Abstract
Postterm pregnancy is a pregnancy that extends to 42 weeks of gestation or beyond. Fetal, neonatal and maternal complications associated with this condition have always been underestimated. It is not well understood why some women become postterm although in obesity, hormonal and genetic factors have been implicated. The management of postterm pregnancy constitutes a challenge to clinicians; knowing who to induce, who will respond to induction and who will require a caesarean section (CS). The current definition and management of postterm pregnancy have been challenged in several studies as the emerging evidence demonstrates that the incidence of complications associated with postterm pregnancy also increase prior to 42 weeks of gestation. For example the incidence of stillbirth increases from 39 weeks onwards with a sharp rise after 40 weeks of gestation. Induction of labour before 42 weeks of gestation has the potential to prevent these complications; however, both patients and clinicians alike are concerned about risks associated with induction of labour such as failure of induction and increases in CS rates. There is a strong body of evidence however that demonstrates that induction of labour at term and prior to 42 weeks of gestation (particularly between 40 & 42 weeks) is associated with a reduction in perinatal complications without an associated increase in CS rates. It seems therefore that a policy of induction of labour at 41 weeks in postterm women could be beneficial with potential improvement in perinatal outcome and a reduction in maternal complications.
Keywords: Body mass index, induction of labour, perinatal complications, postterm pregnancy, ultrasound
Introduction
Post term pregnancy is associated with an increased risk of fetal and neonatal mortality and morbidity (Olesen et al., 2003a;2003b) as well as an increased maternal morbidity (Caughey et al., 2007). Antepartum stillbirth at and beyond term (37-43 weeks gestation) is a major public health problem accounting for a greater contribution to perinatal mortality than either deaths from complications of prematurity or the sudden infant death syndrome (Cotzias et al., 1999). Increased fetal mortality from postterm pregnancy could therefore be prevented by induction of labour (IOL) at term, however, both clinicians and patients alike are concerned about the risks of induction of labour including uterine hyper-stimulation, failed induction and increased Caesarean section rates. Postterm pregnancy is also associated with increased costs related to antenatal fetal monitoring and induction of labour (Allen et al., 2005; Fonseca et al., 2003) and can be a source of significant anxiety for the pregnant woman (ACOG, 1997). Optimisation of these conflicting pressures is a clinical challenge.
Definitions
Postterm pregnancy is defined as pregnancy that has extended to or beyond 42 weeks of gestation (294 days), or estimated date of delivery (EDD) + 14 days (ACOG, 2004). The terms prolonged pregnancy, postdates and postdatism are synonymously used to describe the same condition. The terms postdate and prolonged pregnancy are ill-defined and best avoided (ACOG, 2004).
Postmaturity, postmaturity syndrome and dysmaturity are not synonymous terms to postterm pregnancy. They are often used to describe the features of a neonate who appears to have been in utero longer than 42 weeks of gestation. They describe the effects of intrauterine growth restriction (IUGR) secondary to utero-placental insufficiency encountered in a postterm pregnancy (Shime et al., 1986).
Epidemiology
The incidence of postterm pregnancy is about 7% of all pregnancies (Martin et al., 2007). The prevalence varies depending on population characteristics and local management practices. Population characteristics that affect the prevalence include: the percentage of primigravidas in the studied population, the prevalence of obesity, a prior postterm pregnancy as well as genetic predisposition. The proportion of women with pregnancy complications and the frequency of spontaneous preterm labour also influence the rate of postterm pregnancy. The link between ethnicity and overall duration of pregnancy is not well established (Collins et al., 2001; Caughey et al., 2009).
Local management practices such as scheduled IOL, differences in the use of early ultrasound (US) for pregnancy dating, and elective Caesarean section (CS) rates will affect the overall prevalence of postterm pregnancy. In the United States for example, the increase in the incidence of IOL in the last decade was associated with a drop in the number of pregnancies continued beyond 41 and 42 weeks from 18%&10% respectively in 1998 (Ventura et al., 1998) to 14%& 4% respectively in 2005 (Martin et al., 2005). Similarly, the use of early US for pregnancy dating has been associated with a significant reduction in the incidence of postterm pregnancy from 12% to 3% (Savitz et al., 2002).
Aetiology and risk factors
The most common cause of prolonged pregnancies is inaccurate dating (Neilson, 2000; Crowley, 2004). The use of standard clinical criteria to determine the estimated delivery date (EDD) tends to overestimate gestational age and consequently increases the incidence of postterm pregnancy (Gardosi et al., 1997; Taipale and Hiilermaa, 2001). Clinical criteria which are commonly used to confirm gestational age include last menstrual period (LMP), the size of the uterus as estimated by bimanual examination in the first trimester, the perception of fetal movements, auscultation of fetal heart tones, and fundal height in a singleton pregnancy.
When postterm pregnancy truly exists the cause is usually unknown. Common risk factors include primiparity, previous postterm pregnancy (Alfirevic and Walkinshaw, 1994; Mogren et al., 1999; Olesen et al., 1999), male fetus (Divon et al., 2002), obesity (Usha Kiran et al., 2005; Stotland et al., 2007), hormonal factors and genetic predisposition (Laursen et al., 2004).
It is not known how body mass index (BMI) affects the duration of pregnancy and timing of delivery, but interestingly obese women have a higher incidence of postterm pregnancy (Usha Kiran et al., 2005), while women with low BMI have a higher incidence of preterm labour (delivery before 37 weeks of gestation) (Hickey et al., 1997). Because adipose tissue is hormonally active (Baranova et al., 2006), and because obese women may have an altered metabolic status, it is possible that endocrine factors involved in the initiation of labour are altered in obese women.
Perhaps amongst all the factors which could influence the incidence of postterm pregnancy obesity is the one modifiable risk factor which could theoretically improve by dietary and exercise behavioural modifications before or during pregnancy. Such modifications would have an impact on other health outcomes as well, but because postterm pregnancy is associated with a number of perinatal complications, its prevention would be clearly beneficial (Ingemarsson and Kallen, 1997).
Using a number needed to treat calculation, it was found that for approximately every 20 women who successfully decreased BMI below the obesity range, one fewer woman would go past 41 weeks of gestation (adjusted odds ratio of 1.26; based on baseline risk of reaching 41 weeks gestation of approximately 20%) (Caughey et al., 2009).
Altered levels of circulating hormones that are thought to play a role in spontaneous labour may also play a role in the causation of postterm pregnancy. Placental sulphatase deficiency for example, is a rare X-linked recessive disorder that can prevent spontaneous labour due to a defect in placental sulphatase activity and the resulting decreased oestriol levels (E3). Fetal adrenal insufficiency and fetal adrenal hypoplasia as well as fetal anencephaly (in the absence of polyhydramnios), despite being rare, are all associated with postterm pregnancies (Doherty and Norwitz, 2008).
Genetic factors may be involved with prolongation of pregnancy. Women who were themselves products of a prolonged pregnancy are at higher risk of postterm pregnancy (relative risk is 1.3) (Mogren et al., 1999). Women with prior prolonged pregnancy have an increased risk of subsequent postterm pregnancy (27% with one prior prolonged pregnancy & 39% with 2 prior prolonged pregnancies) (Kistka et al., 2007). Twin studies also support a genetic predisposition. Rates of prolonged pregnancy are increased in women whose twin sister has had a previous postterm birth. This association is greater in monozygotic than in dizygotic twins (Laursen et al., 2004). There also appears to be a paternal role in the recurrence risk of prolonged pregnancy. The risk of recurrence of postterm pregnancy was reduced from 19.9% to 15.4% when the father of the baby changed between the first and second pregnancy (Olesen et al., 2003).
Pathogenesis
The pathogenesis of postterm pregnancy is not clearly understood. As demonstrated above some risk factors associated with postterm pregnancy were identified with some possible explanations, however, the pathogenesis of the condition is not yet clear. Despite improved understanding of parturition in recent years, we still lack clarity about the exact mechanisms which initiate labour and allow its progression. To have a better understanding of the pathogenesis of postterm pregnancy it is essential to shed some lights on the pathophysiology of parturition and try to understand why these mechanisms fail to be triggered in postterm pregnancies or conversely are triggered earlier in preterm labour. It seems logical that a common ground or a link does exist between these three conditions. The mechanisms of parturition include interactions between hormonal, mechanical and inflammatory processes, in which placenta, mother and fetus each play a vital role.
Placental production of the peptide corticotrophin releasing hormone (CRH) has been related to the length of gestation (McLean et al., 1995). Synthesis of CRH by the placenta increases exponentially as pregnancy advances and peaks at the time of labour. In women who deliver prematurely the exponential rise is more rapid than those delivering at term, while in women who deliver postterm the rate of rise is slower (Ellis et al., 2002; Torricelli et al., 2006). This data suggests that postterm delivery is due to a change in the biological mechanisms regulating the length of gestation. This may be due to an inherited predisposition due to polymorphisms in the genes on the physiological pathway linking CRH to birth. It is also possible that the maternal phenotype may change the response of maternal tissues to the usual hormonal signals to birth as may occur in the obese woman.
CRH can directly stimulate fetal adrenal production of DHEAs, the precursor for placental oestriol synthesis (Smith et al., 1998). Maternal plasma CRH concentrations correlate with oestriol concentrations (Smith et al., 2009). The rising oestriol driven by CRH increases at the end of gestation more rapidly than oestradiol levels leading to an increase in the oestriol to oestradiol ratio which has been postulated to produce an estrogenic environment in the last weeks of pregnancy. Concurrently the rise in maternal plasma progesterone concentrations that occurs across gestation slows at the end of pregnancy or even falls. This may be due to CRH inhibition of placental progesterone synthesis (Yang et al., 2006). Thus the pro-pregnancy effect of progesterone (promoting relaxation) is declining as the pro-labour actions of oestriol (promoting contraction) are increasing. These changes in ratios have been observed in preterm births, singletons delivering at term and in twin gestations (Smith et al., 2009). The situation in postterm pregnancies is unknown. It is likely to be similar in postterm women who go into spontaneous labour or those who respond to IOL, based on one study of postterm women (Torricelli et al., 2011).
Complications of postterm pregnancies
Postterm pregnancies are associated with increased fetal and neonatal motality and morbidity as well as maternal morbidity. These risks are greater than it was originally thought. Risks have been underestimated in the past for two reasons. First, earlier studies on postterm pregnancy were published before the routine use of ultrasound for pregnancy dating. As a result many pregnancies included in the studies were not actually postterm. The second reason rests within the definition of stillbirth itself. Stillbirth rates were traditionally calculated using pregnancies delivered at a given gestational age rather than ongoing (undelivered) pregnancies. This would lower the stillbirth rates in postterm pregnancies as once the fetus is delivered it is no longer at risk of intra-uterine fetal death (IUFD). The appropriate denominator is therefore not all deliveries at a given gestational age but ongoing (undelivered) pregnancies (Rand et al., 2000; Smith, 2001; Caughey et al., 2003).
One retrospective study of over 170,000 singleton births, using the appropriate denominator demonstrated a 6-fold increase in stillbirth rates in postterm pregnancies from 0.35 to 2.12 per 1000 ongoing pregnancies (Hilder et al., 1998).
Fetal and neonatal complications
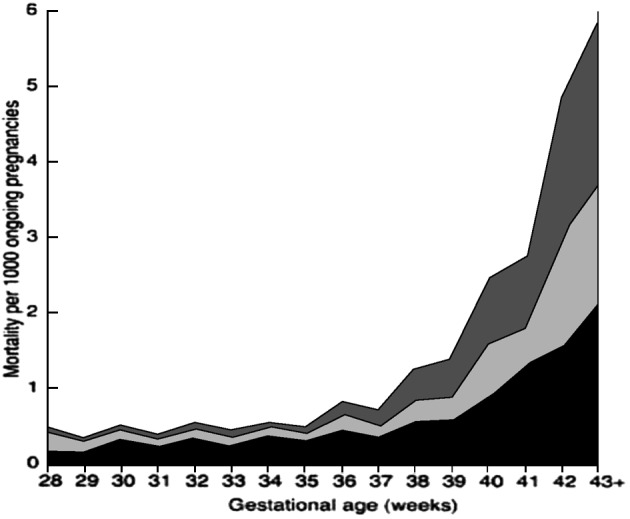
The perinatal mortality rate, defined as stillbirths plus early neonatal deaths, at 42 weeks of gestation is twice as high as that at term (4-7 versus 2-3 per 1000 deliveries, respectively). It increases 4-fold at 43 weeks and 5-7-fold at 44 weeks (Bakketeig and Bergsjo, 1989; Feldman, 1992; Hilder et al., 1998; Cotzias et al., 1999). These data also demonstrate that when calculated per 1000 ongoing pregnancies, fetal and neonatal mortality rates increase sharply after 40 weeks (Hilder et al., 1998) (Fig. 1). It is believed that utero-placental insufficiency, meconium aspiration and intrauterine infection are the underlying causes of the increased perinatal mortality rates in these cases (Hannah, 1993).
Fig. 1. Perinatal mortality per 1000 ongoing pregnancies. Reproduced from BJOG Volume 105 (Hilder L, et al., 1998).
Fetal morbidity is also increased in postterm pregnancies and pregnancies that progress beyond 41 weeks gestation. This includes passage of meconium, meconium aspiration syndrome, macrosomia and dysmaturity. Post term pregnancy also is an independent risk factor for low umbilical cord pH levels (neonatal acidaemia), low 5-minute Apgar scores (Kitlinski et al., 2003), neonatal encephalopathy (Badawi et al., 1998), and infant death in the first year of life (Hilder et al., 1998; Cotzias et al., 1999; Rand et al., 2000). Although some of these infant deaths clearly result from peripartum complications such as meconium aspiration syndrome, most have no known cause.
Meconium aspiration syndrome refers to respiratory compromise with tachypnea, cyanosis, and reduced pulmonary compliance in newborn infants exposed to meconium in utero. It is seen in higher rates in postterm neonates (Kabbur et al., 2005). In the United States the incidence of meconium aspiration syndrome has shown a 4-fold reduction between 1990 and 1998 (from 5.8% to 1.5% in infants more than 37 weeks; P < 0.003). This has been attributed primarily to a reduction in postterm pregnancy rates (Yoder et al., 2002). Conventional interventions such as amnio-infusion (Hofmeyr, 2002; Fraser et al., 2005)) or routine nasopharyngeal and oro-pharyngeal suction of meconium at the perineum at the time of delivery, have made very little contribution to this improvement (Vain et al., 2004).
Postterm infants are larger than term infants and have a higher incidence of fetal macrosomia (2.5-10% in postterm versus 0.8-1% at term) (Spellacy et al., 1985; Rosen and Dickinson, 1992). Fetal macrosomia, defined as an estimated fetal weight ≥ 4.5 kg (ACOG, 2000), is associated with prolonged labour, cephalo-pelvic disproportion and shoulder dystocia. Shoulder dystocia is associated with risk of orthopaedic injury (e.g. fractured humerus and clavicle) as well as neurological injury such as brachial plexus injury and cerebral palsy (Spellacy et al., 1985; Rosen and Dickinson, 1992). However, there is no evidence that IOL as a preventative measure in these cases is associated with a reduction in complication rates or improvement in perinatal outcome (ACOG, 2004).
About 20% of postterm fetuses have dysmaturity syndrome, which refers to infants with characteristics resembling chronic intrauterine growth restriction from utero-placental insufficiency (Vorherr, 1975; Mannino, 1988). This includes thin wrinkled peeling skin (excessive desquamation), thin body (malnourishment), long hair and nails, oligohydramnios and frequently passage of meconium. These pregnancies are at increased risk of umbilical cord compression from oligohydramnios, meconium aspiration, and short-term neonatal complications such as hypoglycemia, seizures, and respiratory insufficiency. They also have an increased incidence of non-reassuring antepartum and intrapartum fetal testing (Knox et al., 1979). Whether such infants are at increased risk of long-term neurologic sequelae is not clear. In a large, prospective, follow-up study of children at ages 1 and 2 years, the general intelligence quotient, physical milestones, and frequency of intercurrent illnesses were not significantly different between normal infants born at term and those born postterm (Shime et al., 1986).
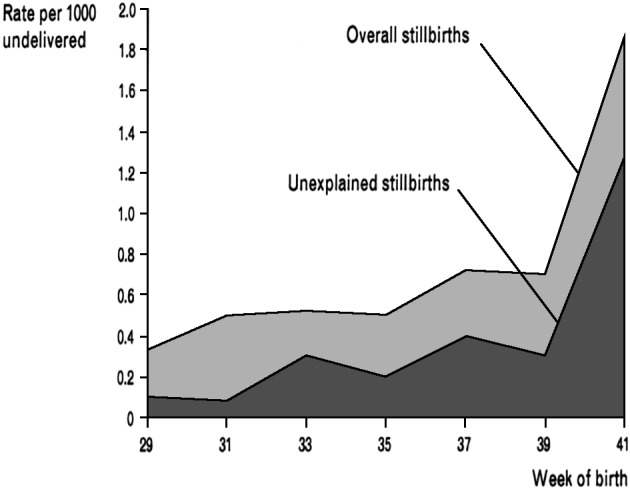
Although much of the work above has been conducted in postterm pregnancies, some risks such as stillbirth, passage of meconium, and neonatal acidaemia have been described as being greater at 41 and even 40 weeks of gestation as compared to 39 weeks gestation (Caughey et al., 2005; Caughey and Musci, 2004). A study from Scotland published in 2010 demonstrated increased risk of stillbirth (both overall and unexplained stillbirth) as pregnancy advances especially after 39 weeks of gestation (Sutan et al., 2010) (Fig. 2). Yudkin et al. (1987) also demonstrated that the risk of unexplained stillbirth rises fourfold after 39 weeks to a maximum at 41 weeks (Fig. 3). The rates of meconium aspiration and neonatal acidaemia both increase as term pregnancies progress beyond 38 weeks (Bruckner et al., 2008). Neonatal morbidity including birth injuries seems to nadir at around 38 weeks and increase in a continuous fashion thereafter (Nicholson et al., 2006). Therefore, 42 weeks of gestation does not represent a thershold below which risks are uniformly distributed. Hence, the definition and management of postterm pregnancy have been questioned and challenged in several studies in recent years (Caughey et al., 2007; Doherty et al., 2008).
Fig. 2. Risk of Stillbirth by week of gestation (rate calculated per 1000 ongoing pregnancies) (Sutan et al. 2010).
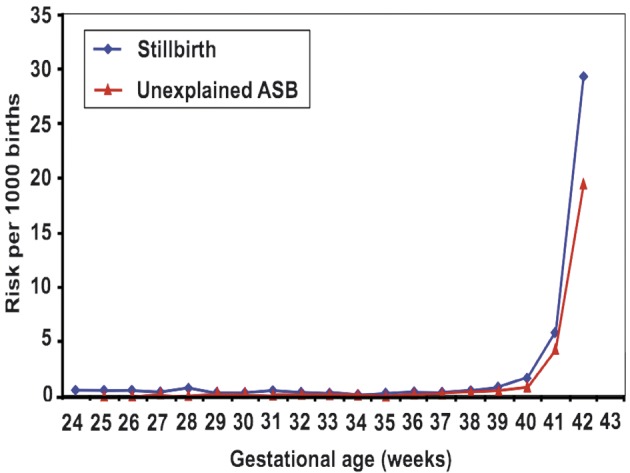
Fig. 3. Relationship between stillbirth and gestational age (Yudkin et al., 1987).
Maternal risks
Postterm pregnancy is associated with significant risks to the mother. There is an increased risk of: 1) labour dystocia (9-12% versus 2-7% at term); 2) severe perineal lacerations (3rd & 4th degree tears), related to macrosomia (3.3% versus 2.6% at term); 3) operative vaginal delivery; and 4) doubling in caesarean section (CS) rates (14% versus 7% at term) (Rand et al., 2000; Campbell et al., 1997; Alexander et al., 2000; Treger et al., 2002). Caesarean delivery is associated with higher incidence of endometritis, haemorrhage, and thrombo-embolic disease (Alexander et al., 2001; Eden et al., 1987).
The emotional impact of prolonged pregnancy should not be underestimated either. In one randomised controlled trial of women at 41 weeks of gestation, women who were induced desired the same management 74% of the time, whereas women with serial antenatal monitoring desired the same management only in 38% of the time (P < 0.001) (Heimstad et al., 2007).
Similar to neonatal outcomes, maternal morbidity also increases in term pregnancies before 42 weeks of gestation. Complications such as chorioamnionitis, severe perineal lacerations, Caesarean delivery, postpartum haemorrhage, and endomyometritis all increase progressively after 39 weeks of gestation (Yoder et al., 2002; Caughey and Bishop, 2006; Heimstad et al., 2006; Caughey et al., 2007; Bruckner et al., 2008;).
A large retrospective study (Caughey et al., 2007), which included 119,254 singleton-low risk pregnancies, demonstrated a statistically significant increase in the rate of maternal complications beyond 40 weeks of gestation and even beyond 39 weeks’ gestation for some morbidities.
The study also showed that the increase in maternal complications persisted at statistically and clinically significant levels even allowing for the increase in operative deliveries. This is true for all of the trends except for the rate of endomyometritis among women undergoing vaginal delivery. For this complication alone, the increase in and among women experiencing caesarean deliveries accounted for the bulk of the increase by gestational age (Caughey et al., 2007).
Management of postterm pregnancy
A) Pregnancy Dating
Accurate pregnancy dating is crucial to the diagnosis and management of postterm pregnancy (Mandruzzato et al., 2010). Last menstrual period has traditionally been used to calculate the expected date of delivery (EDD). But many inaccuracies could exist because of cycle irregularity, recent use of hormonal contraception or because of bleeding early in pregnancy.
Routine ultrasound examination for pregnancy dating demonstrated a reduction in the rate of false positive diagnosis and thereby the overall rate of postterm pregnancy from 10-15% to approximately 2-5%, and thereby minimized unnecessary intervention (Bennett et al., 2004; Caughey et al., 2008a; 2009). A Cochrane systematic review in 2000 found a similar reduction in the overall rates of induction of labour for postterm pregnancy (OR, 0.68; 95% CI, 0.57-0.82) among women who underwent sonographic gestational age assessment before 24 weeks of gestation (Neilson, 2000).
When using ultrasound for dating it is necessary to understand the margin of error reported at various times during gestation. The variation by ultrasonography generally is ± 7 days up to 20 weeks of gestation, ± 14 days between 20 and 30 weeks of gestation, and ± 21 days beyond 30 weeks of gestation (ACOG, 2004).
A calculated gestational age by ultrasound must be therefore considered as an estimate and must take into account the range of possibilities. If the estimated gestational age by a patient’s last menstrual period differs from the ultrasound estimate by more than these accepted variations, the ultrasound estimate of gestational age should be used instead of the patient’s menstrual cycle estimate (ACOG, 2004).
Due to the lower margin of error first trimester ultrasonography seems to be superior to mid-trimester ultrasound for pregnancy dating (Mandruzzato et al., 2010). In a small prospective randomised controlled trial, routine first trimester ultrasound for pregnancy dating reduced the incidence of postterm pregnancy from 13% to 5% when compared with second trimester ultrasound (Bennett et al., 2004). Another study of this issue, showed that prolonged pregnancy is less common in women dated by ultrasound before 12 weeks compared with women scanned between 12 and 24 weeks (2.7 versus 3.7% respectively; P=0.02). Another interesting finding of this study was that better dating revealed a greater difference in the rate of perinatal complications between term and postterm pregnancies (Caughey et al., 2008a).
B) Prevention of postterm pregnancy
Prevention of postterm pregnancies seems to be the best management. Induction of labour at term is the most decisive way of prevention. However, clinicians and patients alike are concerned about risks associated with induction of labour including an increased caesarean section rate. To avoid formal induction and encourage spontaneous onset of labour at term, several minimally invasive interventions have been recommended. This includes membrane sweeping, unprotected sexual intercourse, nipple stimulation and acupuncture.
Membrane sweeping or stripping is a relatively simple technique usually performed without admission to hospital. it has the potential to initiate labour by increasing local production of prostaglandins and, thus, reduce pregnancy duration or pre-empt formal induction of labour with either oxytocin, prostaglandins or amniotomy.
Some studies show that membrane sweeping may reduce the interval to spontaneous onset of labour and in turn the proportion of women with postterm pregnancy. However, there is no consistent evidence that it reduces the incidence of operative vaginal delivery, Caesarean section rates, maternal or neonatal complications (Kashanian et al., 2006; de Miranda et al., 2006).
A Cochrane review (Boulvain et al., 2005) on membrane sweeping for induction of labour in 2010 concluded that sweeping of the membranes, performed as a general policy in women at term, was associated with reduced duration of pregnancy and reduced frequency of pregnancy continuing beyond 41 & 42 weeks. To avoid one formal induction of labour, sweeping of membranes must be performed in eight women (NNT = 8). There was no evidence of a difference in the risk of maternal or neonatal infection. Discomfort during vaginal examination and other adverse effects (bleeding, irregular contractions) were more frequently reported by women allocated to sweeping. Studies comparing sweeping with prostaglandin administration are of limited sample size and do not provide evidence of benefit.
Sexual intercourse is widely believed to facilitate the onset of labour (Schaffir, 2002). The action of sexual intercourse in stimulating labour is unclear, it may in part be due to the physical stimulation of the lower uterine segment, endogenous release of oxytocin as a result of orgasm, uterine activity which is thought to be provoked by orgasm (Chayen et al., 1986), or from the direct action of prostaglandins in semen (Taylor and Kelly, 1974) as human semen is the biological source that is presumed to contain the highest prostaglandin concentration. Some studies show that unprotected sexual intercourse results in earlier onset of labour, reduction in postterm pregnancy rates and fewer interventions with labour induction (Tan et al., 2006). However, a Cochrane review concluded that the role of sexual intercourse as a method of induction of labour is uncertain and that further studies of sufficient power are needed to assess its value (Kavanagh et al., 2001). Another study in 2009 reported that women who had coitus were less likely to go into spontaneous labour prior to their scheduled induction date (Tan et al., 2009).
Acupuncture has long been used in China and other Asian countries for pregnancy-related conditions, including breech presentation (Tiran, 2004), labour pains (Qu and Zhou, 2007), and hyperemesis gravidarum (Helmreich et al., 2006). The Shanghai College of Traditional Medicine recommends acupuncture for labour induction (John Ed O’Conner, 1981), and it is used routinely for labour induction in some societies (West, 1997). Additionally, there do not appear to be significant maternal or fetal risks associated with acupuncture (Neri et al., 2002; Scharf et al., 2003). A Cochrane review on this issue concluded that fewer women receiving acupuncture required induction compared to standard care (RR 1.45, 95% CI 1.08, 1.95; three trials) (Smith and Crowther, 2004). In conclusion, acupuncture cannot be definitely assessed because of the paucity of trial data and the need for further evaluation (Rabl et al., 2001; Smith and Crowther, 2004).
Breast stimulation is thought to promote labour onset and has been suggested as a means of inducing labour. It is a non-medical intervention allowing women greater control over the induction process. A Cochrane review on breast stimulation for cervical ripening and induction of labour (Kavanagh et al., 2005) concluded that breast stimulation appears beneficial in reducing the number of women not in labour after 72 hours, and reducing postpartum haemorrhage rates. However, until safety issues have been fully evaluated it should not be used in high-risk women. Further studies are required before recommending its adoption in practice.
C) Antepartum fetal surveillance
Women who reached 42 weeks gestation and opt to continue their pregnancy with conservative management should undergo antenatal fetal surveillance. Despite the fact that there is no evidence to suggest that antepartum fetal surveillance in postterm pregnancies decreases perinatal mortality, antenatal fetal surveillance has become a common practice in these cases on the basis of universal acceptance. The reasons are: 1) data suggest a gradual increase in perinatal morbidity and mortality during this period (Fig. 3) (Hilder et al., 1998); 2) there is no evidence that antenatal fetal monitoring adversely affects postterm women; 3) the published studies are of insufficient power to demonstrate a benefit of monitoring in these cases; 4) because of ethical and medico-legal considerations, no studies have included postterm patients who were not monitored, and it is unlikely that any future studies will do so.
Women who have passed their EDD but who have not yet reached 42 weeks of gestation constitute another group for whom antenatal fetal surveillance has been proposed. Despite the lack of evidence demonstrating a beneficial effect, antenatal fetal surveillance is often performed during this period. Some studies report a greater complication rate among women giving birth during the latter half of this 2-week period (Bochner et al., 1988; Guidetti et al., 1989; Alexander et al., 2000; Alexander et al., 2001; Treger et al., 2002). Although the data are inconsistent, there is a suggestion that antenatal testing at 40 to 42 weeks of gestation may be associated with improvements in perinatal outcome. In one retrospective study, women with routine antenatal testing from 41 weeks had lower rates of caesarean delivery for non-reassuring fetal testing than women in whom testing was started at 42 weeks (2.3% vs. 5.6%, respectively; P < 0.01) (Bochner et al., 1988). Furthermore, the group with delayed antenatal testing experienced 3 stillbirths and 7 other neonatal morbidity events as compared with none in the 41-week antenatal testing group (P < 0.05). However, no randomized controlled trial has demonstrated an improvement in perinatal outcome attributable to fetal surveillance between 40 and 42 weeks of gestation (Usher et al., 1988).
The literature is inconsistent regarding both the type and frequency of antenatal surveillance among postterm patients (Cardozo et al., 1986; Martin et al., 1989; Hannah et al., 1992; Almstrom et al., 1995; Crowley, 2004). Options for evaluating fetal wellbeing include nonstress testing (CTG), biophysical profile (BPP) or modified BPP (CTG plus amniotic fluid volume estimation), contraction stress testing, and a combination of these modalities. Practices vary widely and no single method has been shown to be superior (Crowley, 2004).
Ultrasound assessment of amniotic fluid volume appears to be important. Delivery should be considered if there is evidence of fetal compromise or oligohydramnios (Crowley et al., 1984; Phelan et al., 1985). Adverse pregnancy outcome (e.g. non-reassuring fetal heart rate tracing, neonatal intensive care unit admission, low Apgar score) is more common when oligohydramnios is present (Bochner et al., 1987; Tongsong and Srisomboon, 1993). Oligohydramnios may result from feto-placental insufficiency or increased renal artery resistance (Oz et al., 2002) and may predispose to umbilical cord compression, thus leading to intermittent fetal hypoxemia, meconium passage, or meconium aspiration. Frequent (twice weekly) screening in postterm pregnancies is suggested because amniotic fluid can become drastically reduced within 24 to 48 hours (Clement et al., 1987). However, there is no consistent definition of oligohydramnios in the postterm pregnancy. Options include 1) largest vertical fluid pocket < 2 cm in depth or 2) amniotic fluid index (AFI) <5cm (Crowley et al., 1984; Chamberlin et al., 1984). A prospective, double blind, cohort study of 1584 women after 40 weeks of gestation found that AFI < 5cm, but not largest vertical fluid pocket < 2cm, was associated with birth asphyxia and meconium aspiration, although the sensitivity for adverse outcome was low (Morris et al., 2003).
Umbilical artery Doppler velocimetry has no proven benefit in monitoring the postterm fetus and is not recommended for this indication (Guidetti et al., 1987; Stokes et al., 1991). Although no firm recommendation can be made on the basis of published research regarding the frequency of antenatal surveillance among postterm women, it seems that twice-weekly testing is widely acceptable by many clinicians (ACOG, 2004). It also seems that testing, using CTG and AF volume assessment, constitutes an acceptable standard by many clinicians.
D) Induction of labour
Induction of labour is indicated when the benefits of delivery outweighs the risks associated with induction. The main concern around induction of labour in postterm low risk pregnancies is related to uterine overstimulation, fetal distress, failure of induction and increase in caesarean section rates. There are also risks associated with induction in particular groups of patients with specific risk factors such as risk of uterine rupture in women with previous caesarean section. Induction of labour is more likely to succeed when the cervix is favourable. Several techniques have been evaluated to assess cervical favourability and to predict the likelihood of success in women undergoing labour induction. These include digital cervical examination (Bishop score), ultrasound assessment of cervical length and more recently biochemical markers (oestriol/oestradiol ratio).
A favourable cervix is defined as a cervix with Bishop score of ≥ 6. Digital cervical assessment has been shown to be superior to trans-vaginal ultrasound assessment of cervical length at term to predict the time interval from IOL to delivery (Rozenberg et al., 2005). However, digital cervical assessment remains subjective and could lack reproducibility.
Oestrogens have been demonstrated to be important hormones involved in the regulation of several functions during pregnancy (Goodwin, 1999). Oestriol (E3), oestradiol (E2), and the oestriol/ oestradiol ratio in particular play an important role in the control of parturition by creating a specific oestrogenic environment at the onset of labour (Smith et al., 2009). Oestrogens were therefore, studied on the basis that they may contribute to a better assessment of women with postterm pregnancy that are at risk of unsuccessful induction, such as women with an unfavourable cervix. In one study, E3/E2 ratio was presented as a biochemical marker to predict the responsiveness to IOL (Torricelli et al., 2011).
It was found that maternal serum E3/E2 ratio is significantly higher in women responding to IOL (Torricelli et al., 2011). These data was in accord with other studies (Walsh et al., 1984; Al-Shawarby et al., 2006). The study suggested that when pregnancy is approaching labour the levels of oestriol and oestradiol change in maternal circulation causing an increase in their ratio (E3/E2 ratio). These data also suggested that oestrogen activation in human parturition is mediated at the functional level by an increase in myometrial oestrogen responsiveness. The study concluded that a combination of ultrasound assessment of cervical length and the E3/E2 ratio shows a good performance in the prediction of successful IOL in postterm pregnancy (Torricelli et al., 2011).
Induction of labour in women with a favourable cervix
Clinicians are less concerned about IOL in women with a favourable cervix; these women are more likely to go into spontaneous labour on their own, and if induced, induction is more likely to succeed. It seems therefore that IOL in this group could be less cost effective as the intervention might not be required in the first place. Most studies of postterm pregnancy comparing outcomes of labour induction with those of expectant management excluded women with a favourable cervix (Dyson et al., 1987; Heden et al., 1991; Hannah et al., 1992; Shaw et al., 1992; NICHHD, 1994). Moreover, when women in the expectant management group experienced a spontaneous change in their cervical status, expectant management ceased and labour was induced (Augensen et al., 1987; Witter et al., 1987; NICHHD, 1994).
In postterm pregnancy studies in which women with a favourable cervix were managed expectantly, there was no indication that expectant management had a deleterious effect on the outcome of the pregnancy, but results were not stratified according to the condition of the cervix (Cardozo et al., 1986; Bergsjo et al., 1989; James et al., 2001; Chanrachakul and Herabutya, 2003). When the ongoing risk of stillbirth is weighted against the very low risk of failed induction in this group, it is suggested that elective IOL may be a reasonable option for such women at 39-41 weeks of gestation. However, such a conclusion requires a large well designed trial to determine whether this approach would reduce complications and improve fetal, neonatal and/or maternal outcomes. At 41-42 weeks of gestation it seems that the risks of IOL are outweighed by the benefits and it is a common practice to offer IOL to such patients (Caughey et al., 2008b).
Induction of labour in women with a unfavourable cervix
As many as 80% of women who reach 42 weeks gestation have an unfavourable cervix (Bishop Score < 6). Using cervical ripening prior to induction in these cases appears to have some advantage in terms of outcome regardless of parity or method of induction. Pre-induction cervical ripening has resulted in fewer failed inductions, reduced fetal and maternal morbidity, reduced medical cost, and possibly a reduced rate of caesarean delivery in the general obstetric population (Xenakis et al., 1997; Poma, 1999; Sanchez-Ramos et al., 2002).
Cochrane systematic reviews demonstrated that prostaglandins (PGs) improve cervical ripeness and could initiate uterine contractions (Boulvain et al., 2007; Kelly et al., 2009). However, their value in reducing induction-delivery interval and CS rate in postterm women is debatable (Rayburn et al., 1988; Papageorgiou et al., 1992; Sawai et al., 1994).
Although multiple studies have used PG to induce labour in postterm pregnancies, no standardized dose or dosing interval has been established. Overall, the medications were well tolerated with few reported side effects. Higher doses of PG (especially PGE1) have been associated with an increased risk of uterine tachysystole and hyper-stimulation leading to non-reassuring fetal testing results (How et al., 2001). As such lower doses (e.g. 25 microgram intravaginal misoprostol) are preferable to 50 microgram (Sanchez-Ramos et al., 2002). When PG is used, fetal heart rate monitoring should be performed routinely to assess fetal well-being because of the risk of uterine hyper-stimulation.
Although postterm pregnancy is defined as a pregnancy of 42 weeks or more of gestation, several large multi-centre randomized studies of management of pregnancy beyond 40 weeks of gestation reported favourable outcomes with routine IOL as early as the beginning of 41 weeks of gestation (Hannah et al., 1992; NICHHD, 1994; Crowley, 2004). The largest study to date randomly assigned 3,407 low-risk women with uncomplicated singleton pregnancies at 41 weeks of gestation to labour induction (with or without cervical ripening agents) within 4 days of randomization or expectant management until 44 weeks of gestation (Hannah et al., 1992). Elective induction resulted in a lower caesarean delivery rate (21.2% versus 24.5%), primarily related to fewer surgeries performed for non-reassuring fetal heart rate tracings. Patient satisfaction was significantly higher in women randomly assigned to labour induction.
A meta-analysis of 19 trials of routine versus selective labour induction in postterm patients found that routine induction after 41 weeks of gestation was associated with a lower rate of perinatal mortality (OR, 0.2; 95% CI, 0.06-0.7) and no increase in the caesarean delivery rate (OR, 1.02; 95% CI, 0.75-1.38) (2). Routine labour induction also had no effect on the instrumental delivery rate, use of analgesia, or incidence of fetal heart rate abnormality. The risk of meconium-stained amniotic fluid was reduced, but the risks of meconium aspiration syndrome and neonatal seizures were unaffected (Crowley, 2004). The actual risk of stillbirth during the 41st week of gestation is estimated at 1.04-1.27 per 1,000 undelivered women, compared with 1.55-3.1 per 1,000 women at or beyond 42 weeks of gestation (Caughey et al., 2008b). Taken together, these data suggest that routine induction at 41 weeks of gestation has fetal benefit without incurring the additional maternal risks of a higher rate of caesarean delivery (Rand et al., 2000; Crowley, 2004). This conclusion has not been universally accepted (Cardozo et al., 1986; Witter et al., 1987; Heden et al., 1991; NICHHD, 1994).
Induction of labour in postterm women with previous caesarean section
Vaginal birth after caesarean delivery (VBAC) has been promoted as a reasonable alternative to elective repeat caesarean delivery for some women. The risk of uterine rupture does not appear to increase substantially after 40 weeks of gestation (Callahan et al., 1999; Zelop et al., 2001), but the risk appears to be increased with IOL with prostaglandins or syntocinon regardless of gestational age (Zelop et al., 2001; Lydon-Rochelle, 2001). In a population-based, retrospective cohort analysis, the risk of uterine rupture with VBAC was 1.6 per 1000 women with previous one caesarean delivery without labour, 5.2 per 1000 women with spontaneous onset of labour, 7.7 per 1000 women with IOL without PG, and 24.5 per 1000 women with PG induction of labour (Lydon-Rochelle, 2001). There is limited evidence on the efficacy or safety of VBAC after 42 weeks of gestation. As such, no firm recommendation can be made for this particular group (ACOG, 2004).
Conclusion
Postterm pregnancy is associated with fetal, neonatal and maternal complications including morbidity and perinatal mortality. These risks were originally underestimated because of inaccurate pregnancy dating and the denominator used to define stillbirth. The use of routine ultrasound for dating in the first trimester has decreased the overall rate of postterm pregnancy and demonstrated higher complication rates in postterm pregnancies due to better distinction between term and postterm gestation. Also the use of ongoing pregnancies as a denominator for stillbirth rather than pregnancies delivered has shown a six-fold increase in perinatal complications in postterm women.
Forty two weeks of gestation does not represent a threshold under which risks are uniformly distributed, and there is emerging evidence that fetal, neonatal and maternal complications do increase before 42 weeks (from 38-39 weeks onwards with an obvious rise after 40&41 weeks gestation). Therefore the definition and management of postterm pregnancy have been challenged in several studies in recent years. In the light of the current evidence earlier intervention with IOL at 41 weeks appears appropriate management.
We conclude that in the light of the current evidence IOL at 41 weeks is justified to minimise both fetal and maternal complications.
References
- Alexander JM, McIntire DD, Leveno KJ. Forty weeks and beyond: pregnancy outcomes by week of gestation. Obstet Gynecol. 2000;96:291–294. doi: 10.1016/s0029-7844(00)00862-0. [DOI] [PubMed] [Google Scholar]
- Alexander JM, McIntire DD, Leveno KJ. Prolonged pregnancy: induction of labor and cesarean births. Obstet Gynecol. 2001;97:911. doi: 10.1016/s0029-7844(01)01354-0. [DOI] [PubMed] [Google Scholar]
- Alfirevic Z, Walkinshaw SA. Management of post-term pregnancy: to induce or not? Br J Hosp Med. 1994;52:218–221. [PubMed] [Google Scholar]
- Allen VM, Connell CM, Farrell SA. Economic implications of method of delivery. Am J Obstst Gynecol. 2005;193:192–197. doi: 10.1016/j.ajog.2004.10.635. [DOI] [PubMed] [Google Scholar]
- Almstrom H, Granstrom L, Ekman G. Serial antenatal monitoring compared with labor induction in post-term pregnancies. Acta Obstet Gynecol Scand. 1995;74:599–603. doi: 10.3109/00016349509013469. [DOI] [PubMed] [Google Scholar]
- Shawarby SA, Connell RJ. Induction of labour at term with vaginal prostaglandins preparations: a randomized controlled trial of Prostin vs Propess. J Obstet Gynaecol. 2006;26:627–630. doi: 10.1080/01443610600903362. [DOI] [PubMed] [Google Scholar]
- ACOG (American College of Obstetricians and Gynecologists) Management of Postterm Pregnancy. ACOG Practice Bulletin No. 6 (1997) Int J Gynaecol Obstet. 1998;60:86–91. [PubMed] [Google Scholar]
- ACOG (American College of Obstetricians and Gynecologists) Fetal Macrosomia. Washington, DC; ACOG; 2000. ACOG Practice Bulletin #22 [Google Scholar]
- ACOG (American College of Obstetricians and Gynecologists) Management of Postterm Pregnancy. ACOG Practice Bulletin No. 55. Obstet Gynecol. 2004;104:639–646. doi: 10.1097/00006250-200409000-00052. [DOI] [PubMed] [Google Scholar]
- Augensen K, Bergsjo P, Eikeland T, et al. Randomised comparison of early versus late induction of labour in post-term pregnancy. Br Med J. (Clin Res Ed) 1987;(294):1192–1195. doi: 10.1136/bmj.294.6581.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. Br Med J. 1998;317:1549–1553. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakketeig LS, Bergsjo P. nkin M, Keirse MJ, Chalmers I, eds. Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press; 1989. Post-term pregnancy: magnitude of the problem; pp. 765–775. [Google Scholar]
- Baranova A, Gowder SJ, Schlauch K, et al. Gene expression of leptin, resistin, and adiponectin in the white adipose tissue of obese patients with non-alcoholic fatty liver disease and insulin resistance. Obes Surg. 2006;16:1118–1125. doi: 10.1381/096089206778392149. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Crane JM, Shea P, et al. First trimester ultrasound screening is effective in reducing postterm labor induction rates: a randomized controlled trial. Am J Obstet Gynecol. 2004;190:1077–1081. doi: 10.1016/j.ajog.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Bergsjo P, Huang GD, Yu SQ, et al. Comparison of induced versus non-induced labor in postterm pregnancy. A randomized prospective study. Acta Obstet Gynecol Scand. 1989;68:683–687. doi: 10.3109/00016348909006139. [DOI] [PubMed] [Google Scholar]
- Bochner CJ, Medearis AL, Davis J, et al. Antepartum predictors of fetal distress in postterm pregnancy. Am J Obstet Gynecol. 1987;157:353–358. doi: 10.1016/s0002-9378(87)80170-9. [DOI] [PubMed] [Google Scholar]
- Bochner CJ, Williams J 3rd, Castro L, et al. The efficacy of starting postterm antenatal testing at 41 weeks as compared with 42 weeks of gestational age. Am J Obstet Gynecol. 1988;159 doi: 10.1016/s0002-9378(88)80005-x. [DOI] [PubMed] [Google Scholar]
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database Syst Rev. 2005;1(CD000451) doi: 10.1002/14651858.CD000451.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochran Database Syst Rev. 2007;(CD006971) doi: 10.1002/14651858.CD006971. [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Cheng YW, Caughey AB. Increased neonatal mortality among post-term births in California. Am J Obstet Gynecol. 2008;199:421. doi: 10.1016/j.ajog.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Callahan C, Chescheir N, Steiner BD. Safety and efficacy of attempted vaginal birth after cesarean beyond the estimated date of delivery. J Reprod Med. 1999;44:606–610. [PubMed] [Google Scholar]
- Campbell MK, Ostbye T, Irgens LM. Post-term birth: risk factors and outcomes in a 10-year cohort of Norwegian births. Obstet Gynecol. 1997;89:543–548. doi: 10.1016/s0029-7844(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Cardozo L, Fysh J, Pearce JM. Prolonged pregnancy: the management debate. Br Med J. (Clin Res Ed) 1986;293:1059–1063. doi: 10.1136/bmj.293.6554.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey AB, Bishop J. Maternal complications of pregnancy increase beyond 40 weeks of gestation in low risk women. J Perinatol. 2006;26:540–545. doi: 10.1038/sj.jp.7211560. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Musci TJ. Complications of term pregnancies beyond 37 weeks of gestation. Obstet Gynecol. 2004;103:57–62. doi: 10.1097/01.AOG.0000109216.24211.D4. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Nicholson JM, Washington AE. First versus second trimester ultrasound: the effect on pregnancy dating and perinatal outcomes. Am J Obstet Gynecol. 2008;198(703):e1–e5. doi: 10.1016/j.ajog.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey AB, Sengovskikh VV, Norwitz ER. Postterm pregnancy: How Can We Improve Outcomes? Obstet Gynecol Survey. 2008;63:715–724. doi: 10.1097/OGX.0b013e318186a9c7. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Stotland NE, Escobar GJ. What is the best measure of maternal complications of term pregnancy: ongoing pregnancies or pregnancies delivered? Am J Obstet Gynecol. 2003;189:1047–1052. doi: 10.1067/s0002-9378(03)00897-4. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Stotland NE, Washington AE, et al. Who is at risk for prolonged and postterm pregnancy. Am J Obstet Gynecol. 2009;200(6):683e1–683e5. doi: 10.1016/j.ajog.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Stotland NE, Washington AE, et al. Maternal obstetric complications of pregnancy are associated with increasing gestational age at term. Am J Obstet Gynecol. 2007;196:155.e1–e6. doi: 10.1016/j.ajog.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey AB, Washington AE, Laros RK. Neonatal complications of term pregnancies: rates increase in a continuous, not threshold fashion. Am J Obstet Gynecol. 2005;192(1):185–189. doi: 10.1016/j.ajog.2004.06.068. [DOI] [PubMed] [Google Scholar]
- Chamberlain PF, Manning FA, Morrison I, et al. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstet Gynecol. 1984;150:245–249. doi: 10.1016/s0002-9378(84)90359-4. [DOI] [PubMed] [Google Scholar]
- Chanrachakul B, Herabutya Y. Postterm with favorable cervix: is induction necessary? Eur J Obstet Gynecol Reprod Biol. 2003;106:154–157. doi: 10.1016/s0301-2115(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Chayen B, Tejani N, Verma UL, et al. Fetal heart rate changes and uterine activity during coitus. Acta Obstet Gynecol Scand. 1986;65:853–855. doi: 10.3109/00016348609157037. [DOI] [PubMed] [Google Scholar]
- Clement D, Schifrin BS, Kates RB. Acute oligohydramnios in postdate pregnancy. Am J Obstet Gynecol. 1987;157:884–886. doi: 10.1016/s0002-9378(87)80078-9. [DOI] [PubMed] [Google Scholar]
- Collins JW, Schulte NF, George L, et al. Postterm delivery among African Americans, Mexican Americans and Whites in Chicago. Ethn Dis. 2001;11:181–187. [PubMed] [Google Scholar]
- Cotzias CS, Brown S, Fisk NM. Prospective risk of unexplained stillbirth in singleton pregnancies at term: population based analysis. Br Med J. 1999;319:287–288. doi: 10.1136/bmj.319.7205.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley P, Herlihy C, Boylan P. The value of ultrasound measurement of amniotic fluid volume in the management of prolonged pregnancies. Br J Obstet Gynaecol. 1984;91:444–448. doi: 10.1111/j.1471-0528.1984.tb04781.x. [DOI] [PubMed] [Google Scholar]
- Crowley P. The Cochrane Library, Issue 2. Chicester, UK: John Wiley & Sons, Ltd; 2004. Interventions for preventing or improving the outcome of delivery at or beyond term (Cochrane review) [Google Scholar]
- de Miranda E, van der Bom JG, Bonsel GJ, et al. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomised controlled trial. Br J Obstet Gynaecol. 2006;113:402–408. doi: 10.1111/j.1471-0528.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- Divon MY, Ferber A, Nisell H, et al. Male gender predisposes to prolongation of pregnancy. Am J Obstet Gynecol. 2002;187:1081–1083. doi: 10.1067/mob.2002.126645. [DOI] [PubMed] [Google Scholar]
- Doherty L, Norwitz R. Prolonged Pregnancy: When should we intervene? Curr Opin Obstet Gynecol. 2008;20:519–527. doi: 10.1097/gco.0b013e328314b6f8. [DOI] [PubMed] [Google Scholar]
- Dyson DC, Miller PD, Armstrong MA. Management of prolonged pregnancy: induction of labor versus antepartum fetal testing. Am J Obstet Gynecol. 1987;156:928–934. doi: 10.1016/0002-9378(87)90359-0. [DOI] [PubMed] [Google Scholar]
- Eden RD, Seifert LS, Winegar A, et al. Perinatal characteristics of uncomplicated postdate pregnancies. Obstet Gynecol. 1987;69:296–299. [PubMed] [Google Scholar]
- Ellis MJ, Livesey JH, Inder WJ, et al. Plasma corticotrophin-releasing hormone and unconjugated estriol in human pregnancy: gestational patterns and ability to predict preterm delivery. Am J Obstet Gynecol. 2002;186:94–99. doi: 10.1067/mob.2002.119188. [DOI] [PubMed] [Google Scholar]
- Feldman GB. Prospective risk of stillbirth. Obstet Gynecol. 1992;79:547–553. [PubMed] [Google Scholar]
- Fonseca L, Monga M, Silva J. Postdates pregnancy in an indigent population: the financial burden. Am J Obstet Gynecol. 2003;188:1214–1216. doi: 10.1067/mob.2003.287. [DOI] [PubMed] [Google Scholar]
- Fraser WD, Hofmeyr J, Lede R, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005;(353):909. doi: 10.1056/NEJMoa050223. [DOI] [PubMed] [Google Scholar]
- Gardosi J, Vanner T, Francis A. Gestational age and induction of labor for prolonged pregnancy. Br J Obstet Gynaecol. 1997;104:792–797. doi: 10.1111/j.1471-0528.1997.tb12022.x. [DOI] [PubMed] [Google Scholar]
- Goodwin TM. A role for estriol in human labor, term and preterm. Am J Obstet Gynecol. 1999;180:S208–213. doi: 10.1016/s0002-9378(99)70702-7. [DOI] [PubMed] [Google Scholar]
- Guidetti DA, Divon MY, Cavalieri RL. Fetal umbilical artery flow velocimetry in postdate pregnancies. Am J Obstet Gynecol. 1987;157:1521–1523. doi: 10.1016/s0002-9378(87)80255-7. [DOI] [PubMed] [Google Scholar]
- Guidetti DA, Divon MY, Langer O. Postdate fetal surveillance: is 41 weeks too early? Am J Obstet Gynecol. 1989;161:91–93. doi: 10.1016/0002-9378(89)90240-8. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Hannah WJ, Hellmann J, et al. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326:1587–1592. doi: 10.1056/NEJM199206113262402. [DOI] [PubMed] [Google Scholar]
- Hannah ME. Postterm pregnancy: should all women have labour induced? A review of the literature. Fetal Matern Med Rev. 1993;5:3. [Google Scholar]
- Heden L, Ingemarsson I, Ahlstrom H, et al. Induction of labor versus conservative management in prolonged pregnancy: controlled study. Int J Feto-Maternal Med. 1991;4:231–236. [Google Scholar]
- Heimstad R, Romundstad PR, Nes SH, et al. Outcomes of pregnancy beyond 37 weeks of gestation. Obstet Gynecol. 2006;108:500–508. doi: 10.1097/01.AOG.0000227783.65800.0f. [DOI] [PubMed] [Google Scholar]
- Heimstad R, Romundstad PR, Hyett , et al. Women’s experiences and attitudes towards expectant management and induction of labor for post-term pregnancy. Acta Obstet Gynecol Scand. 2007;86:950–956. doi: 10.1080/00016340701416929. [DOI] [PubMed] [Google Scholar]
- Helmreich RJ, Shiao SY, Dune LS. Meta-analysis of acustimulation effects on nausea and vomiting in pregnant women. Explore (NY) 2006;2:412–421. doi: 10.1016/j.explore.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Herabutya Y, Prasertsawat PO, Tongyai T, et al. Prolonged pregnancy: the management dilemma. Int J Gynaecol Obstet. 1992;37:253–258. doi: 10.1016/0020-7292(92)90325-d. [DOI] [PubMed] [Google Scholar]
- Hickey CA, Cliver SP, McNeal SF, et al. Low pregravid body mass index as a risk factor for preterm birth: variation by ethnic group. Obstet Gynecol. 1997;89:206–212. doi: 10.1016/S0029-7844(96)00486-3. [DOI] [PubMed] [Google Scholar]
- Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: evaluating gestation-specific risks of fetal and infant mortality. Br J Obstet Gynaecol. 1998;105:169–173. doi: 10.1111/j.1471-0528.1998.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev. 2002;1(CD000014) doi: 10.1002/14651858.CD000014.pub3. [DOI] [PubMed] [Google Scholar]
- How HY, Leaseburge L, Khoury JC, et al. A comparison of various routes and dosages of misoprostol for cervical ripening and the induction of labor. Am J Obstet Gynecol. 2001;185:911–915. doi: 10.1067/mob.2001.117358. [DOI] [PubMed] [Google Scholar]
- Ingemarsson I, Kallen K. Stillbirths and rate of neonatal deaths in 76,761 postterm pregnancies in Sweden, 1982-1991: a register study. Acta Obstet Gynecol Scand. 1997;76:658–662. doi: 10.3109/00016349709024606. [DOI] [PubMed] [Google Scholar]
- James C, George SS, Gaunekar N, Seshadri L. Management of prolonged pregnancy: a randomised trial of induction of labour and antepartum foetal monitoring. Natl Med J. India. 2001;14:270–273. [PubMed] [Google Scholar]
- John Ed O. Acupuncture: A Comprehensive Text. Seattle: Eastland Press; 1981. [Google Scholar]
- Kabbur PM, Herson VC, Zaremba S, et al. Have the year 2000 neonatal resuscitation program guidelines changed the delivery room management or outcome of meconium-stained infants? J Perinatol. 2005;25:694–697. doi: 10.1038/sj.jp.7211385. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Akbarian A, Baradaran H, et al. Effect of membrane sweeping at term pregnancy on duration of pregnancy and labor induction: a randomized trial. Gynecol Obstet Invest. 2006;62:41–44. doi: 10.1159/000091842. [DOI] [PubMed] [Google Scholar]
- Kavanagh J, Kelly AJ, Thomas J. Cochrane Database Syst Rev. 2005;((3):CD003392) doi: 10.1002/14651858.CD003392.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2001;((2):CD003093) doi: 10.1002/14651858.CD003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AJ, Malik S, Smith L, et al. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2009;((4):CD003101) doi: 10.1002/14651858.CD003101.pub2. [DOI] [PubMed] [Google Scholar]
- Kistka ZA, Palomar L, Boslaugh SE, et al. Risk for postterm delivery after previous postterm delivery. Am J Obstet Gynecol. 2007;196:241e1–241e6. doi: 10.1016/j.ajog.2006.10.873. [DOI] [PubMed] [Google Scholar]
- Kitlinski ML, Kallen K, Marsal K, et al. Gestational age-dependent reference values for pH in umbilical cord arterial blood at term. Obstet Gynecol. 2003;102:338–345. doi: 10.1016/s0029-7844(03)00512-x. [DOI] [PubMed] [Google Scholar]
- Knox GE, Huddleston JF, Flowers CE Jr. Management of prolonged pregnancy: results of a prospective randomized trial. Am J Obstet Gynecol. 1979;134:376–384. doi: 10.1016/s0002-9378(16)33078-2. [DOI] [PubMed] [Google Scholar]
- Laursen M, Bille C, Olesen AW, et al. Genetic influence on prolonged gestation: a population-based Danish twin study. Am J Obstet Gynecol. 2004;190:489–494. doi: 10.1016/j.ajog.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Rochelle M, Holt VL, Easterling TR, et al. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3–8. doi: 10.1056/NEJM200107053450101. [DOI] [PubMed] [Google Scholar]
- Magann EF, Chauhan SP, McNamara MF, et al. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. J Perinatol. 1999;19:88–91. doi: 10.1038/sj.jp.7200133. [DOI] [PubMed] [Google Scholar]
- Magann EF, Chauhan SP, Nevils BG, et al. Management of pregnancies beyond forty-one weeks’ gestation with an unfavorable cervix. Am J Obstet Gynecol. 1998;178:1279–1287. doi: 10.1016/s0002-9378(98)70334-5. [DOI] [PubMed] [Google Scholar]
- Mandruzzato G, Alfirevic Z, Chervenak F, et al. Guidelines for the management of postterm pregnancy. J Perinat Med. 2010;38(2):111–119. doi: 10.1515/jpm.2010.057. [DOI] [PubMed] [Google Scholar]
- Mannino F. Neonatal complications of postterm gestation. J Reprod Med. 1988;33:271–276. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- Martin JN Jr, Sessums JK, Howard P, et al. Alternative approaches to the management of gravidas with prolonged-postterm-postdate pregnancies. J Miss State Med Assoc. 1989;30:105–111. [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, et al. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Mogren I, Stenlund H, Hogberg U. Recurrence of prolonged pregnancy. Int J Epidemiol. 1999;28:253–257. doi: 10.1093/ije/28.2.253. [DOI] [PubMed] [Google Scholar]
- Morris JM, Thompson K, Smithey J, et al. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: A prospective blinded observational study. BJOG. 2003;110:989–994. [PubMed] [Google Scholar]
- Neilson JP. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2000;(2:CD000182) doi: 10.1002/14651858.CD000182. [DOI] [PubMed] [Google Scholar]
- Neri I, Fazzio M, Menghini S, et al. Non-stress test changes during acupuncture plus moxibustion on BL67 point in breech presentation. J Soc Gynecol Investig. 2002;9:158–162. [PubMed] [Google Scholar]
- NICHHD. A clinical trial of induction of labor versus expectant management in post-term pregnancy. Am J Obstet Gynecol. 1994;170:716–723. [PubMed] [Google Scholar]
- Nicholson JM, Kellar LC, Kellar GM. The impact of the interaction between increasing gestational age and obstetrical risk on birth outcomes: evidence of a varying optimal time of delivery. J Perinatol. 2006;26:392–402. doi: 10.1038/sj.jp.7211528. [DOI] [PubMed] [Google Scholar]
- Olesen AW, Basso O, Olsen J. An estimate of the tendency to repeat postterm delivery. Epidemiology. 1999;10:468–469. doi: 10.1097/00001648-199907000-00026. [DOI] [PubMed] [Google Scholar]
- Olesen AW, Basso O, Olsen J. Risk of recurrence of prolonged pregnancy. Br Med J. 2003;326:476. doi: 10.1136/bmj.326.7387.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: A national register-based study, 1978-1993. Am J Obstet Gynecol. 2003;189:222–227. doi: 10.1067/mob.2003.446. [DOI] [PubMed] [Google Scholar]
- Oz AU, Holub B, Mendilcioglu I, et al. Renal artery Doppler investigation of the etiology of oligohydramnios in postterm pregnancy. Obstet Gynecol. 2002;100:715–718. doi: 10.1016/s0029-7844(02)02203-2. [DOI] [PubMed] [Google Scholar]
- Papageorgiou I, Tsionou C, Minaretzis D, et al. Labor characteristics of uncomplicated prolonged pregnancies after induction with intracervical prostaglandin E2 gel versus intravenous oxytocin. Gynecol Obstet Invest. 1992;34:92–96. doi: 10.1159/000292734. [DOI] [PubMed] [Google Scholar]
- Phelan JP, Platt LD, Yeh SY, et al. The role of ultrasound assessment of amniotic fluid volume in the management of the postdate pregnancy. Am J Obstet Gynecol. 1985;151:304–308. doi: 10.1016/0002-9378(85)90291-1. [DOI] [PubMed] [Google Scholar]
- Poma PA. Cervical ripening. A review and recommendations for clinical practice. J Reprod Med. 1999;44:657–668. [PubMed] [Google Scholar]
- Qu F, Zhou J. Electro-acupuncture in relieving labor pain. Evid Based Complement Alternat Med. 2007;4:125–130. doi: 10.1093/ecam/nel053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl M, Ahner R, Bitschnau M, et al. Acupuncture for cervical ripening and induction of labor at term--a randomized controlled trial. Wien Klin Wochenschr. 2001;113:942–946. [PubMed] [Google Scholar]
- Rand L, Robinson JN, Economy KE, et al. Post-term induction of labor revisited. Obstet Gynecol. 2000;96:779–783. doi: 10.1016/s0029-7844(00)01002-4. [DOI] [PubMed] [Google Scholar]
- Rayburn W, Gosen R, Ramadei C, et al. Outpatient cervical ripening with prostaglandin E2 gel in uncomplicated postdate pregnancies. Am J Obstet Gynecol. 1988;158:1417–1423. doi: 10.1016/0002-9378(88)90376-6. [DOI] [PubMed] [Google Scholar]
- Rosen MG, Dickinson JC. Management of post-term pregnancy. N Engl J Med. 1992;326 doi: 10.1056/NEJM199206113262409. [DOI] [PubMed] [Google Scholar]
- Rozenberg P, Chevret S, Ville Y. Comparison of pre-induction ultrasonographic cervical length and Bishop score in predicting risk of cesarean section after labor induction with prostaglandins. Gynecol Obstet Fertil. 2005;33:17–22. doi: 10.1016/j.gyobfe.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos L, Kaunitz AM, et al. Labor induction with 25 micro versus 50 micro intravaginal misoprostol: a systematic review. Obstet Gynecol. 2002;99:145–151. doi: 10.1016/s0029-7844(01)01644-1. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Terry JW Jr, Dole N, et al. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- Sawai SK, Brien WF, Mastrogiannis DS, et al. Patient-administered outpatient intravaginal prostaglandin E2 suppositories in post-date pregnancies: a double-blind, randomized, placebo-controlled study. Obstet Gynecol. 1994;84:807–810. [PubMed] [Google Scholar]
- Schaffir J. Survey of folk beliefs about induction of labor. Birth. 2002;29:47–51. doi: 10.1046/j.1523-536x.2002.00047.x. [DOI] [PubMed] [Google Scholar]
- Scharf A, Staboulidou I, Gunter HH, et al. Influence of antenatal acupuncture on cardiotocographic parameters and maternal circulation -a prospective study. Z Geburtshilfe Neonatol. 2003;207:166–172. doi: 10.1055/s-2003-43416. [DOI] [PubMed] [Google Scholar]
- Shaw KJ, Medearis AL, Horenstein J, et al. Selective labor induction in postterm patients. Observations and outcomes. J Reprod Med. 1992;37:157–161. [PubMed] [Google Scholar]
- Shime J, Librach CL, Gare DJ, et al. The influence of prolonged pregnancy on infant development at one and two years of age: a prospective controlled study. Am J Obstet Gynecol. 1986;154:341–345. doi: 10.1016/0002-9378(86)90668-x. [DOI] [PubMed] [Google Scholar]
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews. 2004;(CD002962) doi: 10.1002/14651858.CD002962.pub2. [DOI] [PubMed] [Google Scholar]
- Smith GC. Life-table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol. 2001;184:489–496. doi: 10.1067/mob.2001.109735. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, Chan EC, et al. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulphate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J clin Endocrinol Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- Spellacy WN, Miller S, Winegar A, et al. Macrosomia – maternal characteristics and infant complications. Obstet Gynecol . 1985;66:158–161. [PubMed] [Google Scholar]
- Stokes HJ, Roberts RV, Newnham JP. Doppler flow velocity waveform analysis in postdate pregnancies. Aust N Z J Obstet Gynaecol. 1991;31:27–30. doi: 10.1111/j.1479-828x.1991.tb02759.x. [DOI] [PubMed] [Google Scholar]
- Stotland NE, Washington AE, Caughey AB. Pre-pregnancy body mass index and length of gestation at term. Am J Obstet Gynecol. 2007;197:378.e1–e5. doi: 10.1016/j.ajog.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Sutan R, Campbell D, Prescott GJ, et al. The risk factors for unexplained antepartum stillbirths in Scotland, 1994 to 2003. J Perinatology. 2010;30:311–318. doi: 10.1038/jp.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale P, Hiilermaa V. Predicting delivery date by ultrasound and last menstrual period on early gestation. Obstet Gynecol. 2001;97:189–194. doi: 10.1016/s0029-7844(00)01131-5. [DOI] [PubMed] [Google Scholar]
- Tan P C, Yow C M, Omar S Z. Coitus and orgasm at term: effect on spontaneous labour and pregnancy outcome. Singapore Med J. 2009;50:1062–1068. [PubMed] [Google Scholar]
- Tan PC, Andi A, Azmi, et al. Effect of coitus at term on length of gestation, induction of labor, and mode of delivery. Obstet Gynecol. 2006;108:134–140. doi: 10.1097/01.AOG.0000223229.83920.af. [DOI] [PubMed] [Google Scholar]
- Taylor PL, Kelly RW. 19-Hydroxylated E prostaglandins as the major prostaglandins of human semen. Nature. 1974;250:665–667. doi: 10.1038/250665a0. [DOI] [PubMed] [Google Scholar]
- Tiran D. Breech presentation: increasing maternal choice. Complement Ther Nurs Midwifery. 2004;10:233–238. doi: 10.1016/j.ctnm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Tongsong T, Srisomboon J. Amniotic fluid volume as a predictor of fetal distress in postterm pregnancy. Int J Gynaecol Obstet. 1993;40:213–217. doi: 10.1016/0020-7292(93)90833-i. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Ignacchiti E, Giovannelli A, et al. Maternal plasma corticotrophin-releasing factor and urocortin levels in postterm pregnancies. Eur J Endocrinol. 2006;154:281–285. doi: 10.1530/eje.1.02091. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Novembri R, Voltolini C, et al. Biochemical and biophysical predictors of the response to the induction of labour in nulliparous postterm pregnancy. Am J Obstet Gynecol. 2011;204:39.e1–6. doi: 10.1016/j.ajog.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Treger M, Hallak M, Silberstein T, et al. Post-term pregnancy: should induction of labor be considered before 42 weeks? . J Matern Fetal Neonatal Med. 2002;11:50–53. doi: 10.1080/jmf.11.1.50.53. [DOI] [PubMed] [Google Scholar]
- Usha Kiran TS, Hemmadi S, Bethel J, et al. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768–772. doi: 10.1111/j.1471-0528.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Usher RH, Boyd ME, McLean FH, et al. Assessment of fetal risk in postdate pregnancies. Am J Obstet Gynecol. 1988;158:259–264. doi: 10.1016/0002-9378(88)90134-2. [DOI] [PubMed] [Google Scholar]
- Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597. doi: 10.1016/S0140-6736(04)16852-9. [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Martin JA, Curtin SC, et al. Births: final data for 1998. Natl Vital Stat Rep. 2000;48:1–100. [PubMed] [Google Scholar]
- Vorherr H. Placental insufficiency in relation to postterm pregnancy and fetal postmaturity. Evaluation of fetoplacental function; management of the postterm gravida. Am J Obstet Gynecol. 1975;123:67–103. doi: 10.1016/0002-9378(75)90951-5. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab. 1984;58:929–939. doi: 10.1210/jcem-58-4-629. [DOI] [PubMed] [Google Scholar]
- West Z. Acupuncture within the National Health Service: a personal perspective. Complement Ther Nurs Midwifery. 1997;3:83–86. doi: 10.1016/s1353-6117(97)80041-8. [DOI] [PubMed] [Google Scholar]
- Witter FR, Weitz CM. A randomized trial of induction at 42 weeks gestation versus expectant management for postdates pregnancies. Am J Perinatol. 1987;4:206–211. doi: 10.1055/s-2007-999774. [DOI] [PubMed] [Google Scholar]
- Wong SF, Hui SK, Choi H, et al. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour? BJOG. 2002;109:632–636. doi: 10.1111/j.1471-0528.2002.01193.x. [DOI] [PubMed] [Google Scholar]
- Xenakis EM, Piper JM, Conway DL, et al. Induction of labor in the nineties: conquering the unfavorable cervix. Obstet Gynecol. 1997;90:235–239. doi: 10.1016/S0029-7844(97)00259-7. [DOI] [PubMed] [Google Scholar]
- Yang R, You X, Tang X, et al. Corticotropin-releasing hormone inhibits progesterone production in cultured human placental trophoblasts. J Mol Endocrinol. 2006;37(3):533–540. doi: 10.1677/jme.1.02119. [DOI] [PubMed] [Google Scholar]
- Yoder BA, Kirsch EA, Barth WH, et al. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002;99:731. doi: 10.1016/s0029-7844(02)01942-7. [DOI] [PubMed] [Google Scholar]
- Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1(8543):1192–1194. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- Zelop CM, Shipp TD, Cohen A, et al. Trial of labor after 40 weeks’ gestation in women with prior cesarean. Obstet Gynecol. 2001;97:391–393. doi: 10.1016/s0029-7844(00)01175-3. [DOI] [PubMed] [Google Scholar]


