Abstract
Purpose
We previously found several individual FDG/PET-based prognostic factors for cervical cancer, specifically cervical tumor SUVmax, tumor volume, and highest level of lymph node (LN) involvement. For this study, we evaluate the combined use of these three prognostic factors assessed on pretreatment FDG-PET for predicting recurrence-free survival (RFS), disease-specific survival (DSS), and overall survival (OS).
Patients and methods
The study included 234 cervical cancer patients, FIGO stage Ib1–IVa, treated with definitive radiation or chemoradiation therapy. All patients underwent FDG-PET or FDG-PET/CT at diagnosis, from which cervical tumor volume, SUVmax, and LN status were recorded. Using these PET-based factors, prognostic nomograms were created for RFS, DSS, and OS, and their prediction accuracies were measured using the concordance index (c-statistic).
Results
Fifty-three percent of patients had FDG-avid LN on PET; the highest level of nodal involvement was pelvic in 84, para-aortic in 41, and supraclavicular in 10. The average cervix tumor SUVmax was 12.4 (range, 2.1–50.4) and PET tumor volume average was 66.4 cm3 (range, 3.0–535.7 cm3). The median follow-up was 40.7 months for patients alive at last follow-up. PET LN status had the greatest influence on outcome. The c-statistics for the 3 nomograms were 0.741 for RFS, 0.739 for DSS, and 0.658 for OS. The PET-based nomograms performed better than FIGO stage with c-statistics of 0.605, 0.600 and 0.559 for RFS, DSS and OS, respectively.
Conclusions
Pretreatment FDG-PET LN status, cervical tumor SUVmax, and tumor volume combined in a nomogram create good models for predicting cervical cancer RFS, DSS, and OS.
Keywords: FDG-PET, Lymph node, Prognosis, Cervix, Nomogram
Introduction
It is estimated that in 2012, in the United States, 12,170 women will be diagnosed with cervical cancer and over 4000 women will die of the disease [1]. Being able to identify, at diagnosis, the patients at highest risk of recurrence or death from cervical cancer could be particularly valuable. Several studies have evaluated patient- and treatment-specific prognostic factors for cervical cancer disease response and survival. Some of the main negative prognostic factors include advanced FIGO stage, large tumor size or volume, lymph node status, young patient age, poor performance status, and lack of brachytherapy [2–5].
For this study, we aimed to create a PET-based prognostic model for cervical cancer. Recently, positron emission tomography (PET) with the glucose analogue F-18 fluorodeoxyglucose (FDG) has been shown to be a useful diagnostic tool in the evaluation of patients with cervical cancer, aiding in identifying the extent of the primary cervical tumor, lymph node involvement, and distant disease [6–9]. Additionally, FDG-PET has helped further define new prognostic factors for cervical cancer. Early research found that cervical cancer lymph node status, as detected on FDG-PET, was more predictive of patient outcome than traditional prognostic factors, including FIGO stage [7]. Additional research has demonstrated that cervical tumor volume can be accurately measured by FDG-PET and that PET tumor volume predicts for progression-free survival and overall survival (OS) [10]. Other research found that cervical tumors with high FDG uptake, measured as the maximum standardized uptake value (SUVmax) had an increased risk of lymph node metastasis at diagnosis, persistent disease after treatment and pelvic recurrence, as well as worse OS [11]. Together these studies suggest that FDG-PET findings have significant value for cervical cancer prognosis and may represent new metrics for assessing and predicting the likelihood of treatment response and patient survival.
The prognostic value of PET tumor volume, lymph node status, and SUVmax has been shown individually. Their use in combination has not been evaluated. In this present study, our aim was to create a combined prognostic model including PET tumor volume, SUVmax, and PET lymph node status to assess how these 3 factors together can predict for treatment response, disease-specific survival (DSS) and OS. Using the pretreatment PET data of lymph node status, tumor volume and SUVmax from 234 patients with cervical cancer, we created and evaluated 3 distinct prognostic nomograms for recurrence-free survival (RFS), DSS, and OS. We also evaluated the predictive value of the PET-based nomograms for RFS, DSS, and OS, as compared to FIGO stage.
Patients and methods
Patients
Patients included in this study were selected from a database of 482 consecutive patients with newly diagnosed cervical cancer, who underwent FDG-PET or FDG-PET/CT at Washington University in St. Louis between July 1998 and October 2008. Selection criteria for inclusion into this current retrospective study included patients who were treated with definitive radiation or chemoradiation therapy and for which PET lymph node status, tumor volume and SUVmax were prospectively collected in the database, which created a cohort of 234 patients. The study was approved by the Washington University Human Research Protection Office and a waiver of informed consent was obtained.
All patients were evaluated by history and physical examination, examination under anesthesia, and FDG-PET or integrated PET/CT before initiating treatment. All but one patient also underwent a contrast-enhanced CT of the abdomen and pelvis. Patients were staged clinically, according to FIGO staging (AJCC 2002, 6th edition).
PET imaging
All patients underwent FDG-PET or PET/CT for staging before treatment. Before November 2002 (n=50), FDG-PET was performed with a conventional PET scanner and interpreted in a routine manner as previously described [7]. Thereafter, all FDG-PET studies were performed with a hybrid PET/CT (n=184) scanner as previously described by Wright et al. [12]. The PET and PET/CT were calibrated to ensure accuracy and reproducibility of SUV between the 2 scanners. The PET/CT images were interpreted in standard clinical fashion, both separately and in fused mode. The PET study was typically deferred if blood glucose concentration exceeded 200 mg/dL. Urinary tract activity was minimized by the placement of a Foley catheter before injection of FDG and by administration of furosemide and intravenous fluids after the injection of FDG. FDG-PET data were collected prospectively at the time of the imaging study. From the pretreatment diagnostic FDG-PET, lymph node status, SUVmax and tumor volume were recorded for all patients. A lymph node was considered PET-positive if its FDG uptake was greater than blood pool activity or surrounding background tissues, as described previously [13]. PET tumor volume was calculated using the 40% threshold method, as initially described by Miller and Grigsby [10].
Treatment
All patients were treated with definitive radiation therapy, which included external irradiation to the pelvis and intracavitary brachytherapy. Concurrent weekly cisplatin chemotherapy was administered to 90% of patients.
Outcome evaluation
Patients had follow-up examinations approximately every 2 months for the first 6 months, every 3 months for the next 2 years and then every 6 months. FDG-PET or FDG-PET/CT was repeated when warranted by clinical examination or symptoms and generally at three months after completion of radiation. The sites and timing of any recurrence were recorded.
Nomogram creation
Nomograms are graphical representations of prognostic models that facilitate estimation of prognosis directly from individual patient characteristics without requiring complex computations. Using the PET-based prognostic factors of lymph node status, SUVmax, and tumor volume, prognostic nomograms based on Cox proportional hazard regression models were created for RFS, DSS, and OS. Additionally, models for RFS, DSS and OS using only FIGO stage were created for comparison. The prediction accuracies of the nomograms were measured using the concordance index (c-statistic). The c-statistic is a measure of the model's ability to discriminate between high-risk and low-risk subjects. It varies from 0.0 (the model's predictions are no better than chance) to 1.0 (perfect predictive power). To guard against overfit, the nomogram predictions were assessed using the 10-fold cross-validation re-sampling method, in which 9/10 of the samples were held for training and 1/10 were used for testing in a round-robin fashion [14]. The nomogram representation we selected follows the work by Kattan et al. [15].
Results
Patient and tumor characteristics
The patient population consisted of 234 cervical cancer patients. The tumor and patient characteristics are shown in Table 1. The highest level of lymph node involvement seen on FDG-PET was as follows: 109 patients (46.6%) had no FDG-avid lymph nodes; 84 patients (35.9%) had pelvic lymph nodes only; 31 patients (13.2%) had pelvic and para-aortic lymph nodes; and 10 patients (4.3%) had pelvic, para-aortic, and supraclavicular lymph nodes. The average cervical tumor SUVmax was 12.4 (range, 2.1–50.4). The average PET volume for the primary cervical tumor was 66.4 cm3 (range, 3.0–535.7 cm3).
Table 1.
Patient and tumor characteristics.
| Average age at diagnosis | 52 (range, 24–94) |
| FIGO stage | |
| Ib1 | 29 |
| Ib2 | 36 |
| IIa | 5 |
| IIb | 102 |
| IIIa | 3 |
| IIIb | 56 |
| IVa | 3 |
| FDG-PET lymph node status | |
| None | 109 (47%) |
| Pelvic | 125 (53%) |
| Para-aortic | 41 (18%) |
| Supraclavicular | 10 (4%) |
| Histology | |
| Squamous | 207 (88%) |
| Adenocarcinoma | 16 (7%) |
| Adenosquamous | 4 (2%) |
| Other (small cell, clear cell, poorly differentiated) | 7 (3%) |
The mean follow-up for patients alive at the time of last follow-up was 40.7 months (range, 5–125 months). At the time of last follow-up, the disease status of patients included: 131 patients with no evidence of disease, 60 patients dead of disease, 12 patients dead of intercurrent disease, 29 patients alive with disease, and 2 patients dead from treatment-related toxicity.
Nomograms
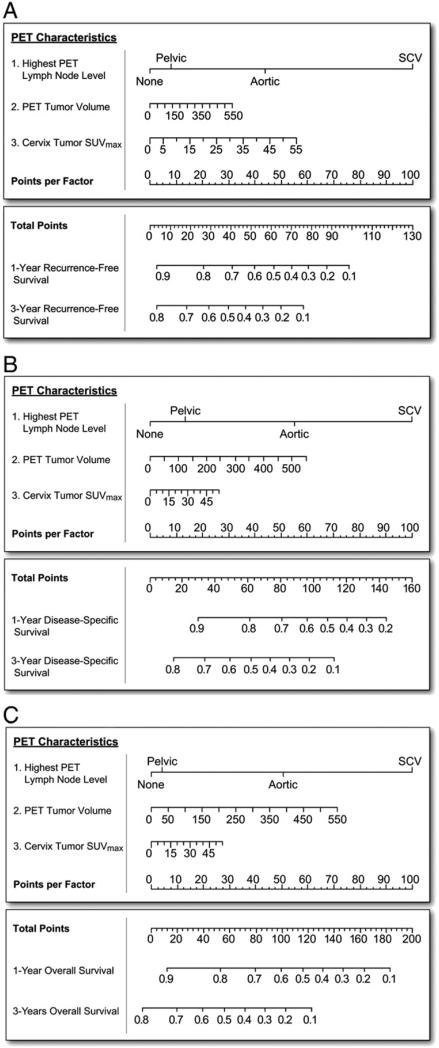
Using the PET-based prognostic factors, prognostic nomograms based on Cox regression were created for RFS, DSS, and OS (Figs. 1A–C). For all 3 nomograms, lymph node status had the greatest influence on outcome. The FDG-PET prognostic nomogram for RFS showed that cervix tumor SUVmax was the second most important factor, after lymph node status (Fig. 1A). The RFS nomogram had a training c-statistic of 0.740±0.011 (standard deviation) while the testing c-statistic was 0.741±0.099 (standard deviation). Fig. 1B shows the DSS nomogram, which demonstrates a more significant influence for PET tumor volume, than SUVmax. The OS nomogram is shown in Fig. 1C. The training and testing c-statistics for the three PET-based nomograms and for FIGO stage alone are shown in Table 2A and B. FIGO stage alone had lower c-statistics. Table 3 lists the hazards ratios and 95% confidence intervals for the components of the PET-based nomograms. Nomograms for RFS, DSS, and OS combining FIGO stage, tumor histology, and patient age at diagnosis had c-statistics similar to models based on FIGO stage alone (data not shown). Combining clinical factors of FIGO stage, histology, and age along with the 3 PET factors did not create superior nomograms with the combined clinical and PET nomograms showing lower c-statistics than the PET-based factors alone (data not shown).
Fig. 1.
FDG-PET-based prognostic nomograms using PET lymph node involvement, cervical tumor SUVmax, and PET tumor volume for A) recurrence-free survival, B) disease-specific survival and C) overall survival.
Table 2.
Concordance index and standard deviation for the training and testing of recurrence-free survival, cause-specific survival, and overall survival for the (A) PET model nomograms and (B) FIGO stage.
| Training | Testing | |
|---|---|---|
| A. PET-based nomograms | ||
| Recurrence-free survival | 0.740 ± 0.011 | 0.741 ± 0.099 |
| Disease-specific survival | 0.727 ± 0.015 | 0.739 ± 0.153 |
| Overall survival | 0.675 ± 0.014 | 0.658 ± 0.105 |
| B. FIGO stage | ||
| Recurrence-free survival | 0.626 ± 0.009 | 0.605 ± 0.086 |
| Disease-specific survival | 0.647 ± 0.013 | 0.600 ± 0.132 |
| Overall survival | 0.602 ± 0.011 | 0.559 ± 0.152 |
Table 3.
Hazard ratios and 95% confidence intervals for PET lymph node status, volume, and SUVmax for the recurrence-free survival (RFS), disease-specific survival (DSS), and overall survival (OS) PET nomogram models.
| PET lymph node status | Volume | SUVmax | |
|---|---|---|---|
| RFS | Pelvic 1.291 (0.750–2.221) | 1.002 (0.999–1.004) | 1.033 (1.005–1.062) |
| Aortic 4.091 (2.257–7.413) | |||
| SCV 24.868 (10.978–56.335) | |||
| DSS | Pelvic 1.291 (0.750–2.221) | 1.003 (1.000–1.005) | 1.011 (0.722–1.417) |
| Aortic 4.091 (2.257–7.413) | |||
| SCV 10.732 (4.505–25.567) | |||
| OS | Pelvic 1.077 (0.611–1.898) | 1.002 (1.000–1.005) | 1.009 (0.977–1.042) |
| Aortic 2.479 (1.273–4.827) | |||
| SCV 6.073 (2.563–14.391) |
Example patient: how to use the nomograms
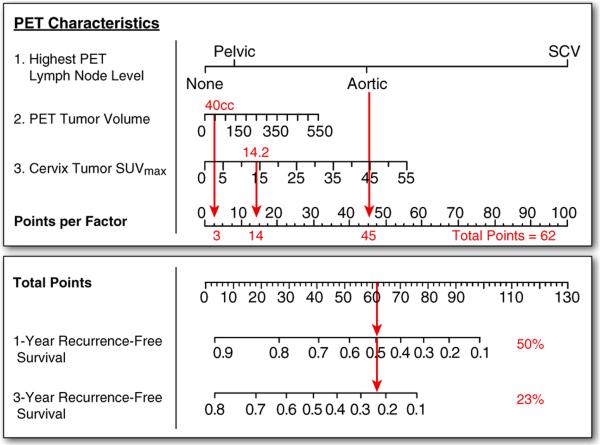
To help demonstrate the utility of the prognostic nomograms, Fig. 2 shows the RFS prediction for a 57-year-old woman who presented with clinical FIGO stage Ib2 disease. Her pretherapy diagnostic FDG-PET revealed pelvic and para-aortic lymph node involvement, a primary cervical tumor volume of 40 cm3, and a cervical tumor SUVmax of 14.2. She was treated with concurrent cisplatin and external irradiation and brachytherapy. The nomogram predicts a 1-year RFS of 50% and a 3-year RFS of 23%. This patient was found to have persistent disease 3 months following treatment, and she died from her disease shortly thereafter.
Fig. 2.
Example risk point estimation for the recurrence-free survival nomogram for a patient with FDG-PET showing para-aortic lymph node involvement, PET tumor volume of 40 cm3, and SUVmax of 14.2.
Discussion
In this study, we created and evaluated nomograms for RFS, DSS, and OS using PET-based data, including lymph node status, SUVmax and tumor volume, from 234 patients. These nomograms, especially for RFS and DSS, showed good prediction accuracies, as indicated by the c-statistics of 0.741 and 0.739, respectively. c-Statistics greater than 0.7 suggest that the RFS and DSS outcomes are well modeled by the PET-based nomograms. Additionally, the PET-based nomograms performed better than FIGO stage alone, which has traditionally been the main means of stratifying patient prognosis.
Approximately one-third of cervical cancer patients will have persistent disease following treatment or develop a recurrence and then eventually die of the disease [16]. For the patient with FIGO stage Ib2 disease discussed in the example above, one would estimate a 5-year OS of approximately 80% and a 5-year local control rate of 60–80%, based on FIGO stage alone. With use of the PET-based prognostic nomograms that take into account lymph node status, SUVmax, and tumor volume, dramatically different outcomes are predicted and these more accurately reflected the disease course. Being able to identify the patients at highest risk of recurrence prior to initiating therapy could provide an opportunity for modifying therapy or encouraging enrollment in a clinical trial to evaluate more aggressive therapy for this high-risk population. The PET-based prognostic nomograms offer a more accurate prediction of disease prognosis than FIGO stage and could also serve as a means of stratifying patients on clinical trials.
Historical factors connoting a poor prognosis for cervical cancer include advanced FIGO stage, large tumor size or volume, lymph node involvement by tumor, young patient age, poor performance status, and lack of brachytherapy [3–5]. Other groups have created prognostic models for cervical cancer. An analysis combining data for three GOG studies utilizing surgical staging and including 626 patients identified tumor size, lymph node status, age, and performance status as significantly associated with progression-free interval on multivariate analysis [2].
Nomograms have been used for risk stratification in a number of other disease sites, and generally it has been found that nomograms outperform expert clinicians in predicting outcome [17]. Nomograms are a visual representation of a statistical model and offer some advantages over univariate or multivariate analysis. Nomograms allow for differential weighting of various factors, while multivariate analyses generally use dichotomous categorization and equally weighted factors. Various prognostic factors may go into stratifying patients’ disease outcome, but particular prognostic factors may be more important for overall prognosis. For example, all three nomograms we present show PET lymph node status to be the strongest determinant of outcome, but for patients with no lymph node involvement on PET or pelvic lymph nodes only, PET volume or SUVmax may become more important prognostic factors.
For cervical cancer, the use of nomograms has been more limited. In a recent study involving 251 stage IIb–IVa cervical cancer patients, Tseng et al. created an OS nomogram using the prognostic factors of age, tumor size, lymph node metastases, parametrial invasion, hydronephrosis, bladder/rectum invasion, and serum squamous cell carcinoma antigen level [18]. This study emphasized the heterogeneity of their cervical cancer population and the utility of a risk stratification method such as a nomogram. With a focus on OS, as opposed to DSS or RFS, their model found older age to be the highest weighted negative prognostic factor. Seo and colleagues created a nomogram for 5-year survival for cervical cancer patients treated with radiation only incorporating age, pre-treatment hemoglobin level, FIGO stage, tumor diameter, LN status, and point A dose [19]. This study seems less widely applicable as over 30% of patients in the Seo study received less than 85 Gy to point A and no patients received concurrent chemotherapy, which has been recognized as standard of care for FIGO stage Ib2 and above since 1999. Our study had the goal of evaluating the combined prognostic value of three significant PET-based pretreatment factors and included RFS and DSS, in addition to OS, as these survival measures are specifically related to the cancer.
The factors included in the prognostic nomograms presented in this paper match well with historic prognostic factors for cervical cancer, while at the same time allowing for construction of a superior prognostic tool based on information obtained noninvasively by imaging. PET lymph node status was the most important prognostic factor in all three nomograms, and multiple studies have shown cervical cancer lymph node status, by surgery or CT, to be a significant determinant of outcome [2,4,5]. More recently, FDG-PET, with its high sensitivity and specificity for detecting lymph node metastasis, has proved useful for determining the extent of disease in patients with cervical cancer [7,8,20]. Additionally, FDG-PET allows for a noninvasive assessment of lymph node status, in contrast to surgical lymph node staging. Studies from our institution and others have shown that PET lymph node staging is an important cervical cancer prognostic factor and a better predictor of outcome than FIGO stage [7,13,21].
Although evaluated in different manners, such as clinical stage or tumor diameter by physical examination, cervical tumor size or volume has also been a consistent prognostic factor for cervical cancer outcome [3,21]. In previous work, our group has found the 40%-threshold volume on PET to be a good measure of tumor volume and prognostic for progression-free survival and OS [10]. This 40% threshold volume has been validated pathologically for early stage cervical cancer [22]. Another study including 63 cervical cancer patients and defining MTV in a slightly different manner found MTV to be correlated with lymph node metastasis, FIGO stage, and SUVmax [23]. FDG-PET allows for a 3-dimensional assessment of the primary tumor volume, while clinical examination only assesses a portion of the tumor size, which may explain the additional prognostic value of PET tumor volume. A study of head and neck cancer found that PET-based parameters of MTV and lymph node status were predictive of PFS and OS [24]. High FDG uptake, measured as the SUVmax, has been shown to predict worse outcome for a number of cancers. We and others have shown high SUVmax to be a negative prognostic factor for cervical cancer, with high FDG uptake predicting for more aggressive disease and worse disease-free survival [11,25]. The nomogram for RFS showed a greater influence of SUVmax, as compared to the DSS and OS nomograms. We had previously found that cervical cancer patients with a high SUVmax at diagnosis were at an increased risk of persistent disease following treatment (as detected by FDG-PET at 3 months posttherapy). This persistent disease, which would be scored as a recurrence, may be amenable to salvage therapy because of its earlier detection by PET. Using pre-treatment FDG-PET data, the nomograms allow for earlier risk stratification.
While the RFS, DSS and OS PET nomograms represent valuable new tools for risk stratifying patients with cervical cancer and for identifying high-risk populations, there are some limitations to our study. This study was performed at a single institution, which provides for some uniformity of PET and treatment standards, but does not necessarily ensure that these nomogram models are transferable to another population. Before applying these models in a clinical setting, it would be valuable to test the nomograms’ predictive ability in a separate population of cervical cancer patients who have undergone pretreatment FDG-PET imaging and for whom follow-up information is available. Our study included patients with a range of stages (Ia2–IVa), which could also influence the variability in outcome. Additionally, there is likely some interaction between the factors, as our previous research has shown that high primary cervical tumor SUVmax predicted for increased risk of lymph node involvement at diagnosis [11].
The RFS, DSS and OS PET-based prognostic nomograms represent a valuable new tool for managing and treating cervical cancer. Some of the strengths of these PET-based nomograms include 1) the large cervical cancer population used for the creation and testing of the nomograms; 2) the inclusion of multiple PET-based risk factors that had individually shown significant prognostic value and that reflect a modernization relative to historical prognostic factors; 3) the higher c-statistics of the PET-based nomograms compared with those based on FIGO stage alone; 4) the ability to differentially weight risk factors by using a nomogram model; and 5) the high c-statistics, specifically for RFS and DSS, indicating the high predictive accuracy of the PET-based prognostic nomograms. By combining PET-based risk factors that parallel historically poor prognostic factors for cervical cancer, the PET-based nomograms offer a modernization for cervical cancer risk models. Being able to identify noninvasively, prior to therapy, those patients with cervical cancer at highest risk of recurrence or compromised survival, allows for targeting this high risk population with more aggressive therapy, such as adjuvant chemotherapy or participation in a clinical trial. Additionally, the prognostic information offered by the PET-based prognostic nomograms could be used for risk stratification in clinical trials.
Footnotes
Conflict of interest statement
Elizabeth A. Kidd, MD, Issam El Naqa, PhD, Farrokh Dehdashti, MD and Perry W. Grigsby, MD — nothing to disclose. Barry A. Siegel, MD — Advisory Board, Siemens Molecular Imaging; Advisory Board, GE Healthcare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Stehman F, Bundy B, DiSaia P, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with irradiation therapy. I. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–85. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Grigsby PW, Chao KS, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–17. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 4.Kapp KS, Stuecklschweiger GF, Kapp DS, Poschauko J, Pickel H, Lahousen M, et al. Prognostic factors in patients with carcinoma of the uterine cervix treated with external beam irradiation and IR-192 high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1998;42:531–40. doi: 10.1016/s0360-3016(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 5.Yalman D, Aras AB, Ozkok S, Duransoy A, Celik OK, Ozsaran Z, et al. Prognostic factors in definitive radiotherapy of uterine cervical cancer. Eur J Gynaecol Oncol. 2003;24:309–14. [PubMed] [Google Scholar]
- 6.Grigsby PW, Dehdashti F, Siegel BA. FDG-PET evaluation of carcinoma of the cervix. Clin Positron Imaging. 1999;2:105–9. doi: 10.1016/s1095-0397(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 7.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 8.Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, Miraldi F. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol. 1999;17:41–5. doi: 10.1200/JCO.1999.17.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Wong TZ, Jones EL, Coleman RE. Positron emission tomography with 2-deoxy-2-[(18)F] fluoro-D-glucose for evaluating local and distant disease in patients with cervical cancer. Mol Imaging Biol. 2004;6:55–62. doi: 10.1016/j.mibio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:353–9. doi: 10.1016/s0360-3016(02)02705-0. [DOI] [PubMed] [Google Scholar]
- 11.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Dehdashti F, Herzog TJ, Mutch DG, Huettner PC, Rader JS, et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. 2005;104:2484–91. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 13.Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010:2108–13. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 15.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 16. http://seer.cancer.gov/statfacts/html/cervix.html.
- 17.Ross PL, Gerigk C, Gonen M, Yossepowitch O, Cagiannos I, Sogani PC, et al. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol. 2002;20:82–8. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JY, Yen MS, Twu NF, Lai CR, Horng HC, Tseng CC, et al. Prognostic nomogram for overall survival in stage IIB–IVA cervical cancer patients treated with concurrent chemoradiotherapy. Am J Obstet Gynecol. 2010;202:e1–7. doi: 10.1016/j.ajog.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Seo Y, Yoo SY, Kim MS, Yang KM, Yoo HJ, Kim JH, et al. Nomogram prediction of overall survival after curative irradiation for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2011;79:782–7. doi: 10.1016/j.ijrobp.2009.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Sugawara Y, Eisbruch A, Kosuda S, Recker BE, Kison PV, Wahl RL. Evaluation of FDG PET in patients with cervical cancer. J Nucl Med. 1999;40:1125–31. [PubMed] [Google Scholar]
- 21.Narayan K, Fisher RJ, Bernshaw D, Shakher R, Hicks RJ. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int J Gynecol Cancer. 2009;19:912–8. doi: 10.1111/IGC.0b013e3181a58d3f. [DOI] [PubMed] [Google Scholar]
- 22.Showalter TN, Miller TR, Huettner P, Rader J, Grigsby PW. 18F-fluorodeoxyglucose-positron emission tomography and pathologic tumor size in early-stage invasive cervical cancer. Int J Gynecol Cancer. 2009;19:1412–4. doi: 10.1111/IGC.0b013e3181b62e8c. [DOI] [PubMed] [Google Scholar]
- 23.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park N-H, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–4. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–8. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 25.Lee YY, Choi CH, Kim CJ, Kang H, Kim TJ, Lee JW, et al. The prognostic significance of the SUVmax (maximum standardized uptake value for F-18 fluorodeoxyglucose) of the cervical tumor in PET imaging for early cervical cancer: preliminary results. Gynecol Oncol. 2009;115:65–8. doi: 10.1016/j.ygyno.2009.06.022. [DOI] [PubMed] [Google Scholar]