Abstract
The widespread use of prenatal ultrasound has made the fetus a patient. A number of conditions diagnosed as such may require therapy prior to birth. Herein we describe past, current and potential future procedures designed to treat pulmonary conditions in the antenatal period. When congenital cystic adenomatoid malformation (CCAM) is associated with fetal hydrops, treatment is required. Prior to viability this may be in utero resection of the pathologic lung lobe or shunting of cystic lesions. More recently, fetuses with isolated congenital diaphragmatic hernia (CDH) with lethal lung hypoplasia have been offered percutaneous fetal tracheal occlusion to provoke lung growth. A very rare condition is laryngeal atresia, which requires peripartum re-establishment of the airways. As we get more experience with access to the fetal airways, this may open the doors for novel therapies. One of these is gene delivery to treat fetuses with serious monogenic disorders or to induce transient overexpression of certain proteins. We review the individual hurdles that are being met by researchers when designing fetal gene therapeutic strategies, in particular for the fetal lung. Also the use of stem cells for pulmonary disorders is currently explored.
Introduction
The introduction of high resolution ultrasound and wide offering of screening programmes prompted the advent of fetal medicine, next to ‘maternal’ care. In other words, information gathered as a consequence of prenatal imaging and subsequent other diagnostic procedures make the unborn fetus a true patient (Fig. 1). When fetal malformations, genetic diseases or in utero acquired conditions are suspected, management can usually wait until after birth. However, a number of conditions may benefit from antenatal interventions, whether they are non-invasive (such as transplacental pharmacological therapy of fetal infections or cardiac arrhythmia) or invasive (such as fetal transfusion of blood derivates). In that case, the intervention is done in the prenatal period because it is either life saving or may prevent organ damage, with the assumption that potential fetal benefits outweigh the risks of the prenatal intervention (Deprest et al., 2006). The fetal respiratory system is only one target for candidate prenatal interventions. Herein we will describe past, current and potential future procedures that focus on treating pulmonary conditions in the antenatal period, such as congenital cystic adenomatoid malformation and congenital diaphragmatic hernia. Next to reviewing the first two indications, the paper focuses on an indication to come, i.e. fetal pulmonary gene therapy. Since fetal medicine specialists are typically not (yet) familiar with gene therapy, its concept will be extensively introduced before focussing on the fetal lung.
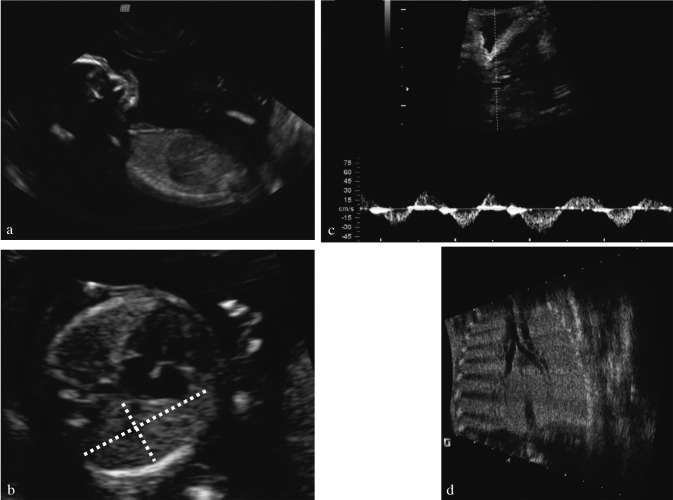
Fig. 1. Normal lungs, airways and diaphragm. (a) Prenatal ultrasound of the normal diaphragm at 15 weeks of gestation in a longitudinal section. (b) Cross-section through the thorax is taken in the four chamber view and one of the lungs is measured as would be done for cases with CDH. (c) Using Doppler, movement of fluid in the upper trachea can be demonstrated. (d) upper airways “bronchogram”, which can be easily seen in a fetus with laryngeal atresia, causing obstruction to lung fluid (from Oepkes et al., 2003; reprinted with permission of ISUOG and Wileys).
Congenital Cystic Adenomatoid Malformation of the Lung
CCAM is a space occupying lesion that, if large enough, causes mediastinal shift and subsequent fetal hydrops as well as pulmonary hypoplasia. In up to 25% of cases these lesions may be hybrid, i.e.; with a component of bronchopulmonary sequestration. When following such patients, one should take into account that the maximal growth of CCAM is around 28 weeks of gestation. Non-hydropic fetuses have an excellent prognosis with in utero transfer, planned delivery, neonatal resuscitation and evaluation. Survival rate is close to 100% for cystic lesions (Cavoretto et al., 2008). Therefore patients should not be needlessly upset (Aite et al., 2009). Actually, about one in five CCAM-lesions decrease or disappear during pregnancy, maybe by decompression into the bronchial tree or by outgrowth. Postnatal imaging is required as they can still cause infection, pneumothorax or even malignant degeneration at a later age. The place of postnatal resection remains a matter of debate (Wilson et al., 2006, 2008).
At present, not much clinical data are available on the potential of predicting lethal pulmonary hypoplasia by different prenatal imaging techniques in case of CCAM. It is therefore difficult to formulate guidelines when to intervene in utero in order to avoid neonatal respiratory insufficiency. The other important aspect is the occurrence of fetal hydrops, which is a poor prognostic factor. This might be caused by impairment of venous return. Mediastinal shift may also contribute to polyhydramnios. Fetal hydrops typically presents as fetal ascites, pleuro-pericardial effusions and subcutaneous edema and is a poor prognostic sign. A large experience gathered by Adzick et al. demonstrated that in case of fetal hydrops, antenatal intervention is needed to prevent in utero fetal death (IUFD) (Adzick, 2003). Moreover, at that time the mother is at risk for the so-called mirror or Balentyne syndrome, particularly in the presence of placentomegaly. It has been proposed to follow up early diagnosed lesions in terms of developing hydrops and IUFD, by using the ratio of the mass of the lesion over the head circumference (CCAM Volume Ratio-CVR) (Crombleholme et al., 2002). When CVR exceeds 1.6, there would be an 80% risk for fetal hydrops.
In case of hydrops, intervention is warranted. As a first treatment step, maternal steroid administration has been described. Its efficacy and wider place in management still remains to be demonstrated, but due to its minimal side effects and their proven benefit in case of prematurity, they seem to be warranted as an initial treatment (Peranteau et al., 2007; Tsao et al., 2003). When hydrops persists, and presents beyond 32 weeks, delivery should take place in appropriate conditions. Earlier than that, fetal intervention can be life saving as reported by several centers now. Roughly spoken the modality is dependent on the morphologic appearance of the thoracic mass. For microcystic masses fetal lobectomy can be carried out by open fetal surgery, with high survival rates in case hydrops resolves. In a series of 22 cases operated between 21-31 weeks, there were 11 long term survivors, who were developmentally normal (up to 12 years of age) (Adzick, 2003). Hydrops resolved in one to two weeks followed by normalization of the position of the mediastinum and the remaining lung underwent impressive catch up growth. Causes of fetal death despite fetal surgery were termination of pregnancy for Ballentyne syndrome (n = 1), preterm labour and/or chorioamnionitis (n = 2), and fetal hemodynamic compromise leading to intra operative death in 6 fetuses and postoperative death in another 2 cases. To prevent intra-operative deaths all means for resuscitation, such as gaining access to the fetal circulation and appropriate fetal monitoring techniques, are now part of the open procedure. It is obvious that the latter procedure can only be offered at centers familiar with open fetal surgery for this and other conditions. When presenting late in pregnancy, fetal lobectomy can be done on placental circulation (Liechty, 2010).
Macrocystic masses can be reduced. Occasionally percutaneous puncture may be successful. However a more permanent drainage by thoraco-amniotic shunting is a minimally invasive procedure that can be successful even if cysts are multilocular, probably because they are all interconnected (Fig. 2, Fig. 3). The largest experience with shunting was published by the Philadelphia group. Shunts reduce the CCAM-volume by 70%, reverse hydrops, and result in a survival rate of 74% (n = 23; (Wilson et al., 2004)). This success rate has since been confirmed by others (Knox et al., 2006). Thoracic shunting has a reported preterm premature rupture of the outer membranes (PPROM) rate of 15% (Picone et al., 2004). Another yet experimental approach is sclerotherapy (Bermudez et al., 2008). Laser coagulation has been used successfully for bronchopulmonary sequestration, where the feeding vessel can be coagulated under ultrasound guidance (Oepkes et al., 2007). Unpublished data from Leiden & Toronto using this modality in CCAM are also encouraging (Oepkes, personal communication). One must realize that this causes collateral thermal damage to surrounding structures, and thoracic deformation has been described already. It can theoretically also worsen fetal hydrops by swelling of the necrotic mass, thus leading to IUFD.
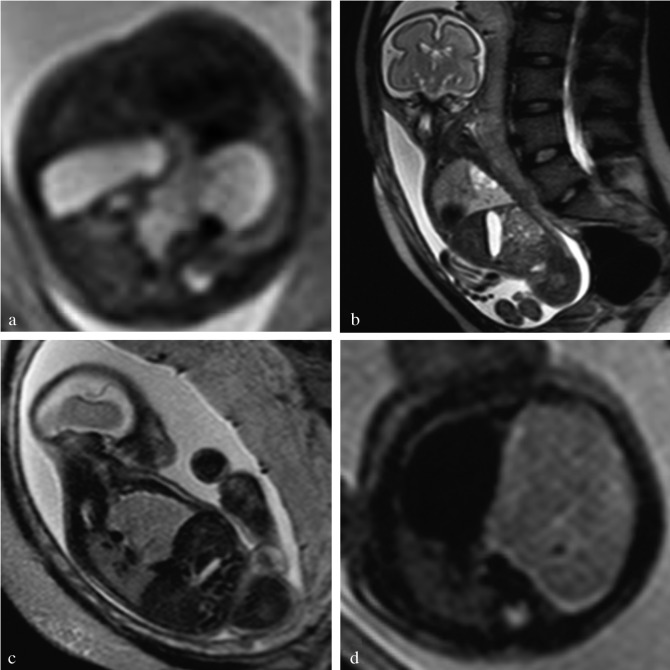
Fig. 3. Fetal MRI (a,d) axial, (b) sagittal and (c) coronal T2-weighted MR-images of a congenital cystic adenomatoid malformation of the fetal lung. The 3 different types are shown: (a) type I CCAM, (b) type II and (c,d) type III CCAM.
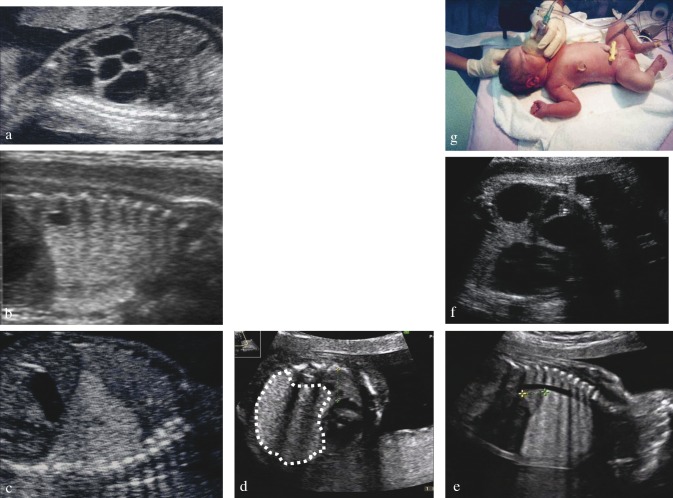
Fig. 2. Cystic Adenomatoid Malformation (CCAM) of the fetal lung. The condition is usually described as either type I (a), II (b) or III (c, d) based on the presence and size of visible cystic areas on the ultrasound image. (d) Dotted line delineates the type III lesion; remnant lung is squeezed between lesion, heart and thorax on transverse and longitudinal section. (e) MR image of cystic lesion. (f) Shunted cystic lesion, with tip of pigtail visible on ultrasound both sides of the thorax wall and at birth (g).
Isolated Congenital Diaphragmatic Hernia
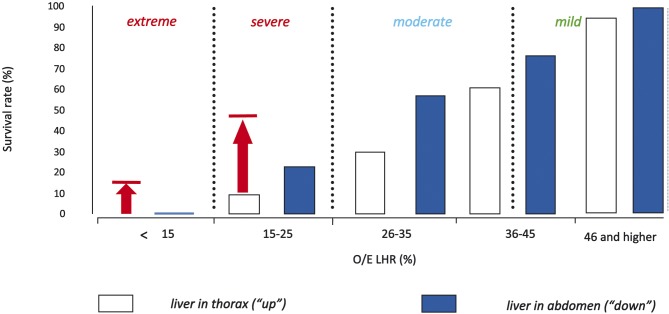
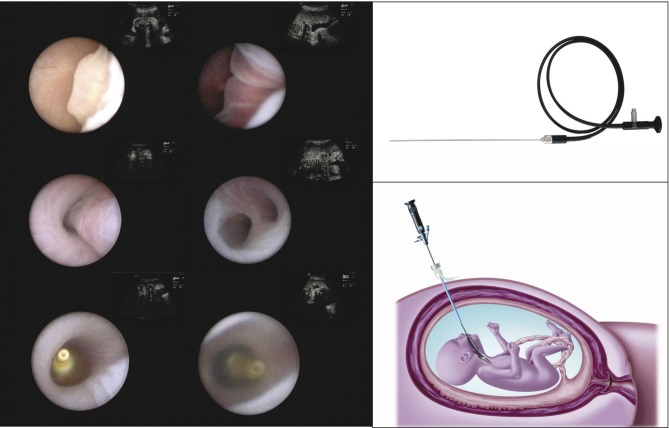
CDH rarely poses an intra-uterine threat to the fetus, except in case of polyhydramnios (leading to preterm labor) or the one percent of cases succumbing to (unexplained) IUFD. The problem of this condition only arises in the neonatal period, when the baby will struggle with the consequences of pulmonary hypoplasia, leading to respiratory deficiency as well as pulmonary hypertension. In essence CDH babies have smaller and less compliant lungs with fewer airway branches and abnormal pulmonary vessels. When isolated, the condition may be lethal in 30-40% of prenatally diagnosed cases in most Western countries (Colvin et al., 2005; Deprest et al., 2006; Deprest et al., 2005; Gallot et al., 2006; Stege et al., 2003). It has been shown that it is possible to predict outcome for left-sided CDH, which constitutes the vast majority (85%). This is done in mid-gestation by documenting the presence of herniation of the liver into the thorax and a low lung-to-head ratio (LHR) (Jani et al., 2006; Metkus et al., 1996) (Fig. 4). For 0.6 LHR < 0.8 survival is 62% and 78% for 0.8 LHR < 1.0 versus 0% resp. 16% in controls treated by standard postnatal care. Correction for gestational age can be done by expressing the observed LHR as a function of what is expected in gestational age matched normal control (O/E LHR) (Jani et al., 2006; Jani et al., 2007). An O/E LHR < 25% identifies a subgroup that is almost certainly deemed to die despite optimal postnatal care (Fig. 5). None of the currently available postnatal therapies can address their underlying problem of severe pulmonary hypoplasia. As a consequence, prenatal therapy aims at stimulating lung growth prior to birth. This is no longer attempted by anatomical repair, but rather by occluding the fetal trachea. This prevents egress of lung liquid, leading to increased pulmonary stretch and accelerated growth of airways and pulmonary vessels. The timing and duration of occlusion are determining the quantitative and qualitative response of airways and pulmonary vessels (Deprest et al., 2006). In experimental lambs cyclical tracheal occlusion yields growth with an optimal balance between type II and I alveolar epithelial cells. Practically the best clinical proxy to this strategy is temporary occlusion (plug-unplug sequence) (Flageole et al., 1998). Tracheal occlusion is achieved by insertion of an endoluminal balloon, which meets both the needs of tracheal growth as well as it allows its reversion either by percutaneous puncture, fetoscopic retrieval or postnatal reversal (Fig. 6). However, prenatal restoration of the airways makes normal vaginal delivery possible at the local referral center and has a beneficial effect on late neonatal survival (Jani et al., 2005). Extrapolating from animal experiments, insertion of the balloon was planned for 26-28 weeks (pseudoglandular phase) and in utero restoration of airways is realized at 34 weeks (saccular to alveolar phase). The procedure is done percutaneously through a 3.3 mm cannula under local or loco-regional anesthesia and fetal immobilization and pain relief.
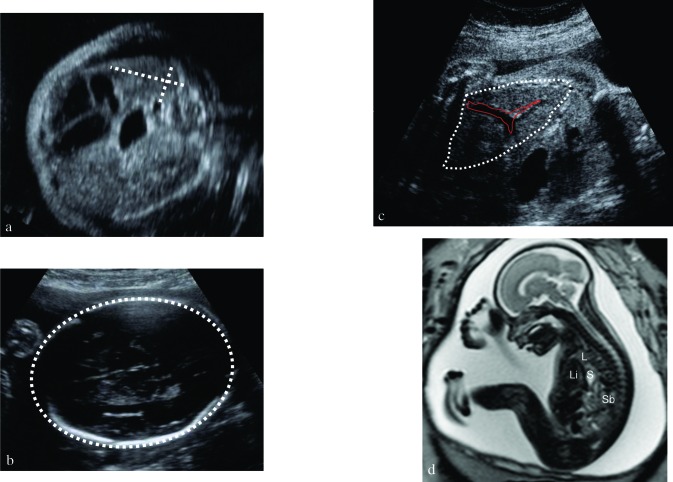
Fig. 4. Fetus with congenital diaphragmatic hernia (CDH). (a) Measurement of the Lung-to-Head Ratio in a section through the so-called four chamber view with the so-called “longest axis method”: the contralateral lung is measured by multiplying the lung’s longest axis by the longest measurement perpendicular to the former one. Its value is proportionated over the head circumference (b), measured in the standard biparietal view, showing two symmetrical hemispheres, the cavum septum pellucidum at one third of the fronto-occipital diameter and the posterior horns of the lateral ventricles. (c) Herniation of the liver. The major vessels help in its identification. (d) MR images of a fetus with CDH in the second trimester. L = lung; Li = liver, S = stomach, Sb = small bowel.
Fig. 5. Survival rates of fetuses with isolated left-sided congenital diaphragmatic hernia as a function of the observed/expected lung:head ratio (O/E LHR) and liver position as in the antenatal congenital diaphragmatic hernia registry. Figure modified from Deprest et al, 2009, with permission. Arrows and horizontal lines indicate observed survival rates following FETO for severity matched fetuses as reported by the FETO consortium (Jani et al., 2006).
Fig. 6. Left: fetoscopic images of landmarks during the operation, from top left to right bottom: epiglottis, vocal cords, trachea, carina, inflated and detached balloon, vocal cords about to close over the balloon (not all images from the same patient - from Nelson et al., 2006). Top right: deported eyepiece fetoscope (reprinted with permission from Karl Storz Endoskope). Bottom right: Schematic drawing of percutaneous FETO (from Deprest et al., 2004; reprinted with permission from ISUOG and John Wiley & Sons, Ltd).
The European Fetoscopic Endoluminal Tracheal Occlusion (FETO) Task force recently reported its clinical experience in 210 consecutive cases (Jani et al., 2009). Overall, 48% of infants were discharged from the hospital alive. On the basis of stratified data from the antenatal CDH registry, FETO therefore increased survival in severe cases with left-sided CDH from 24.1% to 49.1%, and in right-sided from 0% to 35.3% (p < 0.001). The backside of fetal surgery is that PPROM within three weeks occurred in 16.7% cases. In the entire cohort, gestational age at birth was 35.3 weeks (median), with just over 30% of patients delivering prior to 34 weeks. This is far less than in the earlier experience in the NIH trial by Harrison et al. (Harrison et al., 2003) (Table 1). Nevertheless, it remains a problem particularly if PPROM or delivery takes place prior to 32 weeks. Survival rate after FETO increases from 20-25% prior to 32 weeks (what is expected when expectantly managed), to 60% thereafter. Another predictor of survival is the observed/expected LHR prior to the procedure (Jani et al., 2006). Pulmonary response is also less when FETO is done beyond 30 weeks (Cannie et al., 2009). Therefore late FETO is only practiced within a trial involving more moderate forms of hypoplasia (Deprest et al., 2009). Postnatal management of this multicentre trial, as well as the one in severe cases, to be kicking off soon, is standardized by a consensus protocol (www.totaltrial.eu) (Deprest et al., 2009).
Table I. Comparison of recent series of fetoscopic tracheal occlusion. The randomized controlled trial (RCT) by Harrison et al. had very wide selection criteria (Harrison et al., 2003). Only 3 patients met the criteria of the FETO task group (Jani et al., 2009), of whom two were treated in utero.
| Harrison et al. (2003)N = 11 |
FETO consortium (2009) N = 210 |
|
|---|---|---|
| Criteria for surgery (left) | LHR < 1.4 and liver “up” | LHR < 1.0 and liver “up” |
| Anaesthesia | General | Loco-regional or local |
| Access through abdominal wall | Laparotomy | Percutaneous |
| Access diameter | 5 mm cannula | 3.3 mm cannula |
| Occlusive device | Clip or balloon | balloon |
| Operation time | N.R. | 10 (3-93) min |
| Reversal of occlusion | – | Deflation rate: 17/209 (8%) |
| EXIT delivery | In utero reversal | |
| PPROM < 34 weeks | 100% | 25% |
| PPROM < 37 weeks | 100% | 47% |
| Mean gestational age at birth | 30.8 (28-34) | 35.3 wks (25.7-41.0) |
| Survival till discharge (LHR < 1.4) | 73% (8/11) (controls: 77%) | Not eligible |
| Survival till discharge (LHR < 1.0) | 1/3 (33%) | 86/175 (49%) |
| Survival till discharge for Right CDH | – | 12/34 (35%) |
Airway management on placental circulation – perinatal surgery
In the presence of a potential or actual obstruction of the fetal airways, delivery has to be planned such that functionality can be guaranteed. The EXIT (ex utero intrapartum treatment) procedure was initially most frequently used as a delivery technique for safely establishing upper airways following surgical tracheal occlusion. EXIT is however applicable to any fetal condition (potentially) obstructing the upper airways (Mychaliska et al., 1997). The technical details of this treatment modality have been accurately reviewed by Liechty in a recent monography on fetal surgery (Liechty, 2010). Basically EXIT is an operation on placental support (OOPS), as placental circulation allows the surgeon to work safely on the fetal airways, and, more recently, also to enable sufficient time to create vascular access to set up extra-corporeal membrane oxygenation, or to operate on the fetus for other reasons. Using inhalational anesthesia for maximal uterine relaxation, uteroplacental blood flow and gas exchange are maintained, and amnioinfusion and partial delivery of the fetus keep uterine volume normal.
The list of indications for using this technique has grown over the years but essentially includes airway obstruction due to laryngeal atresia, large tumors, or iatrogenic tracheal occlusion. Intrinsic causes of Congenital High Airway Obstruction Syndrome (CHAOS) are stenosis or agenesis of the larynx, cricoid and/or trachea. Associated malformations such as Fraser or DiGeorge syndrome need to be ruled out. Obviously CHAOS is lethal at birth, when not diagnosed prenatally. This should not be a problem as ultrasound reveals signs such as a dilated trachea and main bronchi, bilateral hyperechogenic and enlarged lungs and a flattened to everted diaphragm (Fig. 1). Ascites or hydrops may complicate the condition in utero and even lead to intrauterine fetal death. Prenatal diagnosis enables the organization of this life-saving operation (Oepkes et al., 2003). The level and extent of obstruction can be determined prior to birth and may be of use when securing airways after birth. Essentially this involves establishing a tracheostomy while the neonate is still on placental support. The head and neck of the fetus are exteriorized, and upper airways are explored with a laryngoscope and bronchoscope. If not patent, a tracheostomy is performed.
In utero gene therapy of the lung
Principle of gene therapy
A number of lung disorders are theoretically eligible for gene therapy (Ennist, 1999). Among these, Cystic Fibrosis (CF) has received most interest. CF is the most common autosomal recessive monogenetic disorder in Caucasians and can be readily diagnosed in the prenatal period. CF patients have two out of a series of well identified mutations in the CFTR (Cystic Fibrosis Transmembrane Conductance Regulator)-gene. In the lung, deficient CFTR results in thick mucus, inflammation and secondary bacterial infection, leading to serious morbidity and eventually death early in life. Other candidate pulmonary disorders such as alpha-1 antitrypsin deficiency, surfactant deficiencies (SP-B protein) and primary pulmonary hypertension may benefit from permanent correction, while conditions such as respiratory distress syndrome or perinatal pulmonary infection may benefit from transient gene expression. Today, treatment of those disorders is often symptomatic but not curative.
Although gene therapy is based on a simple concept, there are multiple obstacles (some confined to the lung), preventing successful clinical gene therapy, which will be discussed here. Some of these can be addressed by offering gene therapy in the prenatal period, other issues relate only to the lung and yet other problems are more generic to gene therapy itself. For simplicity we will discuss the concept of gene therapy considering CF as a prototypic disease, but many analogies to other conditions can be made.
Gene transfer agents (GTA) for Cystic Fibrosis
Soon after the cloning of the CFTR gene in 1989, the concept of gene therapy was raised and the first clinical trials started in 1993 (Griesenbach et al., 2009). Clinical trials with non-viral vector systems, i.e. naked DNA, DNA-protein complexes and liposomes showed only transient and partial correction of transepithelial chloride transport, proving the need to optimize the transfer efficiency, improve transgene expression and investigate repeat administrations (Hyde et al., 2000).
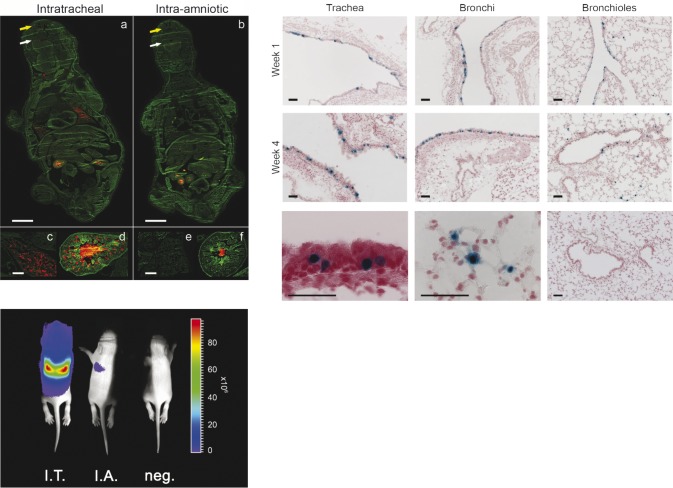
The first viral vector system to be investigated for CF clinical trials was the adenoviral vector (AdV). Also here, transduction efficiency in CF patients was relatively low (Grubb et al., 1994; Joseph et al., 2001). The host developed a specific immune response with substantial inflammation to the vector, with as a consequence loss of transgene expression levels below detection limits already after 2 to 4 weeks (Rosenecker et al., 1996). At present the use of AdV is restricted to preclinical research. In 1999, a first clinical trial with a recombinant adeno-associated viral vector (rAAV) was initiated. The virus from which it originates is a non-pathogenic, replication defective virus with a lesser immunogenic potential than AdV. rAAV are capable of transducing non-dividing cells and achieving medium to long term gene expression. Their packaging capacity however is relatively small, making the incorporation of full-length cDNA of CFTR (4.4 kb) with an accompanying promoter challenging. Up till now, all clinical trials for CF have been conducted with vectors containing the capsid of AAV serotype 2 (rAAV2/2). However, gene transfer efficiency to the airways and reversal of biological activity was limited (Carter 2005; Moss et al., 2007). Therefore clinical trials with rAAV2/2-CFTR have been aborted. Recent data from animal models suggest that several of the newly discovered and engineered serotypes have a better tropism for the airway epithelium which might improve results and could overcome possible problems with repeat administrations (Limberis et al., 2009; Limberis et al., 2006). Also in a fetal/neonatal setting, several AAV serotypes have been used to target the fetal lung of rodents (Fleurence et al., 2005; Garrett et al., 2003) and rabbits (Boyle et al., 2001). rAAV2/6.2, a novel serotype that was shown to be more efficient for airway epithelium transduction in adult mice than other AAV serotypes (Limberis et al., 2009), has recently been tested in fetal mice by direct intratracheal injection and robust expression levels as well as stable gene transfer were evidenced up to 1 postnatal month (Fig. 7) (Carlon et al., 2010).
Fig. 7. Upper left: Comparison of efficiency of intrapulmonary delivery between intratracheal (I.T.) versus intra-amniotic (I.A.) injection at E18 (term E19.5) using red fluorescent microspheres on whole body sections (a,b). Animals were sacrificed 24 h after injection. Fluorescent microspheres are present in the oral (white arrow) and nasal (yellow arrow) cavity, in the fetal lung (c,d) and gastro-intestinal tract (d,e) after I.T. and I.A. injection, respectively. a,b, Bar = 2 mm; c–f, bar = 200 µm. Bottom left: Non-invasive bioluminescence imaging of firefly luciferase expression after rAAV2/6.2 mediated gene delivery (3 × 1010 GC/fetus) in the fetal mouse lung. BLI signal is illustrated at 1 week post injection. The pseudocolor scale depicts the photon flux per second, per square centimeter per steradian (p/s/cm2/sr). Upper right: rAAV2/6.2-mediated transgene expression in murine airway epithelium after fetal I.T. injection of rAAV2/6.2 (3 × 1010 GC/fetus). Representative images of different lung regions are given at 1 and 4 weeks after fetal I.T. injection, respectively, showing the trachea, the bronchi and the bronchioles with the alveoli. A high magnification image depicts β-gal positive ciliated (bottom left image) and alveolar cells (bottom middle image) at week 1. Absence of reporter gene expression in a control lung (bottom right image). Bar = 50 µm. Figure adapted from Carlon et al., 2010.
Retroviruses are able to integrate the transgene into the genome of the targeted cells, providing long-term expression. Lentiviral vectors (LV) are a subdivision of retroviruses with unique characteristics. Human Immunedeficiency Virus (HIV) is the best known and studied virus of this family. HIV-derived vectors are able to transduce slowly and even non-dividing, or terminally differentiated cells, thus theoretically they can incorporate a transgene into the genome of stem cells. LV can also easily be pseudotyped, i.e. adjust their envelope so that their tropism for certain target populations increases. For instance, the affinity for respiratory cells can be adjusted by equipping the vector with an Ebola envelope (Kobinger et al., 2001). LV seem the best candidate vector system for stable transduction of respiratory stem cells, hence correction of CF. An integrating vector system however has the theoretical risk of insertional mutagenesis, i.e. the inadvertent change of normal gene regulation. This was observed in the Severe Combined Immunodeficiency Syndrome (SCID)-trial, where children treated with retroviral vectors developed leukemia (Cavazzana-Calvo et al., 2005). Gene transfer into the germline is another theoretical problem, but none of these complications have so far been described for HIV-based vectors.
The initial clinical CF-gene therapy trials achieved only a modest level of functional CFTR reconstitution in vivo and unveiled the physical and physiological barriers, which protect the airways and restrict exogenous gene delivery. The following paragraph describes these pulmonary defense mechanisms more into detail and where relevant, particular issues or solutions relating to fetal pulmonary gene therapy.
Advantages and risks of fetal gene therapy
When revising the experimental data of postnatal gene therapies, several problems become apparent. These can be summarized as follows: (1) sufficient transgene expression requires prohibitively large amounts of GTA; (2) the underlying defects usually have already caused extensive or even irreversible damage; (3) most adult tissues proliferate less than fetal cells, hence are typically less optimally transduced by integrating vectors; (4) host immune responses, either pre-existing or secondary to vector delivery, may rapidly eliminate transgene expression and prevent future interventions. Early gene transfer, in the neonatal or even fetal period, may overcome some, if not all of these obstacles. Yet a prenatal approach also carries inherent risks regarding germline transduction, developmental aberrations and oncogenesis. These concepts are reviewed in detail below.
Vector dose
From birth to adulthood the human body mass increases approximately 20-fold. Therefore, a relatively small amount of GTA will target a higher percentage of cells when introduced early rather than late in life. This is especially advantageous when viral vector systems are used where titers are limiting, such as LV.
Prevention of abnormalities
Genetic mutations can cause a wide spectrum of abnormalities ranging from asymptomatic to lethal. Many pulmonary diseases such as CF will not result in any pathology during prenatal life. For these and many other genetic diseases prenatal gene therapy, even if only providing partial correction, may have a dramatic effect upon disease onset as well as progression.
Tissue characteristics
The relative abundance of stem and progenitor cells in the fetus make the prenatal environment uniquely suitable for gene therapy. Specifically integrating vectors will maintain stable gene expression and provide restitution in a large proportion of the adult tissue. To gain access to an organ such as the lung, a GTA either has to be injected directly into the airways (Carlon et al. 2010, Peebles et al., 2004) or the parenchyma (Henriques-Coelho et al., 2007).
Fetal immunology
In postnatal or adult life, the organism’s defenses against pathogens may be roughly categorized as innate and adaptive immune mechanisms. An additional defense system in fetal life is provided by the maternal immune system, which performs a delicate balancing act: it has to protect both mother and fetus from microbial infections and at the same time it has to remain tolerant to the fetal semi-allograft (Kammerer et al., 2008). In the fetal lung innate immunity comprises many barriers including immune cells, epithelial cell layers, extracellular matrices and antimicrobial compounds. The fetus is surrounded by amniotic fluid, which not only possesses antibacterial and antifungal properties (Akinbi et al., 2004; King et al., 2007; Yoshio et al., 2003) but also enters the lungs by means of fetal breathing movements. This process washes away the mucus layer, which covers the respiratory epithelium during postnatal life. The developmental immaturity of cells participating in the immune response (Muthukkumar et al., 2000; Takahashi et al., 1995) and the absence of memory cells due to the naivety of the immune system (Adkins et al., 2003) result in an immature adaptive immune response. Tolerance to transgenes after fetal or neonatal transduction has been demonstrated for human factor IX using adenoviral (Waddington et al., 2003), lentiviral (Waddington et al., 2004) or retroviral vectors in mice and dogs (Zhang et al., 2004). In conclusion, although the early adaptive immune system in the fetus appears to be predisposed to tolerance against a transgenic protein, it remains poorly understood compared to the adult immune system. Therefore, careful choice of GTA and rigorous scrutiny of preclinical model systems with attention to differences in immune system development is essential prior to any clinical application.
Adverse effects and risks
In utero gene delivery carries procedural risks that concern the mother as well as the fetus, and include infection, fetal loss, membrane rupture and induction of preterm labor. Other risk factors are specific for fetal gene therapy: germline transmission, developmental aberrations and the possibility of insertional mutagenesis. Fetal somatic gene therapy does not attempt to modify the genetic content of the germline. However, the possible induction of genetic changes to the germline is a concern that is central to the field of in utero gene therapy. It is believed that transduction of germline cells in vivo is unlikely to occur, due to the compartmentalization of these primordial germ cells in the gonads, which is completed in humans by the 7th week of gestation. In utero intraperitoneal gene transfer in male sheep (Park et al., 2009; Porada et al., 2005) resulted in transduction of Sertoli cells but not germ cells. Similarly, evidence for LV transduction of a subpopulation of gonadal cells has been observed in female rhesus fetuses after intraperitoneal vector administration (Lee et al., 2005). However, this was not associated with detectable transgene expression and vector has never been found in purified spermatozoa or in the offspring of these animals.
Another possible complication is the occurrence of oncogenesis due to insertional mutagenesis. The risk of insertional mutagenesis is of particular concern after in utero vector application as the fetal system may be specifically sensitive to such events since integrating vectors prefer to insert their genomes into euchromatin (Ciuffi et al., 2006). Recently, a high postnatal incidence of liver tumors was described in mice following prenatal injection of lentiviral vectors derived from the equine infectious anemia virus (EIAV), but not when using a vector derived from HIV (Themis et al., 2005). Somewhat more surprising are the findings of an increase in hepatocellular carcinoma after rAAV injection in newborn mice (Donsante et al., 2001). Later, integrated vector sequences were found by inverse PCR methods in the tumor cells but not in the surrounding tissue (Donsante et al., 2007). In contrast, many other animal studies where rAAV was injected early in life, did not result in oncogenic events (Russell, 2007). The molecular mechanisms underlying these oncogenic events need further elucidation and will ultimately lead to the design of safer vectors and protocols for both pre- and postnatal gene transfer.
Animal experimentation on prenatal gene therapy for the lung
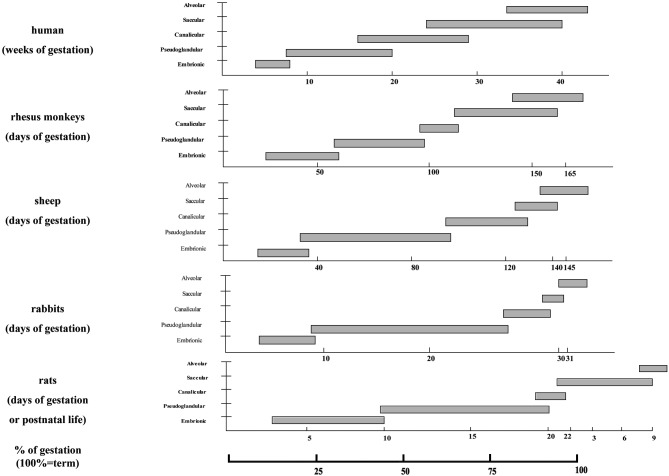
The majority of experiments dealing with fetal gene therapy have been performed in small animal models. The obvious advantages of these include limited ethical and financial constraints, wide availability and low housing demands, well-documented embryogenesis and fetal development, short gestation, large litter size, and in some species the availability of transgenic disease models. As always the mouse has been the irreplaceable model. Yet some aspects of the murine physiology and embryogenesis differ substantially from the human situation. One such example is lung development.
In figure 8 an overview is given of important differences between species regarding the time point of occurrence and duration of the various stages of lung development compared to the human.
Fig. 8. Comparison of lung developmental stages and their length between different species used in experimental surgery. Reference is human lung development (top) and at bottom the % of gestation. Courtesy of Xenia Roubliova.
Extensive research has been performed in small animals as a lot of genetic disease models exist, e.g. CF specific knock-out or mutant mouse models (Scholte et al., 2004). For the delivery of a therapeutic vehicle encoding a correct copy of an affected or protective gene, different access routes have been described to target the fetal lung in preclinical rodent models, including intratracheal injection (Carlon et al., 2010; Skarsgard et al., 2005), intra-amniotic injection (Boyle et al., 2001; Buckley et al., 2008; Davies et al., 2008; Mitchell et al., 2000), (ultrasound-guided) intrapulmonary injection (Henriques-Coelho et al., 2007; Toelen et al., 2007) and intravenous administration into the yolk sac vessels (Waddington et al., 2003; Waddington et al., 2004) or umbilical vein (Senoo et al., 2000). Ideally an animal model should mimic the disease studied. In the context of CF the mouse model is not the most ideal model for investigating the potential of fetal pulmonary gene therapy as most CF mice mainly develop intestinal symptoms, with only few pulmonary problems. Furthermore airway morphology is significantly different (Grubb et al., 1999). Therefore efforts are being done to induce CF in higher species, such as ferret (Li et al., 2003), sheep (Harris, 1997) and pig (Welsh et al., 2009). Large animal models have the advantage of showing more homology with humans in terms of lung development, lung morphology, duration of pregnancy and fetus size. Furthermore, the in utero surgical procedures performed in sheep (David et al., 2003; Peebles et al., 2004; Porada et al., 2004) and primates (Garrett et al., 2003; Tarantal et al., 2001) are less invasive and therefore more directly applicable to the human fetus.
Conclusion
Progress in both genetic prenatal screening and fetal imaging will further increase the demand for fetal interventions in the near future. The fetal respiratory system is only one target for candidate prenatal interventions. CCAM is a condition which can be easily diagnosed in utero. When fetal hydrops presents prior to viability, fetal therapy seems mandatory. Open fetal surgery has successfully salvaged fetuses with microcystic lesions, while larger cystic lesions have been shunted. Obstructed upper airways can be explored or freed at the time of birth, using the placenta as a heart-lung machine during the so called EXIT procedure. Prenatal imaging can also diagnose congenital diaphragmatic hernia, may rule out frequently associated anomalies and determine prognosis. Fetuses with liver herniation into the thorax and a small lung (LHR < 1.0) have a poor prognosis when managed with standard postnatal therapy. FETO may improve survival from < 10% to above 50%. Outcome can be predicted from preoperative lung size. The procedure carries a significant risk for PPROM and preterm delivery. A randomized trial will have to demonstrate whether prenatal therapy is truly beneficial. The above technique has demonstrated the feasibility of accessing the fetal airways, which can even be punctured to deliver pharmacological agents. It seems therefore logic to consider the potential of fetal pulmonary gene therapy. Presently fetal somatic gene therapy is still a purely experimental approach using animal models as substitute. Clinical trials have shown the necessity of extensive preclinical testing before moving towards human test subjects. Once proven to be safe and reliable it might become an alternative treatment option in selected diseases.
Acknowledgments
Work supported by the (Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen) (07/0715) and the European Commission (Eurostec - 6th framework programme, CT-2006-37409). JT and MC are doctoral fellows supported by grants from the IWT Vlaanderen. The Fonds voor Wetenschappelijk Onderzoek Vlaanderen grants JD (1.8.012.07.N.02) and KD a Senior Clinical Investigator position.
References
- Adkins B, Williamson T, Guevara P, et al. Murine neonatal lymphocytes show rapid early cell cycle entry and cell division. J Immunol. 2003;170:4548–4556. doi: 10.4049/jimmunol.170.9.4548. [DOI] [PubMed] [Google Scholar]
- Adzick NS. Management of fetal lung lesions. Clin Perinatol. 2003;30:481–492. doi: 10.1016/s0095-5108(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Aite L, Zaccara A, Trucchi A. When uncertainty generates more anxiety than severity: the prenatal experience with cystic adenomatoid malformation of the lung. J Perinat Med. 2009;37:539–542. doi: 10.1515/JPM.2009.098. [DOI] [PubMed] [Google Scholar]
- Akinbi HT, Narendran V, Pass AK, et al. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191:2090–2096. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bermudez C, Wulff J, Arcadipane M, et al. Percutaneous fetal sclerotherapy for congenital cystic adenomatoid malformation of the lung. Fetal Diagn Ther. 2008;24:237–240. doi: 10.1159/000151345. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Enke RA, Adams RJ, et al. In utero AAV-mediated gene transfer to rabbit pulmonary epithelium. Mol Ther. 2001;4:115–121. doi: 10.1006/mthe.2001.0428. [DOI] [PubMed] [Google Scholar]
- Buckley SM, Waddington SN, Jezzard S, et al. Intra-amniotic delivery of CFTR-expressing adenovirus does not reverse cystic fibrosis phenotype in inbred CFTR-knockout mice. Mol Ther. 2008;16:819–824. doi: 10.1038/mt.2008.26. [DOI] [PubMed] [Google Scholar]
- Cannie MM, Jani JC, De Keyzer F, et al. Evidence and patterns in lung response after fetal tracheal occlusion: clinical controlled study. Radiology. 2009;252:526–533. doi: 10.1148/radiol.2522081955. [DOI] [PubMed] [Google Scholar]
- Carlon M, Toelen J, Van Der Perren A, et al. Efficient gene transfer into the mouse lung by fetal intratracheal injection of rAAV2/6.2. Mol Ther. 2010;18:2130–2138. doi: 10.1038/mt.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Calvo M, Lagresle C, Abina S, et al. Gene therapy for severe combined immunodeficiency. Annu Rev Med. 2005;56:585–602. doi: 10.1146/annurev.med.56.090203.104142. [DOI] [PubMed] [Google Scholar]
- Cavoretto P, Molina F, Poggi S, et al. Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol. 2008;32:769–783. doi: 10.1002/uog.6218. [DOI] [PubMed] [Google Scholar]
- Ciuffi A and Bushman FD. Retroviral DNA integration: HIV and the role of LEDGF/p75. Trends Genet. 2006;22:388–395. doi: 10.1016/j.tig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Colvin J, Bower C, Dickinson JE, et al. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:356–363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg. 2002;37:331–338. doi: 10.1053/jpsu.2002.30832. [DOI] [PubMed] [Google Scholar]
- David A, Cook T, Waddington S, et al. Ultrasound-guided percutaneous delivery of adenoviral vectors encoding the beta-galactosidase and human factor IX genes to early gestation fetal sheep in utero. Hum Gene Ther. 2003;14:353–364. doi: 10.1089/104303403321208952. [DOI] [PubMed] [Google Scholar]
- Davies LA, Varathalingam A, Painter H, et al. Adenovirus-mediated in utero expression of CFTR does not improve survival of CFTR knockout mice. Mol Ther. 2008;16:812–818. doi: 10.1038/mt.2008.25. [DOI] [PubMed] [Google Scholar]
- Deprest J, Gratacos E and Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol. 2004;24:121–126. doi: 10.1002/uog.1711. [DOI] [PubMed] [Google Scholar]
- Deprest J, Jani J, Cannie M, et al. Prenatal intervention for isolated congenital diaphragmatic hernia. Curr Opin Obstet Gynecol. 2006;18:203–215. doi: 10.1097/01.gco.0000193000.12416.80. [DOI] [PubMed] [Google Scholar]
- Deprest J, Jani J, Gratacos E, et al. Deliberately delayed and shortened fetoscopic tracheal occlusion - A different strategy after prenatal diagnosis of life-threatening congenital diaphragmatic hernias (Letter, reply) J Pediatr Surg. 2006;41:1345–1346. [Google Scholar]
- Deprest J, Jani J, Gratacos E, et al. Percutaneous Fetal Endoscopic Tracheal Occlusion (FETO) for severe left sided congenital diaphragmatic hernia. The European Experience. Sem Perinatol. 2005;29:94–103. doi: 10.1097/01.grf.0000184774.02793.0c. [DOI] [PubMed] [Google Scholar]
- Deprest J, Jani J, Lewi L, et al. Fetoscopic surgery: encouraged by clinical experience and boosted by instrument innovation. Semin Fetal Neonatal Med. 2006;11:398–412. doi: 10.1016/j.siny.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Deprest JA, Flemmer AW, Gratacos E, et al. Antenatal prediction of lung volume and in-utero treatment by fetal endoscopic tracheal occlusion in severe isolated congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2009;14:8–13. doi: 10.1016/j.siny.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Deprest JA, Gratacos E, Nicolaides K, et al. Changing perspectives on the perinatal management of isolated congenital diaphragmatic hernia in Europe. Clin Perinatol. 2009;36:329–347. doi: 10.1016/j.clp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Deprest JA, Hyett JA, Flake AW, et al. Current controversies in prenatal diagnosis 4: Should fetal surgery be done in all cases of severe diaphragmatic hernia? Prenat Diagn. 2009;29:15–19. doi: 10.1002/pd.2108. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Donsante A, Vogler C, Muzyczka N, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 2001;8:1343–1346. doi: 10.1038/sj.gt.3301541. [DOI] [PubMed] [Google Scholar]
- Ennist DL. Gene therapy for lung disease. Trends Pharmacol Sci. 1999;20:260–266. doi: 10.1016/s0165-6147(99)01350-4. [DOI] [PubMed] [Google Scholar]
- Flageole H, Evrard VA, Piedboeuf B, et al. The plug-unplug sequence: an important step to achieve type II pneumocyte maturation in the fetal lamb model. J Pediatr Surg. 1998;33:299–303. doi: 10.1016/s0022-3468(98)90451-1. [DOI] [PubMed] [Google Scholar]
- Fleurence E, Riviere C, Masmonteil T, et al. Comparative efficacy of intratracheal adeno-associated virus administration to newborn rats. Hum Gene Ther. 2005;16:1298–1306. doi: 10.1089/hum.2005.16.1298. [DOI] [PubMed] [Google Scholar]
- Gallot D, Coste K, Francannet C, et al. Antenatal detection and impact on outcome of congenital diaphragmatic hernia: a 12-year experience in Auvergne, France. Eur J Obstet Gynecol Reprod Biol. 2006;125:202–205. doi: 10.1016/j.ejogrb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Garrett DJ, Larson JE, Dunn D, et al. In utero recombinant adeno-associated virus gene transfer in mice, rats, and primates. BMC Biotechnol. 2003;3:16. doi: 10.1186/1472-6750-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenbach U, Alton EW. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Pickles RJ, Ye H, et al. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- Harris A. Towards an ovine model of cystic fibrosis. Hum Mol Genet. 1997;6:2191–2194. doi: 10.1093/hmg/6.13.2191. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Keller RL, Hawgood SB SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- Coelho T, Gonzaga S, Endo M, et al. Targeted gene transfer to fetal rat lung interstitium by ultrasound-guided intrapulmonary injection. Mol Ther. 2007;15:340–347. doi: 10.1038/sj.mt.6300057. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Southern KW, Gileadi U, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000;7:1156–1165. doi: 10.1038/sj.gt.3301212. [DOI] [PubMed] [Google Scholar]
- Jani J, Gratacos E, Greenough A, et al. Percutaneous fetal endoscopic tracheal occlusion (FETO) for severe left-sided congenital diaphragmatic hernia. Clin Obstet Gynecol. 2005;48:910–922. doi: 10.1097/01.grf.0000184774.02793.0c. [DOI] [PubMed] [Google Scholar]
- Jani J, Keller RL, Benachi A, et al. Prenatal prediction of survival in isolated left-sided diaphragmatic hernia. Ultrasound Obstet Gynecol. 2006;27:18-–22. doi: 10.1002/uog.2688. [DOI] [PubMed] [Google Scholar]
- Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. 2007;30:67–71. doi: 10.1002/uog.4052. [DOI] [PubMed] [Google Scholar]
- Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:304–310. doi: 10.1002/uog.6450. [DOI] [PubMed] [Google Scholar]
- Jani JC, Nicolaides KH, Gratacos E, et al. Fetal lung-to-head ratio in the prediction of survival in severe left-sided diaphragmatic hernia treated by fetal endoscopic tracheal occlusion (FETO) Am J Obstet Gynecol. 2006;195:1646–1650. doi: 10.1016/j.ajog.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Joseph PM, sullivan BP, Lapey A, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther. 2001;12:1369–1382. doi: 10.1089/104303401750298535. [DOI] [PubMed] [Google Scholar]
- Kammerer U, Kruse A, Barrientos G, et al. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest. 2008;37:499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- King AE, Kelly RW, Sallenave JM, et al. Innate immune defences in the human uterus during pregnancy. Placenta. 2007;28:1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Knox EM, Kilby MD, Martin WL, et al. In-utero pulmonary drainage in the management of primary hydrothorax and congenital cystic lung lesion: a systematic review. Ultrasound Obstet Gynecol. 2006;28:726–734. doi: 10.1002/uog.3812. [DOI] [PubMed] [Google Scholar]
- Kobinger GP, Weiner DJ, Yu QC, et al. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Lee CC, Jimenez DF, Kohn DB, et al. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2005;16:417–425. doi: 10.1089/hum.2005.16.417. [DOI] [PubMed] [Google Scholar]
- Li Z, Engelhardt JF, et al. Progress toward generating a ferret model of cystic fibrosis by somatic cell nuclear transfer. Reprod Biol Endocrinol. 2003;1:83. doi: 10.1186/1477-7827-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty KW. Ex-utero intrapartum therapy. Semin Fetal Neonatal Med. 2010;15:34–39. doi: 10.1016/j.siny.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Vandenberghe LH, Zhang L, et al. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci U S A. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31:148–151. doi: 10.1016/s0022-3468(96)90338-3. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Jerebtsova M, Batshaw Ml-L, et al. Long-term gene transfer to mouse fetuses with recombinant adenovirus and adeno-associated virus (AAV) vectors. Gene Ther. 2000;7:1986–1992. doi: 10.1038/sj.gt.3301332. [DOI] [PubMed] [Google Scholar]
- Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- Muthukkumar S, Goldstein J, Stein KE. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–4813. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- Mychaliska GB, Bealer JF, Graf JL, et al. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg. 1997;32:227–230. doi: 10.1016/s0022-3468(97)90184-6. [DOI] [PubMed] [Google Scholar]
- Nelson S, Cameron A, Deprest J. Fetoscopic surgery for in utero management of Congenital Diaphragmatic Hernia. Fet Mat Med Rev. 2006;17:69–104. [Google Scholar]
- Oepkes D, Devlieger R, Lopriore E, et al. Successful ultrasound-guided laser treatment of fetal hydrops caused by pulmonary sequestration. Ultrasound Obstet Gynecol. 2007;29:457–459. doi: 10.1002/uog.3984. [DOI] [PubMed] [Google Scholar]
- Oepkes D, Teunissen AK, Van De Velde M, et al. Congenital high airway obstruction syndrome successfully managed with ex-utero intrapartum treatment. Ultrasound Obstet Gynecol. 2003;22:437–439. doi: 10.1002/uog.899. [DOI] [PubMed] [Google Scholar]
- Park PJ, Colletti E, Ozturk F, et al. Factors determining the risk of inadvertent retroviral transduction of male germ cells after in utero gene transfer in sheep. Hum Gene Ther. 2009;20:201–215. doi: 10.1089/hum.2007.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles D, Gregory LG, David A, et al. Widespread and efficient marker gene expression in the airway epithelia of fetal sheep after minimally invasive tracheal application of recombinant adenovirus in utero. Gene Ther. 2004;11:70–78. doi: 10.1038/sj.gt.3302130. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Wilson RD, Liechty KW KW, et al. Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagn Ther. 2007;22:365–371. doi: 10.1159/000103298. [DOI] [PubMed] [Google Scholar]
- Picone O, Benachi A, Mandelbrot L, et al. Thoracoamniotic shunting for fetal pleural effusions with hydrops. Am J Obstet Gynecol. 2004;191:2047–2050. doi: 10.1016/j.ajog.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Porada CD, Park P, Porada G, et al. Fetal Diagn Ther. Vol. 19. 30; 2004. The sheep model of in utero gene therapy; p. 23. [DOI] [PubMed] [Google Scholar]
- Porada CD, Park PJ, Tellez J, et al. Male germ-line cells are at risk following direct-injection retroviral-mediated gene transfer in utero. Mol Ther. 2005;12:754–762. doi: 10.1016/j.ymthe.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Rosenecker J, Harms KH, Bertele RM, et al. Adenovirus infection in cystic fibrosis patients: implications for the use of adenoviral vectors for gene transfer. Infection. 1996;24:5–8. doi: 10.1007/BF01780642. [DOI] [PubMed] [Google Scholar]
- Russell DW. AAV vectors, insertional mutagenesis, and cancer. Mol Ther. 2007;15:1740–1743. doi: 10.1038/sj.mt.6300299. [DOI] [PubMed] [Google Scholar]
- Scholte BJ, Davidson DJ, Wilke M, et al. Animal models of cystic fibrosis. J Cyst Fibros. 2004;3(Suppl 2):183–190. doi: 10.1016/j.jcf.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Senoo M, Matsubara Y, Fujii K, et al. Adenovirus-mediated in utero gene transfer in mice and guinea pigs: tissue distribution of recombinant adenovirus determined by quantitative TaqMan-polymerase chain reaction assay. Mol Genet Metab. 2000;69:269–276. doi: 10.1006/mgme.2000.2984. [DOI] [PubMed] [Google Scholar]
- Skarsgard ED, Huang L, Reebye SC SC, et al. Lentiviral vector-mediated, in vivo gene transfer to the tracheobronchial tree in fetal rabbits. J Pediatr Surg. 2005;40:1817–1821. doi: 10.1016/j.jpedsurg.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Stege G, Fenton A, Jaffray B, et al. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Imanishi K, Nishida H, et al. Evidence for immunologic immaturity of cord blood T cells. Cord blood T cells are susceptible to tolerance induction to in vitro stimulation with a superantigen. J Immunol. 1995;155:5213–5219. [PubMed] [Google Scholar]
- Tarantal AF, Lee CI, Ekert JE, et al. Lentiviral vector gene transfer into fetal rhesus monkeys (Macaca mulatta): lung-targeting approaches. Mol Ther. 2001;4:614–621. doi: 10.1006/mthe.2001.0497. [DOI] [PubMed] [Google Scholar]
- Themis M, Waddington SN, Schmidt M, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- Toelen J, Deroose CM, Gijsbers R, et al. Fetal gene transfer with lentiviral vectors: long-term in vivo follow-up evaluation in a rat model. Am J Obstet Gynecol. 2007;196:352. doi: 10.1016/j.ajog.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Tsao K, Hawgood S, Vu L, et al. Resolution of hydrops fetalis in congenital cystic adenomatoid malformation after prenatal steroid therapy. J Pediatr Surg. 2003;38:508–510. doi: 10.1053/jpsu.2003.50089. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Buckley SM, Nivsarkar M, et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101:1359–1366. doi: 10.1182/blood-2002-03-0779. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Mitrophanous KA, Ellard FM, et al. Long-term transgene expression by administration of a lentivirus-based vector to the fetal circulation of immuno-competent mice. Gene Ther. 2003;10:1234–1240. doi: 10.1038/sj.gt.3301991. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Nivsarkar MS, Mistry AR, et al. Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy. Blood. 2004;104:2714–2721. doi: 10.1182/blood-2004-02-0627. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Rogers CS, Stoltz DA, et al. Development of a porcine model of cystic fibrosis. Trans Am Clin Climatol Assoc. 2009;120:149–162. [PMC free article] [PubMed] [Google Scholar]
- Wilson RD, Baxter JK, Johnson MP, et al. Thoracoamniotic shunts: fetal treatment of pleural effusions and congenital cystic adenomatoid malformations. Fetal Diagn Ther. 2004;19:413–420. doi: 10.1159/000078994. [DOI] [PubMed] [Google Scholar]
- Wilson RD, Chitty LS. Anomalies of the fetal thorax and abdomen: diagnosis, management and outcome. Prenat Diagn. 2008;28:567. doi: 10.1002/pd.2042. [DOI] [PubMed] [Google Scholar]
- Wilson RD, Hedrick HL, Liechty KW, et al. Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis, and in utero treatment. Am J Med Genet A. 2006;140:151–155. doi: 10.1002/ajmg.a.31031. [DOI] [PubMed] [Google Scholar]
- Yoshio H, Tollin M, Gudmundsson GH, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53:211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu L, Haskins ME, et al. Neonatal gene transfer with a retroviral vector results in tolerance to human factor IX in mice and dogs. Blood. 2004;103:143–151. doi: 10.1182/blood-2003-06-2181. [DOI] [PubMed] [Google Scholar]