Abstract
Background
With the Dutch population aging, the number of individuals 75 years old or more needing dialysis is growing. This analysis assessed the cost-effectiveness of adding nurse-assisted peritoneal dialysis (aPD) to the usual care pathway in frail Dutch end-stage renal disease (ESRD) patients.
Methods
The current Dutch treatment pathway (conservative management, CM: 40% and PD in nursing home, nhPD: 60%) was compared in a decision-tree model with a new approach where the proportion of patients on dialysis would increase to 80% (i.e. CM: 20%; nhPD: 20%; and aPD: 60%). In-center hemodialysis was added in a secondary analysis. Inputs included survival (from literature), utility (from literature), and costs (2009 official tariffs). A healthcare payer's perspective was used with a 5-year horizon.
Results
The new approach was almost cost neutral in the primary analysis (despite more patients on dialysis) and dominant (more effective and less expensive) in the secondary analysis. The incremental cost-effectiveness ratio was only €52/QALY. In the sensitivity analyses (primary and secondary analyses), the new approach was either dominant or cost-effective in approximately 75% of the simulations.
Conclusions
Despite the investment required, offering aPD to frail elderly ESRD patients is a cost-effective alternative to the current pathway for Dutch healthcare payers.
Keywords: Assisted peritoneal dialysis, Cost-effectiveness, Dialysis, Elderly, End-stage renal disease, Home treatment
Introduction
With the aging population, the number of individuals reaching 75 years of age that are in need of renal replacement therapy (RRT) is steadily growing.1 Studies have shown that dialysis can significantly impact the life of these patients both in terms of duration and quality.2–6 However, hemodialysis (HD) can be perceived as too burdensome as these patients may spend up to 47.5% of their remaining time alive at or in hospital.3 Peritoneal dialysis (PD) is an ideal alternative as it is less aggressive, avoids time loss at the hospital or while travelling to/from the hospital,7 and limits the exposure of these frail patients to hospital pathogens. Individuals 75 years or older are likely to have multiple health or cognitive problems; therefore, help may be needed to assist the patient in performing the procedure. If a spouse, child, or carer is not available for this, Dutch patients can be institutionalized in a nursing home where PD is then performed under the supervision of healthcare professionals. Another alternative is to provide nursing services at home. This is commonly known as assisted PD (aPD). Whether aPD is a cost-effective alternative to healthcare payers remains to be verified.
Methods
Model structure
A decision-tree model constructed in Excel was used to estimate the cost-effectiveness of offering aPD for the management of frail elderly end-stage renal disease (ESRD) patients. The current treatment pathway of these patients in the Netherlands, i.e. 40% managed conservatively (conservative management, CM) and 60% preferring to be institutionalized in a nursing home where PD can be performed under medical care (nhPD) was compared to a new treatment pathway in a primary analysis. In this new treatment pathway, 80% of patients would be treated with dialysis (compared with the current 60% in the current pathway) with 60% choosing aPD, 20% nhPD, and 20% CM. In a secondary analysis, a secondary base case treatment pathway including only 10% of patients managed conservatively, 80% managed through in-center HD (ICHD) and 10% via nhPD was compared to a secondary scenario, where 10% of patients would be managed conservatively, 75% by ICHD, 0% nhPD, and 15% aPD. The structure of the model is described in Fig. 1.
Figure 1:
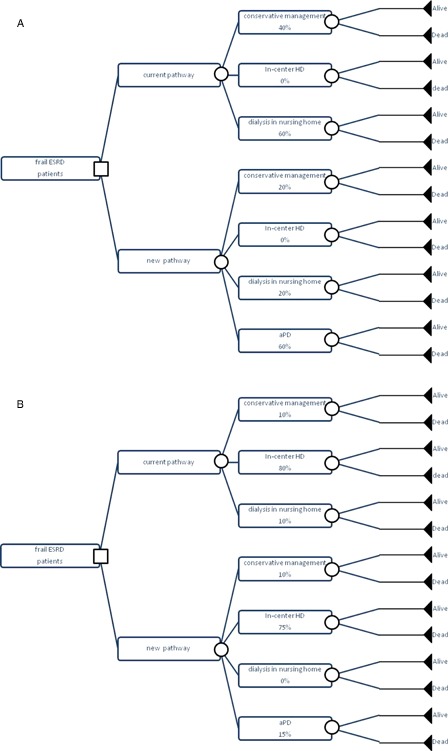
Model structure: (A) primary analysis; (B) secondary analysis.
Time horizon
The model was run with a 5-year horizon.
Inputs
Survival rates and utility (EuroQoL-5 or EQ-5D) values were taken from the medical literature. Five studies comparing survival between dialysis and conservative management were identified (Table 1).2–6 None of the studies specifically studied aPD, however, the survival of dialysis patients observed in these studies was not different than what had been observed in single arm trials in aPD patients.8,9 Four of these studies2–4,6 were felt to be of value for this analysis (based on patient population and sample size). Weighted averages of 1-, 2-, 3-, 4-, and 5-year survival rates were computed and used in the model (Fig. 2). The study by Smith5 was not included because the palliative care population was very small (dialysis = 10; conservative management = 26) and no separate analysis was performed for elderly people. Utilities for HD and PD were taken from a systematic review published by Liem et al. where the utility of PD measured with the EuroQoL 5 dimension scale (EQ-5D) was calculated to be 0.56 and that of PD of 0.58.10 For CM, the estimate from Teerawatananon et al.11 was adjusted for the difference in PD utility values between the two references (see Table 2 for detailed calculations). A utility penalty of 0.02 per year was further assumed for being in a nursing home and a utility premium of 0.01 per year for being at home. As a reference, Sennfalt et al. have estimated that a 14-day infection episode in a dialysis patient would negatively impact the annual utility value of dialysis by 0.02.12 Costs were taken from official tariffs (2009), except for conservative management, where an assumption was made by some of the authors based on their experience of the healthcare resources used. The complete list of inputs and their respective sources can be found in Table 2.
Table 1:
Description of studies reporting survival rates in elderly ESRD patients
| Author and year of publication | Country | Type of study | Sample size | Entry criteria | Survival results |
|---|---|---|---|---|---|
| Chandna et al. 20102 | UK | Retrospective (1990–2008) Single center |
All: RRT = 689 CM = 155 In >75 years old RRT = 77 CM = 106 |
ESRD patients seen at nephrology clinic (all ages) | Estimated from Fig. 3 (1-,2-,3-,4-,5-year survival) CM: 90%, 60%, 41%, 27%, 17% RRT: 90%, 62%, 45%, 33%, 22% |
| Carson et al. 20093 | UK | Retrospective (1997–2005) Single center (including unplanned start) 24% on PD |
All: RRT = 173 CM = 29 Excl unplanned start: RRT = 121 CM = 29 |
ESRD patients seen at Low Clearance Clinic ≥ 70-year old |
Estimated from Fig. 1C (excluding unplanned start) (1-,2-,3-,4-,5-year survival) CM: 60%, 17%, 12%, 0%, 0% RRT: 85%, 68%, 62%, 39%, 39% |
| Murtagh et al. 20074 | UK | Retrospective (2003–2007) Multi-center (unplanned start not included) % on PD unknown |
RRT = 52 (including 16 patients that did not start dialysis by end of study) CM = 77 |
ESRD patients who received dedicated multidisciplinary pre-dialysis care >75-year old |
From Table 2 and Fig. 2 (1-,2-,3-,4-,5-year survival) CM: 68%, 47%, 38%, 20%, 18% RRT: 84%, 76%, 72%, 72%, 72% |
| Smith et al. 20035 | UK | Retrospective (1996–2000) Single center (unplanned start not included) |
RRT = 258 CM = 63 For survival analysis in palliative care: RRT = 10 CM = 26 |
ESRD patients who received dedicated multidisciplinary pre-dialysis care All ages (16–92) |
Median survival: CM = 6.3 months RRT = 8.3 months |
| Joly et al. 20036 | France | Retrospective (1989–2000) Single center |
RRT = 107 CM = 37 |
ESRD patients not yet on dialysis seen at renal unit ≥80-year old |
From Fig. 1 (1-,2-,3-,4-,5-year survival) CM = 52%, 10%, 7%, 0%, 0% RRT = 85%, 70%, 40%, 30%, 27% |
Figure 2:
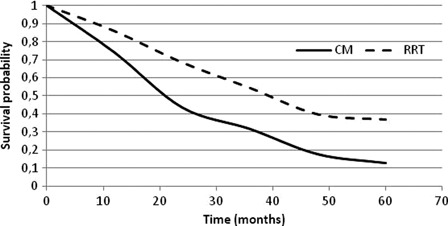
Weighted average of survival probabilities with dialysis (RRT) and CM.
Table 2:
Model inputs
| Variable | Value (range used for probabilistic sensitivity analysis) | Comment | |
|---|---|---|---|
| Conservative management (CM) | Proportion of patients | Primary analysis: Base case: 40% (30–50%) Scenario: 20% (10–30%) Secondary analysis: Base case: 10% (0–20%) Scenario: 10% (0–20%) |
Beta distribution within boundaries used for sensitivity analyses aPD assumed to have no impact on patients' preference for CM in secondary analysis, i.e. percent of patient choosing CM in scenario is the same as in the base case. |
| Survival (proportion) | |||
| Year 1 | 0.741 (0.686–0.795) | Weighted average of: Chandna et al.,2 Murtagh et al.,4 Carson et al.3 and Joly et al.6 95% confidence interval (CI) of proportion; beta distribution |
|
| Year 2 | 0.435 (0.374–0.497) | ||
| Year 3 | 0.316 (0.259–0.374) | ||
| Year 4 | 0.177 (0.129–0.224) | ||
| Year 5 | 0.128 (0.087–0.170) | ||
| Annual costs* | €15 000 (€12 000–18 000) | Assumption based on experience of some authors on the type of resources used ±20% of estimated tariff; beta distribution |
|
| Utility | 0.48 (0.24–0.72) | Adjusted from Teerawattananon et al.9: 0.60 × 0.58/0.72 95% CI; beta distribution |
|
| In-center HD (ICHD) | Proportion of patients | Primary analysis: Base case: 0% (0%) Scenario: 0% (0%) Secondary analysis: Base case: 80% (100%-CM value – nhPD value) Scenario: 75% (base case ICHD-0–10% of ICHD patients preferring aPD) |
ICHD not included in primary analysis In the secondary analysis, 0–10% of ICHD patients prefer aPD in the scenario; beta distribution |
| Survival (proportion) | |||
| Year 1 | 0.858 (0.824–0.892) | Weighted average of: Chandna et al.,2 Murtagh et al.,4 Carson et al.3 and Joly et al.6 95% CI of proportion; beta distribution |
|
| Year 2 | 0.684 (0.639–0.729) | ||
| Year 3 | 0.543 (0.495–0.591) | ||
| Year 4 | 0.397 (0.350–0.445) | ||
| Year 5 | 0.369 (0.322–0.415) | ||
| Annual costs | €50 087 (€40 070–60 104) | As per Dutch official dialysis tariff ±20% of current tariff; beta distribution |
|
| Utility for HD | 0.56 (0.49–0.62) | Liem et al.10 95% CI; beta distribution |
|
| Nursing home PD (nhPD) | Proportion of patients | Primary analysis: Base case: 60% (100%–CM value) Scenario: 20% (0–40%) Secondary analysis: Base case: 10% (0–20%) Scenario: 0% (0–15% of nhPD patients preferring aPD) |
Beta distribution. In the secondary analysis, 0–15% of nhPD prefer aPD in the scenario; beta distribution |
| Survival (proportion) | |||
| Year 1 | 0.858 (0.824–0.892) | Weighted average of: Chandna et al.,2 Murtagh et al.,4 Carson et al.3 and Joly et al.6 95% CI of proportion; beta distribution |
|
| Year 2 | 0.684 (0.639–0.729) | ||
| Year 3 | 0.543 (0.495–0.591) | ||
| Year 4 | 0.397 (0.350–0.445) | ||
| Year 5 | 0.369 (0.322–0.415) | ||
| Annual costs | €99 924 (€79 939–119 909) | Cost of PD (51 926.50€ per year + per diem nursing home, i.e. 160€/day) ±20% of current tariff; beta distribution |
|
| Utility for PD | 0.58 (0.50–0.67) | Liem et al.10
95% CI; beta distribution |
|
| Utility penalty for nursing home | 0.02 (0.016–0.022) | Assumption ± 20% of average; beta distribution |
|
| aPD | Proportion of patients | Primary analysis: Base case: 0 Scenario: 0.6 (1-CM-nhPD) Secondary analysis: Base case: 0 Scenario: 0.15 (1-CM-ICHD-nhPD) |
|
| Survival (proportion) | As per nhPD | As per nhPD | |
| Annual costs | €69 835 (€55 868–83 803) | Dutch tariff of PD (51 926.50€/year + assistance for APD, i.e. 17 908.50€/year) ±20% of estimated tariff; beta distribution |
|
| Utility premium for home | 0.01 (0.008–0.012) | Assumption ±20% of average; beta distribution |
*Ministry of Health perspective.
Perspective
Dutch healthcare (using the highest boundary of willingness-to-pay for the Netherlands, i.e. €80 000/QALY).13
Sensitivity analyses
Sensitivity analyses were performed by varying simultaneously all model parameters (Table 2). Monte-Carlo simulation (1000 simulations) was used to randomly select the value of each parameter (according to pre-specified probabilities and value boundaries, Table 2) and to re-calculate the estimated incremental cost-efficacy ratio (probabilistic sensitivity analysis). Multiple univariate sensitivity analyses were also performed by varying variables one at a time using the lower and upper boundaries of the estimate in order to identify the variables having the greater impact on the cost-effectiveness ratio and the conclusions.
Discounting
A 4% discounting rate was applied to costs while the discounting rate for benefits was 1.5%, in line with the recommendations of the Dutch Health Technology Assessment Agency.14 An analysis without discounting was also performed.
Results
Primary analysis
The primary analysis showed that the new approach including aPD was a cost-effective option despite having an additional 20% of patients on dialysis (Table 3). The incremental QALY per patient over the 5-year period was 0.1904 when discounted 1.5% per year (0.1981 undiscounted), while the incremental costs per patient over the same 5-year period amounted to only €10 when discounted 4% per year (€115 undiscounted). Savings were seen in the first year (€564 per patient) and then the new pathway was slightly becoming more expensive (€15–327 per patient) over the following years as the proportion of frail elderly patients alive on dialysis increased. The incremental cost-effectiveness ratio (ICER, i.e., difference in costs/difference in benefits of the two pathways) was therefore €52/QALY when discounting was applied to costs (4%) and benefits (1.5%) (€580 /QALY undiscounted), well below the Dutch willingness-to-pay (€80 000/QALY).
Table 3:
Results of the primary and secondary analyses
| Primary analysis |
Secondary analysis |
|||
|---|---|---|---|---|
| 5-year cumulative costs* | 5-year cumulative QALY* | 5-year cumulative costs* | 5-year cumulative QALY* | |
| Current pathway** | ||||
| Discounted | €166 918 | 1.2629 | €133 477 | 1.4740 |
| Not discounted | €181 718 | 1.3030 | €145 426 | 1.5232 |
| New pathway*** | ||||
| Discounted | €166 928 | 1.4533 | €128 189 | 1.4864 |
| Not discounted | €181 833 | 1.5011 | €139 663 | 1.5360 |
| Incremental analysis (new pathway – current pathway) | ||||
| Discounted | €10 | 0.1904 | −€5 288 | 0.0124 |
| Not discounted | €115 | 0.1981 | −€5 763 | 0.0128 |
| Incremental cost-effectiveness ratio (€/QALY) | ||||
| Discounted | 52 | −426 093 | ||
| Not discounted | 580 | −449 222 | ||
*Values rounded for presentation in the table.
**40% CM and 60% nhPD in the primary analysis; 10% CM, 80% ICHD, and 10% nhPD in the secondary analysis.
***20% CM, 20% nhPD, and 60% aPD in the primary analysis; 10% CM, 75% ICHD, 0% nhPD, and 15% aPD in the secondary analysis.
Secondary analysis
In the secondary analysis (Table 3), the new pathway was dominant (i.e., more effective, less costly) with an incremental QALY per patient of 0.0124 (0.0128 undiscounted), savings of €5288 (€5763 undiscounted), and an ICER of −€426 093/QALY (−€449 222/QALY undiscounted).
Sensitivity analyses
The new pathway was dominant in 44.4% of the 1000 simulations (discounted values) in the primary analysis, while it was cost-effective in 29.8% of the cases. In the secondary analysis, the new pathway was dominant in 20.4% of the simulations (discounted values) and cost-effective in 54.2%. The undiscounted analysis showed similar results. The scatter-plot graphs of the discounted values are shown in Fig. 3. The variables having the greatest impact on the ICER in the primary analysis (discounted and undiscounted analysis) were in decreasing order of impact: the proportion of CM patients in the base case, the cost of aPD, the cost of nhPD, the proportion of CM patients in the scenario, and the proportion of nhPD in the scenario (Fig. 4A). All except the percent of CM patients in the scenario could potentially generate an ICER above the Dutch willingness-to-pay (i.e. €80 000/QALY). In the secondary analysis, the variables having the greatest impact on the ICER were in decreasing order of impact: the PD utility, the cost of ICHD, the percent of patients on CM, and the percent of nhPD patients in the base case (Fig. 4B). Only one of these variables (i.e. the cost of ICHD) could potentially generate an ICER above the Dutch willingness-to-pay.
Figure 3:
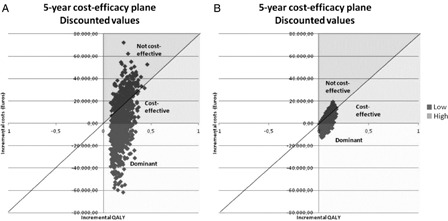
Probabilistic sensitivity analyses: (A) primary analysis; (B) secondary (discounted values).
Figure 4:
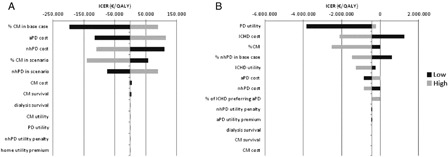
Tornado diagram of multiple univariate analysis: (A) primary analysis; (B) secondary analysis.
Discussion
This analysis shows that offering home nursing assistance to frail ESRD patients is a cost-effective alternative to the current pathway that can prolong life of these patients while maintaining them at home and allowing them to be independent from the hospital system. With the assumption that an additional 20% of patients would opt for having dialysis if aPD was available, the new pathway was more or less cost-neutral to the Dutch healthcare system. Should the proportion of additional patients opting for dialysis be lower than 20%, the new pathway would be cost-saving.
As with any health economic model, this analysis is as good as the inputs it uses to estimate the costs and benefits of the therapies compared. Only a few studies exist showing the impact of dialysis on the survival of frail ESRD patients. All these studies report combined survival rates for HD and PD. If survival rates were dependent on the dialysis modality in this age group, our model could either overestimate or underestimate the differences between treatment pathways. Earlier analyses of large patient databases suggested that the risk of death was higher for some sub-groups of PD patients.15 However, significant improvements in the survival of PD patients have been seen over the last decade16,17 and nowadays, the overall survival of PD patients is at least as good as that of HD patients.17,18 There seems to remain a difference in favour of HD in older diabetic patients, in particular for those with additional comorbidities.17 However, this is the group of patients for which dialysis (PD or HD) may not bring significant benefit.2,4 None of the studies used for this analysis were performed in aPD patients; however, there is no evidence that patients receiving nursing assistance for PD have a different survival than those who do not. In a retrospective single-arm study, Povlsen and Ivarsen8 observed 1- and 2-year survival rates in Danish aPD patients who were not different than the ones from Carson et al.3 used for this analysis. The survival rates observed by Castrale et al.9 in a retrospective single-arm study of aPD patients are also in line with the survival rates from the four studies used in this analysis. Therefore, using mixed HD and PD survival rates is unlikely to have impacted the conclusion from the model.
For some inputs (e.g. utility associated with nursing home or home assistance, cost of CM) no information could be found in the literature and some assumptions had to be made. The utility estimate used for PD came from a meta-analysis of the medical literature (i.e. 0.5800);10 however, this analysis did not differentiate PD from aPD. It was assumed that being institutionalized in a nursing home would have a negative impact on patient's quality of life and an arbitrary decrease of 0.02 was applied to the utility value. As a reference, this is equivalent to the annual impact of an infection episode in dialysis patients.12 On the contrary, maintaining the patient at home and providing home nursing assistance was assumed to positively impact patient's quality of life and the utility value was increased by 0.01. One could argue that these corrections were purely speculative and maybe overly optimistic (or pessimistic). However, patients' preference for home-based treatment is consistently seen in the literature19 and furthermore, sensitivity analyses on the utility values did not change the conclusions. On the cost side, the current tariffs were used (except for CM), thus reducing the variability of estimates. Nonetheless, these tariffs were varied by 20% in the sensitivity analyses. The aPD tariff assumed that all patients would need a nurse visit twice a day for as long as they are on PD. This may be an overestimation of the costs, as some may need only a few visits per week or assistance for only a few weeks or months. Using lower estimates of aPD costs would have further improve the cost-effectiveness of the new pathway. For the cost of CM, the authors made an estimate based on their experience in managing these patients and the costs were varied by ±20% in the sensitivity analyses. In addition, the costs of ICHD in the secondary analysis did not include transport to and from the dialysis center. This population of frail elderly patients is likely to need organized transport. If these costs had been added, the new pathway would have been even more cost-saving.
It may seem extraordinary to some nephrologists that 40% of frail elderly patients would choose CM in the Netherlands. Murtagh et al.4 in the UK reported than in 2003–2004, 59% of their elderly patients chose conservative management. Joly et al.6 on the other hand reported a rate of 26% in her French cohort in the years 1989–2000. A weighted average of the two cohorts would give a rate of 42%, thus in line with the 40% used in this analysis. Obviously, this is different in other parts of the world such as North America or Australia where conservative management has been reported to be preferred by only 10–14% of individuals.2,19 A lower percentage of patients preferring conservative management was used in the secondary analysis and in this analysis, aPD was dominant, i.e. more effective, less expensive.
In the Netherlands, frail elderly patients needing RRT are also offered ICHD but as shown by Carson et al.,3 the clinical benefit remains limited as these patients are likely to spend up to 47.5% of their time at or in hospital. Furthermore, frail elderly patients often have multiple comorbidities and contra-indications to HD. Therefore, only very few elderly ESRD patients would have the option to choose HD in the Netherlands, hence the exclusion of HD in the primary analysis current treatment pathway. The current options for frail elderly ESRD patients in the Netherlands are therefore conservative management or being institutionalized in a nursing home for appropriate renal replacement therapy. This pathway is very specific to the Netherlands and may limit the relevance of this analysis to other countries. However, a secondary analysis where most of these frail elderly patients were treated by ICHD showed that aPD was dominant (i.e. more effective and less expensive).
aPD represents for these patients a possibility to remain at home for a longer period of time without heavy medical treatment. Thus, it was felt that more patients would prefer being assisted at home rather than choosing one of the two current options, thereby increasing the overall number of patients on dialysis in the primary analysis (from 60% in the current pathway to 80% in the new pathway). Despite the increased costs associated with a larger number of patients choosing to have dialysis, the new treatment pathway was cost-effective over the current pathway. Other proportions of patients on the three treatment modalities were tested in the sensitivity analysis without significantly impacting the conclusions.
Lastly, the willingness-to-pay in the Netherlands is high and although the cost of healthcare is likely to be higher as well, both the cost of resources and the willingness-to-pay threshold would need to be customized before making a conclusion for other countries.
In conclusion, dialysis has been shown to significantly improve the survival and quality of life of frail elderly patients (over 75-year old). However, these patients are likely to need help to perform the procedure at home. In the absence of a spouse, child, or carer, paid help may be necessary. Despite the required investment in home nursing activities and the likely higher proportion of patients who would opt for dialysis treatment rather than conservative management, in the Netherlands, aPD in these patients is cost-effective. Secondary and sensitivity analyses confirm the robustness of these conclusions with the new pathway being either cost-saving or cost-effective in approximately 75–80% of the simulations.
Biographies
Suzanne Laplante is a pharmacist with a master in pharmaceutical sciences and a diploma in Public Health working for Baxter Healthcare Corporation as a health economist since 2008.
Harmen Krepel currently works as a physician and nephrologist in the Franciscus Hospital Roosendaal. Prior to his current position, he worked in West-Africa in epidemiological research. He published various articles on renal replacement therapy.
Bregje Simons is a dialysis nurse and the team leader of the Roosendaal Franscisus Hospital dialysis unit since 2007. In her role she coordinates care innovation projects such as nocturnal in-centre dialysis and dialysis at home.
Aafke Nijhoff is a nurse who worked at the dialysis unit of several hospitals in the Netherlands, where among other things she implemented patient self-management programs. She is now Therapy and Clinical manager at Baxter Healthcare Corporation.
Rens van Liere owns a master in business economics and is the president of Healthcare Management School, a healthcare consultant founded in 2001. One of his activities is to help the healthcare industry develop new models for care pathway.
Michel Simons worked in genetics research and development after finishing his studies in biochemistry. He is currently working at Baxter Healthcare Corporation in the Netherlands.
References
- 1.Kramer A, Stel V, Zoccali C, Heaf J, Ansell D, Groenhagen-Riska C, et al. An update on renal replacement therapy in Europe: ERA-EDTA registry data from 1997 to 2006. Nephrol Dial Transplant 2009;24:3557–66 [DOI] [PubMed] [Google Scholar]
- 2.Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011;26:1608–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009;4:1611–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007;22:1955–62 [DOI] [PubMed] [Google Scholar]
- 5.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 2003;95:c40–c46 [DOI] [PubMed] [Google Scholar]
- 6.Joly D, Anglicheau D, Alberti C, Nguyen AT, Toua M, Gruenfeld JP, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 2003;14:1012–21 [DOI] [PubMed] [Google Scholar]
- 7.Brown EA. PD in the elderly. Should older patients be offered peritoneal dialysis? Perit Dial Int 2008;28:444–8 [PubMed] [Google Scholar]
- 8.Povlsen JV, Ivarsen P. Assisted automated peritoneal dialysis (AAPD) for the functionally dependent and elderly patient. Perit Dial Int 2005;25Suppl. 3:S60–S63 [PubMed] [Google Scholar]
- 9.Castrale C, Evans D, Verger C, Fabre E, Aguilera D, Ryckelynck JP, et al. Peritoneal dialysis in elderly patients: report from the French peritoneal dialysis registry (RDPLF). Nephrol Dial Transplant 2010;25(1):255–62 [DOI] [PubMed] [Google Scholar]
- 10.Liem YS, Bosch JL, Hunink M. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11(4):733–41 [DOI] [PubMed] [Google Scholar]
- 11.Teerawattananon Y, Mugford M, Tangcharoensathlen V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decision in Thailand. Value Health 2007;10(1):61–72 [DOI] [PubMed] [Google Scholar]
- 12.Sennfalt K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis – a cost-utility analysis. Peritoneal Dial Intnl 2002;22:39–47 [PubMed] [Google Scholar]
- 13.College voor zorgverzekeringen (CVZ). A background study on the cost-effectiveness package principle for the benefit of the appraisal phase in package management. Publication number 291. 17 Nov 2010. [Accessed 2011 Jan 4]. Available from: http://www.cvz.nl/binaries/content/documents/cvzinternet/en/documents/assessments/asm1011-cost-effectiveness-principle.pdf
- 14.College vor zorgverzekeringen (CVZ). Guidelines for pharmacoeconomic research, updated version. March 2006. [Accessed 2012 Jan 4]. Available from: http://www.cvz.nl/binaries/content/documents/cvzinternet/en/documents/procedures/guidelines-pharmacoeconomic-research.pdf
- 15.Vonesh EF, Snyder JJ, Foley RN, Collins AJ. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Intnl 2004;66:2389–401 [DOI] [PubMed] [Google Scholar]
- 16.Kramer A, Stel V, Zocalli C, Heaf J, Ansell D, Gronhagen-Riska C, et al. An update on renal replacement therapy in Europe: ERA-EDTA registry data from 1997 to 2006. Nephrol Dial Transplant 2009;24:3557–66 [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra R, Chiu YW, Kalatar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011;171(2):110–18 [DOI] [PubMed] [Google Scholar]
- 18.Van de Luijtgaarden MWM, Noordzij M, Wanner C, Jager KJ. Renal replacement therapy in Europe – a summary of the 2009 ERA-EDTA registry annual report. Clin Kidney J 2012;5:109–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012;60(1):102–11 [DOI] [PubMed] [Google Scholar]


