Abstract
A new quinoline-based tripodal thiourea has been synthesized, which exclusively binds fluoride anion in DMSO, showing no affinity for other anions including, chloride, bromide, iodide, perchlorate, nitrate and hydrogen sulfate. As investigated by 1H NMR, the receptor forms both 1:1 and 1:2 complex yielding the binding constants of 2.32(3) (in log β1) and 4.39(4) (in log β2), respectively; where quinoline groups are protonated by the fluoride-induced proton transfer from the solution to the host molecule. The 1:2 binding is due to the interactions of one fluoride with NH binding sites of urea sites and another fluoride with secondary +NH binding sites within the tripodal pocket. The formation of both 1:1 and 1:2 complexes has been confirmed by the theoretical calculations based on density functional theory (DFT).
Keywords: Fluoride sensor, Thiourea, Anion complex, Molecular recognition, Acyclic receptor
The development of selective fluoride receptors has attracted significant attention because of the key roles played by fluoride anions in environmental, biological, and health-related processes.1 The human consumption of fluoride over a prolonged period of time increases the risk of several health related problems including dental fluorosis, reproductive defects and gastrointestinal infections.2 However, the fluoride anion is distinct from other halides, showing strong basicity and high hydration energy due to the considerably high bond strength (569 kJmol−1) of its conjugated acid (HF).3 Therefore, fluorides, particularly hygroscopic tetraalkylammonium fluorides, can extract protons from weakly acidic aprotic solvents like DMSO and CH3CN.3,4 In addition, the binding of fluoride anion can be hampered with highly acidic receptors including ureas and thioureas due to the potential ability of the fluoride to deprotonate NH groups in aprotic solvents.5 Such deprotonations often can lead to an intense color change (red) that is due to the formation of the highly stable [HF2]− species, and not related to host–guest complexation.5 In order to design effective fluoride receptors, it is important to have NH groups serve as the primary recognition units that should interact with fluoride without deprotonation.
While there are several classes of anion receptors containing neutral NH groups including ureas, thioureas, amides, pyrroles, imidazoles and indoles,6–11 exclusive fluoride receptors that selectively recognize fluorides over other halides are indeed rare.6 In most cases, such receptors bind other anions also, thereby hampering the selectivity for fluoride. Herein we report a new quinoline based tripodal tris-thiourea receptor L that exclusively binds fluoride over other halides via NH interactions in DMSO. Furthermore, we demonstrate that quinoline groups can be protonated due to the fluoride-induced proton transfer from the solvent to the host molecule, providing secondary binding sites for fluoride anions.
L was synthesized in a two-step reaction as shown in Scheme 1. Tripodal amine (tren) 1 was reacted with carbon disulfide under nitrogen atmosphere at cold temperature to yield the tren-based isothiocyanate 2. The final product L was synthesized from the reaction of 2 and 8-aminoquinoline at low temperature.
Scheme 1.
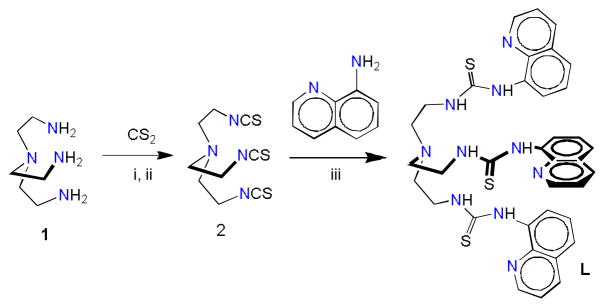
Synthetic pathway to the tripodal thiourea L: (i) N,N′-dicyclohexylcarbodiimide; (ii) Dry THF; (iii) CH2Cl2.
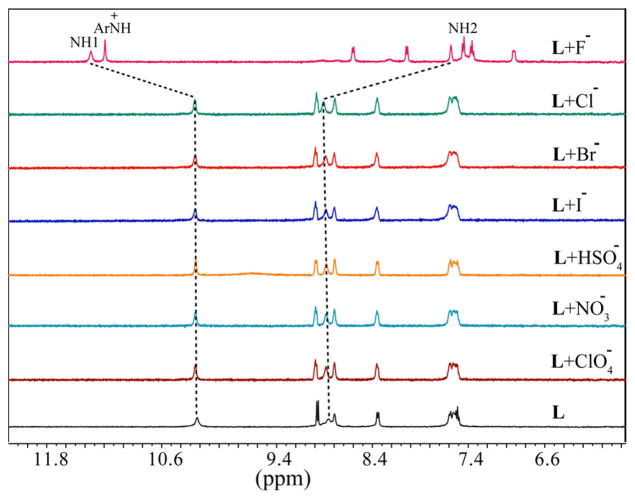
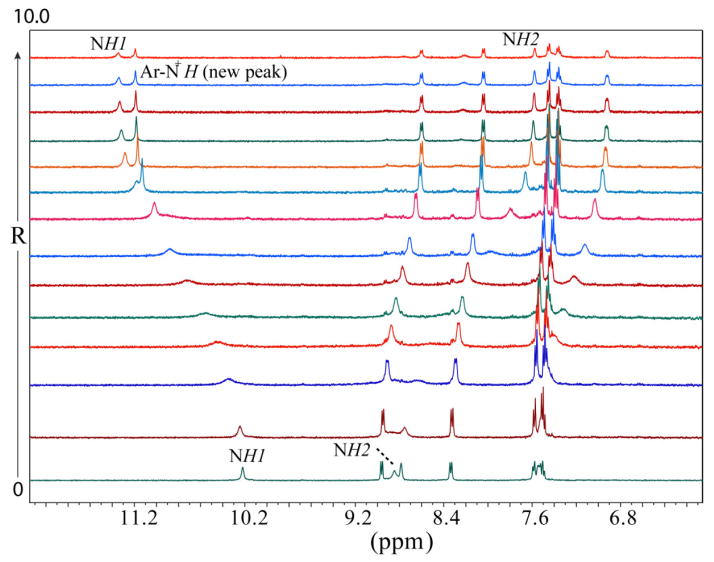
Solution binding studies of L for anions were performed with 1H NMR titrations using [n-Bu4N]+A− (A− = F−, Cl−, Br−, I−, ClO4−, NO3− and HSO4−) in DMSO-d6. At first, the halide binding ability of L was screened by the addition of an excess amount of the respective anion (five equivalents) to the host solution. As shown in Figure 1, the free L shows two peaks for two NH groups (NH1 = 10.21 ppm and NH2 = 8.84 ppm) that remain almost unchanged in the presence of Cl−, Br−, I−, ClO4−, NO3− and HSO4−, which could be the effect of the attached electron-donating groups reducing the NH acidity of thiourea groups. In contrast, both NH resonances shift significantly after the addition of fluoride anion: the NH1 proton shifts downfield (ΔδNH1 = 1.13 ppm) and the NH2 proton shifts upfield (ΔδNH2 = 1.27 ppm. In addition, all other aromatic protons are observed to shift upfield in the presence of fluoride. These results indicate that the ligand interacts exclusively with fluoride among other halide anions via hydrogen bonding interactions. Interestingly, a new sharp peak appears at 11.18 ppm, which could be the result of the formation of quinoline NH+ - a potential secondary binding site formed from the interactions of basic quinoline N groups and HF. The appearance of NH+ at 11 to 12 ppm is well documented for the related organic salts (e.g. pyridinium chlorides).12 Presumably, the very hygroscopic TBAF abstracts protons in DMSO solution from the crystalline salt (TBAF·3H2O)/moisture rather than from the thiourea groups. In general, the removal of NH protons causes the disappearance of NH peaks, which has not been observed in the present case. It is assumed that the quinoline moieties act as electron-donating groups, making the N-H bonds strong enough for the interactions with the fluoride without deprotonation. The change in the chemical shift of NH resonances of L, as recorded with an increasing amount of TBAF at room temperature (Figure 2), gave the best fit for a 1:2 (host/guest) binding model (Figure 3),13 yielding the binding constants, Log β1 = 2.32(3), and Log β2 = 4.39(4). The binding stoichiometry was confirmed by a Job’s plot analysis (Figure S11). The 1:2 binding could be due to the interactions of one fluoride with NH binding sites and another fluoride with secondary +NH binding sites within the tripodal pocket, as verified by DFT calculations (discussed later).
Figure 1.
Partial 1H NMR spectra of L (2mM) in the presence of 5 equivalents of different anions in DMSO-d6 (NH1 =CSNHAr, NH2 = CH2NHCS).
Figure 2.
1H NMR spectra of L (2 mM) with an increasing amount of TBAF(R = [TBAF]0/[L]0) in DMSO-d6.
Figure 3.
1H NMR titration plots of L (2 mM) with an increasing amount of TBAF (R = [TBAF]0/[L]0) in DMSO-d6.
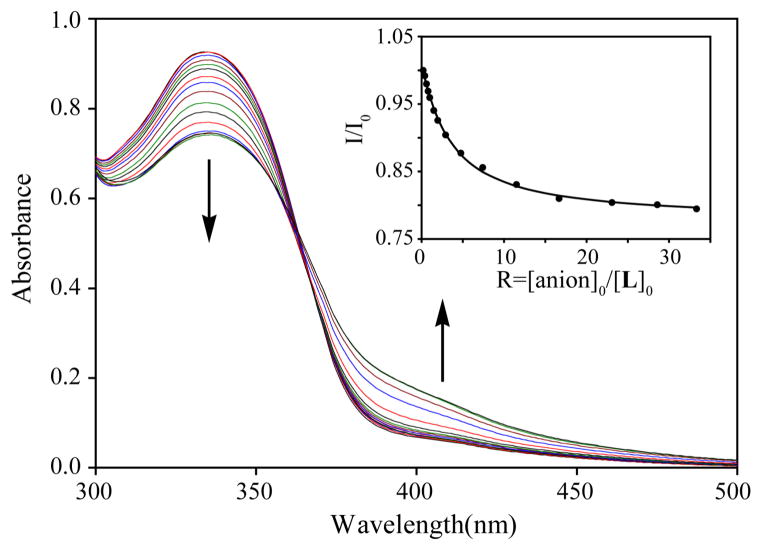
UV-Vis studies were further performed to evaluate the binding ability of L for anions in DMSO. The free receptor displayed an absorption band (λmax) at 335 nm in the absence of an anion. With the exception for fluoride, the receptor did not show any change in its absorption patterns for other anions in DMSO (Figure 4). This observation is fully consistent with the 1H NMR data showing no interactions for Cl−, Br−, I−, ClO4−, NO3− and HSO4−. The addition of TBAF to the receptor solution resulted in a gradual decrease in the absorbance of λmax (335 nm), displaying a new peak at ~ 400 nm, which could be ascribed to the formation of an intramolecular charge transfer (ICT) complex (Figure 5).14 The relative absorbance I/I0 of L (where I0 and I represent the absorbance of L before and after the addition of an anion, respectively) upon the incremental addition of TBAF provided the best fit for a 1:1 association model (inset in Figure 5), yielding a binding constant of Log K= 3.81(5). However, this 1:1 binding model is in contrast to the 1:2 complex observed from the 1H NMR titrations, which could be the effect of initial concentrations used for two different techniques. The host molecule was further investigated for naked eye colorimetric studies with anions in DMSO. As shown in Figure 6, a visible colour change was observed after the addition of fluoride, which is consistent with the results of UV titration studies. Notably, the host does not result in a colour change after the addition of other anions in DMSO; this observation is consistent with results of UV titrations.
Figure 4.
Changes in absorption spectra of L (5 × 10−5 M) in the presence of 10 equivalents of different anions in DMSO.
Figure 5.
Changes in absorption spectra of L (5 × 10−5 M) with an increasing amount of TBAF in DMSO. (inset) Titration plot of change in relative absorbance of L with an increasing amount of the anion (R = 0 – 35) at λmax = 335 nm.
Figure 6.
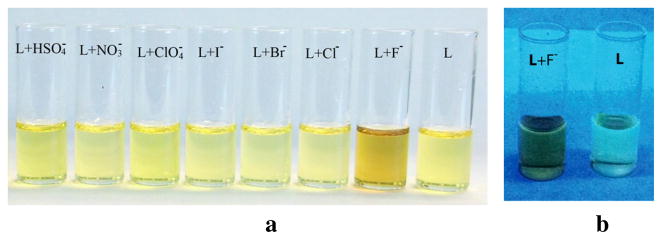
(a) Colorimetric studies of L (1 × 10−2 M) after the addition of one equivalent of different anions in DMSO, showing a noticeable color change only for fluoride. (b) Color change of L after the addition of one equivalent of TBAF in DMSO under UV light at 365 nm.
In order to understand the fluoride binding patterns of the receptor, theoretical calculations based on density functional theory (DFT) were performed using L (unprotonated) with a single fluoride (1:1 complex) and [H3L]3+ (protonated) with two fluorides (1:2 complex) in gaseous phase. Each of these complexes was optimized at the M06-2X level of theory with a polarized 6–31g(d,p) basis set.15 In order to make a meaningful comparison of binding energies for both of these complexes, we calculated the average binding energy per fluoride anion defined by the expression:
where, n = 1 for the 1:1 complex and n = 2 for the 1:2 complex. With this expression, we obtained an average binding energy of 395.8 kcal/mol (per fluoride) for the single-fluoride complex and 459.4 kcal/mol (per fluoride) for the double-fluoride complex. The optimized structures of both 1:1 and 1:2 complexes are shown in Figure 7. In the 1:1 complex (Figure 7a), the anion is encapsulated within the cavity and held by six NH···F bonds (NH···F = 2.677 to 2.822 Å). These H-bonding distances are comparable to the reported experimental values (NH···F = 2.584 (3) to 2.724 (3) Å)16 and calculated values (NH···F = 2.700(3) to 2.884(3) Å)17 observed in the fluoride complexes. On the other hand, the protonated [H3L]3+ species, as presumed from the 1H NMR experiments due to the fluoride-induced proton transfer, is stabilized with two fluorides inside the cavity: one (F1) with six NH···F bonds (NH···F = 2.716 to 2.7333 Å) from thiourea groups and another (F2) with three NH···F bonds (NH···F = 2.504 Å from quinoline NH+ groups (Figure 7b). In the 1:2 complex, the quinoline groups are twisted significantly in order to encapsulate the second fluoride.
Figure 7.
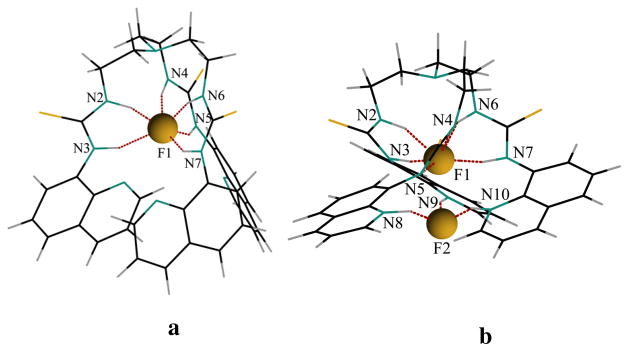
Optimized structure of (a) [L(F)]− showing a 1:1 complex, and [H3L(F)2]+ showing a 1:2 complex calculated at M06-2X level of theory with a polarized 6–31g(d,p) basis set.
In summary, we have synthesized a new quinoline-based thiourea receptor that exclusively binds fluoride without exhibiting any affinity for other anions in DMSO. The attached quinoline moieties act as both bases and electron-donating groups, making the N-H bonds of thiourea groups strong enough for the interactions with fluoride without deprotonation, but limiting its effectiveness for other anions. In addition, an elegant application of solution chemistry is presented for fluoride, which extracts protons from the DMSO solution and transfers them to the basic quinoline groups, effectively making these groups as potential secondary binding sites for fluorides. This effect, along with the natural anion ability of NHs of thiourea groups, results in a receptor that is extremely selective for fluorides.
Supplementary Material
Acknowledgments
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to M.A.H. The NMR core facility at Jackson State University was supported by the National Institutes of Health (G12RR013459). Ismet Basaran was supported by a grant from the Council of Higher Education of Turkey, being on leave from Balikesir University, Turkey. B.M.W. acknowledges the National Science Foundation for supercomputing resources through the Extreme Science and Engineering Discovery Environment (XSEDE), Project No. TG-CHE130052.
Footnotes
Experimental procedure for the synthesis of L, characterizations, and NMR and UV-Vis binding studies. Supplementary data associated with this article can be found in the online version, at doi:xxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cametti M, Rissanen K. Chem Soc Rev. 2013;42:2016–2038. doi: 10.1039/c2cs35439j. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PTC. J Fluor Chem. 2005;126:1448–1456. [Google Scholar]
- 3.Clark JH. Chem Rev. 1980;80:429–452. [Google Scholar]
- 4.Kang SO, VanderVelde D, Powell DR, Bowman-James K. J Am Chem Soc. 2004;126:12272–12273. doi: 10.1021/ja046078n. [DOI] [PubMed] [Google Scholar]
- 5.Gomez DE, Fabbrizzi L, Licchelli M, Monzani E. Org Biomol Chem. 2005;3:1495–1400. doi: 10.1039/b500123d. [DOI] [PubMed] [Google Scholar]
- 6.Cametti M, Rissanen K. Chem Commun. 2009:2809–2829. doi: 10.1039/b902069a. [DOI] [PubMed] [Google Scholar]
- 7.(a) Hossain MA, Llinares JM, Powell D, Bowman-James K. Inorg Chem. 2001;40:2936–2937. doi: 10.1021/ic015508x. [DOI] [PubMed] [Google Scholar]; (b) Kang SO, Day VW, Bowman-James K. Org Lett. 2009;11:3654–3657. doi: 10.1021/ol9014249. [DOI] [PubMed] [Google Scholar]
- 8.(a) Russ TH, Pramanik A, Khansari ME, Wong BM, Hossain MA. Nat Prod Commun. 2012;7:301–304. [PMC free article] [PubMed] [Google Scholar]; (b) Ravikumar I, Lakshminarayanan PS, Arunachalam M, Suresh E, Ghosh P. Dalton Trans. 2009:4160–4168. doi: 10.1039/b820322a. [DOI] [PubMed] [Google Scholar]; (c) Emami Khansari M, Wallace KD, Hossain MA. Tetrahedron Lett. 2014;55:438–440. doi: 10.1016/j.tetlet.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kim J, Juwarker H, Liu X, Lah MS, Jeong KS. Chem Commun. 2010;46:764–766. doi: 10.1039/b919519j. [DOI] [PubMed] [Google Scholar]; (b) Gale PA, Hiscock JR, Jie CZ, Hursthouse MB, Light ME. Chem Sci. 2010;1:215–220. [Google Scholar]
- 10.Wu B, Liang J, Yang J, Jia C, Yang XJ, Zhang H, Tang N, Janiak C. Chem Commun. 2008:1762–1764. doi: 10.1039/b719019k. [DOI] [PubMed] [Google Scholar]
- 11.Schulze B, Friebe C, Hager MD, Günther W, Köhn U, Jahn BO, Görls H, Schubert US. Org Lett. 2010;12:2710–2713. doi: 10.1021/ol100776x. [DOI] [PubMed] [Google Scholar]
- 12.Shuppert JW, Angell CA. J Phy Chem. 1977;67:3050–3056. [Google Scholar]
- 13.Hynes MJ. J Chem Soc Dalton Trans. 1993:311–312. [Google Scholar]
- 14.Hossain MA, Morehouse M, Powell DR, Bowman-James K. Inorg Chem. 2005;44:2143–2149. doi: 10.1021/ic048937e. [DOI] [PubMed] [Google Scholar]
- 15.Truhlar DG. J Chem Theory Comput. 2008;4:1849–1868. doi: 10.1021/ct800246v. [DOI] [PubMed] [Google Scholar]
- 16.Hossain MA, Saeed MA, Pramanik MAA, Wong MM, Haque SA, Powell DR. J Am Chem Soc. 2012;134:11892–11895. doi: 10.1021/ja3043855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pramanik A, Powell DR, Wong BM, Hossain MA. Inorg Chem. 2012;51:4274–4284. doi: 10.1021/ic202747q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.