plasma levels of circulating hormones reflecting adiposity stores, such as leptin and insulin.
CONCEPTUALIZING A CORE FEEDING NEURAXIS
The first part of this review will outline nodes of convergence where interoceptive, cognitive and arousal inputs (Figure 1, red arrows) could be integrated and relayed to coordinately regulate the output of somatomotor, visceromotor and secretomotor systems (Figure 1, blue arrows). Key elements of these pathways are highlighted in Figure 1, hereafter referred to as the “core feeding neuraxis”. Actions of the core feeding neuraxis are sufficient to regulate both involuntary as well as volitional aspects of feeding behavior.
Figure 1.
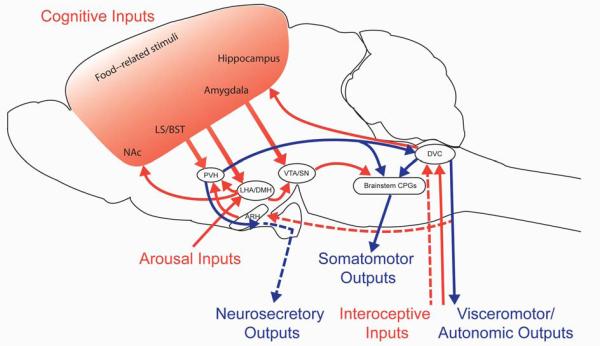
Outline of circuits involved in controlling feeding behavior. The diagram depicts a subset of the neuronal connections reported to influence feeding, chosen to highlight opportunities for integration between different classes of inputs (red arrows) and outputs (blue arrows). These inputs and outputs are transmitted via neuronal projections (solid lines) as well as humoral factors (dotted lines). Note that arrows reflect connectivity between two brain regions and not an actual anatomical pathway. Interoceptive inputs transmit information about energy availability that is essential for homeostatic regulation of food intake. Sensory meal-related signals from the alimentary tract are relayed to the brain through autonomic and sensory nerves, as well as humoral factors. Cognitive inputs are required for volitional feeding; food-related cues are processed in cortico-limbic circuits to confer learned aspects of feeding as well as to determine the reward value of food. A state of arousal is transmitted by neurons in the LHA to a network of highly interconnected neurons in the hypothalamus and brainstem. Feeding behavior is directed through the coordinated signals of three classes of output pathways. Somatomotor outputs from brain stem CPGs direct the muscular contractions needed to seek and consume food. Visceromotor outputs through the autonomic nervous system regulate the secretion of factors involved in nutrient processing, metabolism and storage. Neurosecretory outputs regulate the release of pituitary hormones that influence feeding behavior. Abbreviations: ARH, arcuate nucleus of the hypothalamus; BST, bed nuclei of the stria terminalis; CPG, central pattern generator; DMH, dorsomedial nucleus of the hypothalamus; DVC dorsovagal complex (area postrema, nucleus of the solitary tract, and dorsal motor nucleus of the vagus); LHA, lateral hypothalamic area; LS, lateral septum; NAc, nucleus accumbens; PVH, paraventricular nucleus of the hypothalamus; SN, substantia nigra; VTA, ventral tegmental area.
FEEDING CIRCUIT OUTFLOWS
Feeding behavior may be construed as the consequence of activating three distinct classes of effector pathways that lie downstream of circuits in the central nervous system (CNS) (9; 119)(Figure 1, blue arrows). Somatomotor outputs determine the contraction of muscles in the face, mouth, jaw and upper gastrointestinal (GI) tract required to consume and digest food. Visceromotor outputs are transmitted via sympathetic and parasympathetic innervation of the smooth muscle of the GI tract, as well as other organs that secrete factors involved in nutrient processing, metabolism and storage. Finally, neurosecretory outputs regulate the release of pituitary hormones that influence feeding behavior.
Somatomotor Outputs
The consumption of food involves coordinated muscular contractions of jaw, facial, and lingual muscles as well as the upper esophagus. Key nodes in the somatomotor system controlling feeding are outlined in Figure 1. Chewing is associated with rhythmic opening and closing of jaw in conjunction with repetitive movements of the lips, tongue, and cheeks. These behaviors are coordinately regulated by central pattern generators (CPGs) in the caudal pons and medulla, which serve as critical points of integration between higher cortical inputs and motor neuron outputs (75). Swallowing involves complex patterns of contraction and relaxation in 25 muscle pairs (78). The swallowing CPG is composed of two groups of neurons - neurons in the nucleus of the solitary tract (NTS) integrate gustatory and visceral inputs with cortico-limbic inputs and relay this information to neurons in the ventrolateral medulla (VLM), which provide the drive to motor nuclei in the brainstem and uppermost levels of cervical spinal cord (58). Thus, the NTS represents a key node of integration of inputs regulating consummatory aspects of feeding; processed inputs are then relayed to coordinately direct somatomotor outputs of the brainstem CPGs (58). It has also been reported that inputs from some hypothalamic sites (e.g., paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA)) and cortico-limbic sites (e.g., amygdala and substantia nigra (SN)) influence food consumption, although it is unclear whether these influences are mediated via the NTS or via direct projections to brainstem CPGs (58; 119).
Autonomic/Visceromotor Outputs
As food is conveyed down the GI tract via peristalsis, it is processed by a series of enzymes secreted from the stomach and intestines, as well as organs such as the pancreas and liver. Pre-ganglionic sympathetic and parasympathetic neurons innervate endocrine and exocrine glands, smooth musculature and fat. Sympathetic outflow is transmitted via preganglionic neurons in the spinal cord, which modulate the activity of motor neurons in sympathetic ganglia, adjacent to spinal cord, and then project to their final targets. In contrast, parasympathetic outflow is transmitted via long range projections from preganglionic neurons in the dorsal motor nucleus of the vagus (DMX) that project to ganglia adjacent to the gut, as well as other organs that contribute to digestion (such as the pancreas). In addition to metabolizing food, digestive processes also result in the generation of new nutrient, hormonal and mechanosensory stimuli (such as gastric distension), which provide feedback to the system to regulate meal size and frequency. The dorsovagal complex (DVC), composed of the NTS, DMX and area postrema (AP), is a major site of cross-talk between feeding circuits and the autonomic nervous system. In turn, DVC function is influenced by descending projections from neurons in the autonomic compartment of the PVH (3; 12; 46; 119). Notably, inhibition of the activity of caudally-projecting PVH neurons expressing oxytocin (OXY), either by gamma-aminobutyric acid (GABA) and peptide signaling from AgRP/NPY neurons or by optogenetic manipulations, is sufficient to drive robust feeding behavior (3).
Neuroendocrine/Secretomotor Outputs
In addition to autonomic regulation of food intake and digestive metabolism, populations of neurosecretory cells in the hypothalamus influence food intake by secreting factors into the blood. Magnocellular neurons in the PVH and supraoptic nucleus project to the posterior pituitary, where they release OXY and arginine vasopressin (AVP) into the blood via hypophyseal capillaries. Parvicellular neurons in the PVH project to the median eminence, where they release hormones in the hypophyseal portal system to regulate hormone release from all endocrine cell types in the anterior pituitary. Whereas the action of several pituitary hormones can indirectly influence food intake via effects on energy expenditure, hormones of the hypothalamus-pituitary-adrenal (HPA) axis have been reported to modulate feeding behaviors directly. The PVH is the main site of integration for visceral inputs from the sympathetic nervous system and NTS, cortico-limbic inputs from the lateral septal nuclei (LS) and bed nucleus of the stria terminalis (LS and BST), and interoceptive inputs from the arcuate nucleus of the hypothalamus (ARH).
FEEDING CIRCUIT INPUTS
Inputs influencing feeding behavior are widely distributed throughout the central and peripheral nervous system and thus are not as easily parsed into anatomically distinct tracts as feeding circuits outflows. Instead, they are often divided into three classes on the basis of the type of information they transmit (119). Interoceptive inputs, a complex array of signals reflecting endogenous energy availability, are collectively essential for the control of food intake in the service of energy homeostasis. Cognitive inputs mediate volitional aspects of feeding, including motivation and reward, which guide behavioral strategies to meet energy requirements in an efficient manner. Finally, arousal inputs to feeding circuits promote adaptability to the changing needs of the organism. Each of these classes of inputs has been the subject of excellent and detailed reviews (9; 119). In this review, we will highlight neuronal nodes in the core feeding network where feeding circuit inputs are integrated (Figure 1, red arrows).
Interoceptive Inputs
Meal-related inputs are transmitted by neuronal as well as secreted signals and are key determinants of meal duration and size. Orosensory signals arising from the taste, texture and temperature of foods in the oral cavity are transmitted to the brain via gustatory visceral and somatic nerves supplying gustatory, thermal and tactile sensory transducers distributed along the tongue and palate. Mechanical aspects of digestion, such as gastric distension, are transmitted to the brain via vagal and spinal nerves. The NTS is a major target of these visceral and somatic inputs. In turn, these meal-sensitive NTS neurons project to additional brainstem sites, such as the lateral parabrachial nucleus (lPB), hypothalamic sites such as the PVH, and cortical sites, such as the insular cortex (97). Cortical sites that respond to and process visceral signals project to the amygdala and are relayed to the autonomic division of the PVH (via the BST) and as well as to parasympathetic preganglionic neurons.
Meal-Related Peptide Signals: CCK
Cholecystokinin (CCK), a gut peptide released by enteroendocrine cells in response to the presence of intestinal nutrients, exemplifies how peripheral meal-related signals generated at the time of food intake drive circuit activity, both by modulating vagal inputs as well as by direct effects on the central brainstem-hypothalamic feeding core circuit. The prandial nature of CCK as a meal-related signal is consistent with findings that plasma CCK levels rise rapidly within minutes of meal onset, as ingested food fills the stomach and reaches the intestine during gastric filling (71), and circulating CCK is rapidly degraded following release. Peripheral administration of CCK reduces food intake in multiple mammalian species by selectively reducing meal size (113).
CCK Modulates Interoceptive/Vagal Inputs
Feeding-inhibitory effects of CKK are blocked by surgical or chemical interruption of subdiaphragmatic vagal afferent nerve fibers (e.g. (114)). Intestinal vagal afferents are localized in close apposition to CCK-expressing gut enteroendocrine cells (11). These close approximations make it possible for local paracrine release of CCK from enteroendocrine cells to act on vagal afferents. Cell bodies of the afferent vagus nerve express CCKA receptors (83) and these receptors have been demonstrated to be transported along gut vagal afferent axons. Furthermore, local administration of CCK-8 into the gastrointestinal arterial blood supply rapidly and dose-dependently increases action potential generation in vagal afferents supplying the stomach and small intestine via CCKA receptors (e.g. (105)).
Peripheral vagal afferents also have the ability to integrate more than one class of meal-related stimulus. CCK-sensitive vagal afferents are activated by mechanical distension or stroking of the intestinal mucosa, two events that normally occur during meal ingestion. In addition, vagal afferent firing patterns can encode interactions among these diverse mechanical, chemical and peptidergic stimuli, as combinations of gastric or intestinal distension combined with local gut vascular administration of CCK drive single vagal afferents to a greater degree than any single one of these individual stimuli (e.g. (104)). From a functional perspective, these vagal afferent interactions likely contribute significantly to the negative feedback control of food intake during a meal, as combinations of such gastric or duodenal meal-related stimuli are more effective in promoting satiety when presented in combination with peripheral CCK agonists (107).
CCK influences on the brainstem-hypothalamic core feeding neuraxis
Peripheral CCK at doses and times that reduce meal size also are effective in activating c-fos in gut-recipient regions of the caudomedial NTS. The magnitude of CCK-induced feeding suppression correlates well with the degree of CCK-induced increases in c-fos expression (e.g. (42)). These c-fos responses are also mediated by vagal afferents, as vagotomy or systemic capsaicin treatment using the sensory neurotoxin capsaicin blocks peripheral CCK-induced c-fos activation (80). Gut vagal afferent meal-related signals such as CCK, reach the caudal brainstem at the level of the caudomedial NTS, where they terminate in part on N-Methyl-D-Aspartate Receptor (NMDAR) positive neurons (10). Accordingly, local surgical or neurochemical interruption of gut vagal afferent traffic at the level of the brainstem, either by surgical rhizotomy or by administration of NMDAR antagonists, not only block the ability of CCK to reduce food intake (82; 138), but can also increase spontaneous meal size (56). Finally, there is also good evidence for convergence and integration of meal-related signals within the NTS, as combinations of gastric loads and duodenal nutrient infusion or gastric loads and peripheral CCK generate greater c-fos expression than any one of these individual stimuli when administered alone (e.g. (129).)
Cognitive Inputs
Cortico-limbic circuits that process feeding-related cues and determine the reward value of food are critical components of circuits regulating feeding behavior (9; 119). Remembering past experiences with foods is important for successful foraging behavior as well as for avoidance of noxious foods. Representations of food-related cues are relayed from the cortex (notably insular, cingulate and orbitofrontal regions) to the PVH via the LS and BST (119; 131). In parallel, information about the sensory properties of food (i.e. visual, olfactory, taste) is processed in the hippocampus and amygdala to encode memories or learned aspects of feeding, which are then conveyed to the PVH via the LS and BST (119).
Reward pathways strongly influence feeding behavior, as they convey information about “liking” or “wanting” of food (9; 119). Cortical inputs related to food experiences are also transmitted to important nodes in circuits regulating reward and motivated behavior, including the nucleus accumbens (NAc) and ventral tegmental area (VTA). The LHA, an important node in circuits related to arousal and motivation, receives learning-related inputs from the hippocampus and amygdala, as well as reward-related inputs from the NAc. Descending cortical inputs to the SN influence motivated aspects of feeding behavior through projections to the medullary reticular formation and NTS, which mediate motor and autonomic outflows (119).
Arousal Inputs
Animals consume more food when they are awake and aroused. Orexin (ORX) neurons in the LHA play a central role in promoting arousal through excitatory projections to hypothalamic and brainstem nuclei (99; 102; 140). As neuronal substrates of arousal communicate with key nodes in feeding circuits, such as the ARH and PVH, they are well-positioned to influence consummatory components of feeding behavior. In addition, projections from distinct sets of ORX neurons to important nodes in cortico-limbic circuits, such as the VTA and NAc, are reported to modulate reward-related aspects of feeding (51).
CONTEXTUAL INPUTS
The drive to eat in response to a given stimulus is not constant- it will vary depending on factors such as availability of food, energy stores, time of day and levels of arousal. In the second part of this review, we outline a framework for conceptualizing how the activity of the core feeding neuraxis is coordinately modulated in response to different conditions in the internal and external environment (outlined in Figure 2). We propose that “contextual inputs” provide cues that coordinate adaptations in circuit activity that determine the metabolic and motivational value of feeding behaviors. We define “contextual inputs” as signals that influence feeding behavior by modulating the strength of interoceptive, cognitive or arousal inputs. As these signals often simultaneously impinge on more than one node of the core feeding neuraxis, they promote flexible and coordinated responses to different environmental conditions. We will use several exemplar signals to illustrate how these three classes of contextual inputs influence the function of the core feeding neuraxis (Figure 2): metabolic (green arrows), circadian (blue arrows) and stress (red arrows).
Figure 2.
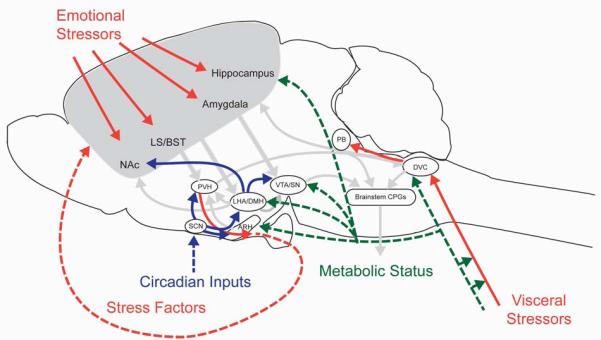
Outline of pathways mediating contextual cues to feeding circuits. Several classes of contextual signals ensure that the circuit response to a given stimulus is appropriate for the internal and external conditions. Contextual inputs (outlined in color) facilitate adaptations in circuit activity to coordinately modulate the strength of interoceptive, cognitive and arousal inputs (arrows from Figure 1 outlined in gray). These modulatory influences are conveyed by neuronal projections (solid lines) as well as humoral factors (dotted lines). Nutrient and peptide hormone signals of short- and long-term energy status (green arrows) are relayed from the viscera. Circadian inputs (blue arrows) are relayed from light-activated circuits in the SCN, which transmits humoral and neuronal signals to hypothalamic nuclei implicated in feeding regulation and arousal. Visceral stress signals (red arrows) are integrated with interoceptive inputs in the brainstem that regulate feeding and are also transmitted to midbrain nuclei regulating gastrointestinal malaise (PB). Emotional stressors (red arrows) are integrated with other cognitive inputs in corticolimbic circuits to the PVH; stress factors produced in the brain and periphery feed back to cognitive circuits to encourage or suppress motivated feeding behavior. Abbreviations: ARH, arcuate nucleus of the hypothalamus; BST, bed nuclei of the stria terminalis; CPG, central pattern generator; DMH, dorsomedial nucleus of the hypothalamus; DVC dorsovagal complex (area postrema, nucleus of the solitary tract, and dorsal motor nucleus of the vagus); LHA, lateral hypothalamic area; LS, lateral septum; NAc, nucleus accumbens; PB, parabrachial nucleus; PVH, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus; SN, substantia nigra; VTA, ventral tegmental area.
Metabolic Context
Humoral Signals of Energy Status: Leptin and Ghrelin
As discussed above, the characterization of direct and indirect controls of feeding includes the idea that feeding behavior in response to short-term signals of nutrient availability is modulated by signals reflecting the long-term availability of nutrients. Thus, the behavioral response to a feeding stimulus is different under fed versus fasted conditions. Food deprivation poses a direct threat to survival; thus, signals of inadequate nutrient supply promote food-seeking behaviors (17). Under conditions where energy supply is replete, negative feedback signals to the system ensure that an organism does not engage in food-seeking behaviors to the exclusion of all other activities. The circulating levels of numerous peptide and nutrient factors are regulated by energy availability, and their aggregate actions determine a distinct metabolic context that modulates the functions of the core feeding neuraxis.
Ghrelin and leptin, which exert opposing influences on feeding, provide instructive examples of the ways in which neurohumoral signals reflecting metabolic status can act at brainstem/hypothalamic sites, as well as in cognitive and arousal pathways to coordinately regulate feeding behaviors appropriate for the “metabolic context” (67; 76; 109). Ghrelin is an orexigenic hormone that is primarily secreted from gastric X/A cells; circulating levels are elevated during fasting, and decline upon refeeding, especially during duodenal exposure to nutrients (34; 126). Leptin is an adipokine secreted in proportion to body fat, and thus provides information about long-term energy stores (143). Circulating ghrelin and leptin reach the central nervous system by crossing the blood brain barrier (4-5). Peripheral and central ghrelin administration rapidly promote appetitive and consummatory ingestive behaviors (86; 91; 126; 137), while leptin suppresses fasting-induced increases in feeding (37). Central administration of both leptin and ghrelin selectively modulate meal size without affecting meal frequency, supporting the idea that the central pattern generator is not the target of their actions.
Influences on Interoceptive Inputs
The afferent vagus expresses receptors for ghrelin and leptin, and has been implicated as a neural substrate important for the ability of peripheral intravenous administration of these factors to affect food intake (e.g. (93)). Local leptin administration to the celiac artery, but not the jugular vein, dose-dependently limits meal size, without significantly elevating plasma leptin levels (93). Because the celiac artery supplies the upper gastrointestinal tract, including the stomach and the duodenum, these data support a local gut action for leptin in driving the neural circuitry mediating the control of meal size. Both subdiaphragmatic vagotomy and perivagal application of the sensory neurotoxin capsaicin blocked the ability of peripheral ghrelin and leptin to modulate feeding (33; 93). Consistent with a functional role for vagal afferent leptin receptor (LEPR) expression, leptin activates short-latency, transient depolarizations, action potentials, and increases in cytosolic calcium in neuroanatomically-identified cultured nodose ganglion neurons that arise from gastric and duodenal sites (94). Conversely, ghrelin has been reported to reduce neurophysiological spike activity in gastric vagal afferents sensitive to CCK (33). These findings demonstrate that peripheral signals of energy status can act at a peripheral node of the brainstem/ hypothalamic feeding neuraxis to modulate neural activity in ways consistent with their effects on meal size. Thus, they support the idea that gut vagal leptin and ghrelin receptor expression acts as part of an afferent limb of the brainstem/ hypothalamic core neuraxis sensitive to the metabolic context provided by local ghrelin and leptin levels.
Influences on the Brainstem/Hypothalamic Feeding Neuraxis
Within the CNS, intracerebroventricular administration of ghrelin rapidly increases food intake, while leptin suppresses fasting-induced feeding (86). Receptors for ghrelin and leptin have been localized to multiple mediobasal hypothalamic (MBH) and brainstem nuclei implicated in the control of food intake, including ARH, ventromedial nucleus of the hypothalamus (VMH), dorsomedial nucleus of the hypothalamus (DMH) and PVH, as well as the NTS, DMX and AP (38; 108; 137; 144).
Direct administration of ghrelin or leptin to the caudal brainstem fourth ventricle or parenchymally into the DVC, as well as adenovirally-mediated knockdown of LEPRb restricted to the caudal brainstem AP/NTS, modulate food intake by influencing meal size and each modulates the neuroanatomical extent of meal-related c-fos neuronal activation as well (41; 52; 62). The brainstem is also a neurophysiological target of ghrelin action, as ghrelin application to the fourth circumventricular area postrema has been shown to both hyperpolarize as well as depolarize dissociated AP neurons (43).
Direct administration of ghrelin into multiple, distinct hypothalamic sites has revealed that it most potently increases short term 1-hour food intake following hypothalamic arcuate nucleus injections, with the PVH as an alternate effective site of action (28). Ghrelin stimulates the activity of arcuate NPY neurons, and mimics the effect of NPY in the PVH (28; 86), consistent with the finding that central ghrelin’s feeding effects are mediated in part through NPY Y1 receptors (28) in selective brain regions. Moreover, ghrelin is necessary and sufficient to modulate fasting-induced increases in excitatory currents in NPY/AgRP neurons in acute hypothalamic preparations (142).
Local administration of leptin directly into the parenchyma of the MBH reduces food intake by selectively reducing meal size (15). The neuronal populations responsible for these effects have not been fully determined, but ARH neurons expressing AgRP/NPY and pre-opiomelanocortin (POMC) have been implicated. Mice that lack leptin receptor (LEPR) function in POMC and AGRP neurons exhibit increased food intake and meal size (128), supporting the idea that leptin’s effects on feeding are mediated directly on these ARH populations. However, due to off-target Pomc-Cre-mediated recombination (89), these effects cannot be exclusively attributed to leptin signaling deficits solely within in AgRP and/or POMC neurons. Additional studies involving conditional loss of Lepr in AgRP or POMC neurons have called these initial assumptions into question. Genetic disruption of leptin signaling in POMC neurons does not affect inhibitory currents in POMC neurons at baseline, nor does it impair leptin’s well-established ability to suppress fasting-mediated increases in inhibitory currents in these neurons (132). Fasting-induced increases in NPY/AgRP activity are inhibited by leptin (47; 95) through the suppression of fasting-mediated increases in excitatory transmission (142). Leptin attenuates Ca2+ activation responses to ghrelin in ARH AgRP/NPY neurons (63). These observations are consistent with the idea that leptin’s effects on hypothalamic neurons of the core feeding neuraxis are mediated via pre-synaptic modulation of the activity of key populations in the ARH and PVH. Leptin sensing neurons in the MBH are positioned to modulate other inputs to the core feeding neuraxis, as they also project to nodes that process cognitive (LS/BST) and gustatory (lPB) inputs (18; 139).
Influences on Cortico-Limbic Circuits
Ghrelin and leptin signaling can directly modulate the reward value of palatable food by engaging forebrain structures that project to the core neuraxis (36; 45). Central or peripheral ghrelin administration can mimic the ability of fasting to increase the motivation to eat, as measured by operant responding on a progressive ratio (88), whereas leptin is able to suppress the ability of food restriction to increase motivation to consume palatable foods (36).
The effects of ghrelin and leptin on reward-related inputs to the core feeding neuraxis are likely mediated by influences on the mesolimbic dopamine system. The VTA has been implicated as an important site for mediating ghrelin’s and leptin’s effects on the mesolimbic dopamine system. Receptors for both hormones have been localized to subpopulations of VTA neurons that project to the NAc (44; 109). VTA injections of ghrelin can stimulate food reward behavior and extracellular dopamine concentration in the NAc ; these effects are likely mediated via VTA to NAc projections, as direct injections into the NAc do not affect feeding behavior (1; 59; 110). Similarly, acute reduction in LEPR signaling in the VTA leads to increased dopamine transporter activity in the NAc (92), locomotor activity and consumption of palatable food (54). Diminished leptin signaling, due to either caloric restriction or genetic impairments, is associated with decreased tyrosine hydroxylase expression in the VTA as well as diminished dopamine levels and electrically-stimulated release from the NAc, phenotypes that are reversible by leptin (44; 54; 96).
Ghrelin and leptin signaling have been reported in key nodes processing cognitive inputs to regulate motivated feeding, including prefrontal and insular cortices, as well as the hippocampus (49; 109; 144). Intravenous ghrelin administration in healthy human volunteers is associated with increases in the neural responses to pictures of foods. These increases have been identified in multiple regions that project to the core feeding neuraxis, including the amygdala, orbitofrontal cortex, insula and NAc, and such infusions increase the self-reports of hunger and perceived pleasantness of foods (76) . Notably, several of these sites overlap with regions that are activated in response to pictures of food in weight-reduced humans and are restored to baseline levels by leptin administration (98). Ghrelin activates hippocampal neurons and stimulates long term potentiation (LTP) and enhances spatial learning (22; 35), adaptations which could increase the likelihood of success in securing food. In contrast, leptin signaling in the ventral hippocampus limits conditioned place preferences for palatable foods (61).
Influences on Arousal Inputs
In response to food scarcity, mammals increase wakefulness and activities that would promote food seeking (17). ORX neurons in the LHA have been implicated as a critical source of signals determining the levels of arousal and wakefulness, and fasting-mediated increases in arousal and activity require activation of ORX neurons (140). Accordingly, ghrelin administration stimulates Prepro-orexin expression in the LHA (20; 100) and the neuronal activity of ORX neurons (67; 140). Moreover, ghrelin microinjection into the LHA elicits increased arousal and food intake (121); stimulatory effects on food intake could be mediated, in part, through activation of AgRP/NPY neurons (63). Neurotensin-expressing neurons in the LHA express Lepr and could mediate some of leptin’s feeding suppressive effects by inhibiting arousal inputs of ORX neurons (69), as well by modulating mesolimbic dopamine signaling in the VTA (68).
Leptin and Ghrelin Influence Feeding by Modulating the Potency of Orexigenic and Anorexigenic Stimuli
Studies of the mechanisms by which leptin and ghrelin influence feeding are consistent with the idea that they do not signal through the core feeding neuraxis directly, but rather modulate the sensitivity of responses to interoceptive, cognitive and arousal inputs. In the periphery, gut vagal neurophysiological activation produced by CCK is enhanced by leptin (94; 134) and suppressed by ghrelin (33). Similarly, third ventricular administration of subthreshold doses of leptin augments the suppression of meal size produced by either gastric nutrient preloads or peripheral CCK, and this is paralleled by increased PVH and NTS c-fos expression in each case (39-40; 106; 135). There is also evidence to support the idea that enhanced responsiveness to feeding-inhibitory stimuli can be modulated by leptin action in the NTS (62), as well as the ARH (84).
Activation of AgRP/NPY neurons by ghrelin is important component of its effect on feeding (67; 86). As AgRP/NPY neurons express ghrelin receptor (136), it was often assumed that ghrelin-mediated effects on feeding were mediated through direct action on this important orexigenic stimulus. Recent studies support the idea that ghrelin (and/or food deprivation) promote feeding by enhancing the strength and duration of pre-synaptic excitatory inputs onto a subpopulation of AgRP/NPY neurons that project to autonomic OXY neurons in the PVH (3; 74; 142). Moreover, it has been proposed that leptin-stimulated beta- endorphin release from POMC neurons could provide negative feedback to these excitatory pre-synaptic inputs (142). By simultaneously acting at multiple circuit nodes in the core feeding neuraxis to modulate the potency of anorexigenic and orexigenic stimuli, collective signaling from the full spectrum of neurohumoral signals of “metabolic context” can rapidly and reversibly regulate feeding behaviors in response to changes in energy status.
Nutrient Signals: Leucine
In addition to the neural and humoral inputs to the core hypothalamic-brainstem core neuraxis, and importantly for the present discussion, both of these core sites are anatomically bounded in part by circumventricular organs with specialized vasculature and fenestrated capillaries that facilitate access of circulating factors within the cerebrospinal fluid to adjacent neuronal tissue. This structural feature raises the additional possibility whereby neuronal populations in these two regions may be exposed to and respond to alterations in circulating nutrient availability as a function of meal ingestion and digestion. An illustrative example comes from recent work of Blouet et al. (14; 16) demonstrating that plasma levels of the essential amino acid l-leucine are rapidly elevated after high leucine meals. Moreover, direct leucine stimulation of the MBH or the NTS quickly activate POMC-ergic populations in both of these regions, and reduce food intake by selectively reducing meal size. There is also evidence that the proposed hypothalamic/brainstem core neuraxis functions as an ensemble to mediate the ability of MBH leucine to control feeding behavior. MBH leucine stimulates OXY neurons within the PVH, which in turn project strongly to the NTS. Selective brainstem application of the oxytocin receptor antagonist at doses that have no effect on food intake when administered alone, are sufficient to block the ability of MBH leucine to reduce feeding. Thus, an ARH-PVH-NTS circuit can mediate the effects of direct hypothalamic nutrient sensing in the negative feedback control of food intake and meal size (14).
Circadian Context
Food seeking behavior is often temporally constrained, either because of restricted availability of prey/food, or to avoid predators. Circadian systems promote survival by synchronizing the activity of circuits regulating diverse biobehavioral processes, such as feeding, arousal and locomotor activity, to ensure that they match the timing for optimal foraging (117). Moreover, circadian signals modulate circuits that help the body prepare for impending food-seeking activity, such as the HPA axis (81). Circadian influences on feeding behavior are mediated by a combination of sources - the master light-entrained oscillator in the suprachiasmatic nucleus (SCN), as well as secondary oscillators in hypothalamic neurons (ARH, DMH) cortico-limbic structures (BST, hippocampus and amygdala), and peripheral organs (i.e. adrenal gland, liver) (65; 102). Loss of function mutations in several key components of the transcriptional machinery regulating circadian rhythms – including Clock (127) and Per2 (141) – abolish diurnal fluctuations in feeding (due to increased intake during light cycle and decreased intake in dark cycle), without affecting total daily caloric intake. Moreover, SCN lesions abolish free-running diurnal rhythms of food intake, locomotor activity and plasma corticosterone/cortisol (CORT) (81; 85; 118), consistent with important contributions from light-entrained oscillators.
The SCN transmits humoral (AVP) and neuronal signals to nuclei implicated in feeding regulation, including PVH, ARH and DMH (60; 66; 102), as well as to the LHA, a critical node in arousal circuits (102). AVP is released from the SCN during the light period in both nocturnal and diurnal species (32), but it is not clear how this light-stimulated signal is differentially transmitted in both types of species. It has been proposed that the SCN projects to GABAergic interneurons in nocturnal species, and glutamatergic interneurons in diurnal species (60), however this possibility has not yet been tested directly.
Influences on the Brainstem/Hypothalamic Feeding Neuraxis
Influences of circadian inputs on the core feeding neuraxis to modulate diurnal patterns of feeding could be mediated via direct projections to the PVH and DMH, as well as indirect effects via glucocorticoid (GC) release. Direct projections from SCN to PVH neurons participating in the control of feeding behaviors have not been explicitly identified, but circadian fluctuations in plasma CORT are mediated by excitatory SCN projections to OXY neurons in the autonomic division of PVH, which inhibit sympathetic output to the adrenal medulla during the light cycle (19). As OXY neurons in the autonomic PVH have recently been implicated as a key component of circuits that promote feeding downstream of AgRP neurons (3), it is possible that direct inputs from the SCN to caudally-projecting OXY neurons similarly act to suppress feeding.
Influences of Glucocorticoids on Cortico-Limbic Circuits
As diurnal fluctuations in feeding and CORT are synchronous, and GC infusions increase caloric intake in humans and rodents (30; 123), circadian influences on feeding could also be mediated via GC actions. GCs coordinately activate many sites in the brain and periphery to promote feeding behavior, including pathways regulating learning, memory, reward and arousal. It has been proposed that regulation of CORT release by the central clock in the SCN could provide a means to synchronize rhythmicity in physiological processes controlled by peripheral clocks. The Per2 gene has GC responsive elements in its promoter; eliminating them abolishes CORT’s effects on glucose homeostasis and leptin (115). In addition, it has been reported that PER2 rhythms in the amygdala and BST are sensitive to CORT (65), raising the possibility that circadian fluctuations of CORT modulate the activity of cortico-limbic circuits as well. Effects of GCs on motivational aspects of feeding are supported by observations that GC infusions have been reported to increase caloric intake in humans and rodents when highly palatable foods are offered but not under chow-fed conditions (6; 30; 123). Circadian influences on cortico-limbic circuits could underlie reports that sleep deprivation is associated with increased subjective ratings of hunger and appetite in humans (87; 116) as well as decreased motivation for food reward and suppressed dopamine function in the NAc in rodents (50).
Influences on Arousal Inputs
The DMH may represent another node where circadian inputs are integrated with information about available energy stores to regulate feeding behavior. The DMH projects directly to the PVH (26; 102; 125), although the contribution of these projections to defined neuronal groups to regulate feeding has not been examined. In addition, glutamatergic inputs from the DMH to ORX and melanin-concentrating hormone neurons in the LHA are thought to promote wakefulness and feeding (26; 102). DMH lesions are reported to decrease feeding in the dark cycle without a compensatory increase in the light cycle, leading to reductions in daily caloric intake (8; 26). DMH lesions similarly dampen circadian fluctuations in wakefulness, locomotor activity and CORT due to dark cycle decreases (7; 26). Together, these observations are consistent with a role for the DMH in promoting dark cycle food intake, locomotor activity and CORT, which is inhibited by SCN signals during the light cycle. Neuroendocrine signals of nutrient availability acting at the level of the DMH could contribute to a network of food-entrained oscillators; during periods of restricted nutrient availability, food-entrained oscillators override light-entrained rhythms to establish new patterns of activity and CORT release to coincide with feeding bouts (79; 102; 117).
Stress-Related Context
Whereas mild elevations in GCs, such as those observed at the diurnal peak of plasma CORT levels or in response to a mild stressor, promote feeding (101), chronic GC elevation and acute stress repress food intake through decreases in both the amount of time spent consuming a meal, as well as in the amount of food consumed within a meal (21). Stress-related influences on feeding are mediated via a dispersed network of neurons that express corticotropin-releasing factor (CRF) or a closely-related factor urocortin (UCN). CRF is necessary and sufficient to induce a wide range of stress-associated behaviors, including food intake suppression (53; 77; 111).
Influence on Neuroendocrine/Secretomotor Outputs
Stress-activated CRF neurons in the PVH drive HPA axis activation, as assessed by rapid elevations in plasma levels of CORT and adrenocorticotropic hormone within minutes of stressor onset (24; 31). In addition, GCs initiate negative feedback on the HPA axis, largely at the level of the pituitary and hypothalamus (27; 31).
Influences on the Brainstem/Hypothalamic Feeding Neuraxis
CRF and UCN have been reported to act at multiple forebrain and hindbrain nodes of the core feeding axis to regulate ingestive behavior. Decreased food intake that occurs after restraint stress is abolished by intracerebroventricular infusion of a CRF antagonist (111) or in Crf2 receptor null mutants (122). Forebrain administration of CRF2R receptor agonists (CRF/UCN) reduces acute food intake in rats and mice (29; 57; 133). CRF action within the PVH has been reported to oppose NPY-induced feeding signals (29; 53; 77; 133). In addition to acting at forebrain sites, delivery of stress factors to the brainstem reduces short term and 24-hour food intake, perhaps by modulating the strength of negative feedback signals (i.e. CCK) stimulated by visceral oral, gut neural afferent signals, or gut hormone signals that act on the NTS (48).
Influences on Cortico-Limbic and Arousal Circuits
CRF modulates reward and arousal pathways independent of its effects on the HPA axis (53; 77; 111). Under conditions of acute or chronic stress, the normally stimulatory influence of the reward pathway switches to an aversive state, suppressing motivated behaviors and promoting actions to mitigate the environmental threat (27). Environmental stressors come in many forms – emotional, physical and gastrointestinal malaise resulting from bad-tasting or toxic food. Thus, they can impinge on feeding circuits at many levels, including spinal nociceptive, mechanosensory, and chemosensory afferents to the DVC, and oral, vagal and non-vagal visceral/somatic inputs to the DVC, as well as descending corticolimbic projections from the amygdala and NAc to the LS, BST and PVH. Stress activates CRF expression in many brain regions involved in processing visceral sensory information, including the hippocampus, amygdala, NAc, LS, BST, lPB, VLM, and NTS (13; 70; 103; 119-120; 130). In addition, CRF neurons are found in midbrain cell groups that regulate arousal (13; 130).
There is a growing body of evidence to support a role for CRF action in cortico-limbic reward circuits in mediating stress-induced suppression of food intake, most notably in the NAc. Medium spiny neurons expressing the D1 dopamine receptor (D1 MSNs) in the NAc are thought to promote motivated behaviors by producing an appetitive/rewarding state (90). CRF and CRFR1/2 are expressed in the NAc, and CRF action via co-activation of CRFR1/2 in NAc facilitates dopamine release and motivated behaviors (70). Under conditions of chronic stress, CRF no longer facilitates dopamine release, and an aversive state is produced (70).
Melanocortin signals have been implicated in mediating some stress-induced symptoms of depression (23). Recent studies support the idea that chronic stress suppresses food intake via action of melanocortin signals from ARH POMC neurons onto D1 MSNs (72). POMC neurons project to NAc (72) and melanocortin receptor 4 (MC4-R) is expressed in D1 MSNs (55; 72). Chronic restraint stress or URN3 delivery into the VMH (25) activates POMC neurons (without alterations in hypothalamic NPY or CRF) (21; 73), and increases MC4R expression in the NAc. As both stress and the POMC product alpha-melanocyte-stimulating hormone produce the same synaptic changes in D1 neurons that decrease excitatory drive onto these neurons, these effects would be predicted to reduce motivated behavior. Interestingly, MC4R function in D1 neurons in the NAc is necessary and sufficient for stress-mediated suppression of food intake, but does not affect other stress-induced behaviors (72).
CONCLUSIONS AND FUTURE DIRECTIONS
This review introduces and describes a neuroanatomical framework designed to capture critical features of a neural network essential for feeding behavior. Important elements of this framework include the identification of distinct interoceptive, cognitive, and arousal network inputs that contribute to the coordinated production of somatomotor, autonomic, and neuroendocrine outputs that together determine feeding and its nutritional consequences. The neuronal functions of this core feeding neuraxis are modulated by metabolic, circadian, and stress-related contexts to promote adaptive feeding responses to internal and external environmental stimuli. It is anticipated that this framework will facilitate the incorporation of new findings, and will foster the development and testing of new hypotheses designed to identify and characterize the neurobiological controls of feeding.
An important area for future research is to understand how these diverse signals are integrated within and across circuit nodes to modulate motor outputs and thus feeding behavior. For this to occur, we will need better tools to identify and manipulate functionally distinct subpopulations within key nodes of the core feeding neuraxis. Two recent research directions hold promise. First, as evidence mounts for the existence of molecular heterogeneity across neurochemically similar neurons, it will be important to design studies to reveal the intracellular signaling cascades underlying the integration of adiposity hormone signals and short-term meal related signals at the level of the single neuron. Second, the development of tools that will allow for inducible, site-specific manipulation of defined neuronal projections (3; 64; 139), hold great potential to advance our understanding of the connectivity and integrative capacity of core feeding circuits across the three modulatory contexts.
ACKNOWLEDGMENTS
The writing of this article was supported by grants from the NIH (G.J.S. DK 020541 EINSTEIN DRTC and DK 026687 NYORC; L.M.Z. DK 089038).
Footnotes
The authors have no financial interest to disclose.
LITERATURE CITED
- 1.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012 doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 6.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–9. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger LL, Bernardis LL, Mendel VE. Effect of ventromedial and dorsomedial hypothalamic lesions on circadian corticosterone rhythms. Neuroendocrinology. 1976;22:216–25. doi: 10.1159/000122628. [DOI] [PubMed] [Google Scholar]
- 8.Bellinger LL, Mendel VE, Bernardis LL, Castonguay TW. Meal patterns of rats with dorsomedial hypothalamic nuclei lesions or sham operations. Physiol Behav. 1986;36:693–8. doi: 10.1016/0031-9384(86)90356-2. [DOI] [PubMed] [Google Scholar]
- 9.Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neuroscience & Biobehavioral Reviews. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud HR, Earle T, Zheng H, Patterson LM, Phifer C. Food-related gastrointestinal signals activate caudal brainstem neurons expressing both NMDA and AMPA receptors. Brain Res. 2001;915:143–54. doi: 10.1016/s0006-8993(01)02826-8. [DOI] [PubMed] [Google Scholar]
- 11.Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–31. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- 12.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–56. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–11. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–67. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blouet C, Schwartz GJ. Brainstem Nutrient Sensing in the Nucleus of the Solitary Tract Inhibits Feeding. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borbely AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res. 1977;124:457–71. doi: 10.1016/0006-8993(77)90947-7. [DOI] [PubMed] [Google Scholar]
- 18.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–44. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 20.Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, et al. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–7. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- 21.Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, et al. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104:675–83. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Carlini VP, Monzon ME, Varas MM, Cragnolini AB, Schioth HB, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–43. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 23.Chaki S, Okuyama S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides. 2005;26:1952–64. doi: 10.1016/j.peptides.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Vaughan J, Donaldson C, Vale W, Li C. Injection of Urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels, and hypothalamic POMC gene expression but not the HPA axis. Am J Physiol Endocrinol Metab. 2010;298:E337–45. doi: 10.1152/ajpendo.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–66. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–86. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 29.Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, et al. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 2001;916:222–8. doi: 10.1016/s0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- 30.Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995;771:730–42. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- 31.Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, et al. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- 32.Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–51. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 34.Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–10. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 36.Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–8. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;275:R186–93. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- 38.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–47. [PubMed] [Google Scholar]
- 39.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav. 2001;72:123–8. doi: 10.1016/s0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 40.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–9. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 41.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 42.Fraser KA, Davison JS. Cholecystokinin-induced c-fos expression in the rat brain stem is influenced by vagal nerve integrity. Exp Physiol. 1992;77:225–8. doi: 10.1113/expphysiol.1992.sp003579. [DOI] [PubMed] [Google Scholar]
- 43.Fry M, Ferguson AV. Ghrelin: central nervous system sites of action in regulation of energy balance. Int J Pept. 2010 doi: 10.1155/2010/616757. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 46.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–99. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glaum SR, Hara M, Bindokas VP, Lee CC, Polonsky KS, et al. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol Pharmacol. 1996;50:230–5. [PubMed] [Google Scholar]
- 48.Grill HJ, Markison S, Ginsberg A, Kaplan JM. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res. 2000;867:19–28. doi: 10.1016/s0006-8993(00)02193-4. [DOI] [PubMed] [Google Scholar]
- 49.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 50.Hanlon EC, Benca RM, Baldo BA, Kelley AE. REM sleep deprivation produces a motivational deficit for food reward that is reversed by intra-accumbens amphetamine in rats. Brain Res Bull. 2010;83:245–54. doi: 10.1016/j.brainresbull.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 52.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- 54.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 55.Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, et al. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–42. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R642–51. doi: 10.1152/ajpregu.00641.2005. [DOI] [PubMed] [Google Scholar]
- 57.Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, et al. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–93. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- 58.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 59.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 60.Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–9. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–70. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab. 2012;303:E496–503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–56. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 64.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A. 2005;102:4180–4. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582:142–51. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–62. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 68.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–23. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–6. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liddle RA, Green GM, Conrad CK, Williams JA. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol. 1986;251:G243–8. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- 72.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–40. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu T, Kong D, Shah BP, Ye C, Koda S, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–22. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006;21:167–74. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- 76.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, Koob GF. Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Res. 1993;618:76–82. doi: 10.1016/0006-8993(93)90431-l. [DOI] [PubMed] [Google Scholar]
- 78.Miller AJ. Deglutition. Physiol Rev. 1982;62:129–84. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- 79.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–45. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770:277–88. doi: 10.1016/s0006-8993(97)00865-2. [DOI] [PubMed] [Google Scholar]
- 81.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 82.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 1997;272:R1245–51. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 83.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res. 1990;526:95–102. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- 84.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–10. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagai K, Nishio T, Nakagawa H, Nakamura S, Fukuda Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978;142:384–9. doi: 10.1016/0006-8993(78)90648-0. [DOI] [PubMed] [Google Scholar]
- 86.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 87.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol. 2012;303:R259–69. doi: 10.1152/ajpregu.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Padilla SL, Reef D, Zeltser LM. Defining POMC Neurons Using Transgenic Reagents: Impact of Transient Pomc Expression in Diverse Immature Neuronal Populations. Endocrinology. 2012;153:1219–31. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perry ML, Leinninger GM, Chen R, Luderman KD, Yang H, et al. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. J Neurochem. 2010;114:666–74. doi: 10.1111/j.1471-4159.2010.06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–84. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- 94.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–9. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- 95.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 96.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 100.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. page following. [DOI] [PubMed] [Google Scholar]
- 101.Samarghandian S, Ohata H, Yamauchi N, Shibasaki T. Corticotropin-releasing factor as well as opioid and dopamine are involved in tail-pinch-induced food intake of rats. Neuroscience. 2003;116:519–24. doi: 10.1016/s0306-4522(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 102.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–52. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- 103.Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, et al. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol. 1991;261:R64–9. doi: 10.1152/ajpregu.1991.261.1.R64. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz GJ, McHugh PR, Moran TH. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol. 1993;265:R872–6. doi: 10.1152/ajpregu.1993.265.4.R872. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–84. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz GJ, Netterville LA, McHugh PR, Moran TH. Gastric loads potentiate inhibition of food intake produced by a cholecystokinin analogue. Am J Physiol. 1991;261:R1141–6. doi: 10.1152/ajpregu.1991.261.5.R1141. [DOI] [PubMed] [Google Scholar]
- 108.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–6. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 111.Smagin GN, Howell LA, Redmann S, Jr., Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–8. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 112.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–6. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 113.Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity (Silver Spring) 2006;14(Suppl 4):168S–70S. doi: 10.1038/oby.2006.300. [DOI] [PubMed] [Google Scholar]
- 114.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–41. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 115.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 2009;106:17582–7. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 117.Stephan FK. The "other" circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 118.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–64. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 120.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 121.Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R575–85. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 122.Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, et al. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26:2303–14. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–25. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 124.Teubner BJ, Keen-Rhinehart E, Bartness TJ. Third ventricular coinjection of subthreshold doses of NPY and AgRP stimulate food hoarding and intake and neural activation. Am J Physiol Regul Integr Comp Physiol. 2012;302:R37–48. doi: 10.1152/ajpregu.00475.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 126.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 127.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–85. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;289:R695–703. doi: 10.1152/ajpregu.00809.2004. [DOI] [PubMed] [Google Scholar]
- 130.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 131.Verhagen JV, Engelen L. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev. 2006;30:613–50. doi: 10.1016/j.neubiorev.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 132.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr., Lowell BB. Leptin Action on GABAergic Neurons Prevents Obesity and Reduces Inhibitory Tone to POMC Neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Mullet MA, Glass MJ, Billington CJ, Levine AS, Kotz CM. Feeding inhibition by urocortin in the rat hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R473–80. doi: 10.1152/ajpregu.2001.280.2.R473. [DOI] [PubMed] [Google Scholar]
- 134.Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 135.Wang L, Martinez V, Barrachina MD, Tache Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–66. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- 136.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 137.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 138.Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2011;301:R448–55. doi: 10.1152/ajpregu.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–7. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 141.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–60. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 144.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]


