Abstract
Puberty is characterized by hormonal, physical and psychological transformation. The human brain undergoes significant changes between childhood and adulthood, but little is known about how puberty influences its structural development. Using a longitudinal sample of 711 magnetic resonance imaging scans from 275 individuals aged 7–20 years, we examined how subcortical brain regions change in relation to puberty. Our regions of interest included the amygdala, hippocampus and corpus striatum including the nucleus accumbens (NA), caudate, putamen and globus pallidus (GP). Pubertal development was significantly related to structural volume in all six regions in both sexes. Pubertal development and age had both independent and interactive influences on volume for the amygdala, hippocampus and putamen in both sexes, and the caudate in females. There was an interactive puberty-by-age effect on volume for the NA and GP in both sexes, and the caudate in males. These findings suggest a significant role for puberty in structural brain development.
Abbreviations: NA, Nucleus Accumbens; GP, Globus Pallidus
Keywords: Puberty, Adolescence, MRI, Subcortex
Highlights
-
•
Subcortical regions continue to develop through puberty in females and males.
-
•
The developmental trajectories vary between subcortical regions.
-
•
Puberty and age have independent and interactive influences on this development.
Introduction
The past 15 years have seen a major expansion in research on the structural development of the human adolescent brain, based largely on the results of cross-sectional and longitudinal magnetic resonance imaging (MRI) studies (Brain Development Cooperative Group, 2012; Giedd et al., 1996; Lenroot et al., 2007; Østby et al., 2009; Raznahan et al., 2011; Sowell et al., 2002). Studies of brain growth trajectories over adolescence to date have predominantly considered growth in relation to chronological age, with few exceptions (Paus et al., 2010; Raznahan et al., 2010). However, this approach ignores the cascade of developmental processes during adolescence that are related to puberty, including sexual maturation, linear (height) growth, body fat re-distribution and maturation of many other physiological systems (Patton and Viner, 2007; Wheeler, 1991). Since there is a normal variation of 4–5 years in the timing of onset of puberty in healthy humans (Parent et al., 2003), pubertal development is partially dissociable from chronological age. Examining brain development in relation to pubertal maturation may provide additional information regarding the mechanisms associated with adolescent brain development. In this study, we investigated how the developmental trajectories of subcortical regions that are linked to stereotypical behaviours are associated with pubertal development (Forbes and Dahl, 2010; Steinberg, 2008): the amygdala and hippocampus, which play an important role in emotion and mood regulation (Davidson et al., 2002); and the corpus striatum including the nucleus accumbens (NA), caudate, putamen and globus pallidus (GP), which are involved in decision-making and reward-seeking behaviours (Gottfried, 2011).
It has been hypothesised that the brain restructuring and development seen in adolescence may be specifically related to the hormonal influences that control the onset of and progression through puberty (Giedd et al., 1999; Lenroot et al., 2007; Peper et al., 2011; Sowell et al., 2002). Sex steroids such as testosterone (an androgen) and oestradiol (an oestrogen) have been shown to be capable of inducing both synaptogenesis and synaptic pruning in rats and nonhuman primates (Ahmed et al., 2008; Hajszan et al., 2008; Sato et al., 2008) with differential effects of androgens and oestrogens on different brain areas, which may be related to hormone receptor distribution (Clark et al., 1988; Sholl and Kim, 1989). These differential effects across brain areas may provide an explanation for the diverging growth trajectories of particular brain structures between males and females documented across studies, and the resultant increasing sexual dimorphism in adolescence reported in some regions (Brain Development Cooperative Group, 2012; Lenroot et al., 2007; Neufang et al., 2009; Sowell et al., 2002).
Cross-sectional studies have reported that puberty is associated with aspects of brain development in adolescence. A study focussing on the association between brain volumes and both pubertal stage and testosterone concentration found that males and females in later stages of puberty, and with higher circulating testosterone concentration, had larger amygdala volumes and smaller hippocampal volumes than their less well developed peers (Neufang et al., 2009). In contrast, a second study investigating puberty and pubertal hormone correlations with grey matter volume replicated the positive association between amygdala volume and pubertal stage in boys, but showed decreasing amygdala volume with increasing testosterone levels in girls (Bramen et al., 2011). These studies have limited power due to their relatively small sample sizes and cross-sectional methods. To date, no longitudinal studies incorporating pubertal measures have been published. Longitudinal analysis allows comparison of brain volumes both between-subjects and also within each subject over time, and therefore can provide a measure not just of brain volume at a particular time-point, but also of developmental trajectories for each of these subcortical brain regions by following what happens to each participant. This is particularly advantageous when looking at brain volumes, which vary substantially between individuals (Brain Development Cooperative Group, 2012; Giedd et al., 1999; Tamnes et al., 2013).
Using a large sample of scans from 275 individuals scanned longitudinally, we examined how subcortical brain regions change in relation to puberty as measured by Tanner stage (Tanner and Whitehouse, 1976), and compared developmental trajectories in females and males. We used a large dataset containing information on pubertal stage and chronological age to examine growth trajectories over adolescence in each of these subcortical regions. Based on previous cross-sectional findings, we predicted that the volume of the amygdala and hippocampus would increase between 7 and 20 years (Giedd et al., 1999; Østby et al., 2009), whilst the volumes of the corpus striatum – the NA, caudate, putamen and GP – would decrease (Brain Development Cooperative Group, 2012; Østby et al., 2009; Sowell et al., 2002). We hypothesized that volume change for all structures of interest would be related to pubertal development as measured by Tanner stage, and that puberty and age would have independent effects on volume in these regions. Therefore, we hypothesized that models incorporating both Tanner stage and chronological age as explanatory variables for volume change would provide a significant fit for the developmental data from the structures examined.
Experimental procedures
Participants
The sample used for the analysis was taken from the NIMH Child Psychiatry Branch Section on Brain Imaging longitudinal dataset of structural MRI scans (Giedd et al., 1996). This large dataset consists of more than 6500 scans from more than 3000 participants of which approximately half are typically developing and half are from various diagnostic groups. Inclusion and exclusion criteria for the overall dataset can be found on the NIMH website (http://clinicalstudies.info.nih.gov/cgi/detail.cgi?A_1989-M-0006.html). For each participant, the outcomes of interest that were measured included ethnicity, socioeconomic status (using Hollingshead scales), IQ (estimated using age-appropriate Wechsler Intelligence Scales) and handedness (using Physical and Neurological Examination of Soft Signs inventory (Denckla, 1985)), each of which was collected at the time of the first scan, and pubertal status, ascertained at the time of each scan using Tanner stage diagrams (Taylor et al., 2001). Tanner staging is the most widely used technique for assessing physical puberty stage. For each participant at each time-point, a single combined Tanner stage score was assigned based on the overall stage that the participant felt best described themselves from looking at the separate breast/genital and pubic hair scores.
The subgroup used for the current analysis consisted of 275 unrelated individuals (117 females), and incorporated the scans of all individuals who met the following criteria:
-
1.
Healthy individuals at the time of scanning. Participants were screened for neurological or psychiatric illness using a telephone screening interview and completion of a parent-report screening questionnaire (CBCL) at each time-point, and only healthy participants were included in the analysis as established by standardized scoring (Achenbach, 1991). All participants included had an IQ of greater than 80.
-
2.
Two or more MRI scans between the ages of 7.0 and 20.0 years. This age range was estimated using two large US population-based studies on pubertal timing (Herman-Giddens et al., 1997; Sun et al., 2002), and incorporating ages from two 2 SD below the mean age of being in Tanner stage 2 (6.3–7.8 years for females and 6.9–9.7 years for males depending on the measure (breast/pubic hair/gonadal development) used), to 2 SD above the mean age of being in Tanner stage 5 (20.5–21.0 years for females, 19.6–20.1 years for males depending on the measure used). This range was refined based on our dataset to 7.0 to 20.0 years, as this incorporated the ages at which there was variation in Tanner stage between individuals.
-
3.
Complete data for age and self-reported Tanner stage for each MRI scan. Individuals who reported regression of puberty during adolescence (as indicated by reducing Tanner score) were excluded from the analysis (N = 1; female), since pubertal regression is essentially biologically implausible and likely represents error. Pubertal arrest, which may be perceived as regression, is associated with significant systemic illness or malnutrition.
-
4.
Only one individual per family. The original dataset incorporates a high number (> 400 individuals) of monozygous and dizygous twin pairs, as well as siblings. The heritability of brain structure and development is still not well-understood, but there are likely to be both genetic and environmental effects on structural brain volume and developmental trajectory, as well as pubertal timing, which are not independent between family members, and therefore might bias the analysis. Where data were available for more than one family member, only one individual was included in the analysis; the family member included was determined by the participant with a higher number of high quality scans, more complete demographic data, or, if these were equal, by random selection.
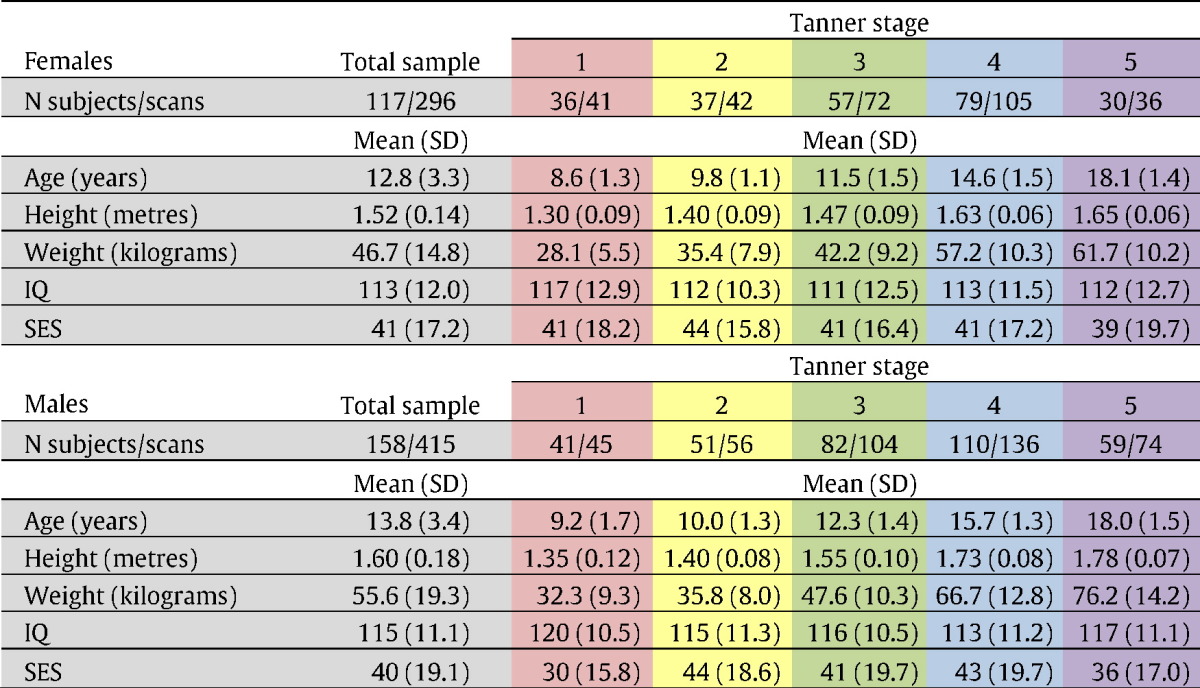
Of the 275 participants whose data were included in the study, 87.3% were right-handed (7.3% left-handed, 5.4% mixed). The majority (89.5%) were Caucasian (5.1% African–American; 2.2% Hispanic; 0.7% Asian; 2.5% other). Details of socioeconomic status, IQ, puberty status and number of scans can be seen in Table 1.
Table 1.
Showing participants' demographics.
*This shows the number of participants who underwent an MRI scan at each Tanner stage, and the total number of scans collected of participants at each Tanner stage. Since some participants had more than one scan at a single Tanner stage, there are more scans than participants.
Participants were recruited from the community through local advertisement and reimbursed for their participation in the study. The study research protocol was approved by the Institutional Review Board of the National Institutes of Health and written informed consent and assent to participate in the study were obtained from parents/adult participants and children respectively.
Image acquisition
All MRI scans were T-1 weighted images with contiguous 1.5 mm axial slices and 2.0 mm coronal slices, obtained on the same 1.5-T General Electric Signa scanner (Milwaukee, WI) using a 3D spoiled gradient recalled echo sequence with the following parameters: echo time: 5 ms; repetition time: 24 ms; flip angle: 45°; acquisition matrix: 256 × 192; field of view: 24 cm. The same scanner, hardware and software were used throughout the scanning period. All scans were assessed by a clinical neuroradiologist for gross abnormalities. All scans performed as part of the NIH project have been rated for motion-related quality by trained technicians. Only scans given a high-quality rating were included in the analysis.
Image processing
Subcortical volume estimation was performed with the Freesurfer 5.1 image analysis suite using the programme's automated segmentation procedure (http://surfer.nmr.mgh.harvard.edu/). This procedure has been described in detail previously (Fischl et al., 2002), and is summarized here. An optimal linear transform is computed that maximises the likelihood of the input image, given an atlas constructed from manually labelled images. A nonlinear transform is then initialized with the linear one, and the image is allowed to further deform to better match the atlas. Finally, a Bayesian segmentation procedure is carried out, and the maximum a posteriori estimate of the labelling is computed. The segmentation uses three pieces of information to disambiguate labels: (1) the prior probability of a given tissue class occurring at a specific atlas location; (2) the likelihood of the image given that tissue class; and (3) the probability of the local spatial configuration of labels given the tissue class. The automated segmentations have been found to be statistically indistinguishable from manual labelling (Fischl et al., 2002), and correlations between Freesurfer segmentation and manual labelling of hippocampal volume by trained researchers reached 0.85 in a study by Tae et al. (2008).
Our analysis focussed on the following subcortical brain regions, based on a priori hypotheses regarding changes in volume over adolescence and pubertal effects: the amygdala, hippocampus, NA, caudate, putamen and GP. Each of these regions is defined by the automated Freesurfer segmentation procedure based on location, likelihood of tissue class and spatial configuration. An example of T1W scans showing both the raw data and with Freesurfer's automated segmentation can be seen in the Supplementary information (Fig. S1).
Statistical analysis
We conducted our analysis using the average volume across hemispheres, to produce one value for each ROI. Previous studies have demonstrated no evidence of developmental difference between hemisphere in these regions (Brain Development Cooperative Group, 2012; Østby et al., 2009), and our own dataset shows high correlations between hemispheres for all volumes (r = 0.5–0.9, p < 0.001). We analysed raw volumes for each region and percentage change for each volume over time. Mixed effects modelling was used (R version 3.1-102; nlme package (Pinheiro et al., 2011)) to analyse the data, thereby allowing an estimation of the fixed effects of measured variables on volume change, whilst incorporating the longitudinal nature of the data by including within-person variation as nested random effects. Age was centred on 7 years, which represented the minimum age included in the sample. Tanner stage was treated as a continuous variable for this analysis, allowing the model to incorporate changing Tanner stage and changing brain volume for each individual.
For each structure, volume was first modelled against Tanner stage (Tanner-only model), and linear, quadratic or cubic developmental trajectories were modelled. Males and females were modelled separately, to allow for different trajectories of growth through adolescence (Lenroot et al., 2007). Tanner stage was treated as a continuous variable to allow the model to account for movement of individuals between stages and to maintain the ordinal nature of the data. The equations for volume growth of a structure in relation to Tanner stage are:
where β1, β2 and β3 represent the constant terms defining the effects of each fixed term. To determine whether a cubic, quadratic or linear growth model with Tanner stage best fit the sample, an F test was performed on models where the marginal p-value of the highest order variable was significant (p < 0.05).
Given that pubertal development is a developmental process, Tanner stage is necessarily highly correlated with chronological age, and this was found to be the case in our sample (r = 0.89 for males and r = 0.88 for females). It was therefore necessary to consider the effects of age on the developmental trajectories of the subcortical volumes, to see whether this could explain all of the puberty-related effects, and to establish whether models including age with puberty were a better fit than puberty models alone. Therefore, age was incorporated into the model to ascertain whether there were (i) dissociable main effects of Tanner stage and age, and/or (ii) a Tanner stage by age interactive effect. The model including the main effects of both Tanner stage and age and the tanner by age interaction is referred to as the combined model (Volume = Intercept + β1(Tanner) + β2 (Age) + β3 (Tanner ∗ Age)), whilst the model including only the Tanner stage by age interaction is referred to as the interaction-only model (Volume = β1 (Tanner ∗ Age). Lastly, an age-only model was estimated (using linear, quadratic and cubic growth options as above).
Comparison of Tanner-only, combined, interaction-only and age-only models was undertaken using likelihood ratio tests where possible. Where LR tests could not be performed, i.e. if the models were not nested, Akaike Information Criterion (AIC) values were used to compare models. Models were considered to be a significantly improved fit if the difference between AIC values was 5.9 or greater, equating to an Akaike weight of the poorer model of less than 0.05. If the difference exceeded this, the inferior model was discarded, leaving only the models that were equally valid based on relative likelihood (Wagenmakers and Farrell, 2004).
Results
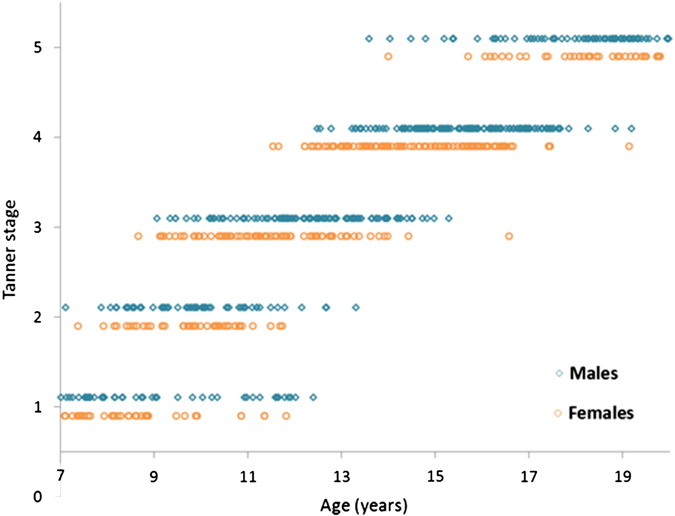
Demographic details for all participants are included in Table 1 and Fig. 1. The raw data for each sex and each structure are displayed in Fig. S2 (Supporting Information).
Fig. 1.
Showing the age and Tanner stage of each participant at each study time-point. Males are shown in turquoise, and females are shown in orange.
Models using Tanner stage as an explanatory variable for subcortical volume change
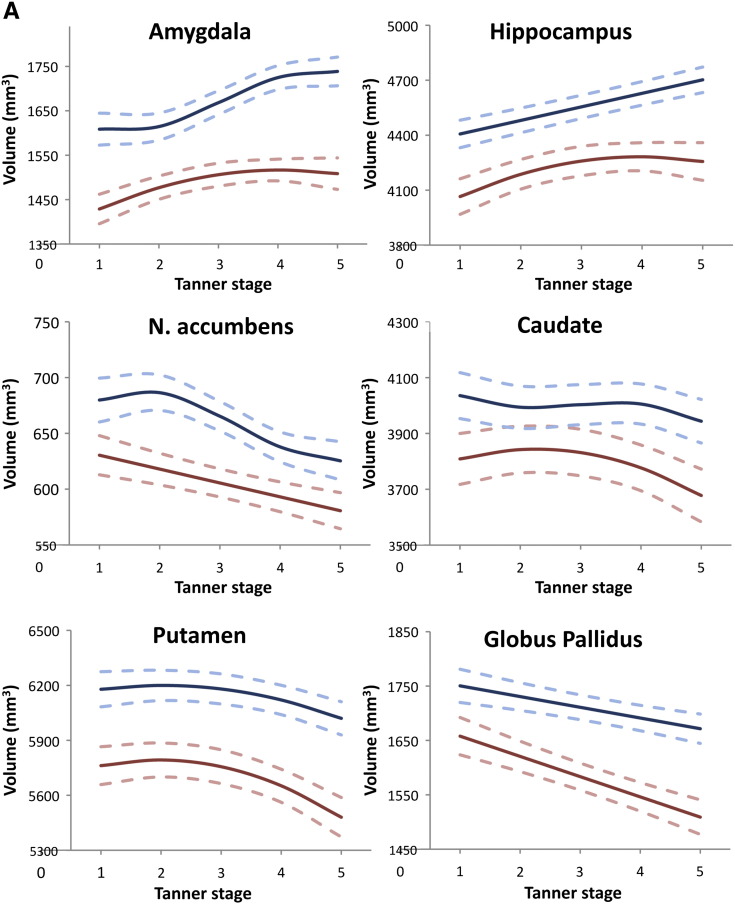
Pubertal development, as measured by Tanner stage, was significantly related to the structural development of all six subcortical regions in both males and females (See Table 2 and Fig. 2). In both sexes, amygdala and hippocampus volume increased across puberty, whilst the other structural volumes (NA, caudate, putamen and GP) decreased (See Fig. 2). In females, the growth trajectories were either linear (NA, GP) or quadratic (amygdala, hippocampus, caudate and putamen), whilst in males the trajectories were linear (hippocampus, GP), quadratic (putamen) or cubic (amygdala, NA, caudate) (See Table 2). The proportional volume change over puberty varied between structures from a 7.5% increase in male amygdala volume, to a 9.9% reduction in female GP volume, with some regions showing more modest volume changes e.g. caudate 2.3% reduction in males. See Table 2 and Fig. 2 for full results.
Table 2.
Showing the Tanner-only best fit model for each of the six subcortical regions in females and males with the F test and p-value of the highest order variable. For each region, volume change across puberty is given in absolute (mm3) and relative (% change for the structure compared to initial volume) terms.
| Best-fitting model | Volume change across puberty |
Significance of highest order variable | p-value | ||
|---|---|---|---|---|---|
| Absolute (mm3) | % change | ||||
| Females | |||||
| Amygdala | Quadratic | 80 | 5.3% | F(1, 177) = 6.38 | 0.012 |
| Hippocampus | Quadratic | 191 | 5.1% | F(1, 177) = 6.26 | 0.013 |
| Nucleus accumbens | Linear | − 50 | − 8.6% | F(1, 178) = 18.73 | < 0.0001 |
| Caudate | Quadratic | − 131 | − 3.6% | F(1, 177) = 10.78 | 0.001 |
| Putamen | Quadratic | − 282 | − 5.5% | F(1, 177) = 16.15 | 0.0001 |
| Globus pallidus | Linear | − 149 | − 9.9% | F(1, 178) = 45.02 | < 0.0001 |
| Males | |||||
| Amygdala | Cubic | 130 | 7.5% | F(1, 254) = 7.40 | 0.007 |
| Hippocampus | Linear | 296 | 6.3% | F(1, 256) = 69.01 | < 0.0001 |
| Nucleus accumbens | Cubic | − 55 | − 8.7% | F(1, 254) = 4.44 | 0.036 |
| Caudate | Cubic | − 92 | − 2.3% | F(1, 254) = 4.21 | 0.041 |
| Putamen | Quadratic | − 159 | − 2.6% | F(1, 255) = 7.91 | 0.005 |
| Globus pallidus | Linear | − 79 | − 4.7% | F(1, 256) = 19.22 | < 0.0001 |
Fig. 2A.
Showing the growth trajectories for each subcortical region modelled against Tanner stage in females and males. For both sexes, the amygdala and hippocampus increase in volume over puberty, whilst the NA, caudate, putamen and GP decrease in volume. Shows the models for each region separately. Pink lines represent females and blue lines represent males. The solid line represents the best model fit, with 95% confidence intervals shown by the dashed lines.
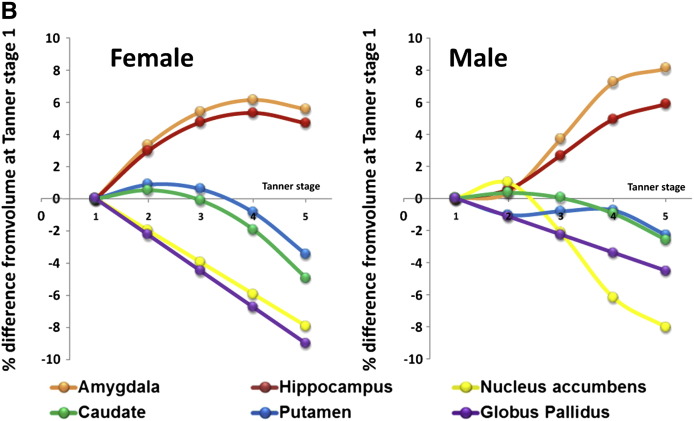
Fig. 2B. Showing the growth trajectories for each subcortical region modelled against Tanner stage in females and males. For both sexes, the amygdala and hippocampus increase in volume over puberty, whilst the NA, caudate, putamen and GP decrease in volume. Shows the models in terms of % volume change to allow comparison between structures. For each structure, the percentage volume was calculated for each pubertal stage as a proportion of prepubertal volume (at Tanner stage 1).
Models using Tanner stage and chronological age as explanatory variables for subcortical volume change
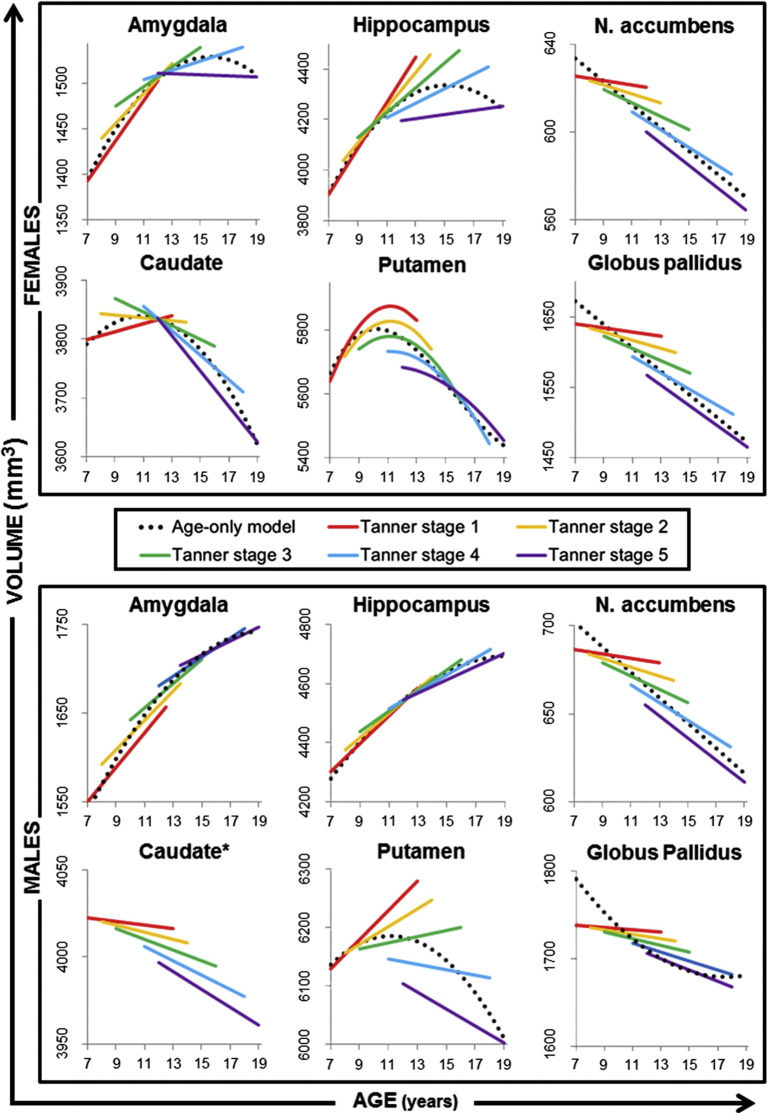
For the amygdala, hippocampus and putamen in both sexes, as well as the caudate in females, a combined model (including Tanner stage and age as main effects, and a Tanner stage by age interaction) provided a significantly better fit than the Tanner-only model (See Table 3). For each of these structures, individuals in later stages of puberty (i.e. higher Tanner stage score) than their age-matched peers had volumes that are further along the developmental trajectory than peers in earlier stages of puberty. For females, individuals who were more pubertally mature than their age-matched peers had a larger volume in these structures in late childhood and early adolescence, an earlier peak volume and then a smaller volume until the end of puberty than their less mature, age-matched peers (see Fig. 3). In males, for the amygdala and hippocampus, individuals in later stages of puberty (i.e. higher Tanner stage score than age-matched peers) had larger structural volumes throughout our studied age range (7–20 years) than age-matched peers in earlier stages of puberty (see Fig. 3). In contrast, for the putamen in males, individuals in later stages of puberty had smaller structural volumes throughout our studied age range than age-matched peers in earlier stages of puberty (see Fig. 3).
Table 3.
Showing best fit models using a combination of Tanner stage and chronological age variables, with likelihood ratios and differences in AIC. Structures in BOLD show structures where the mixed Tanner stage and age model is a significantly better fit than the Tanner stage only model.
a: Combined model refers to a model incorporating independent effects of Tanner stage and chronological age as well as an interactive Tanner stage by age effect. Interactive model refers to a model using only an interactive Tanner stage by age effect.
b: In females, the best fit model for the putamen was a combined model including independent effects of Tanner stage, linear and quadratic chronological age as well as an interactive Tanner stage by age effect.
* For these structures, a likelihood ratio test is not valid as the models are not nested and have the same number of degrees of freedom. Therefore, significance of the models has been judged using AIC differences. If AIC difference ≥ 5.9, the model is a significantly better fit (equivalent to Akaike weight of < 0.05).
| Structure | Best-fitting Tanner and age modela | Likelihood ratio test compared to Tanner only model | p-value | Difference between AIC |
|---|---|---|---|---|
| Females | ||||
| Amygdala | Combined | 7.67 | 0.006 | |
| Hippocampus | Combined | 23.96 | < 0.0001 | |
| Nucleus accumbens | Interactive | 3.21* | * | 3.21 |
| Caudate | Combined | 14.16 | 0.0002 | |
| Putamen | Combinedb | 28.13 | < 0.0001 | |
| Globus pallidus | Interactive | 3.43* | * | 3.43 |
| Males | ||||
| Amygdala | Combined | 9.48* | * | 9.48 |
| Hippocampus | Combined | 23.06 | < 0.0001 | |
| Nucleus accumbens | Interactive | 4.63 | 0.099 | |
| Caudate | Interactive | 5.29 | 0.071 | |
| Putamen | Combined | 5.16 | 0.023 | |
| Globus pallidus | Interactive | 1.51* | * | 1.51 |
Fig. 3.
Showing how subcortical volumes change with age and puberty stage in (A) females and (B) males using the best-fit combined or interactive model for each structure.
Age is presented on the x-axis, whilst puberty stage is indicated by the coloured lines (Red — Tanner stage 1, Yellow — Tanner stage 2, Green — Tanner stage 3, Blue — Tanner stage 4, Purple — Tanner stage 5). Data for each graph were extracted from the combined models by calculating Intercept + (coefficient for main effect of Tanner x Tanner stage) + (coefficient for main effect of Age-centred x Age-centred) + (coefficient for interactive effect of Tanner-by-age x Tanner stage x Age). Age ranges for each Tanner stage line were decided using the ages and pubertal variation in our sample (see Fig. 1 for range). The age-only model for each structure is included (black dotted line) to aid interpretation.
*For the caudate in males, there is no significant model that explains the developmental trajectory.
For the NA and GP in both sexes, and for the caudate in males, an interaction-only model provided a significant fit to the data, but the model was no improvement over the Tanner stage only model (See Table 3). Based on this interaction model, individuals in a later stage of puberty than their age-matched peers had a smaller NA or GP (both sexes) or caudate (males only) volume throughout the age range investigated (7–20 years) (Fig. 3).
Models using chronological age as an explanatory variable for subcortical volume change
For some structures in males, the hippocampus and the GP, age-only models gave a significantly better fit of the data than models incorporating Tanner stage (hippocampus LR test compared to interactive model = 4.69, p = 0.030; GP LR test compared to interactive model = 9.29, p = 0.002). There was no statistically significant age-only model for volume change in the caudate in males over our age range. For the remaining structures in males (amygdala, NA and putamen) and all the six investigated structures in females, an age-only model did not improve the model fit over the models incorporating puberty measures.
Discussion
In the current study, we examined the relationship between puberty and growth trajectories of subcortical regions during adolescence using a large longitudinal dataset. Each of the regions studied showed significant longitudinal associations with puberty (Fig. 2). For many of the structures (Females: amygdala, hippocampus, caudate, putamen; Males: amygdala, putamen), models including both age and Tanner stage modelled development better than Tanner-only models. This suggests that both variables are important factors when modelling volume change over adolescent development. Some structures in males (hippocampus, GP) were best modelled using only age as an explanatory variable, whilst the caudate in males was best modelled using only Tanner stage.
Despite the close proximity of the subcortical structures explored, there were clear differences in their structural development during adolescence. In both males and females, the amygdala and hippocampus continued to increase in volume during puberty, whilst the other structures examined decreased in volume, with structures changing between 2.3% and 9.9% across adolescence (Fig. 2). These results may reflect the different mechanisms that influence macroscopic volume changes between structures. Alternatively, the regions may undergo similar growth patterns, but do so at different chronological time-points, resulting in different growth patterns within our restricted age and developmental range.
Observation of pubertal development during adolescence provides an indirect marker of an individual's systemic sex steroid hormone exposure. The principal hormones involved are testosterone and dehydroepiandrosterone (DHEA), both androgens, and oestradiol, an oestrogen. Rising hormone levels, triggered by re-activation of the hypothalamic-pituitary-gonadal axis, leads to the series of physical changes classically associated with puberty. Androgens signal the development of adult-type body hair and skin changes in both females and males, in addition to gonadal development in males, whilst oestradiol primarily affects females, and causes breast and gonadal development. Tanner staging categorises pubertal maturation by describing five stages of development from pre-pubertal to full maturation for each of pubic hair development, genital development and breast development. An individual's puberty stage is related both to how long they have been exposed to the sex steroid hormones, and to their current level of hormones (Dorn and Biro, 2011; Shirtcliff et al., 2009). Our results, showing that growth trajectories of subcortical structures were related to pubertal development, suggest that these same pubertal hormones influence structural brain growth.
Systemic pubertal hormones cross the blood-brain barrier in small concentrations (Marynick et al., 1976), and systemic concentrations of testosterone have been shown to be related to amygdala volume in both males and females (Neufang et al., 2009). Both androgens and oestrogens induce synaptogenesis and synaptic pruning in rat and non-human primates (Ahmed et al., 2008; Hajszan et al., 2008; Sato et al., 2008) and it is likely that this process also occurs in humans, modulating brain growth across puberty (Matsumoto, 1991). Sex hormone receptors for both oestrogens and androgens are found throughout the brain in varying concentrations, with high levels in subcortical regions, particularly the hippocampus and amygdala (Abdelgadir et al., 1999; Clark et al., 1988; Sholl and Kim, 1989). The difference in regional receptor concentrations in the brain potentially helps to explain why different patterns of growth are seen across structures, and the resultant sexual dimorphism reported to emerge during adolescence in some regions (Brain Development Cooperative Group, 2012; Lenroot et al., 2007; Neufang et al., 2009; Sowell et al., 2002).
We found that both males and females demonstrated pubertal effects related to amygdala growth, with increases in volume over puberty in both sexes. Although the overall volume change was similar between the sexes, the growth trajectories were quite different (Fig. 2), with females showing a large increase in volume in early puberty before peaking and decreasing, and males showing an increasing volume until the end of puberty. This is consistent with the different patterns of testosterone concentration in puberty, with males showing larger increases in concentration and a longer period of increase, and females showing a much smaller rise and earlier plateau of testosterone concentration (Ankarberg and Norjavaara, 1999). The smaller increase and earlier peak in testosterone concentration in females compared with males might explain the differences in trajectories between the two sexes, where the changes in neural structure that are seen are being modulated by systemic testosterone concentrations (Zuloaga et al., 2008). This connection might reflect a direct effect of testosterone on amygdala volume, via the testosterone receptors found in high concentrations in the amygdala, or indirect effects e.g. via aromatisation and effects on oestrogen receptors (Schwarz and McCarthy, 2008), or through interaction with growth hormone (Meinhardt and Ho, 2006) and its receptors present in the brain. Previous cross-sectional studies investigating changes in amygdala volume with puberty have reported volume increases with increasing pubertal stage (and testosterone level) in males (Bramen et al., 2011; Neufang et al., 2009), but have had conflicting results in females, where both increases (Neufang et al., 2009) and decreases (Bramen et al., 2011) in amygdala volume have been reported. The different findings between these two papers may result from the relatively small sample sizes or the differing age ranges of the two studies (Neufang et al., 2009: n = 46, 23 male, age 8–15 years; Bramen et al., 2011: n = 80, 32 male, ages 10.7–14.0 years). As our sample shows, there is large variation in brain volumes between participants, and small samples may not be able to reflect the variation in the population, and within each group (early vs late puberty or Tanner stages 1–5). Our results indicate that both age and puberty impact on the structural development of the amygdala in males and females, and differences in the findings of these two previous studies may reflect that they were sampling participants of different ages.
In the hippocampus, the best model to explain growth in females was the combined model incorporating puberty and age, whilst in males the age-only model provided a better fit. This is consistent with an oestrogen-modulated growth pattern. During puberty, oestrogen concentrations in females increase by 4–9 times (Ikegami et al., 2001), and oestrogens are dominant in many pubertal changes. In contrast among males, relatively minor rises in oestrogens are seen during puberty due to aromatization of testosterone. The hippocampus in non-human primates has high levels of oestrogen receptors (Sholl and Kim, 1989), and the increasing concentration of oestrogen that occurs predominantly in females coincides with the growth trajectories seen in the hippocampus in this study.
In our analysis, both males and females showed a decrease in NA volume with puberty (see Fig. 2), and both sexes show on-going development throughout adolescence. Both androgens and oestrogens modulate the function of the NA, changing levels of dopamine release (Thompson and Moss, 1994), and these results are consistent with the existence of macroscopic structural effects of both hormones on NA volume. For the NA, there was no statistical difference between the amount of volume change explained by the model based on puberty status alone, the model based on chronological age alone, or the model incorporating the interactive effects of both puberty and age. This may relate to the high correlation between age and pubertal stage. Note that such collinearity reduces the precision of the estimates of coefficients but does not affect estimates of model fit. Importantly, all the models appear to show on-going structural changes across adolescence in the NA.
The caudate, putamen, and GP lie in close proximity to one another, and have related functions. Growth trajectories of these three regions across puberty are similar, with decreases in volume seen with greater pubertal development. The relative changes in volume for the caudate and putamen are the smallest of all the structures over the course of puberty (Fig. 2). The caudate in males was the only structure analysed that showed no significant relationship with age, emphasizing the importance of considering alternative variables that influence development over this age range. Previous cross-sectional studies of the caudate have shown similar findings, with weak or no age-related changes (Brain Development Cooperative Group, 2012; Østby et al., 2009; Sowell et al., 2002); our results using a longitudinal dataset support this. Less is known about the sex hormone receptor concentrations in the caudate, putamen and GP. Sex hormones have been shown to impact upon receptor densities in these regions (Sumner and Fink, 1998), giving one potential indirect mechanism for the changes in structure seen, but further work exploring how sex hormones influence macroscopic structural changes is needed to help ascertain this.
One of the major strengths of this study is the longitudinal nature of the dataset, which enabled us to model growth trajectories based on the trajectories of real individuals instead of using cross-sectional points. There is wide variability in structural volumes in the brain between individuals, and repeated measurements of the same individual will therefore produce more accurate trajectories than assuming that cross-sectional data can be extrapolated to define trajectories, making the analysis more powerful to detect small but significant changes in brain volume. The structural development of the brain is likely to be affected by a number of variables and the use of a large longitudinal dataset maximises our ability to characterise the relationships of different variables with changing structural volume. The longitudinal nature of the data, the replication of previous cross-sectional findings and the presence of plausible biological mechanisms allow us to hypothesize that puberty may have causal effects on brain development. However our study does not allow us to address whether the effects of puberty on structural brain growth are direct, via hormonal action on the brain tissue, or indirect, with the brain structure being shaped by how young people undergoing puberty may be treated differently in society than their less well-developed peers, or how they might perceive themselves differently (Blakemore et al., 2010). To our knowledge, previous research has not tackled this subject, and further work exploring how pubertal hormones influence brain structural development in both animal and human studies, and combining hormonal and pubertal stage variables, may help to establish how this relationship is modulated.
Our data are subject to a number of limitations. Self-reported Tanner stage, whilst the most widely used and clinically validated measure of pubertal development, is nevertheless a relatively crude measure which subjectively categorises puberty into broad developmental stages. It is therefore limited in its capacity to document accurately small developmental changes, and has significantly poorer resolution than age as an explanatory variable. This may mean that our puberty-related effects on growth trajectories are in fact underestimates, and further studies may help to validate our results and explore further their implications. Despite the large sample size, and longitudinal nature of the current dataset, there are limited numbers of participants at the extreme ages for each Tanner stage. This would be expected because our dataset is representative of a normal population undergoing typical development, with a normal distribution of ages for each puberty stage, and therefore relatively small numbers of individuals at the extreme ends of normal puberty timing. These smaller numbers may reduce the accuracy of the model at these extreme ages and further research targeting narrower age ranges, and focusing on these extremes of normal pubertal development, and using different methods to measure pubertal maturation, would help to validate and further expand on our findings. Polynomial models, as used in this study, have been shown to be susceptible to the age range and age-centring used (Fjell et al., 2010) when performing age-based analyses of development. For this reason, we developed a clear a priori method for our analysis, based on our primary aim to explore the relationship between puberty and structural development of subcortical regions. Our age range was clearly defined, based on the largest available recent US-population based studies of pubertal timing (see page 6) to incorporate the reasonable ages associated with pubertal development. We included the full range of pubertal variation, our primary variable of interest, in the analysis, which should reduce any impact on the reliability of the model fit. A further limitation is our use of automated segmentation software to extract structural volumes for our six regions of interest. This method was chosen in view of the large number of scans (711) included in the study, and is widely accepted to be appropriate for very large scale-studies where manual tracing techniques are prohibitively time-consuming and resource intensive. Correlations between amygdala and hippocampus volumes for Freesurfer vs. manual tracing are high (Fischl et al., 2002; Morey et al., 2009; Tae et al., 2008).
Conclusion
We have shown that the structural development of subcortical brain regions is related to pubertal development during adolescence. This relationship likely reflects the effects of systemic sex hormones on structural brain development. Examining brain development in relation to pubertal development may provide additional information regarding the control mechanisms behind adolescent brain development, and in particular may shed light on how many of the behaviours classically associated with puberty come to arise. It may also help explain the development of a marked sexual dimorphism in psychiatric disorders around the time of puberty (Zahn-Waxler et al., 2008), as there is emerging evidence of associations between volumes of subcortical structures and psychiatric diagnoses (Karchemskiy et al., 2011; Rigucci et al., 2010).
Acknowledgments
The authors gratefully acknowledge the continued participation of all families and individuals involved in this longitudinal study. We thank F Lalonde for his valuable contribution in processing the imaging data for this analysis. The authors of this study are funded by grants from the MRC (AG: Clinical Training Research Fellowship), the Royal Society (SJB: University Research Fellowship) and the National Institutes of Health (JNG: Intramural Research Program; KLM: Graduate Partnership Program).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2013.09.073.
Appendix A. Supplementary data
Supplementary figures.
References
- Abdelgadir S.E., Roselli C.E., Choate J.V.A., Resko J.A. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biol. Reprod. 1999;60:1251–1256. doi: 10.1095/biolreprod60.5.1251. [DOI] [PubMed] [Google Scholar]
- Achenbach T.M. University of Vermont; Burlington: 1991. Child behavior checklist/4–18. [Google Scholar]
- Ahmed E.I., Zehr J.L., Schulz K.M., Lorenz B.H., DonCarlos L.L., Sisk C.L. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarberg C., Norjavaara E. Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17β-estradiol and dehydroepiandrosterone sulfate. JCEM. 1999;84:975–984. doi: 10.1210/jcem.84.3.5524. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb. Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W., Dinov I.D., Worthman C.M., Sowell E.R. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb. Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.S., MacLusky N.J., Goldman-Rakic P.S. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Lewis D.A., Alloy L.B., Amaral D.G., Bush G., Cohen J.D., Drevets W.C., Farah M.J., Kagan J., McClelland J.L., Nolen-Hoeksema S., Peterson B.S. Neural and behavioral substrates of mood and mood regulation. Biol. Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Denckla M.B. Revised neurological examination for subtle signs (1985) Psychopharmacol. Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- Dorn L.D., Biro F.M. Puberty and its measurement: a decade in review. J. Res. Adolesc. 2011;21:180–195. [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Westlye L.T., Østby Y., Tamnes C.K., Jernigan T.L., Gamst A., Dale A.M. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Snell J.W., Lange N., Rajapakse J.C., Casey B.J., Kozuch P.L., Vaituzis A.C., Vauss Y.C., Hamburger S.D., Kaysen D., Rapoport J.L. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A. CRC Press; Boca Raton (FL): 2011. Neurobiology of Sensation and Reward, Frontiers in Neuroscience. [PubMed] [Google Scholar]
- Hajszan T., MacLusky N.J., Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm. Behav. 2008;53:638–646. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens M.E., Slora E.J., Wasserman R.C., Bourdony C.J., Bhapkar M.V., Koch G.G., Hasemeier C.M. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the pediatric research in office settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Moriwake T., Tanaka H., Inoue M., Kubo T., Suzuki S., Kanzakili S., Seino Y. An ultrasensitive assay revealed age-related changes in serum oestradiol at low concentrations in both sexes from infancy to puberty. Clin. Endocrinol. (Oxf.) 2001;55:789–795. doi: 10.1046/j.1365-2265.2001.01416.x. [DOI] [PubMed] [Google Scholar]
- Karchemskiy A., Garrett A., Howe M., Adleman N., Simeonova D.I., Alegria D., Reiss A., Chang K. Amygdalar, hippocampal, and thalamic volumes in youth at high risk for development of bipolar disorder. Psychiatry Res. 2011;194:319–325. doi: 10.1016/j.pscychresns.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynick S.P., Havens W.W., II, Ebert M.H., Loriaux D.L. Studies on the transfer of steroid hormones across the blood–cerebrospinal fluid barrier in the rhesus monkey. Endocrinology. 1976;99:400–405. doi: 10.1210/endo-99-2-400. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Synaptogenic action of sex steroids in developing and adult neuroendocrine brain. Psychoneuroendocrinology. 1991;16:25–40. doi: 10.1016/0306-4530(91)90069-6. [DOI] [PubMed] [Google Scholar]
- Meinhardt U.J., Ho K.K.Y. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. (Oxf.) 2006;65:413–422. doi: 10.1111/j.1365-2265.2006.02676.x. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Xu Y., Hayes J.P., Wagner H.R., Lewis D.V., LaBar K.S., Styner M., McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Güntürkün O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A.-S., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J.-P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Patton G.C., Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T., Nawaz-Khan I., Leonard G., Perron M., Pike G.B., Pitiot A., Richer L., Susman E., Veillette S., Pausova Z. Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm. Behav. 2010;57:63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Hulshoff Pol H.E., Crone E.A., van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D. 2011. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3. 1-104. [Google Scholar]
- Raznahan A., Lee Y., Stidd R., Long R., Greenstein D., Clasen L., Addington A., Gogtay N., Rapoport J.L., Giedd J.N. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Lerch J.P., Lee N., Greenstein D., Wallace G.L., Stockman M., Clasen L., Shaw P.W., Giedd J.N. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigucci S., Serafini G., Pompili M., Kotzalidis G.D., Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J. Biol. Psychiatry. 2010;11:165–180. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Sato S.M., Schulz K.M., Sisk C.L., Wood R.I. Adolescents and androgens, receptors and rewards. Horm. Behav. 2008;53:647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., McCarthy M.M. Cellular mechanisms of estradiol-mediated masculinization of the brain. J. Steroid Biochem. Mol. Biol. 2008;109:300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl S.A., Kim K.L. Estrogen receptors in the rhesus monkey brain during fetal development. Brain Res. Dev. Brain Res. 1989;50:189–196. doi: 10.1016/0165-3806(89)90194-6. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner B.E.H., Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Mol. Brain Res. 1998;59:205–214. doi: 10.1016/s0169-328x(98)00148-x. [DOI] [PubMed] [Google Scholar]
- Sun S.S., Schubert C.M., Chumlea W.C., Roche A.F., Kulin H.E., Lee P.A., Himes J.H., Ryan A.S. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Tae W.S., Kim S.S., Lee K.U., Nam E.-C., Kim K.W. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Ostby Y., Grydeland H., Richardson G., Westlye L.T., Roddey J.C., Hagler D.J., Jr., Due-Tønnessen P., Holland D., Fjell A.M. Brain development and aging: overlapping and unique patterns of change. Neuroimage. 2013;68C:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J.M., Whitehouse R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.J., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Thompson T.L., Moss R.L. Estrogen regulation of dopamine release in the nucleus accumbens: genomic-and nongenomic-mediated effects. J. Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E.-J., Farrell S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004;11:192–196. doi: 10.3758/bf03206482. [DOI] [PubMed] [Google Scholar]
- Wheeler M.D. Physical changes of puberty. Endocrinol. Metab. Clin. North Am. 1991;20:1–14. [PubMed] [Google Scholar]
- Zahn-Waxler C., Shirtcliff E.A., Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu. Rev. Clin. Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zuloaga D.G., Puts D.A., Jordan C.L., Breedlove S.M. The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation. Horm. Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.