Highlights
-
•
We examined epoxygenase product formation and regulation in endothelial cells.
-
•
The epoxygenase CYP2J2 is an LPS (TLR-4) inducible enzyme in endothelial cells.
-
•
The endothelial cell line EA.Hy926 synthesises epoxygenase products.
-
•
Inhibition of endothelial epoxygenases increases TNFα secretion.
-
•
Soluble epoxide hydrolase inhibitors reduce inflammation-induced TNFα and NFκB.
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; DHET, dihydroxy eicosatrienoic acid; DHOME, dihydroxy-octadecenoic acid; DiHDPA, dihydroxy-docosapentaenoic acid; EET, epoxyeicosatrienoic acid; EPA, eicosapentaenoic acid; EPOME, epoxy-octadecenoic acid; HETE, hydroxyeicosatetraenoic acid; LA, linoleic acid; sEH, soluble epoxide hydrolase
Keywords: Endothelial, TNFα, Epoxygenase, Soluble epoxide hydrolase
Abstract
The roles of CYP lipid-metabolizing pathways in endothelial cells are poorly understood. Human endothelial cells expressed CYP2J2 and soluble epoxide hydrolase (sEH) mRNA and protein. The TLR-4 agonist LPS (1 μg/ml; 24 h) induced CYP2J2 but not sEH mRNA and protein. LC–MS/MS analysis of the stable commonly used human endothelial cell line EA.Hy926 showed active epoxygenase and epoxide hydrolase activity: with arachidonic acid (stable epoxide products 5,6-DHET, and 14,15-DHET), linoleic acid (9,10-EPOME and 12,13-EPOME and their stable epoxide hydrolase products 9,10-DHOME and 12,13-DHOME), docosahexaenoic acid (stable epoxide hydrolase product 19,20-DiHDPA) and eicosapentaenoic acid (stable epoxide hydrolase product 17,18-DHET) being formed. Inhibition of epoxygenases using either SKF525A or MS-PPOH induced TNFα release, but did not affect LPS, IL-1β, or phorbol-12-myristate-13-acetate (PMA)-induced TNFα release. In contrast, inhibition of soluble epoxide hydrolase by AUDA or TPPU inhibited basal, LPS, IL-1β and PMA induced TNFα release, and LPS-induced NFκB p65 nuclear translocation. In conclusion, human endothelial cells contain a TLR-4 regulated epoxygenase CYP2J2 and metabolize linoleic acid > eicosapentaenoic acid > arachidonic acid > docosahexaenoic acid to products with anti-inflammatory activity.
1. Introduction
Arachidonic acid is metabolised to families of biologically active mediators by cyclooxygenase, lipoxygenase and cytochrome P450 (CYP) pathways [1,2]. Unlike COX and lipoxygenase products, the roles of CYP pathways in vascular biology remains poorly understood. CYPs metabolise arachidonic acid in several ways: (i) epoxygenases metabolise arachidonic acid to epoxyeicosatrienoic acids (EETs); (ii) lipoxygenase-like CYPs generate mid-chain hydroxyeicosatetraenoic acids (HETEs); and (iii) the ω- and ω-1-hydroxylase CYPs produce ω-terminal HETEs [2]. In addition to arachidonic acid, these lipid metabolising CYPs can also metabolise linoleic acid (LA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), to structurally related epoxy-products [3]. CYP2 family members are the main fatty acid-metabolising CYPs. Of this larger family, the CYP2J and CYP2C subfamilies represent the most important fatty acid epoxygenases [2–6].
In most organs, epoxygenase products are short-lived due to rapid metabolism. The major pathway of EET metabolism is via epoxide hydrolases [7], which convert EETs to dihydroxy derivatives called DHETs [8]. Although other enzymes possess epoxide hydrolase activity, soluble epoxide hydrolase (sEH) appears to be the key enzyme in this pathway, as genetic disruption or pharmacological inhibition of sEH elevates EETs, and reduces neointima formation [9], atherosclerosis, abdominal aortic aneurysm, and dyslipidaemia in hyperlipidaemic mice [10]. Knockout or inhibition of she also causes hypertension [11] and diabetes [12] in mouse models. The elevation of EETs by sEH inhibitors is now considered a potentially important therapeutic goal for management of cardiovascular and inflammatory disorders [13].
Primary endothelial cells use complex growth media and change phenotype with passage. EA.Hy926 are commonly used stable human cell line derived from human umbilical vein endothelial cells fused to A549 human lung epithelial cells, which retain many endothelial cell characteristics [14]. Here we have characterized the lipid metabolizing CYP, lipoxygenase and cyclo-oxygenase pathways in EA.Hy926 cells, and investigated whether they represent a simple model to test the anti-inflammatory actions of sEH inhibitors. Our results show human endothelial cells contain TLR-4 inducible CYP2J2. As a model for this induction, EA.Hy926 actively metabolizes AA, LA, DHA, and EPA to epoxygenase metabolites, and sEH inhibitors strongly inhibit inflammation-induced TNFα release.
2. Materials and methods
2.1. Materials
MS-PPOH (N-(methylsulfonyl)-2-(2-propynyloxy)-benzene hexanamide), AUDA (12-[[(tricyclo[3.3.1.13,7]dec-1-ylamino)carbonyl]amino]-dodecanoic acid) and TPPU (N-[1-(1-oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl)-urea) were from Cayman Chemical Company (Cambridge Bioscience, Cambridge, UK). SKF525A (α-phenyl-α-propyl-2-(diethylamino)ethyl ester-benzeneacetic acid) was from Biomol (Affiniti Research Products, Exeter, UK). Human TNFα ELISA was from eBioscience (Hatfield, UK). Rabbit anti-p65 was from Santa Cruz (Heidelberg, Germany), while rabbit anti-CYP2J2 and anti-sEH were from Biorybt (Cambridge, UK). Taqman primers and reagents were from Invitrogen (Paisley, Renfrewshire, UK). Recombinant human IL-1β was from R&D Systems (Abingdon, Oxfordshire, UK). Unless stated, all other reagents were from Sigma–Aldrich (Poole, Dorset, UK).
2.2. Cell and tissue culture
EA.Hy926 (ATCC; LGC Standards, Middlesex, UK) were cultured in M199 supplemented with antibiotic/antimycotic mix, and 10% FBS; 37 °C; 5% CO2; 95% air. HAEC used at passage 3–4, were from PromoCell and grown according the manufacturer’s recommended conditions. Human blood out-growth endothelial cells were a gift from Prof. Jane Mitchell (Imperial College London). Since FBS interferes with lipid substrate composition, and the release and detection of eicosanoids (M. Edin, unpublished observations), all experiments were performed with M199 supplemented with antibiotic/antimycotic mix, without FBS. Detection of eicosanoids by liquid chromatography tandem mass spectrometry was performed as previously described [27].
2.3. RT-PCR and real-time qRT-PCR
Total RNA was extracted using Qiagen’s QiaAmp RNA Blood Mini Kit as per the manufacturer’s instructions. qRT-PCR for CYP2J2, and sEH (ephx2) was measured by the Taqman qRT-PCR ddCt method and normalized to GAPDH levels, using a Corbett Rotor-Gene 6000 machine.
2.4. Immunoassays, and EET measurements
TNFα ELISAs were performed according to manufacturer’s instructions. In these experiments cell viability by MTT assays were performed as previously described [15]; and there were no significant changes between treatments groups. Immunofluorescence was performed essentially as previously described using primary antibody dilutions of 1:50–1:100 [15]. LC–MS/MS analysis of epoxygenase products in culture supernatants was as previously described [16].
3. Results
3.1. Profile of lipid metabolizing CYPs and their products in EA.Hy926
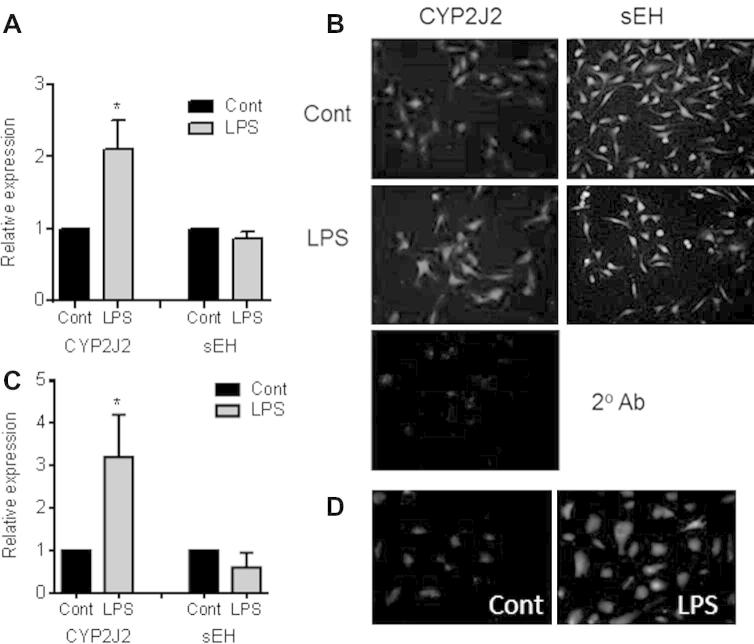
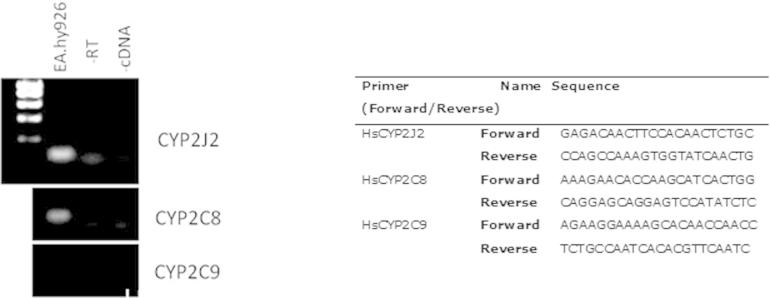
EA.Hy926, human aortic endothelial cells, and human blood outgrowth endothelial cells contained CYP2J2 and sEH mRNA and protein (Fig. 1). The TLR-4 ligand LPS (1 μg/ml) induced CYP2J2 mRNA (4 h) and protein (24 h) (Fig. 1). In contrast, sEH mRNA and protein levels remained relatively stable following LPS treatment (Fig. 1).
Fig. 1.
Expression and regulation of CYP2J2 and sEH by LPS in human endothelial cells. (A) qRT-PCR (4 h) and (B) immunofluorescent analysis (24 h) of CYP2J2 and sEH expression in EA.Hy926 treated with LPS (1 μg/ml). qRT-PCR data represent relative expression compared to GADPH as mean ± SEM from n = 4 separate experiments. (C) qRT-PCR (4 h) of human blood out growth endothelial cells and (D) immunofluorescent analysis (24 h) of CYP2J2 in human primary aortic endothelial cells treated with LPS (1 μg/ml). qRT-PCR data represent relative expression compared to GADPH as mean ± SEM from n = 3 separate donors. ∗ indicates p < 0.05 by one-sample t-test between control and LPS treatment. Immunofluorescent micrographs are representative of n = 3 experiments. As a negative control, in some experiments primary antibody was omitted (2° Ab) which showed no specific staining.
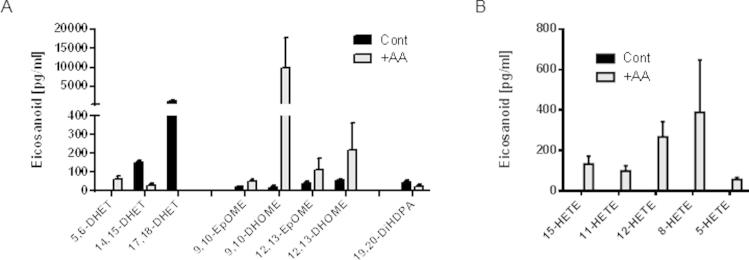
As a stable endothelial cell line we chose to study EA.Hy926 further. Over 24 h EA.Hy926 released detectable levels of the epoxygenase products of AA [14,15-DHET]; LA [9,10-epoxy-octadecenoic acid (EPOME), 12,13-EPOME and their respective diol products 9,10-DHOME, and 12,13-DHOME]; DHA [19,20-dihydroxy-docosapentaenoic acid (DiHDPA)]; and EPA [17,18-DHET; the most abundant epoxygenase product detected under basal culture conditions] (Fig. 2A). LA but not AA lipoxygenase products 9-, and 13-HODE were also detected basally (Fig. 2B), as were the cyclo-oxygenase products PGF2α, PGD2 and PGE2 (Fig. 2C).
Fig. 2.
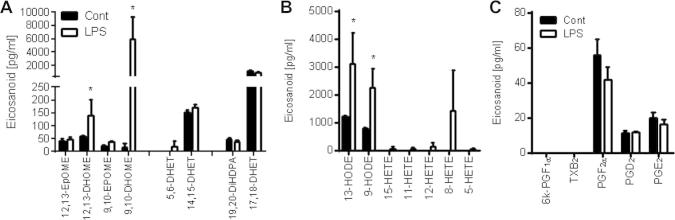
Oxylipin product profile of control and LPS stimulated EA.Hy926 endothelial cells. LC/MS/MS analysis of the (A) epoxygenase, (B) lipoxygenase and (C) cyclo-oxygenase products release (pg/ml) from EA.Hy926 untreated or treated with LPS (1 μg/ml) for 24 h. Data represents the mean ± SEM from n = 3. ∗ indicates p < 0.05 for each analyte by unpaired t-test between control and LPS treatment.
When stimulated with the TLR-4 ligand LPS (1 μg/ml; 24 h), 12,13-DHOME, 5,6-DHET and in particular 9,10-DHOME release was stimulated (Fig. 2A). LPS also stimulated release of 9- and 13-HODE, and 12- and 8-HETE (Fig. 2B), but not COX products (Fig. 2C).
3.2. Endogenous epoxygenase activity inhibits TNFα release in EAHy926 cells
EA.Hy926 cells were treated with vehicle (0.01% DMSO) or the chemically distinct epoxygenase inhibitors MS-PPOH or SKF525A (10 μM each; 7 h). Vehicle treated EA.hy926 cells produced 19.8 pg/ml of TNFα. Treatment with either MS-PPOH or SKF525A induced an approximate 3-fold increase in TNFα production (Fig. 3A). In the presence of high concentrations of the inflammatory stimuli IL-1β (10 ng/ml), PMA (5 nM) or LPS (1 μg/ml), MS-PPOH co-incubation had no additional effect on TNFα release (Fig. 3B).
Fig. 3.
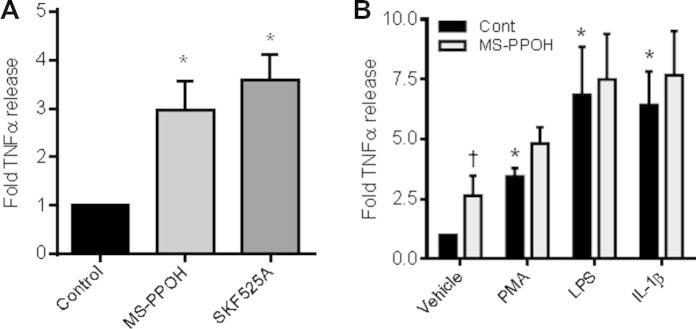
Epoxygenase inhibition induces TNFα release from EAHy926 endothelial cells. (A) Fold TNFα release (7 h) from EA.Hy926 treated with vehicle control (0.01% DMSO), or epoxygenase inhibitor, MS-PPOH (10 μM) or SKF525A (10 μM). Data represents mean ± SEM from n = 7 replicates from 4 separate experiments. ∗ indicates p < 0.05 by one-sample t-test between control and epoxygenase inhibitors. (B) Fold TNFα release (7 h) from EA.Hy926 treated with vehicle control (0.01% DMSO), 5 nM PMA, 1 μg/ml LPS or 10 ng/ml IL-1β in the presence of absence of 10 μM MS-PPOH. Data represents mean ± SEM from n = 5 replicates from 4 separate experiments. ∗ indicates p < 0.05 by one-sample t-test between control and inflammatory stimuli inhibitors. † indicates p < 0.05 by one-sample t-test between treatment in presence or absence of MS-PPOH.
3.3. Soluble epoxide hydrolase inhibitors are anti-inflammatory in EAHy926 cells
Treatment with 10 μM AUDA significantly reduced TNFα secretion in the absence of inflammatory stimuli, and abolished PMA (5 nM), LPS (1 μg/ml) or IL-1β (10 ng/ml) stimulated TNFα release (7 h; Fig. 4A). Moreover, treatment with AUDA (10 μM) or TPPU (1 μM) inhibited LPS (1 μg/ml; 2 h)-induced nuclear localization of the NFκB p65 subunit (Fig. 4B).
Fig. 4.
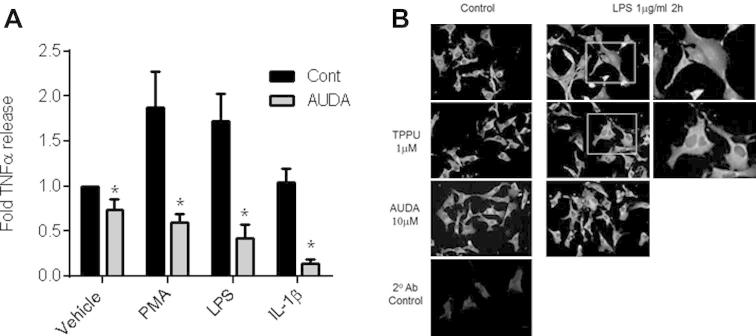
sEH inhibition limits basal and inflammation induced TNFα release from EAHy926 endothelial cells. (A) TNFα release (fold from control; 7 h) from EA.Hy926 treated with vehicle control (0.01% DMSO), 5 nM PMA, 1 μg/ml LPS or 10 ng/ml IL-1β in the presence of absence of the sEH inhibitor AUDA (10 μM). Data represents mean ± SEM from n = 8 replicates from 4 separate experiments. ∗ indicates p < 0.05 by paired t-test between treatment in presence or absence of AUDA. (B) EA.Hy926 were treated with LPS (1 μg/ml; 2 h) to induce p65 nuclear localization. Scale bar 50 μ. LPS induced p65 nuclear localization is absent or strongly diminished in cells treated with sEH inhibitors TPPU (1 μM; middle panels) or AUDA (10 μM; lower panels). 2nd Ab control shows immunofluorescence levels when primary antibody was omitted. Each panel was enhanced by 20% brightness and 20% contrast in PowerPoint to aid visualization. Data is representative of n = 3 experiments.
4. Discussion
Here we show human endothelial cells contain a TLR-4 inducible CYP2J2 epoxygenase. In addition, we show EA.hy926 are a useful, simple, and reproducible human model system to study this inducible CYP epoxygenase enzyme pathway and sEH inhibitors.
Under basal conditions, the release of AA epoxygenase/sEH product 14,15,DHET, the linoleic acid products 9,10-EPOME and 12,13-EPOME (and 9,10-DHOME and 12,13-DHOME), the DHA product 19,20-DiHDPA, and the EPA product 17,18-DHET were all detectable in EA.Hy926 conditioned media. All these products are also produced by vascular tissue (e.g., rat aorta; DBB, ME, DCZ unpublished observations), and in the circulation of healthy volunteers [17]; however the actions of many of these lipid metabolites, including those produced in the largest amounts (e.g., 17,18-DHET) are still poorly understood. Exogenous 17,18-EET acts as a vasodilator [18] via actions on the BK channel alpha subunit [19], inhibits ristocetin-induced thrombus formation [20], and exerts negative chronotropic effects and protects neonatal rat cardiomyocytes against Ca2+-overload [21]. Any effects on endothelial cells themselves have yet to be described, so warrant further investigation.
We recently showed CYP2J2 is induced by LPS in human peripheral blood monocytes and monocyte cell lines [22]. CYP2J2 is expressed in endothelial cells under basal conditions and is similarly induced by the TLR-4 ligand LPS in endothelial cells. This induction of CYP2J2 was accompanied by increases in 9,10-DHOME, 12,13-DHOME and 5,6-DHET formation. These findings indicate that CYP2J2 is a more general inflammation-induced enzyme than perhaps previously thought. EA.Hy926 cells also express CYP2C8 but not CYP2C9 mRNA (Fig. 1), which showed no sign of induction with LPS. Although EPA and DHA may be rate limiting in this system for their own metabolite formation, our results indicate that the LPS inducible eicosanoids are from an inducible CYP such as CYP2J2, while the non-inducible products (e.g., 17,18-EET) may derive more from non-TLR-4 regulated enzymes such as CYP2C8.
Epoxygenase pathway metabolites regulate endothelial cell adhesion molecule expression [3]. Our results now show suppression of TNFα can be added to the repertoire of anti-inflammatory actions of endothelial epoxygenases. TNFα is a potent inflammatory activator of endothelial cells [23] and is critical to the pathogenesis of many inflammatory diseases, most notably rheumatoid arthritis [24]. sEH inhibitors with their anti-inflammatory and analgesic profile [25,26] may therefore represent a viable treatment for highly inflammatory disorders such as rheumatoid arthritis. Although we may assume the actions of sEH inhibition in endothelial cells is due to the direct elevation of epoxygenase products acutely, it is also important to note that following sEH inhibition, endothelial epoxygenase products also get incorporated into cell membranes (potentially for future release) as well as get metabolized via β-oxidation to form novel lipid species such as 10,11-epoxy-hexadecadienoic acid (16:2) and 8,9-epoxy-myristoleic acid (14:1) whose functions are not known [27], and are rarely measured.
Primary endothelial cells are responsive to IL-1β, LPS and PMA [28]. The fact that EA.Hy926 respond similarly further supports their use as an in vitro cell line to model physiological effects of EETs. Chemically distinct epoxygenase inhibitors SKF525A and MS-PPOH increased TNFα production. This suggests, similar to our recent report on monocytes [29], that EET production helps to maintain inflammatory homeostasis. The stronger TNFα release induced by the classical endothelial cell activators IL-1β, LPS or PMA, was not further elevated by epoxygenase inhibition, suggesting a common pathway e.g., NFκB, critical for TNFα release, is being used by both pathways.
Although EA.Hy926 cells appear to be a good model to study epoxygenase products and activity, they are not without their limitations when compared to primary endothelial cells. Importantly, in our hands the cyclooxygenase system was relatively inactive, and no prostacyclin could be detected under any condition, including following LPS stimulation (Fig. 2C).
Endothelial cells and EA.Hy926 cells actively metabolize different fatty acid substrates to a variety of biologically active fatty acyl/oxy-lipins. There has been intensive interest in the mechanistic basis for the effect of diets rich in different sources of fatty acids (e.g., the Mediterranean diet, diets rich in ω6-polyunsaturated fatty acids, or fish oil supplementation), and their effects on cardiovascular biology [30]. Modifying substrates and diet may therefore change the balance of oxylipins and may be responsible for the purported benefits of these lifestyle modifications. Moreover, changing the substrate itself not only alters mediator production but also can change the balance of mediators produced by regulating phospholipase activation. Interestingly, when we gave AA (10 mM) acutely to EA.Hy926 cells, we did induce AA products 5,6-DHET and HETEs, however we also strikingly induced LA product EPOMEs and DHOMEs (Fig. 2). In contrast, 14,15-DHET, 17,18-DHET, and 19,20-DiHDPA were all reduced. Therefore in this simple system, just substrate delivery to the epoxygenase enzymes is far more complex than would be originally predicted.
In summary, endothelial cells contain an anti-inflammatory TLR-4 inducible epoxygenase. In addition, EA.Hy926 cells represent a useful tool to study the anti-inflammatory actions of the epoxygenase pathway metabolites and soluble epoxide hydrolase inhibitors in vascular endothelial cells.
Acknowledgments
This work was funded by grants from the British Heart Foundation, United Kingdom (PG/11/39/28890), and the Intramural Research Programs of the National Institutes of Health, NIEHS (D.C.Z.).
Appendix A. Supplementary data
Supplementary Figure 1.
Supplementary figure.
Supplementary Figure 2.
Supplementary figure.
References
- 1.Bishop-Bailey D., Wray J. Peroxisome proliferator-activated receptors: a critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003;71:1–22. doi: 10.1016/s0090-6980(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 2.Zeldin D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 3.Askari A., Thomson S.J., Edin M.L., Zeldin D.C., Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell W.B., Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capdevila J.H., Falck J.R., Harris R.C. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 6.Spiecker M., Liao J.K. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Zeldin D.C., Kobayashi J., Falck J.R., Winder B.S., Hammock B.D., Snapper J.R., Capdevila J.H. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J. Biol. Chem. 1993;268:6402–6407. [PubMed] [Google Scholar]
- 8.Zeldin D.C., Wei S., Falck J.R., Hammock B.D., Snapper J.R., Capdevila J.H. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch. Biochem. Biophys. 1995;316:443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 9.Revermann M., Schloss M., Barbosa-Sicard E., Mieth A., Liebner S., Morisseau C., Geisslinger G., Schermuly R.T., Fleming I., Hammock B.D., Brandes R.P. Soluble epoxide hydrolase deficiency attenuates neointima formation in the femoral cuff model of hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:909–914. doi: 10.1161/ATVBAHA.110.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L.N., Vincelette J., Cheng Y., Mehra U., Chen D., Anandan S.K., Gless R., Webb H.K., Wang Y.X. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2009;29:1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.R., Imig J.D., Edin M.L., Foley J., DeGraff L.M., Bradbury J.A., Graves J.P., Lih F.B., Clark J., Myers P., Perrow A.L., Lepp A.N., Kannon M.A., Ronnekleiv O.K., Alkayed N.J., Falck J.R., Tomer K.B., Zeldin D.C. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo P., Chang H.H., Zhou Y., Zhang S., Hwang S.H., Morisseau C., Wang C.Y., Inscho E.W., Hammock B.D., Wang M.H. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J. Pharmacol. Exp. Ther. 2010;334:430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imig J.D., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discovery. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouis D., Hospers G.A., Meijer C., Molema G., Mulder N.H. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4:91–102. doi: 10.1023/a:1012259529167. [DOI] [PubMed] [Google Scholar]
- 15.Piqueras L., Sanz M.J., Perretti M., Morcillo E., Norling L., Mitchell J.A., Li Y., Bishop-Bailey D. Activation of PPAR {beta}/ {delta} inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J. Leukocyte Biol. 2009;86:115–122. doi: 10.1189/jlb.0508284. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y., Edin M.L., Theken K.N., Schuck R.N., Flake G.P., Kannon M.A., DeGraff L.M., Lih F.B., Foley J., Bradbury J.A., Graves J.P., Tomer K.B., Falck J.R., Zeldin D.C., Lee C.R. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuchardt J.P., Schmidt S., Kressel G., Dong H., Willenberg I., Hammock B.D., Hahn A., Schebb N.H. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot. Essent. Fatty Acids. 2013;89:19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauterbach B., Barbosa-Sicard E., Wang M.H., Honeck H., Kargel E., Theuer J., Schwartzman M.L., Haller H., Luft F.C., Gollasch M., Schunck W.H. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39:609–613. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- 19.Hercule H.C., Salanova B., Essin K., Honeck H., Falck J.R., Sausbier M., Ruth P., Schunck W.H., Luft F.C., Gollasch M. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp. Physiol. 2007;92:1067–1076. doi: 10.1113/expphysiol.2007.038166. [DOI] [PubMed] [Google Scholar]
- 20.Jung F., Schulz C., Blaschke F., Muller D.N., Mrowietz C., Franke R.P., Lendlein A., Schunck W.H. Effect of cytochrome P450-dependent epoxyeicosanoids on ristocetin-induced thrombocyte aggregation. Clin. Hemorheol. Microcirc. 2012;52:403–416. doi: 10.3233/CH-2012-1614. [DOI] [PubMed] [Google Scholar]
- 21.Falck J.R., Wallukat G., Puli N., Goli M., Arnold C., Konkel A., Rothe M., Fischer R., Muller D.N., Schunck W.H. 17(R),18(S)-epoxyeicosatetraenoic acid, a potent eicosapentaenoic acid (EPA) derived regulator of cardiomyocyte contraction: structure-activity relationships and stable analogues. J. Med. Chem. 2011;54:4109–4118. doi: 10.1021/jm200132q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bystrom J., Thomson S.J., Johansson J., Edin M.L., Zeldin D.C., Gilroy D.W., Smith A.M., Bishop-Bailey D. Inducible CYP2J2 and its product 11,12-EET promotes bacterial phagocytosis: a role for CYP2J2 deficiency in the pathogenesis of Crohn’s disease? PLoS ONE. 2013;8:e75107. doi: 10.1371/journal.pone.0075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piqueras L., Reynolds A.R., Hodivala-Dilke K.M., Alfranca A., Redondo J.M., Hatae T., Tanabe T., Warner T.D., Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 24.Vinay D.S., Kwon B.S. Targeting TNF superfamily members for therapeutic intervention in rheumatoid arthritis. Cytokine. 2012;57:305–312. doi: 10.1016/j.cyto.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Hwang S.H., Wecksler A.T., Wagner K., Hammock B.D. Rationally designed multitarget agents against inflammation and pain. Curr. Med. Chem. 2013;20:1783–1799. doi: 10.2174/0929867311320130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisseau C., Hammock B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang X., Kaduce T.L., Weintraub N.L., Harmon S., Teesch L.M., Morisseau C., Thompson D.A., Hammock B.D., Spector A.A. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J. Biol. Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 28.Loppnow H. LPS, recIL1 and smooth muscle cell-IL1 activate vascular cells by specific mechanisms. Prog. Clin. Biol. Res. 1994;388:309–321. [PubMed] [Google Scholar]
- 29.Bystrom J., Wray J.A., Sugden M.C., Holness M.J., Swales K.E., Warner T.D., Edin M.L., Zeldin D.C., Gilroy D.W., Bishop-Bailey D. Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS ONE. 2011;6:e26591. doi: 10.1371/journal.pone.0026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum S.J., Kris-Etherton P.M., Willett W.C., Lichtenstein A.H., Rudel L.L., Maki K.C., Whelan J., Ramsden C.E., Block R.C. Fatty acids in cardiovascular health and disease: a comprehensive update. J. Clin. Lipidol. 2012;6:216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]