Highlights
-
•
Simultaneous quantification of eight polyamines in biological samples.
-
•
High-throughput analytical SPE–LC/MS method.
-
•
Sample pre-treatment minimized to a single derivatization step.
-
•
The method can be used for urine, blood, and solid tissue.
Keywords: Biogenic amines, Online solid phase extraction, Tandem mass spectrometry, Polyamine, Spermidine, Spermine
Abstract
Polyamines are ubiquitous active biogenic amines which contribute to basic cellular functions. Hence, their quantification in samples of diverse biological origins is essential for understanding how they function, especially in disease-relevant conditions. We present here a robust, high-throughput solid-phase extraction online coupled to a liquid chromatography–tandem mass spectrometry (SPE–LC/MS/MS) approach for the simultaneous quantification of eight polyamines in various biological samples. The polyamines include 1,3-diaminopropane, putrescine, cadaverin, N-acetyl-putrescine, spermidine, spermine, N1-acetyl-spermine, and l-ornithine. The novelty of the work is the use of two SPE columns online coupled to LC/MS/MS, which minimizes the sample pretreatment to a single derivatization step. The analysis is complete within 4 min, making the method highly suitable for routine clinical analysis and high throughput screenings. The method was fully validated with serum samples. Dynamic ranges were 0.03 to 15 μg/ml for ornithine and 1 to 500 ng/ml for other polyamines, which cover physiological concentrations in serum samples. Lower limits of quantification (LLoQ) were found to be between 0.1 and 5 ng/ml. As a proof of concept, we investigated gender differences in polyamine levels by analyzing the serum levels of 102 subjects.
1. Introduction
Polyamines are ubiquitous polycationic compounds associated with a variety of biological processes [1,2]. These active biogenic amines contribute to basic cellular functions, including cell growth, proliferation and differentiation, as well as regulating cellular stress-response and survival mechanisms. Spermidine and spermine are two polyamines reported to have anti-inflammatory as well as anti-oxidant activities [3], and have thus been implicated in cellular and organismal stress defense during aging and disease [4,5]. For instance, spermine was shown to provide protection from lethal sepsis in mice when applied intraperitoneally [6], and from paraquat-induced death in the fruit fly Drosophila melanogaster when added to ordinary food [7]. Indeed, spermidine supplementation extends life span and reduces markers of cell stress in various model organisms, including yeast, flies, worms and human immune cells [8], while polyamine-enriched food has been shown to reduce mortality and prolong the health span of aging mice [8,9]. The longevity-promoting effects of spermidine in lower model organisms requires the induction of autophagy, a degradation pathway that contributes to cellular self-renewal and cell protection [8,10,11]. In fact, we recently revealed spermidine to be a novel, conserved, and potent inducer of autophagy in model organisms, in human cell cultures, and in vivo in various tissues of mice [8,12]. On the other hand, polyamines can also be toxic to cells at high levels, and can facilitate cell death, mainly by oxidative mechanisms [13,14]. In this regard, polyamines are important clinical biochemical markers for malignancy [15]. This was first indicated in the urine of cancer patients, where polyamines were present at higher levels than in healthy humans [15].
To understand the relevance of changes in polyamine profiles detected in a given tissue, cell population or organism (obtained from, for instance, patient samples or basic research models), the biosynthetic activities of the polyamines, as well as their catabolic pathways, need to be determined. Hence, metabolic profiling of polyamines and their close derivatives is prerequisite for understanding the potentially complex changes in polyamine content that can be induced by disease-relevant conditions. For such metabolic profiling, an analytical method to accurately identify and quantify a broader group of polyamines and their derivatives in a single analysis would be most useful. Such an advance would also be significant for the potential clinical use of polyamines as biomarkers or pharmacological interventions.
The widespread interest in polyamines has led to the development of several analytical methods for polyamine determination [2,16]. In general, analysis of polyamines obtained from various biological samples is performed by chromatographic separation prior to detection with selective detectors or mass spectrometers [17–23]. Hyphenated techniques, such as LC/MS, GC/MS or capillary electrophoreses coupled to MS are among the most powerful methods for the analysis of metabolites [19–24]. Nonetheless, many published analytical methods suffer from at least one of the following drawbacks: they have long chromatographic run times; they are optimized for determination of polyamines only in one specific matrix; they have higher limits of detection; or they require time-consuming sample pretreatment steps [1,2,16,21,22,24–32].
Hence, there is a need for a more versatile technique that can be used across a range of matrices, so that levels in different tissues or fluids from a single person can be directly compared. Such comparisons between different tissue samples are important, because polyamines are not evenly distributed throughout the human body. For example, a number of polyamines have been shown to be raised in the serum of breast cancer patients, even when the urine shows normal levels [21].
The present study was undertaken to develop a robust and high-throughput analytical method for the simultaneous determination of various polyamines, at levels ranging from trace amounts to high concentrations. In particular, we aimed to design a universal method for quantification of polyamines in a broad range of biological matrices. We report here a novel analytical method applying two SPE columns on-line coupled to a highly sensitive and selective LC/MS/MS that allows the separation and unambiguous determination of eight polyamines in a broad variety of biological samples. Using two SPE columns in parallel means that sample preparation can be performed simultaneously with chromatographic separation and analyte detection, reducing the time for a whole single analysis to just 4 min. Since SPE sample preparation was performed online, sample pre-treatment was significantly accelerated by minimizing it to a single derivatization step before measurement. No further extraction or manual clean-up was necessary. The method is fully validated for human serum samples and could easily be extended to a higher number of polyamines if required.
2. Materials and methods
2.1. Chemicals
Biogenic amines, i.e. 1,3-diaminopropane (1,3-dap, ≥99%), putrescine (put, ≥99%), cadaverine (cad, ≥99%), N-acetyl-putrescine (N-actput, ≥98%), spermidine (spd, ≥98%), spermine (sp, ≥99%), N1-acetyl-spermine (N1-actsp, ≥97%), and l-ornithine (orn, ≥99%), along with four isotopically labeled internal standards d8-putrescine (d8-put, ≥99%), 13C5-ornithine (13C5-orn, 95%), d8-spermin (d8-sp, 95%), and 13C4-spermidine (13C4-spd, ≥95%), as well as acetonitrile and methanol (both HPLC gradient grade), trichloroacetic acid (TCA, ≥99%), sodium carbonate (≥99%), hydrochloric acid (fuming, 37%), acetic acid (99.8–100.5%), isobutyl chloroformate (≥99%), and human serum from clotted human male whole blood, sterile-filtered (mycoplasma tested, virus tested), were purchased from Sigma-Aldrich (Vienna, Austria). Phosphate buffered saline (PBS buffer, pH 7.2–7.3) was purchased from Apotheke LKH–Univ. Klinikum Graz, Austria. Ultra-pure water purified by a Milli-Q Gradient system (resistivity >18 MΩ cm; Millipore, Bedford, USA) was used for all experiments.
2.2. Preparation of standard solutions
Stock solutions of sp, N-actput, N1-act, sp, orn, 1,3-dap, spd, put, and internal standard d8-sp were prepared by dissolving the appropriate amount in water/methanol (7:3, v/v) to yield a concentration of 1 mg/ml for sp, N-actput, N1-actsp, orn, 1,3-dap, 5 mg/ml for spd, and 0.5 mg/ml for put and d8-sp. Stock solutions of internal standards (ISTD) d8-put, 13C5-orn, and 13C4-spd were prepared in water/methanol (1:1, v/v) to achieve a concentration of 1 mg/ml. A stock solution of cad was prepared by dissolving the appropriate amount in water/acetonitrile (7:3, v/v) to give a concentration of 1 mg/ml. All stock solutions were stored at −80 °C.
The concentration of the calibration solution was generally varied from 1 to 500 ng/ml in order to cover as broad a physiological range as possible. Five non-zero calibration standards and one zero sample were used for calibration in each batch. In serum samples, however, the concentration of orn is typically higher than 500 ng/ml, so calibration solutions for orn were 0.03 to 15 μg/ml. The calibration solutions for serum samples were prepared by diluting all stock solutions, except orn, with water/methanol (7:3, v/v) to achieve an intermediate working concentration of 10 μg/ml. The stock solutions of orn and the working standard solutions were further diluted with water (double-distilled) to the desired concentration for the calibration solutions and three quality control (QC) samples. Five non-zero calibration solutions and one zero calibration solution were used for calibration in each batch. The QC samples were prepared from a different set of working stock than the calibration solution. QC concentration levels for orn were 0.5, 5, and 10 μg/ml and for the other polyamines 5, 50, and 100 ng/ml. ISTD stock solution was also diluted with water (double-distilled)/methanol (7:3, v/v) to achieve a working solution with a concentration of 6 μg/ml, and was further diluted with water (double-distilled) to prepare an ISTD mix solution with a concentration of 200 ng/ml. The isotopically labeled d8-put was used as internal standard for N-actput, 1,3-dap, cad and put to calculate peak/area ratios. For sp and N1-actsp, d8-sp was used as internal standard. For Spd and orn, the internal standards 13C4-spd and 13C5-orn, respectively, were used.
2.3. Extraction and derivatization protocols
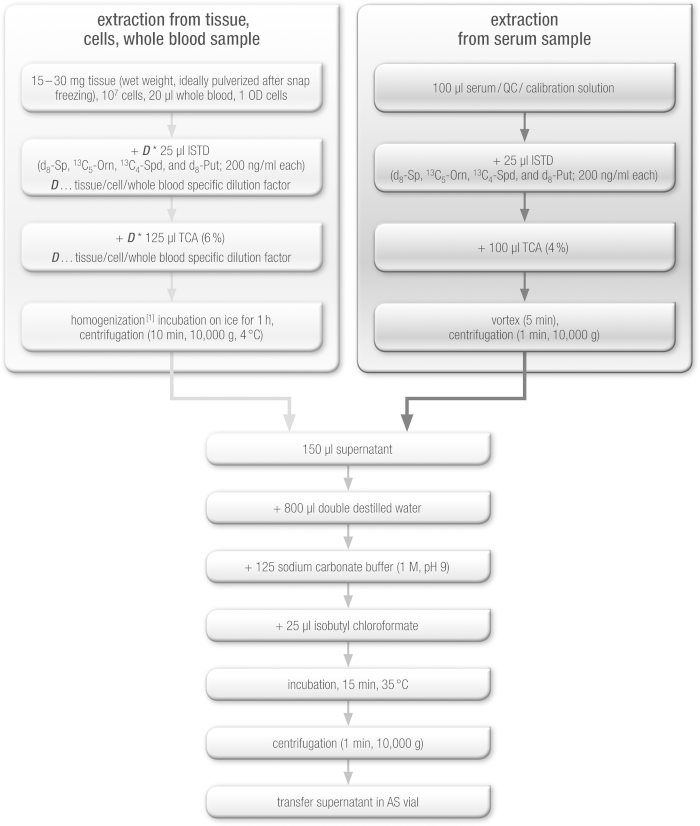
Polyamine extraction from biological sources is commonly performed by acid extraction with cold TCA or perchloric acid (PCA) with subsequent neutralization for analysis of cultured cells [33] and animal and plant tissue [34]. In general, homogenization is required for tissue, and a variety of standard techniques can be used (for a set of references see [34]). For tissues that are difficult to homogenize (e.g. plant tissues or chitin-rich insects), a protocol involving repeated freeze–thaw cycles may be beneficial, especially when larger sample numbers need to be processed [34]. Based on these methods [33,34], the following protocols were developed for the polyamine analyses described in this paper. Extraction protocols for serum, tissue, cell and whole-blood samples are summarized in Fig. 1. For tissue, cell or whole-blood samples, an individual dilution step was required before sample preparation, which enabled polyamine concentrations to fall within the linear calibration range. Table 1 shows the expected concentrations of polyamines in various tissues, organism or cells. Based on this concentration range, samples were individually diluted by using the dilution factor D specific for tissue, cell or whole blood. This dilution factor D was calculated for the analytes put, spd and sp. Sample dilution was always performed by increasing the volume of TCA and IS. D is calculated for 30 mg tissue or 107 cells or 20 μl whole-blood samples. For orn, no dilution was required, since the concentration of orn in different samples was always within the calibration range of orn.
Fig. 1.
Extraction procedure for serum, tissue, cell and whole blood samples.
D indicates specific dilution factor for put, spd and sp in tissue, cell or whole blood samples and is listed in Table 3. D is calculated for 30 mg tissue or 107 cells or 20 μl whole blood samples. 1Homogenization can be omitted if cultured cells in suspension or whole blood is used, but thoroughly vortexing to evenly mix samples is recommended.
Table 1.
Expected values of polyamines extracted from various tissues, organisms or cells. The table presents mean values from 6 to 9 biological replicates (cultured growing cells in synthetic complete medium, worms, flies) or 6–10 young adult (3–5 month old) individuals (mice) ±1.96 × standard deviation (SD), giving an estimate of the expected range (95% confidence interval) of the polyamine content of indicated samples. For details of strains and conditions, see Section 2. n.a. data ‘not available’ (below LLoQ). Weight (g) refers to wet weight of tissues or whole organisms determined before polyamine extraction. In some cases, samples had to be diluted before sample preparation to enables polyamine concentrations to fall within the linear calibration range (1–500 ng/ml). Therefore D indicates the specific dilution factor for put, spd and sp in tissue, cell or whole blood samples. D was calculated for 30 mg tissue, 107 cells, or 20 μl whole-blood samples. Samples were diluted with TCA and IS. For orn, no sample dilution was required since a different calibration range was used (0.03–15 μg/ml).
| Source (strain) | Expected range of polyamines |
Tissue/cell/blood specific dilution factor D for put, spd, sp | ||||
|---|---|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | l-Ornithine | Units2 | ||
| Yeast (S. cerevisiae, BY4741) | 2.2 (±0.5) | 16 (±3) | 8 (±7) | 15 (±3) | (μg/109 cells) | 10 |
| Bacteria (E. coli, XL-1) | 4.2 (±1.1) | 3.6 (±0.9) | 0.9 (±0.1) | 0.08 (±0.04) | (μg/OD600) | 250 |
| Worm (C. elegans, N2) | 51 (±39) | 126 (±86) | n.a. | n.a. | (μg/g sample) | 240 |
| Fruit fly heads (D. melanogaster, w1118) | 2.2 (±2.7) | 125 (±51) | 16 (±8) | 6.6 (±2) | (μg/g sample) | 240 |
| Mouse liver tissue (M. musculus, C57BL/6N) | 1.1 (±0.8) | 134 (±81) | 176 (±103) | 34 (±34) | (μg/g sample) | 330 |
| Mouse heart tissue (M. musculus, C57BL/6N) | 0.4 (±0.3) | 12 (±8) | 38 (±22) | 2.9 (±5) | (μg/g sample) | 100 |
| Mouse whole blood (M. musculus, C57BL/6N) | 0.1 (±0.1) | 4.7 (±2.1) | 0.6 (±0.4) | n.a. | (μg/ml sample) | 10 |
The derivatization of polyamines was carried out according to the method of Byun et al. [21]. Amine carbamoylation derivatization of polyamines was performed by using isobutyl chloroformate. The derivatization protocol is presented in Fig. 1.
2.4. Chromatographic methods
All experiments were carried out on an Ultimate 3000 HPLC system comprising an autosampler and a 10-way switching valve unit coupled to a triple-quadrupole mass spectrometer, a Quantum TSQ Ultra AM, both from Thermo Fisher Scientific (San Jose, CA). The system was controlled by Xcalibur Software 2.1. The oven of the autosampler was maintained at room temperature, and the tray at 5 °C. For online SPE–LC/MS/MS, two Strata-X SPE cartridges (2 × 20 mm, 25 μm particle size) from Phenomenex (California, USA) were installed. Additionally, an in-line filter (KrudKatcher Classic HPLC In-Line Filter, 0.5 μm Depth Filter, Phenomenex (California, USA)) was placed in front of the 10-way switching valve unit to protect both SPE columns.
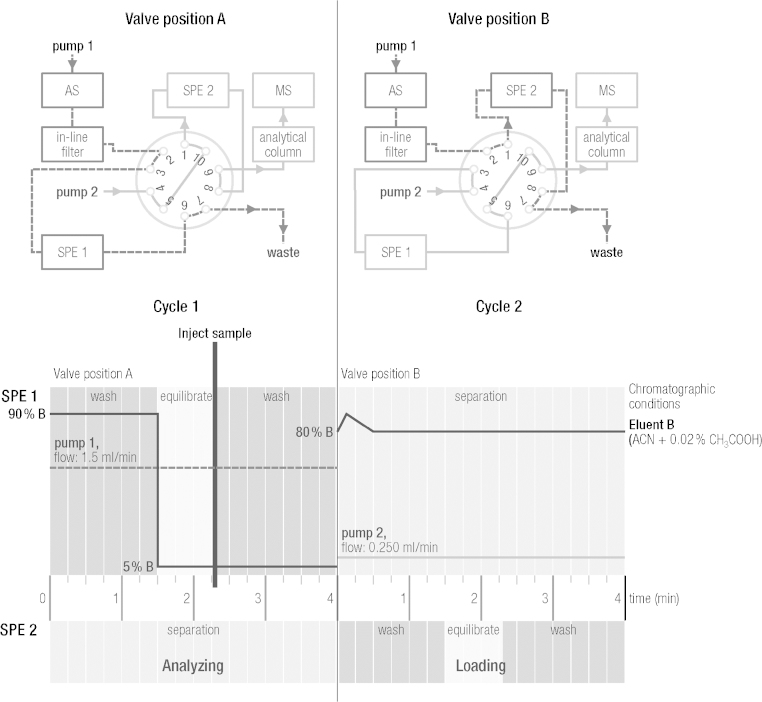
Layout details of the online SPE–LC/MS/MS instrumentation, such as individual flow rates, switching times and switching valve settings, are illustrated in Fig. 2. One ternary pump (pump 1) was connected to the autosampler and delivered solvents to one SPE column. The second ternary pump (pump 2) delivered solvents to the second SPE column and subsequently to the HPLC column. The HPLC column comprised a reversed-phase C18 column (Kinetex reversed phase C18; 50 × 2.1 mm i.d., 2.6 μm particle size) from Phenomenex (California, USA). The outlet of the HPLC column was connected to the ion source of the mass spectrometer. If the HPLC switching unit was set to valve position A, the sample was loaded onto SPE 1 cartridge by using pump 1. In the meantime, the trapped analytes onto SPE 2 column (from previous loading step) were eluted with the solvent system of pump 2 and transferred onto the analytical column, where the analytes were further separated. For both cycles (pump 1 and pump 2) the same solvent composition was used. Mobile phase A consisted of 0.2% acetic acid in water, while mobile phase B consisted of 0.2% acetic acid in acetonitrile. Detailed settings for both cycles 1 and 2, with the 10-way switching valve unit, are shown for SPE 1 in Fig. 2.
Fig. 2.
Connectivity sketch of the 10-port switching valve coupling the SPE and HPLC.
Cycle 1 (SPE 2): The sample was loaded onto the SPE 2 column. The flow rate of pump 1 was set to 1500 μl/min. The six-way switching valve unit from the autosampler was set to loading position for 2.3 min before sample injection. The binary gradient, starting with 90% B (ACN + 0.02% CH3COOH), was held for 1.4 min before decreasing to 5% B over 0.1 min and holding for 4 min. For sample injection, the 6-port valve was set to the analysis position after 2.3 min of the binary gradient. A sample volume of 250 μl was injected and loaded onto the SPE 2, since the 10-port valve was set to loading position for SPE 2.
Cycle 2 (SPE 1): Trapped analytes on the SPE 1 column (from the previous loading step, when 10-port valve was switched to loading position for cycle 2) were eluted from the column and further separated on the analytical column before detection. Chromatographic separation and determination of analytes was performed simultaneously with cycle 1 since the 10-port valve for cycle 2 was set to analysis position. The flow rate of pump 2 was set to 250 μl/min. The binary gradient was increased from 80% to 100% B over 0.1 min, before decreasing over 0.4 min back to 80% B, where it was held for 3.5 min for isocratic separation of polyamines.
2.5. Mass spectrometry
A quantum TSQ Quantum triple quadrupole mass spectrometer equipped with an electrospray ion source was used. The instrument was operated in multiple reaction-monitoring mode (MRM). The ion source was operated in positive electrospray ionization (ESI) mode. The MRM of the precursor-product ion transitions, collision energies and tube lens offset are shown in detail in Supplementary Table 1S. The optimized conditions were: ESI spray voltage 4.0 kV, sheath gas pressure 20 AU, auxiliary gas pressure 15 AU, capillary temperature 270 °C and skimmer offset −24 V. The scan was performed in profile mode with scan width (m/z) 0.1, scan time 0.05 s, and peak width of Q1 and Q3 0.7 (FWHM).
2.6. Validation procedure
The analytical method was fully validated by evaluating specificity, linearity range, intra- and inter-day precision and accuracy, lower limit of quantification (LLoQ), limit of detection (LOD), recovery, and matrix effect. Particular attention was paid to sample stability, in that short-term stability, long-term stability, and freeze–thaw cycles were evaluated. The method was applied to serum samples.
3. Results and discussion
We have developed a method to separate eight polyamines in a biological matrix in 4 min. Our online coupling of SPE with LC/MS/MS allowed us to minimize the sample preparation to a single derivatization step. High sensitivity was maintained by combining the derivatization of the polyamines with reversed-phase separation.
3.1. Derivatization of polyamines
Analysis of trace amounts of polyamines requires a derivatization step to enhance the sensitivity (see also discussion below, in Section 3.2).
Our sample preparation procedure, including the derivatization, builds on the method of Byun et al. [21], with significant modifications. Derivatization of polyamines was performed by carbamoylation with isobutyl chloroformate, which is completed within in 15 min [21]. Moreover, carbamoyl derivates gave sharp peaks upon measurement with LC/MS/MS. Compared to the method of Byun et al., our sample preparation, with its single derivatization step, resulted in a faster procedure, increased sensitivity, and better detectability. No additional extractions or manual clean-up steps were required. The initial liquid–liquid extraction (and protein precipitation) step of Byun et al. [21] was omitted because this led to a depletion of polyamines from the sample (data not shown). Polyamines are known to bind to various macromolecules, which may be the reason for the observed depletion [35–37]. Since TCA is the most effective reagent for the extraction of biogenic amines from various tissue samples, we also used it for serum samples, and observed much higher recoveries than with organic solvents (e.g. recovery of spermidine was 40% higher with TCA than with acetonitrile, data not shown). After carbamoylating the polyamines with isobutyl chloroformate, the sample was directly applied to the online SPE–LC/MS/MS. This avoided the need for the additional liquid–liquid extraction of Byun et al., which also required evaporation of the organic solvent and reconstitution of the sample with an appropriate solvent mixture. In our method, removal of the isobutyl chloroformate residue and other interferences before measurement was fully automated with the online SPE approach (described in detail in online SPE–LC/MS/MS). This is of particular importance, since isobutyl chloroformate in positive ESI mode causes ion suppression effects that result in lower specificity and sensitivity.
3.2. Chromatographic separation of polyamines
There are a number of reversed-phase LC/MS approaches for the determination of polyamines that do not use a derivatization step [19,20]; however those methods have their drawbacks.
Traditional reversed-phase separation of underivatized polyamines is challenging because of the low retention of the polyamines and their susceptibility to undergo severe tailing [19,38]. Ion-pair reagents as additives in the LC separation enable adequate retention time of polar analytes; however, these non-volatile additives are known to cause ion-source contamination and strong signal suppression in ESI–MS [20]. Thus, volatile perfluorinated carboxylic acids, such as heptafluorobutyric acid or perfluoroheptanoic acid are more widely used as ion-pairing reagents in LC/MS to improve peak shape and retention time [20,30]. Unfortunately, due to their combined effect of ion-pairing and surface-tension modification, these additives also induce signal suppression in ESI–MS [39,40]. The combination of heptafluorobutyric acid with isopropanol/propionic acid helps to increase polyamine signal in ESI, but signal suppression cannot be completely prevented. Furthermore, heptafluorobutyric acid has an intensely unpleasant and rather persistent odor.
Hydrophilic-interaction liquid chromatography (HILIC) is a valuable alternative to reversed-phase LC/MS for the analysis of highly polar compounds such as polyamines [41,42]. With HILIC columns, sufficient retention can be achieved to move the more polar analytes away from the solvent front [41,42]. In addition, the highly volatile organic mobile phases used in HILIC increase the sensitivity for ESI–MS [41]. We used HILIC for the determination of polyamines in mice liver and fly tissue with no additional derivatization [8]. The HILIC approach is particularly suitable for the determination of highly concentrated polyamines like intracellular polyamines in tissue probes, but is not sufficiently sensitive for measuring low concentrations of polyamines, such as in serum samples.
3.3. Online SPE–LC/MS/MS
For reproducible ionization efficiency, and to remove interferences such as derivatization reagents from analytes, sample pre-treatment and separation by HPLC are crucial. By using a setup consisting of two online SPE columns coupled to a C-18 analytical column, we were able to omit the liquid–liquid extraction (Fig. 2). Solvent evaporation pre-treatment steps could also be avoided because we injected a high sample volume (250 μl). After derivatization, the sample was directly applied to the online SPE–LC/MS/MS.
Two Strata X columns were used as online SPE columns operating in parallel. Due to the polymeric nature and large particle diameter (25 μm) of the packing material, these columns can tolerate sample matrices that would block other column types. Nonetheless, an in-line filter was installed at the start of the SPE columns to prevent any possible column blockage (Fig. 2) to enhance the lifetime of the SPE columns. Since sample preparation and LC/MS/MS measurement can be done in parallel (cycles 1 and 2) rather than sequentially, the measurement procedure can be completed in half the time.
Briefly, online SPE was performed as follows (Fig. 2). We describe here the cycle for SPE 1 column. The cycles for the SPE 2 column are the same as for SPE 1, but delayed:
3.3.1. Cycle 1 (SPE 1), loading
(i) Prior to sample injection, the SPE 1 column was flushed (pump 1) with mobile phase B (0.2% acetic acid in acetonitrile) for 1.5 min. Since the flow was set to 1500 μl/min, the washing period could be kept very short but was still sufficient to remove interferences from the SPE column prior to sample injection. Thus, the washing step increased the robustness of our system, enabling us to apply our method to different biological matrices. (ii) The SPE column was preconditioned in 0.8 min due to the high flow rate. No shift in the retention time of the analytes was observed. (iii) A high sample volume (250 μl) was injected after 2.3 min and transferred onto the SPE column. Despite the high flow rate, the polyamine samples were retained on the SPE column head, since the acetonitrile content (5%) was very low. (iv) The SPE column wash further flushed with 5% acetonitrile for 4 min to remove any hydrophilic matrix material, including isobutyl chloroformate, which could otherwise lead to ion suppression in positive electrospray ionization LC/MS/MS, if it co-elutes with the polyamines. (v) Elution of polyamines on the analytical column used for chromatographic separation and MS determination was performed by switching the 10-way valve unit to position B.
3.3.2. Cycle 2 (SPE 1), analyzing
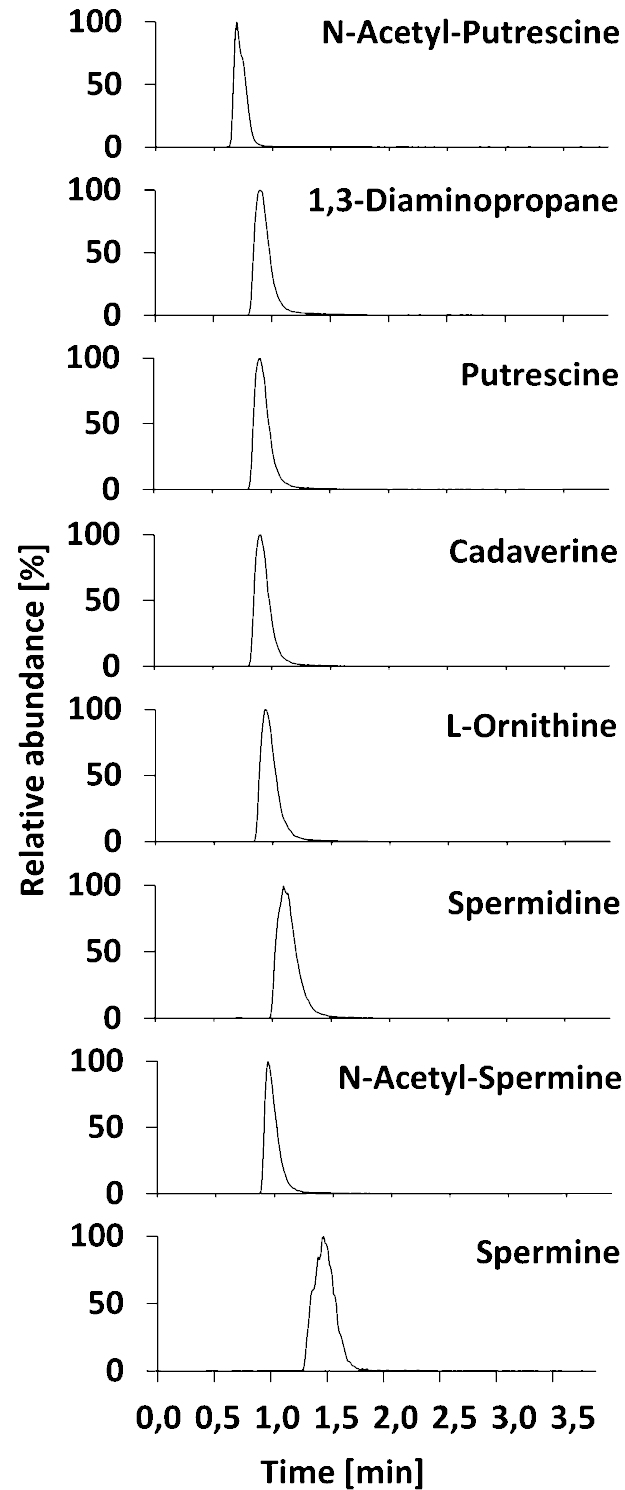
(i) SPE 1 column was flushed with increased acetonitrile for 30 s to transfer the polyamines onto the analytical column, where they were chromatographically separated. This high level of organic solvent at the beginning is required to transfer the polyamines from the SPE to the analytical column to avoid prior separation and peak broadening on the SPE column. (ii) The polyamines are then separated by using 80% acetonitrile. The current method efficiently separates eight polyamines as shown in Fig. 3, although baseline separation could not be achieved for all peaks due to the short run time (4 min). Generally, extensive chromatographic separation of analytes is not necessary for the selected reaction monitoring (SRM) analysis, since there is usually only a single peak for all the analytes in an entire chromatographic run. However, with low molecular-weight compounds such as polyamines, there is an increased probability of observing multiple peaks from endogenous compounds in the complex biological samples sharing the same SRM transitions. Nevertheless, we observed no multiple peaks with the same SRM transitions.
Fig. 3.
LC/MS/MS chromatograms for eight polyamines in positive ESI.
3.4. Method validation
The present method was fully validated with human serum samples to demonstrate the specificity, linearity, accuracy, precision, recovery, and stability of the method. A detailed summary of validation data is shown in Supplementary Tables 2S–7S.
In general, we recommend isotopically labeled internal standards for each polyamine. Isotopically labeled internal standards efficiently compensate for ion suppression effects, which can be observed especially if samples show large variations in orn concentration. However, as shown in the extensive method validation, very good results can also be obtained by using non isotopically labeled standards within the physiological concentration range.
Briefly, the specificity of the method was evaluated by analyzing aqueous samples to investigate the potential interferences with the analytes and internal standards in the LC peak region using the proposed extraction, derivatization procedure and LC/MS/MS conditions. No interfering peaks were detected. Linearity was generated by plotting the measurement signal (peak/area ratio of analyte/internal standard) versus nominal concentration by 1/x weighted least squares linear regression. Evaluation of intra-day and inter-day accuracy and precision was performed with three QC levels. QC samples were assayed in sets of four replicates on the same day to evaluate intra-day accuracy and precision. Inter-day accuracy and precision were carried out in eight replicates on three consecutive validation days. LLoQ and LOD were determined for put and spd in serum samples for which the analytes had signal-to-noise-ratios of >10 and >3, respectively. For all other analytes, the lowest calibration concentration (1 ng/ml) was used as LLoQ. For orn, a higher calibration range was used, since its concentration in serum is much higher than for other polyamines. However, a lower calibration range was also tested for orn, which revealed that the LLoQ for orn was 5 ng/ml (data not shown). The recovery was evaluated for put and spd spiked in serum matrix at three QC levels. The recovery was calculated as Cobs/Cref, where Cobs is the response of the analyte blank serum sample subtracted from the response of spiked serum sample, and Cref is the theoretically spiked standard concentration. Evaluation of the matrix effect in serum was investigated by using the standard addition method for put and spd. The slopes of the external calibration plot and that obtained by the standard addition method were compared. If the slopes have the same gradient, no matrix effect is observable. Stability tests of the analytes put and spd were assessed by using four replicates of spiked serum samples at 50 μg/ml under different conditions: Serum samples and extracted serum samples were measured after three freeze–thaw cycles (approx. 25 to −80 °C); short-term stability was assessed by measuring extracted serum samples left at 2–8 °C for one day and long-term stability by measuring serum samples and extracted serum samples left at −80 °C after 53 days.
3.5. Quantification of polyamines in clinical studies and samples with relevance for basic and biomedical research
We applied our SPE–LC/MS/MS approach to the analysis of gender differences in polyamine levels in human serum samples by analyzing serum samples of 102 obese patients from a clinical study, of whom 38 were male (age 47 ± 11 with a body mass index (BMI) 45 ± 5) and 64 were female (age 42 ± 10 with a BMI of 44 ± 7) (Table 2 and Supplementary Fig. 2S). The polyamine levels detected in serum samples are shown in Table 3 and Supplementary Fig. 3S. Briefly, 1,3-dap could be detected in approximately half of the female and male patients’ serum samples. The polyamines orn, N-actput, put, spd and sp were detected in nearly all serum probes analyzed (detailed information is given in Table 3). Statistical analyses were performed by using the freely available statistical software package R (vs. 2.15.1), which revealed no significant differences in polyamine concentrations between male and female obese patients (linear modeling & t-tests, p-values >0.1).
Table 2.
Anthropometric data of male and female obese patients: mean of age and BMI ± standard deviation.
| Male |
Female |
|
|---|---|---|
| (n = 38) | (n = 64) | |
| Age (years) | 47 ± 11 | 43 ± 10 |
| BMI (kg/m2) | 46 ± 6 | 45 ± 7 |
Table 3.
Mean concentration of six polyamines ± standard deviation in human serum from male and female obese patients.
| Polyamines | Concentration (mean ± SD) (ng/ml) |
|||
|---|---|---|---|---|
| Female | N (female) | Male | N (male) | |
| 1-3-Diaminopropane | 2.1 ± 0.9 | 31 | 2.3 ± 1 | 22 |
| l-Ornithine | 9 × 103 ± 3 × 103 | 64 | 9.8 × 103 ± 2.5 × 103 | 38 |
| N-Act-putrescine | 12 ± 4 | 64 | 13 ± 4 | 38 |
| Putrescine | 20 ± 6 | 64 | 21 ± 7 | 38 |
| Spermidine | 14 ± 10 | 64 | 13 ± 5 | 38 |
| Spermine | 12 ± 20 | 64 | 10 ± 7 | 37 |
N = number of patients where polyamines were detected.
Our SPE–LC/MS/MS method has been already applied for the assessment of a wide variety of highly relevant samples for basic and biomedical research (including samples from model organisms, mice, or human tissues) [43]. The concentrations of put, spd, sp and orn detected in various biological samples are shown in Table 1. This information provides us an overview of polyamine levels in different organisms ranging from yeast to worms, fruit flies and mice. Therefore our SPE–LC/MS/MS-based method is a valuable tool for analyzing polyamine concentrations in samples of diverse origins, and should be especially useful for selecting suitable sample/extraction volume ratios for MS analysis.
4. Conclusion
We have described a fully validated online SPE–LC/MS/MS method that allows high-throughput, rapid, and robust simultaneous determination of various polyamines. This is the first report of the use of two SPE cartridges online-coupled to LC/MS/MS for the determination of polyamines in biological samples. Because the SPE is online-coupled to the LC/MS/MS, the procedures, sample preparation, chromatographic separation and measurement can be performed in parallel, making sample measurement extremely fast (<4 min). Furthermore, the online SPE sample preparation also reduces the polar and nonpolar contamination, while the combination of positive ESI and selective MRM scanning allows polyamines to be quantified under robust and sensitive conditions. The broad dynamic range of the method allows the unambiguous determination of eight polyamines, including 1,3-diaminopropane, putrescine, cadaverine, N-acetyl-putrescine, spermidine, spermine, N1-acetyl-spermine, and l-ornithine, although this could be easily extended to an even greater range. Our method is a valuable tool for analyzing polyamine concentration of samples of diverse origins, including samples from model organisms, mice, or human tissue, as well as for routine clinical analysis, as demonstrated here: we investigated polyamine levels in human serum samples of obese patients, and found no significant differences in polyamine concentration between male and female obese patients.
Acknowledgments
This work was supported by the Austrian Science Fund FWF (grants S-9304-B05, LIPOTOX, P24381-B20 and P23490 to F.M.), the European Commission (Apo-Sys to F.M. and T.E.). T.E. is the recipient of an APART-fellowship of the Austrian Academy of Sciences at the Institute of Molecular Biosciences, University of Graz and BioPersMed: COMET K-Project and IDT4Brain: COIN Project, both funded by the Austrian Research Promotion Agency (FFG). We also thank Alison Green for help with writing the manuscript.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Gugliucci A. Clin. Chim. Acta. 2004;344:23. doi: 10.1016/j.cccn.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Teti D., Visalli M., McNair H. J. Chromatogr. B. 2002;781:107. doi: 10.1016/s1570-0232(02)00669-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M.H., Caragine T., Wang H.C., Cohen P.S., Botchkina G., Soda K., Bianchi M., Ulrich P., Cerami A., Sherry B., Tracey K.J. J. Exp. Med. 1997;185:1759. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minois N., Carmona-Gutierrez D., Madeo F. Aging. 2011;3:716. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moinard C., Cynober L., de Bandt J.P. Clin. Nutr. 2005;24:184. doi: 10.1016/j.clnu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S., Ashok M., Li J., Li W., Yang H., Wang P., Tracey K.J., Sama A.E., Wang H. Mol. Med. 2009;15:275. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minois N., Carmona-Gutierrez D., Bauer M.A., Rockenfeller P., Eisenberg T., Brandhorst S., Sigrist S.J., Kroemer G., Madeo F. Cell Death Dis. 2012;3 doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg T., Knauer H., Schauer A., Buettner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., Deszcz L., Hartl R., Schraml E., Criollo A., Megalou E., Weiskopf D., Laun P., Heeren G., Breitenbach M., Grubeck-Loebenstein B., Herker E., Fahrenkrog B., Froehlich K.-U., Sinner F., Tavernarakis N., Minois N., Kroemer G., Madeo F. Nat. Cell Biol. 2009;11:1305. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 9.Soda K., Dobashi Y., Kano Y., Tsujinaka S., Konishi F. Exp. Gerontol. 2009;44:727. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Madeo F., Eisenberg T., Buettner S., Ruckenstuhl C., Kroemer G. Autophagy. 2010;6:160. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 11.Madeo F., Tavernarakis N., Kroemer G. Nat. Cell Biol. 2010;12:842. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 12.Morselli E., Marino G., Bennetzen M.V., Eisenberg T., Megalou E., Schroeder S., Cabrera S., Benit P., Rustin P., Criollo A., Kepp O., Galluzzi L., Shen S., Malik S.A., Maiuri M.C., Horio Y., Lopez-Otin C., Andersen J.S., Tavernarakis N., Madeo F., Kroemer G. J. Cell Biol. 2011;192:615. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunton V.G., Grant M.H., Wallace H.M. Biochem. Pharmacol. 1990;40:1893. doi: 10.1016/0006-2952(90)90371-q. [DOI] [PubMed] [Google Scholar]
- 14.Morgan D.M.L. Biochem. Soc. Trans. 1990;18:1080. doi: 10.1042/bst0181080. [DOI] [PubMed] [Google Scholar]
- 15.Russell D.H. Nat. New Biol. 1971;233:144. doi: 10.1038/newbio233144a0. [DOI] [PubMed] [Google Scholar]
- 16.Önal A. Food Chem. 2007;103:1475. [Google Scholar]
- 17.Lapa-Guimaraes J., Pickova J. J. Chromatogr. A. 2004;1045:223. doi: 10.1016/j.chroma.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Sherma J. J. Chromatogr A. 2000;880:129. doi: 10.1016/s0021-9673(99)01132-2. [DOI] [PubMed] [Google Scholar]
- 19.Häkkinen M.R., Keinänen T.A., Vepsäläinen J., Khomutov A.R., Alhonen L., Jänne J., Auriola S. J. Pharm. Biomed. Anal. 2007;45:625. doi: 10.1016/j.jpba.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Häkkinen M.R. 2011. Polyamines: Methods and Protocols; p. 505. [DOI] [PubMed] [Google Scholar]
- 21.Byun J.A., Lee S.H., Jung B.H., Choi M.H., Moon M.H., Chung B.C. Biomed. Chromatogr. 2008;22:73. doi: 10.1002/bmc.898. [DOI] [PubMed] [Google Scholar]
- 22.Gosetti F., Mazzucco E., Gianotti V., Polati S., Gennaro M.C. J. Chromatogr. A. 2007;1149:151. doi: 10.1016/j.chroma.2007.02.097. [DOI] [PubMed] [Google Scholar]
- 23.Gosetti F., Mazzucco E., Gennaro M.C., Marengo E. Anal. Bioanal. Chem. 2013;405:907. doi: 10.1007/s00216-012-6269-z. [DOI] [PubMed] [Google Scholar]
- 24.Simo C., Moreno-Arribas M.V., Cifuentes A. J. Chromatogr. A. 2008;1195:150. doi: 10.1016/j.chroma.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Muskiet F.A.J., Dorhout B., Vandenberg G.A., Hessels J. J. Chromatogr. 667. 1995:189. doi: 10.1016/0378-4347(95)00023-c. [DOI] [PubMed] [Google Scholar]
- 26.Khuhawar M.Y., Qureshi G.A. J. Chromatogr. B. 2001;764:385. doi: 10.1016/s0378-4347(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Hara K., Liu J., Yu Y., Gao L., Wang Y., Wang Y. J. Chromatogr. B. 2008;876:257. doi: 10.1016/j.jchromb.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 28.Dziarkowska K., Jönsson J.A., Wieczorek P.P. Anal. Chim. Acta. 2008;606:184. doi: 10.1016/j.aca.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Liu R., Bi K., Jia Y., Wang Q., Yin R., Li Q. J. Mass Spectrom. 2012;47:1341. doi: 10.1002/jms.3084. [DOI] [PubMed] [Google Scholar]
- 30.Liu R., Li Q., Ma R., Lin X., Xu H., Bi K. Anal. Chim. Acta. 2013;791:36. doi: 10.1016/j.aca.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Ubhi B.K., Davenport P.W., Welch M., Riley J., Griffin J.L., Connor S.C. J. Chromatogr. B. 2013;934:79. doi: 10.1016/j.jchromb.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hadithi N.N., Saad B. Anal. Lett. 2011;44:2245. [Google Scholar]
- 33.Balasundaram D., Tabor C.W., Tabor H. PNAS. 1991;88:5872. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minocha R., Shortle W.C., Long S.L., Minocha S.C. J. Plant Growth Regul. 1994;13:187. [Google Scholar]
- 35.Dubeau S., Bourassa P., Thomas T.J., Tajmir-Riahi H.A. Biomacromolecules. 2010;11:1507. doi: 10.1021/bm100144v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berwanger A., Eyrisch S., Schuster I., Helms V., Bernhardt R. J. Inorg. Biochem. 2010;104:118. doi: 10.1016/j.jinorgbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Schuster I., Bernhardt R. Interactions of natural polyamines with mammalian proteins. 2011 doi: 10.1515/bmc.2011.007. [DOI] [PubMed] [Google Scholar]
- 38.Feistner G.J. Biol. Mass Spectrom. 1994;23:784. doi: 10.1002/bms.1200231211. [DOI] [PubMed] [Google Scholar]
- 39.Trufelli H., Palma P., Famiglini G., Cappiello A. Mass Spectrom. Rev. 2011;30:491. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- 40.Gustavsson S.A., Samskog J., Markides K.E., Langstrom B. J. Chromatogr. A. 2001;937:41. doi: 10.1016/s0021-9673(01)01328-0. [DOI] [PubMed] [Google Scholar]
- 41.Naidong W. J. Chromatogr. B. 2003;796:209. doi: 10.1016/j.jchromb.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Xu R.N., Fan L., Rieser M.J., El-Shourbagy T.A. J. Pharm. Biomed. Anal. 2007;44:342. doi: 10.1016/j.jpba.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Gupta V.K., Scheunemann L., Eisenberg T., Mertel S., Bhukel A., Koemans T.S., Kramer J.M., Liu K.S.Y., Schröder S., Stunnenberg H.G., Sinner F., Magnes C., Pieber T.R., Dipt S., Fiala A., Schenck A., Schwaerzel M., Madeo F., Sigrist S.J. Nat. Neurosci. 2013 doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.