Abstract
Tumor spheroids are increasingly recognized as an important in vitro model for the behavior of tumor cells in three dimensions. More physiologically relevant than conventional adherent-sheet cultures, they more accurately recapitulate the complexity and interactions present in real tumors. In order to harness this model to better assess tumor biology, or the efficacy of novel therapeutic agents, it is necessary to be able to generate spheroids reproducibly, in a controlled manner and in significant numbers.
The AggreWell system consists of a high-density array of pyramid-shaped microwells, into which a suspension of single cells is centrifuged. The numbers of cells clustering at the bottom of each microwell, and the number and ratio of distinct cell types involved depend only on the properties of the suspension introduced by the experimenter. Thus, we are able to generate tumor spheroids of arbitrary size and composition without needing to modify the underlying platform technology. The hundreds of microwells per square centimeter of culture surface area in turn ensure that extremely high production levels may be attained via a straightforward, nonlabor-intensive process. We therefore expect that this protocol will be broadly useful to researchers in the tumor spheroid field.
Keywords: Bioengineering, Issue 81, tumor spheroid, size control, scalable, mixed cell types, microwell, forced aggregation, microtissue
Introduction
There is an increasing body of evidence that tumor cells behave differently in three dimensional cultures than they do when cultured on plastic, and that therapeutic agents identified on conventional tissue-culture platforms may lose efficacy when transitioned to a more physiologically-relevant system1. It is therefore desirable to study the behavior of cancer cells under these conditions, both to gain insights into their underlying biology, and also to increase the success rate of transitioning new therapeutic agents from the screening facility to the clinic. One useful model system with a long history employs three-dimensional clusters of cancer cells known as tumor spheroids1,2. Ideally, techniques for spheroid formation would permit production of large numbers of uniform spheroids whose size and composition are controlled by the experimenter. While hanging-drop and well-plate approaches3,4 are able to fulfill some of these requirements, throughput is generally limited, and generation of large numbers of spheroids becomes a labor-intensive task.
We have recently developed a system to resolve similar challenges in the regenerative medicine field5. Employing forced aggregation within a series of densely packed micron-scale wells (see Figure 1), this approach permits the generation of spheroids from arbitrary numbers of cells, including mixtures of multiple cell types6, as well as the incorporation of various biomaterials7. Spheroids are formed in large numbers - thousands to hundreds of thousands or more - and may be extracted immediately or maintained in the microwells in which they were formed with medium exchange for at least one8 to two (unpublished observation) weeks. This system is therefore well suited to the generation of large numbers of uniform and reproducible tumor spheroids for the assessment of the effectiveness of novel antitumor agents or basic biological investigations.
Protocol
NOTE: Microwell plates are available in different formats, depending on the desired results. Specifications as well as approximate guidelines for the minimum and maximum numbers of cells that should be used with each format are shown in Table 1. The smaller microwells taper to a sharp point, and thus there is no lower size limit, although variability between spheroids becomes more significant at smaller sizes. Routine production of spheroids from an average of as little as 20 cells each is straightforward8. The larger microwell size is not fully tapered, therefore attempts to form spheroids from small numbers of cells may result in multiple smaller spheroids in each microwell. Due to slight differences in surface properties, preparation with the surfactant solution (step 1.2) is not optional when using AggreWell 400Ex plates.
This protocol is based on the use of AggreWell 400 plates - for other formats consult Table 1. The number of cells to be loaded in each well is determined by multiplying the number of microwells it contains by the number of cells to be clustered for each spheroid. For example, to generate spheroids from 1000 cells apiece, each well must be loaded with (1,200 spheroids times 1,000 cells each = ) 1.2 x 106 cells. Cell density must be calculated given that the cells will be loaded in a volume of 0.4 ml. So for the example of spheroids formed from 1000 cells each, 1.2 x 106 cells in 0.4 ml translates to a density of 3 x 106 cells per ml.
1. Preparing Microwells to Receive Cells
- Microwell plates are sterile as provided, and should only be removed from their packaging in a sterile environment such as a laminar-flow biosafety cabinet. Sterility should be maintained throughout the protocol. Should sterility be compromised, the wells are resterilized using 70% ethanol in water using the following optional steps.
- Add 0.5 ml of 70% ethanol to each unused well. Some microwells will trap air bubbles, although this can be reduced by adding liquid at one side, and allowing it to spread across the surface, rather than dropping it vertically onto the surface. Replace lid.
- While ethanol vapor should quickly permeate any bubbles, if there are large numbers it may be desirable to remove them. Centrifuge for 2 min at 2,000 x g. Note that it is important to ensure the rotor is properly balanced at this speed.
- Using a low-magnification inverted microscope, verify bubbles have been removed from the microwells.
- Incubate for 5 min with the lid on at room temperature.
- Aspirate ethanol. Plate can now be washed with sterile water or PBS for immediate use, or air-dried for storage.
- In order to ensure that cells do not interact with the microwell surface, it may be prepared using a surfactant. In general it is advisable to perform this step initially, and subsequently compare spheroids generated with and without surfactant-coated microwells.
- Add 0.5 ml of Rinsing Solution to each well. Some microwells will trap air bubbles, although this can be reduced by adding liquid at one side, and allowing it to spread across the surface, rather than dropping it vertically onto the surface. Replace lid.
- Centrifuge for 2 min at 2,000 x g to remove bubbles. Note that it is important to ensure the rotor is properly balanced at this speed.
- Using a low-magnification inverted microscope, verify bubbles have been removed from the microwells.
- Incubate for at least thirty minutes with the lid on at room temperature, or overnight at 4 °C. Plates may be stored at four degrees with the surfactant solution in the wells until needed if properly sealed to prevent drying out.
- Wash plate with sterile water or PBS immediately prior to use. Do not allow plates to dry out after coating.
2. Preparing Cells
NOTE: The details of cell preparation will vary somewhat with the specific cell type being studied. However we have observed these conditions to be effective with a broad range of cells, including the lines discussed here, as well as human and murine embryonic stem cells (ESC), human induced pluripotent stem cells (iPSC), fibroblasts, putative primitive endoderm, and stromal cells. Other groups have employed this system successfully for a wide range of applications including chondrogenesis from mesenchymal stem cells9, standardized generation of neural precursors from pluripotent stem cells10, toxicological analyses in hepatocytes11 and assessment of spinal cord regeneration in salamanders12. The specific protocol for working with HT29 cells is shown here.
Start with a T75 flask of HT29 cells, cultured in DMEM + 10% FBS
Aspirate growth medium from flask, and add 3 ml of trypsin.
Incubate cells for 5 min at 37 °C.
Stop trypsin digestion by adding 3 ml of growth medium. Take an aliquot for cell counting and transfer the remainder into a 15 ml centrifuge tube.
Centrifuge for 5 min at 200 x g.
While the centrifugation is in progress, count cells.
Aspirate trypsin/medium mixture and replace with fresh growth medium, volume determined by the required cell density as calculated above.
(OPTIONAL) Pass the cell suspension through a strainer in order to remove any clumps. NOTE: Cell harvesting conditions will vary somewhat between lines. If a dissociation protocol for e.g. passaging the cells of interest is available, that should be used as a starting point. With sensitive cell lines use of trypsin may result in excessive cell death. In these cases we have observed good results using TrypLE as an alternative dissociation reagent8. If the cells become clumped during dissociation and lose viability, addition of DNAse to the enzyme solution may resolve this. Reducing dissociation times and employing a trituration step to break up cell clumps can also increase viability.
3. Spheroid Formation
NOTE: Spheroids may be generated in a variety of medium formulations, however initial trials should be carried out using the medium in which the cells were cultured, to distinguish consequences of transitioning to a 3-dimensional culture system from consequences of changing medium composition.
Add 0.4 ml of growth medium to each well.
Centrifuge for 2 min at 2,000 x g to remove bubbles. Note that it is important to ensure the rotor is properly balanced at this speed prior to centrifugation.
Using a low-magnification inverted microscope, verify bubbles have been removed from the microwells.
Add 0.4 ml cell suspension at the required density (calculated above). Gently mix by pipetting up and down, without introducing air bubbles into the microwells. It is important to ensure that the cells are evenly distributed throughout the well, in order to obtain consistent spheroids.
Centrifuge for 5 min at 200 x g. If the plate does not self-level during centrifugation, convection currents may arise that result in uneven distribution of cells across the microwells. Therefore, the plate should be balanced internally as well as against the other plate, so that the weight is distributed evenly to both sides of the plate carrier.
NOTE: If evidence of uneven cell distribution is seen, it may be necessary to apply a small amount of lubrication to the pivot points on the plate carrier - consult with centrifuge manufacturer for instructions.
NOTE: Once the cells have been centrifuged into the microwells, they are reasonably resistant to washing out from minor motions of the plate. Abrupt movements that result in sloshing of the medium should be avoided, but transfer of the plate from centrifuge to microscope or incubator should not result in significant cell displacement.
Using an inverted microscope, verify that cells have clustered within the microwells, and that they are evenly distributed across the well.
Incubate overnight at 37 °C.
Using an inverted microscope, observe the process of spheroid formation. Some cell lines will form compact spheroids within 24 hr, others may take longer. The progression from cluster to aggregate in HT29 cells is shown in Figure 2.
- Recover spheroids from the microwells and transfer to suspension culture by gently jetting them out with a pipette. Alternatively, maintain spheroids in situ with medium replacement8 - the duration depends on their initial size and growth rate, which define the time until they outgrow the microwells in which they were formed.
- To replace medium without losing spheroids, aspirate medium at the edge of the well using a Pasteur pipette. Slowly bring the tip down until it begins to draw liquid from the meniscus, and follow the meniscus down as it drops. Then add fresh medium against the well wall in the same place. A few spheroids may be lost, however if medium is always aspirated and replaced in the same position, this number will remain small in comparison to the large numbers in the well.
Representative Results
Spheroids from multiple tumor lines of differing anatomical origins may readily be generated and extracted into suspension culture. Figure 3 illustrates the consistent size control obtainable via this method, with highly uniform spheroid populations under each condition. Clear inter-line differences in behavior are also visible, with HT29 colon cancer cells and TE6 esophageal cancer cells forming densely packed spheroids with sharply defined boundaries, while LNCaP prostate cancer cells gave rise to less coherent spheroids with irregular boundaries (see Discussion for possible reasons). The stronger internal forces in the more coherent aggregates also resulted in collapse to a more symmetrical form even while still in the microwells in which they were formed, whereas the LNCaP aggregates, particularly at the larger size, visibly retain the square-pyramidal geometry of the microwells. The relationship between input cell numbers and the physical size of the resulting spheroid will depend on a number of variables. Theoretical volumes based on the product of the volume per cell and the number of cells employed may be reduced by cell loss, which in turn may include loss of cells during the single-cell suspension stage, as well as loss from excessively small aggregates due to insufficient numbers of neighbors, and from excessively large aggregates due to transport limitations and necrosis in the core. Size will also be influenced by any proliferation that may occur during the aggregation process. As these parameters will vary from cell line to cell line, if spheroids of specific physical dimensions are desired the correct number of cells to introduce must be determined experimentally. As a first approximation, spheroid diameter is expected to increase with the cube root of the number of cells incorporated - e.g. an 8-fold increase in number of cells should result in a doubling of spheroid diameter.
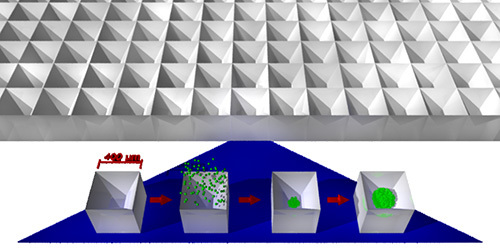 Figure 1. Schematic of spheroid production. The microwell system consists of an array of tightly packed square-pyramidal microwells (625 per cm2 for the 400 µm size), into which cells may be centrifuged. The cells are brought together by the sloping sidewalls, and the size of the resulting spheroid is controlled by varying the density of the cell suspension employed.
Figure 1. Schematic of spheroid production. The microwell system consists of an array of tightly packed square-pyramidal microwells (625 per cm2 for the 400 µm size), into which cells may be centrifuged. The cells are brought together by the sloping sidewalls, and the size of the resulting spheroid is controlled by varying the density of the cell suspension employed.
 Figure 2. Assembly of clustered cells into spheroids. Spheroids were formed from seven hundred HT29 cells each. Immediately after centrifugation (A), cells are loosely clustered in the bottom of each microwell. Note that the clusters are uniformly offset towards the lower right in this case. This is acceptable provided cluster sizes remain consistent across the array. If significant cluster size differences are seen from one side of the array to the other, see Note in step 3.5. After incubation for 24 hr (B), intercellular adhesive forces have caused the clusters to aggregate and form coherent spheroids, which may then be extracted (C).
Figure 2. Assembly of clustered cells into spheroids. Spheroids were formed from seven hundred HT29 cells each. Immediately after centrifugation (A), cells are loosely clustered in the bottom of each microwell. Note that the clusters are uniformly offset towards the lower right in this case. This is acceptable provided cluster sizes remain consistent across the array. If significant cluster size differences are seen from one side of the array to the other, see Note in step 3.5. After incubation for 24 hr (B), intercellular adhesive forces have caused the clusters to aggregate and form coherent spheroids, which may then be extracted (C).
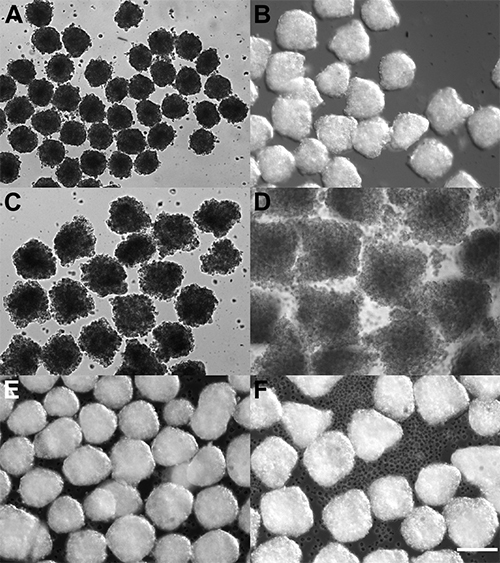 Figure 3. Spheroids generated in microwell plates. Spheroids were formed from seven hundred (A, C, E) or one thousand five hundred (B, D, F) HT29 colon cancer cells (A, B), LNCaP prostate cancer cells (C, D) or TE6 esophageal cancer cells (E, F). Note the consistency of spheroids within a preparation, and also the variation between cell lines - in particular the loose LNCaP aggregates as compared to the more densely packed HT29 and TE6 cells. Scale bar represents 200 μm.
Figure 3. Spheroids generated in microwell plates. Spheroids were formed from seven hundred (A, C, E) or one thousand five hundred (B, D, F) HT29 colon cancer cells (A, B), LNCaP prostate cancer cells (C, D) or TE6 esophageal cancer cells (E, F). Note the consistency of spheroids within a preparation, and also the variation between cell lines - in particular the loose LNCaP aggregates as compared to the more densely packed HT29 and TE6 cells. Scale bar represents 200 μm.
| Microwell format | |||
| AggreWell 400 | AggreWell 40EX | AggreWell 800 | |
| Microwell width (µm) | 400 | 400 | 800 |
| Microwells per cm2 | 625 | 625 | 156.25 |
| Microwells per well | 1,200 | 4,700 | 300 |
| Minimum cells per microwell* | N/A | N/A | 2000 |
| Maximum cells per microwell* | 2,000 | 2,000 | 10,000 |
| Minimum cells per well* | N/A | N/A | 6 x 105 |
| Maximum cells per well* | 2.4 x 106 | 9.4 x 106 | 3 x 106 |
| 1.1.1 - 70% Ethanol volume (ml) | 0.5 | 2.0 | 0.5 |
| 1.2.1 - Rinsing solution volume (ml) | 0.5 | 2.0 | 0.5 |
| 3.2 - Medium preload volume (ml) | 0.4 | 1.6 | 0.4 |
| 3.5 - Cell loading volume (ml) | 0.4 | 1.6 | 0.4 |
| *Numbers of cells per microwell are only an approximation as this value will vary with the size of the specific cells employed, and should be determined empirically |
Table 1. AggreWell options and protocol modifications.
Discussion
We have established a system whereby large numbers of uniform spheroids may be generated from multiple cell lines from different sources. We have yet to encounter an adherent cell line that does not form spheroids under these conditions. We have previously observed cell loss in populations prone to anoikis5,8, however to date this issue has not arisen with tumor lines. The system is arbitrarily scalable with surface area, with behavior consistent across microwells in 24-well and 6-well format, as well as prototype bioreactors containing 50,000 microwells each presently under development.
Should spheroid asymmetry be a concern, the incubation time in step 4.8 may be increased to two or three days. Spheroids may also be incubated for a period after extraction from the microwells to increase symmetry, however in this case care must be taken to keep culture densities sufficiently low, as spheroids in contact with one another will often fuse into larger structures. For this reason, we have previously maintained cultures within the microwells in which the aggregates were formed, with the primary limitation being the size of the spheroid. This in turn is a function of both growth rate and initial size8, and if extended culture within the microwell plate is planned, this should be considered in advance. Options to prevent overgrowth, should it occur, include either starting with smaller spheroids, or employing the larger microwells of the AggreWell 800 plate.
Should spheroids fail to increase in coherence over time, one potential cause may be cell death. Particularly in larger aggregates of highly metabolically active cells, mass transport limitations on the delivery of oxygen and essential nutrients can result in a necrotic core2 - thus a non-cohesive spheroid may simply be a consequence of excessive cell death. Alternatively, measurements of the mechanical cohesion of spheroids have been used to assess intercellular binding forces, in relationship to the metastatic potential of a given cell line13. It would be interesting to investigate the relationship between spheroid shape and metastatic potential, perhaps using morphometric parameters such as roundness and perimeter to area ratio.
In addition to investigating the mechanical and morphometric properties of spheroids, assembly in the microwell system permits large-scale production of mixed-composition spheroids, consisting of combinations of multiple cell types and / or biomaterials6,7. The interactions of tumor cells with other cell types are important to more closely model the behavior of tumors in vivo14, thus it may be of interest to generate spheroids from tumor cells in combination with fibroblasts and endothelial cells, for example. Microparticles of various biomaterials may also be incorporated, and can affect spheroid properties both directly through their interactions with cells7,15, and also as reservoirs for the controlled release of growth factors and cytokines into the interior of the spheroid16.
If there is any concern about sterility, for example when working with an microwell plate in which some wells have previously been used, that may have spent some time in an incubator as part of a previous experiment, the wells may be resterilized with 70% ethanol in water.
Once spheroids have been formed, they may also be separated from residual unincorporated cells by passing the suspension over a cell strainer. Individual cells will pass through, while the spheroids will be retained. If the spheroid is suspected of shedding potentially metastatic cells over the course of culture, it may be desirable to perform this procedure multiple times - initially to remove unincorporated cells, and subsequently to isolate purified populations of the cells given off by the spheroids.
Disclosures
Dr. Ungrin has a financial interest in the AggreWell technology as an inventor.
Acknowledgments
This work was funded by the University of Calgary, under a new investigator start-up grant to Dr. Ungrin.
References
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: An underestimated tool is catching up again. Journal of Biotechnology. 2010;148(1):3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Sherar MD, Noss MB, Foster FS. Ultrasound backscatter microscopy images the internal structure of living tumour spheroids. Nature. 1987;330(6147):493–495. doi: 10.1038/330493a0. [DOI] [PubMed] [Google Scholar]
- Foty R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. 2011. p. 2720. [DOI] [PMC free article] [PubMed]
- Naber HPH, Wiercinska E, Ten Dijke P, Van Laar T. Spheroid Assay to Measure TGF-β-induced Invasion. J. Vis. Exp. 2011. p. e3337. [DOI] [PMC free article] [PubMed]
- Ungrin MD, Joshi C, Nica A, Bauwens C, Reproducible Zandstra PW. ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens CL, Song H, et al. Geometric control of cardiomyogenic induction in human pluripotent stem cells. Tissue Engineering. Part A. 2011;17(15-16):1901–1909. doi: 10.1089/ten.TEA.2010.0563. [DOI] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32(1):48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrin MD, Clarke G, et al. Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnology and Bioengineering. 2012;109(4):853–866. doi: 10.1002/bit.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplantation. 2010;19(1):29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- Kozhich O, Hamilton R, Mallon B. Standardized Generation and Differentiation of Neural Precursor Cells from Human Pluripotent Stem Cells. Stem Cell Reviews and Reports. 2012. pp. 1–6. [DOI] [PMC free article] [PubMed]
- Fey SJ, Wrzesinski K. Determination of Drug Toxicity Using 3D Spheroids Constructed from an Immortal Human Hepatocyte Cell Line. Toxicological Sciences. 2012. [DOI] [PMC free article] [PubMed]
- Mchedlishvili L, Mazurov V, et al. Reconstitution of the central and peripheral nervous system during salamander tail regeneration. Proceedings of the National Academy of Sciences. 2012. [DOI] [PMC free article] [PubMed]
- Butler CM, Foty RA. Measurement of Aggregate Cohesion by Tissue Surface Tensiometry. J. Vis. Exp. 2011. p. e2739. [DOI] [PMC free article] [PubMed]
- Xu K, Buchsbaum RJ. Isolation of Mammary Epithelial Cells from Three-dimensional Mixed-cell Spheroid Co-culture. J. Vis. Exp. 2012. p. e3760. [DOI] [PMC free article] [PubMed]
- Baraniak PR, Cooke MT, Saeed R, Kinney MA, Fridley KM, McDevitt TC. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. Journal of the Mechanical Behavior of Biomedical Materials. 2012;11:63–71. doi: 10.1016/j.jmbbm.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura KA, Bratt-Leal AM, Hammersmith KA, McDevitt TC, Zandstra PW. Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials. 2012. [DOI] [PMC free article] [PubMed]


