Abstract
GATA3 is a transcription factor important in the differentiation of breast epithelia, urothelia, and subsets of T-lymphocytes. It has been suggested useful in the evaluation of mammary or urothelial origin or metastatic carcinomas, but its distribution in normal and neoplastic tissues is incompletely mapped. In this study, we examined normal developing and adult tissues in 2040 epithelial and 460 mesenchymal or neuroectodermal neoplasms for GATA3 expression to explore its diagnostic value in surgical pathology, using monoclonal antibody (clone L50-823) and Leica Bond automated immunohistochemistry. GATA3 was expressed in trophoblast, fetal and adult epidermis, adult mammary and some salivary gland and sweat gland ductal epithelia, urothelia, distal nephron in developing and adult tissues, some prostatic basal cells, and subsets of T-lymphocytes. It was expressed stronger in fetal than adult mesothelia and was absent in respiratory and gastrointestinal epithelia. In epithelial neoplasms, GATA3 was expressed in >90% of primary and metastatic ductal and lobular carcinomas of the breast, urothelial, and cutaneous basal cell carcinomas, and trophoblastic and endodermal sinus tumors. In metastatic breast carcinomas, it was more sensitive than GCDFP. Among squamous cell carcinomas, the expression was highest in the skin (81%) and lower in cervical (33%), laryngeal (16%) and pulmonary tumors (12%). Common positivity was found in skin adnexal tumors (100%), mesothelioma (58%), salivary gland (43%) and pancreatic (37%) ductal carcinomas, whereas frequency of expression in adenocarcinomas of lung, stomach, colon, endometrium, ovary, and prostate was <10%. Chromophobe renal cell carcinoma was a unique renal tumor with frequent positivity (51%), whereas oncocytomas were positive in 17% of cases but other types only rarely. Among mesenchymal and neuroectodermal tumors, paragangliomas were usually positive, which sets these tumors apart from epithelial neuroendocrine tumors. Mesenchymal tumors were only sporadically positive, except epithelia of biphasic synovial sarcomas. GATA3 is a useful marker in characterization not only mammary and urothelial but also for renal and germ cell tumors, mesotheliomas, and paragangliomas. The multiple specificities of GATA3 should be taken into account when using this marker to detect metastatic mammary or urothelial carcinomas.
Keywords: GATA3, breast cancer, urothelial cancer, choriocarcinoma, endodermal sinus tumor, chromophobe renal carcinoma, mesothelioma, immunohistochemistry
INTRODUCTION
GATA3 binding protein, commonly and from here on abbreviated as GATA3, is a transcription factor of the GATA family. These nuclear proteins recognize G-A-T-A nucleotide sequences in target gene promoters and activate or repress those genes. 1,2 GATA3 function is known to be important in the regulation of genes such as MUC1/EMA involved in the luminal differentiation of breast epithelium 3 and genes related to T-cell development. 4,5 Other known functions include gene regulation in the development or maintenance of skin, especially hair shafts 6,7, trophoblast 8-10 and some endothelial cells, especially in the great vessels. 11 Constitutional allelic loss of GATA3 (haploinsufficiency) causes a syndrome characterized by hypoparathyroidism, sensoneurial deafness and renal malformations, abbreviated as HDR based on these manifestations.12
GATA3 has been thus far explored in surgical pathology as a marker for breast and urothelial carcinomas. Most primary and metastatic mammary carcinomas express GATA3 (80-90%), but the expression is reportedly lower in triple-negative tumors (67%). 13 In one study, 82% of female breast carcinomas but only 32% of male breast carcinomas were GATA3-positive. 14 GATA3 expression seems to be more extensive in lower grade tumors and positive cases, and cases with high expression may have a better survival 14,15 However, none of those studies have assessed the specificity of GATA3 for breast cancer. In another study, strong expression of GATA3 (in >50% tumor cells) was detected only in mammary (94%), urothelial (86%), and endometrial (2%) adenocarcinomas, but in none of the pulmonary, pancreatic, colonic, prostatic, and ovarian carcinomas in a relatively small sample. 16
GATA3 was discovered as a urothelial carcinoma marker by CDNA expression microarrays 17 and found downregulated in invasive bladder cancers.18 Since then it has been suggested useful in the distinction between urothelial vs. prostatic adenocarcinoma and metastatic urothelial vs. squamous cell carcinoma in the lung, with 80% of metastatic urothelial carcinomas but none of the pulmonary squamous cell carcinomas or high-grade prostatic carcinomas being positive. 19 In another study, 78% primary invasive urothelial carcinomas and 23% pulmonary squamous cell carcinomas were positive indicating a lesser specificity. 20 Also, transitional cell lesions of ovaries, such as Brenner tumors, have been found as GATA3-positive. 21
In this study we immunohistochemically examined 2500 epithelial and mesenchymal or neuroectodermal tumors for GATA3 to further explore its specificity for mammary and urothelial carcinomas and evaluated its other specificities potential applicable in surgical pathology.
MATERIALS AND METHODS
The tumors and normal tissues were obtained from surgical specimens and arranged in multitissue blocks containing 5-50 cases as previously described. 22 A total of 2040 epithelial neoplasms and 460 mesenchymal and neuroectodermal tumors were examined. In addition, we evaluated a spectrum of normal human adult, fetal, and embryonic tissues from surgical specimens. Lymphomas and related tumors were excluded from the study.
The primary antibody to GATA3 (Clone L50-823) was obtained from Biocare Medical, Concord, CA. The antibody was used in a dilution of 1:500. Immunohistochemical analysis was performed in a Leica Bond Max automated immunostainer (Leica, Bannockburn, IL). High-pH epitope retrieval buffer was used for 25 minutes prior to application of the primary antibody (30 min.). Detection was performed using Leica Bond-detection kit with diaminobenzidine as the chromogen, followed by a light hematoxylin counterstain. Tumor-infiltrating or peritumoral lymphocytes were used as internal controls and renal distal tubules and collecting ducts as external positive controls. Only nuclear staining was scored and the percentage of positive nuclei was estimated. However, cytoplasmic staining was an infrequent finding and such staining was usually weak. Intensity of staining was generally strong and was not scored separately. Tumors studied here were previously extensively characterized with relevant markers.
Metastatic breast cancers were also evaluated for GCDFP-15 (mouse monoclonal antibody, clone D6 from Signet, Dedham, MA, diluted at 1:40, staining preceded by 4 min digestion with Ventana protease 1, primary antibody incubated for 32 min.) and all trophoblastic tumors/components for beta HCG (Dako Cytomation, diluted 1:60000, staining preceded by heat retrieval in a steamer in 1mM EDTA buffer, pH 9, primary antibody incubated for 2 hrs). Both latter antibodies were tested using Ventana Benchmark automation using the Ultra View detection kit (Ventana Medical Systems, Tucson, AZ). The GATA3-negative urothelial carcinomas were also examined for thrombomodulin/CD141 using a Novocastra/Leica antibody (diluted 1:100), with detection performed by Leica automated immunohistochemistry as described above.
RESULTS
Developing tissues
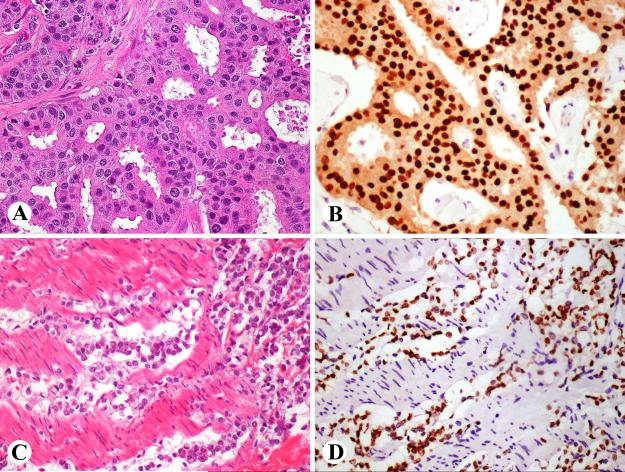
In a 7th week embryo, strong nuclear GATA3-positivity was detected in the epidermis, peritoneal mesothelia, virtually all trophoblastic cells, and amnion epithelia (Fig. 1 A-C). Distinct focal positivity was also detected in the caudal mesenchyme and in some endothelial cells in the great vessels adjacent to the heart. Negative were spinal nervous and muscle tissue, liver, heart muscle, primordia of lung and intestines, mesenchyme in general, and the yolk sac.
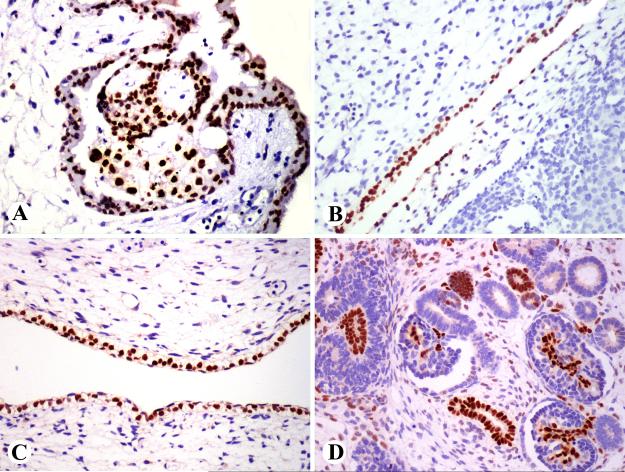
Fig. 1.
GATA3 expression in embryonic tissues. A 7-week embryo shows GATA3-positivity in trophoblast (A), peritoneal mesothelium (B), and skin (C). A 10-week fetus shows GATA3 expression in some renal tubuli and the glomerular mesangium (D).
In a 10th week fetus, epidermis (especially basal portion), epithelia of oral cavity, olfactory plate, periocular mesenchyme, cartilage of the skull base, isolated cells in the brain, and nerve ganglia showed positivity. In the kidney, approximately 1/3 of the tubular structures and glomerular mesangial cells were positive (Fig. 1 D). Choroid plexus, eye, liver, and intestines were negative.
Normal adult tissues
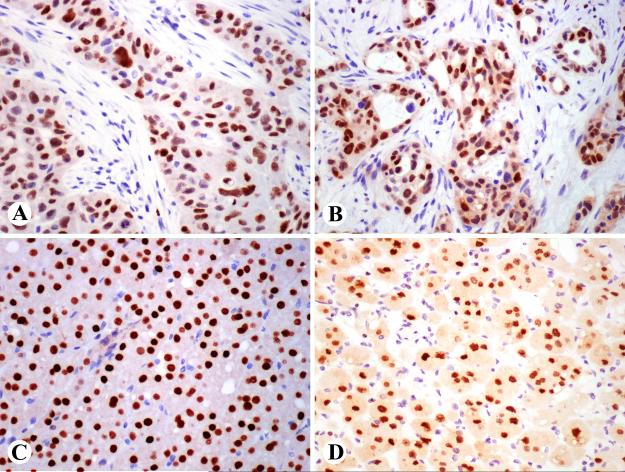
Strong nuclear GATA3-positivity was detected in the epidermis, sebaceous epithelia, and hair shafts (Fig. 2A). Eccrine sweat glands were focally and apocrine glands extensively positive. Uterine ectocervical squamous epithelium was variably positive. Lobular and ductal epithelia of the breast and nipple were positive, but myoepithelial cells were negative (Fig. 2 B). Urothelial epithelia of the renal pelvis, ureter, and urinary bladder were strongly positive (Fig. 2 C). In the kidney, other positive elements included collecting ducts, distal tubules, and mesangial cells (Fig. 2D). Seminal vesicle epithelia were positive, and prostatic basal cells variably positive and luminal cells typically negative. Terminal ducts of normal parotid and small ducts in atrophic glands were extensively positive. In the lymphoid tissue of tonsil, only scattered germinal center cells and interfollicular T-cells were positive, but the thymic cortex contained large numbers of positive thymocytes. Omental peritoneal mesothelium was negative, but positivity was detected in reactive mesothelia.
Fig. 2.
GATA3 expression in adult tissue. A. Epidermal cells are positive. Note also positive dermal lymphocytes. B. Luminal cells of ducts of a breast lobule a positive, whereas myoepithelial cells are negative. C. Ureteral epithelium and some stromal lymphocytes are positive. D. Distal tubular epithelial and mesangial cells are GATA3-positive.
Negative were thyroid, respiratory and pulmonary alveolar epithelia, gastric and intestinal mucosae and mesenchyme, hepatocytes, bile ducts, gallbladder, exo- and endocrine pancreas, testicular seminiferous tubules, Leydig cells of testis, and ovarian stroma. In the endometrium, glands were negative. However, decidual stromal change in the uterus and extra-uterine endometriosis were positive. The latter was evaluated as a non-trophoblast containing tissue.
Epithelial neoplasms
The results on epithelial neoplasms have been summarized in Table 1 and the description below is centered on positive results.
Table 1.
Expression of GATA3 in 2040 epithelial neoplasms.
| Tumor type | Positive/total | % positive |
|---|---|---|
| Adrenocortical carcinoma | 3/27 | (11) |
| Basal cell carcinoma, skin | 61/62 | (98) |
| Benign skin adnexal tumors (see text) | 24/24 | (100) |
| Breast, ductal carcinoma, primary | 164/179 | (92) |
| Breast, ductal carcinoma, metastatic | 49/51 | (96) |
| Breast, lobular carcinoma | 38/38 | (100) |
| Colon, adenocarcinoma | 2/142 | (1) |
| Endometrium, adenocarcinoma | 6/89 | (7) |
| Germ cell tumor, seminoma | 0/76 | |
| Germ cell tumor, choriocarcinoma | 11/11 | (100) |
| Germ cell tumor, endodermal sinus tumor | 6/6 | (100) |
| Germ cell tumor, pure embryonal carcinoma | 0/5* | (40) |
| Liver, hepatocellular carcinoma | 1/47 | (2) |
| Liver, Cholangiocarcinoma | 5/57 | (9) |
| Lung, adenocarcinoma | 6/71 | (8) |
| Lung, small cell carcinoma | 0/30 | |
| Lung, carcinoid | 0/11 | |
| Small, intestine, carcinoid | 0/18 | |
| Malignant mesothelioma | 37/64 | (58) |
| Merkel cell carcinoma | 0/4 | |
| Ovary, serous carcinoma | 4/73 | (6) |
| Ovary, other carcinomas | 0/25 | |
| Pancreas, adenocarcinoma | 23/62 | (37) |
| Pancreas, neuroendocrine tumor | 0/15 | |
| Prostate, adenocarcinoma | 2/95 | (2) |
| Rectum, adenocarcinoma | 0/27 | |
| Renal cell carcinoma, chromophobe | 18/35 | (51) |
| Renal oncocytoma | 6/35 | (17) |
| Renal cell carcinoma, other than chromophobe | 3/154 | (2) |
| Salivary gland, adenoid cystic carcinoma | 5/17 | (29) |
| Salivary gland, ductal carcinoma | 6/14 | (43) |
| Squamous cell carcinoma, skin | 25/31 | (81) |
| Squamous cell carcinoma, cervix | 7/21 | (33) |
| Squamous cell carcinoma, larynx | 8/36 | (16) |
| Squamous cell carcinoma, lung | 9/74 | (12) |
| Stomach, adenocarcinoma | 6/133 | (5) |
| Thymoma | 0/41 | |
| Thyroid, papillary carcinoma | 3/55 | (5) |
| Thyroid, follicular carcinoma | 1/20 | (5) |
| Thyroid, anaplastic carcinoma | 1/11 | (9) |
| Urothelial carcinoma, low-grade | 22/22 | (100) |
| Urothelial carcinoma, high-grade | 27/32 | (84) |
Majority of tumor cells negative, small positive subpopulations of uncertain nature detected in some cases.
A great majority of primary ductal carcinomas of the breast (163/178, 92%), and their metastases (49/51, 96%) including 30 from lymph nodes, 10 from brain (Fig. 3 A, B), and 9 from other sites, were positive. Great majority of cases showed positivity in all tumor tumor cells. All but two negative examples were high-grade ductal carcinomas and all but one of the negative examples tested were also estrogen receptor (ER)-negative (6/7). However, 30/36 of ER-negative ductal carcinomas were GATA3-positive, but they often showed heterogeneous, reduced GATA3-expression, which however exceeded 50% of positive tumor cells in 23/36 cases. The 4 triple-negative cancers (values of ER, PR, and HER2 = 0) had GATA3 expression in 40-70% of tumor cells. However, if <5% of ER, PR, or HER2 was accepted as triple negative, there were 1/13 cases negative for GATA3 and 4 showing reduced < 50% positivity. In a cohort of 37 metastatic ductal carcinomas tested for both markers, GATA3 was more sensitive than GCDFP-15 (92 vs. 78% for overall positivity) and often showed a higher number of positive cells (median values 100% vs. 30%).
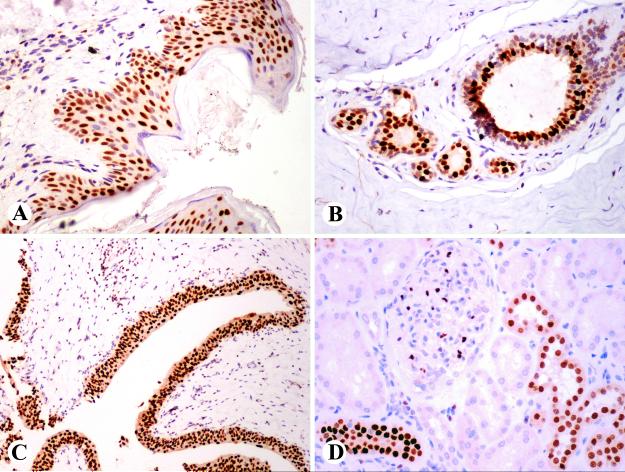
Fig. 3.
Two examples of GATA3-positive metastatic breast cancers. A, B. A ductal carcinoma with apocrine features metastatic to the brain is strongly positive. C, D. Lobular carcinoma metastatic to small intestine in highlighted as GATA3-positive.
All 31 lobular carcinomas of the breast, including 4 metastases, 10 mucinous, and 1 papillary carcinoma of the breast were also positive, but 4/10 mucinous carcinomas showed reduced GATA3-positivity (<50% of tumor cells positive). A metastatic mammary lobular carcinoma in the small intestine was highlighted as a rare GATA3-positive intestinal neoplasm (Fig. 3 C, D).
Most urothelial carcinomas (49/54) were extensively positive, except 5 tumors, which were either poorly differentiated or showed prominent squamous differentiation. All three negative tumors that were studied were also negative for thrombomodulin.
Of skin tumors, cutaneous squamous cell carcinomas were usually positive, 81%, with a median of 50% tumor cells positive (range, 5-100%) (Fig. 4 A). In comparison, other squamous cell carcinomas were less commonly positive: cervical carcinomas (33% of cases, nearly half of them showing positivity in >50% of tumor cells). Laryngeal (16%) and pulmonary ones (12%) were less commonly positive. However, half of the GATA3-positive examples of the pulmonary tumors and 2 of 8 of the laryngeal ones showed positivity in > 50% of tumor cells.
Fig. 4.
A. Examples of GATA3-positive epithelial neoplasms. Strong expression in cutaneous squamous cell carcinoma B. Pancreatic ductal adenocarcinoma cells show nearly uniform labeling. C. Renal chromophobe carcinoma cells are positive. D. Most cells of renal oncocytoma are positive and there is also weak cytoplasmic labeling.
Nearly all cutaneous basal cell carcinomas were strongly and uniformly positive. Similarly positive were trichoepitheliomas, basaloid cells of pilomatricoma, epithelia of pilar cyst, and seborrhoic keratosis (4 cases of each studied). Focal epithelial cell positivity was seen in mixed tumors of the skin, cylindroma, eccrine spiradenoma, and hidradenoma/eccrine acrospiroma (2 of each studied).
Salivary gland and pancreatic ductal adenocarcinomas were the most commonly GATA3-positive non-mammary adenocarcinomas (43% and 37% of cases positive). The salivary gland ductal carcinomas had 100% of positive cells in 4 cases, 60% in one case, and 20% in the remaining case. The positive pancreatic carcinomas included non-mucinous (Fig. 4B) and mucinous adenocarcinomas. The number of positive cells varied from 10-100% (median, 25%). One sweat gland carcinoma was also strongly positive similar to breast cancers.
Among renal carcinomas, chromophobe carcinomas showed a high frequency (51%) of GATA3-positivity (Fig. 4 C), and this was typically extensive and detected in the majority of tumor cells. Oncocytomas were less commonly positive (17%), but also had high numbers of positive nuclei and additionally displayed some cytoplasmic labeling (Fig. 4 D). Two oncocytoma-chromophobe carcinoma hybrid tumors showed variable positivity in 10-20% of tumor cells. In clear cell carcinomas, positivity was rare (<2%). One of the other positive tumors was a type 2 papillary renal carcinoma, whereas all 15 type 1 papillary carcinomas were negative.
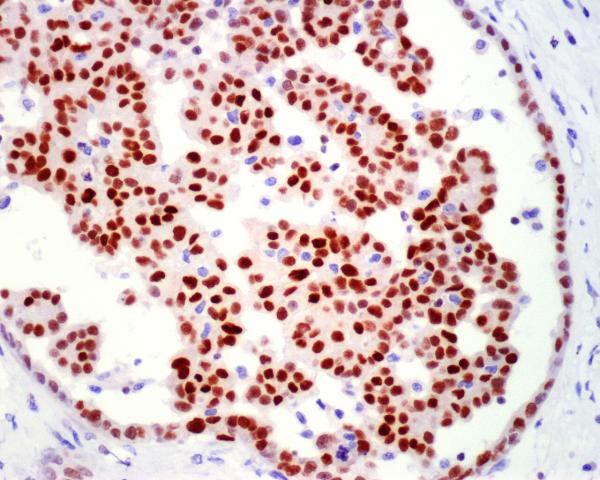
Over half (58%) of malignant mesotheliomas showed nuclear GATA3-positivity. Positivity was detected in both epithelial and sarcomatoid mesotheliomas (Fig. 5). The number of positive cells varied but most positive cases showed a high percentage of positive cells (range: 10-100%, median: 75%).
Fig. 5.
A malignant epithelial mesothelioma with a pseudoglomerular formation within a tubulopapillary pattern shows strong GATA3-positivity.
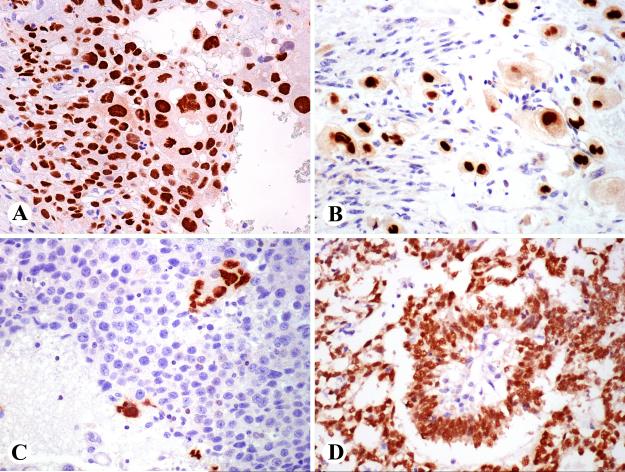
In germ cell tumors, GATA3 positivity was consistently detected in pure choriocarcinomas and choriocarcinoma components of mixed germ cell tumors also shown positive for beta HCG, and in a placental site trophoblastic tumor involving myometrium (Fig. 6 A, B). Also, syncytiotrophoblastic giant cells that were present in 2 seminomas and verified beta HCG-positive were positive for GATA3, whereas seminoma cells were negative in all cases (Fig. 6 C). Endodermal sinus tumors seen as the pure type or as a component of a mixed germ cell tumor were strongly GATA3 positive (Fig. 6 D). Embryonal carcinoma cells were generally negative. However, some embryonal carcinomas contained GATA3-positive focal elements that included thin layers of cuboidal epithelioid cells surrounding embryoid bodies and additionally focal cell clusters in some cases.
Fig. 6.
GATA3 expression in germ cell tumors. A. Choriocarcinomas were strongly positive. B. Myometrial invasion of placental site trophoblastic tumor is positive. C. Seminomas are negative, but isolated syncytiotrophoblastic cells are highlighted. D. Endodermal sinus tumor with Schiller-Duval body in the center is strongly positive.
GATA3-positivity was rare in adenocarcinomas of the lung (6/71). The positive cases were 3 poorly differentiated tumors containing 40-60% of positive cells, 2 mucinous adenocarcinomas with 30 and 60% of positive cells, and one papillary adenocarcinoma with <5% of GATA3-positive cells.
The frequency of GATA3-positivity in carcinomas of stomach, colon, prostate, endometrium, ovary and thyroid did not exceed 7% in any of the above mentioned categories. The percentage of positive cells was 5-30% with the exception of one endometrial and one gastric carcinoma, in which 100% of tumor cells were positive. All gastric signet ring cell carcinomas were negative.
All epithelial neuroendocrine tumors including low-grade tumors such as carcinoids and well-differentiated pancreatic neuroendocrine tumors, and high-grade neuroendocrine carcinomas, such as pulmonary small cell and Merkel cell carcinomas, were negative.
Epithelial components of thymomas were negative but GATA3-positive T-lymphocytes were variably present, especially in type B1 thymomas.
Mesenchymal and neuroectodermal tumors
The results on mesenchymal or neuroectodermal tumors have been summarized in Table 2. Exceptionally prominent GATA3-positivity was detected in pheochromocytoma and extra-adrenal paraganglioma that were GATA3-positive in most cases (>80%) and usually had positivity in >50% of tumor cells. In biphasic synovial sarcoma, glandular epithelial component showed GATA3-positivity in 4/5 cases, whereas only one poorly differentiated spindle cell variant was focally positive. Three of 17 epithelioid sarcomas were also positive.
Table 2.
Expression of GATA3 in 460 mesenchymal and neuroectodermal neoplasms
| Angiosarcoma | 2/30 |
| Clear cell sarcoma | 0/18 |
| Epithelioid sarcoma | 3/17 |
| Fibrothecoma of ovary | 0/14 |
| Gastrointestinal stromal tumor, stomach | 0/24 |
| Granulosa cell tumor of ovary | 0/19 |
| Kaposi sarcoma | 0/25 |
| Leiomyosarcoma, soft tissue (non-visceral) | 5/59 |
| Malignant peripheral nerve sheath tumor | 3/36 |
| Melanoma, metastatic | 0/74 |
| Meningioma | 0/25 |
| Myxofibrosarcoma/undifferentiated sarcoma/MFH | 2/49 |
| Paraganglioma, extra-adrenal | 18/22 |
| Pheochromocytoma | 22/24 |
| Synovial sarcoma | 5/24* |
Expressed in glands of 4 biphasic tumors and one poorly differentiated spindle cell tumor.
Other mesenchymal tumors were only sporadically positive (< 10% of cases in each category). There were examples of myxofibrosarcomas/undifferentiated sarcomas, poorly differentiated angiosarcomas, leiomyosarcomas, and malignant peripheral nerve sheath tumors with focal to extensive nuclear GATA3-positivity. In these categories it was not possible to identify specific features that correlated with GATA3-positivity. All metastatic melanomas and clear cell sarcomas were negative.
DISCUSSION
GATA3 is a multifunctional transcription factor especially linked in the development and function of ductal epithelial cells of breast, urothelia, epidermis, some skin adenxa, and subsets of T-cells. 1-10 In this study we immunohistochemically mapped GATA3 expression in developing, normal adult, and neoplastic tissues to obtain understanding of its distribution and further evaluate its specificity for its common detection targets, mammary and urothelial carcinomas. Furthermore, we explored other potential applications for GATA3 immunohistochemistry in surgical pathology by screening a large number of epithelial and non-epithelial neoplasms.
Despite its complex distribution in epithelial tumors, GATA3 is potentially useful in several specific settings. Strong nuclear labeling obtained with a new monoclonal antibody to GATA3 (Clone L50-823) that is easy to interpret is an additional factor increasing the diagnostic value of this marker. In normal tissues, this antibody yielded similar results as previously noted for breast 3, skin 7, kidney 5, endothelia of great vessels 10, and subsets of lymphocytes 4, which in part validates the used antibody. Presence of GATA3-positive tumor-associated T-lymphocytes also serves as a nearly universal internal control useful in technically validating individual cases.
In the breast, GATA 3 is mainly expressed in luminal epithelia and it has been reported frequently expressed in breast carcinomas and as a potential marker for mammary origin of metastatic carcinoma.13-16 In this study, GATA3 was detected in 92% of primary ductal carcinomas of the breast. Furthermore, it was retained with a high percentage (96%) in metastatic breast cancers. Retention of GATA3 in most metastatic carcinomas makes this marker useful in evaluating metastatic carcinomas, especially when presenting in unusual sites. The frequency of GATA3-positivity found in this study is approximately similar if not higher than that reported in previous studies (80-90%). 14-16
GATA3 was also present in most urothelial carcinomas, but was absent in some high-grade examples. In terms of sensitivity, the findings are similar to a previous study (>80%). 19 GATA3 expression in seminal vesicle epithelia should be taken into account when using this marker in cytologic specimens suspected of urothelial carcinoma. In the differential diagnosis of urothelial vs. pulmonary squamous cell carcinoma one has to consider that some cases of the latter can have a strong GATA3-expression in a majority of tumor cells, so that such positivity is not specific for urothelial carcinoma.
Beyond mammary and urothelial carcinomas, commonly GATA3-positive carcinomas based on this study are basal cell carcinoma (98%), cutaneous squamous cell carcinoma (75%), skin adnexal tumors, malignant mesothelioma (58%), chromophobe carcinoma of the kidney (51%), and ductal carcinomas of salivary gland (43%) and pancreas (37%). In the latter, GATA3 protein expression seems to parallel common expression of GATA3-specific RNA, as previously detected in pancreatic carcinoma. 23 Expression of GATA3 in the above mentioned tumors has to be considered when using this marker to identify mammary or to so degree, urothelial carcinomas, especially in small biopsies or cytologic specimens, in which immunohistochemical findings may have a greater role in diagnosis.
High GATA3-expression in cutaneous squamous cell carcinomas parallels GATA3 expression in developing and adult epidermis. 6,7 Because GATA3 is markedly less commonly detected in cervical, laryngeal, and pulmonary squamous cell carcinomas, GATA3 may have some value in evaluating cutaneous vs. other origin for a squamous cell carcinoma.
In renal cell carcinomas, GATA 3 was expressed in over half of chromophobe carcinomas, while its expression was notably rare in other carcinoma types (<2%), so that GATA3 is of interest in subtyping of renal carcinomas. Also, 2 hybrid chromophobe carcinoma-oncocytoma tumors considered related to chromophobe carcinoma and often occurring in Birt-Hogg-Dubé syndrome 24 were focally GATA3-positive. GATA3-positivity of chromophobe carcinomas and oncocytomas seems to be a new parameter linking these tumors with the distal nephron, which is also GATA3-positive. Older studies considered both of these tumors potentially histogenetically related with the intercalated cells of collecting ducts. 25,26 Electron microscopic studies showed oncocytomas to share distal tubular character in their content of short microvilli. 27 A more recent observation also linking both chromophobe carcinoma and renal oncocytoma with distal nephron is their expression of kidney-specific cadherin 28
Choriocarcinomas are strongly GATA3 positive mirroring the fetal trophoblastic phenotype. In addition, GATA3 labels strongly endodermal sinus tumors, but is absent in seminomas and embryonal carcinoma cells, with exceptional expression around embryoid bodies. However, the latter are believed to have endodermal sinus tumor differentiation29, which seems to explain their GATA3-positivity. Based on these observations, GATA3 can be useful tool in the subtyping of germ cell tumors and especially highlighting trophoblastic and endodermal sinus tumor-like differentiation. A caveat in using GATA3 to identify trophoblasts in the context of uterine tissues is its expression also in decidual stromal cells, as seen in endometrium and extra-uterine endometriosis.
Carcinomas with very infrequent GATA3-expression include pulmonary, gastrointestinal, ovarian and prostatic adenocarcinomas, and adrenocortical and thyroid carcinomas. Rare GATA3-expression in these tumors facilitates detection of metastatic mammary or urothelial carcinomas in those sites. More studies are required to determine biologic significance of the rare GATA3-positive subsets of the above mentioned carcinomas.
Mesenchymal and neuroectodermal tumors of various types generally showed a low frequency of GATA3 expression. An exception was paraganglioma (both adrenal and extra-adrenal), which were positive in >80% of cases. GATA3 may therefore have value in separating paragangliomas and epithelial neuroendocrine tumors, as the latter are uniformly negative, as recently reported. 30
There is little information on GATA3 expression in sarcomas. In this study, glandular elements of biphasic synovial sarcoma were usually positive, and 20% of epithelioid sarcomas were variably positive indicating that GATA3-positivity may reflect epithelial differentiation in mesenchymal tumors. In most groups of other sarcomas, 0-10% of cases showed positivity. However, GATA3 is mentioned as gene expressed in MFH/undifferentiated pleomorphic sarcoma by RNA-expression array studies and suggested as an adverse prognostic factor based on a small cohort. 31
In summary, we examined immunohistochemically a large number of epithelial and mesenchymal tumors for GATA 3. This marker is a sensitive although not totally specific for mammary and urothelial carcinomas. Commonly GATA3 positive other epithelial tumors include basal cell carcinoma, cutaneous squamous cell carcinoma, skin adnexal tumors, choriocarcinoma, endodermal sinus tumor, renal chromophobe carcinoma, malignant mesothelioma, and salivary gland and pancreatic ductal adenocarcinoma. In addition, GATA3 is expressed with a lower frequency (generally <10%) in a wide variety of carcinomas. However, GATA3 is rarely expressed in neuroectodermal and mesenchymal tumors, except in pheochromocytoma/paraganglioma, which helps to distinguish the latter from neuroendocrine carcinomas. Despite its multispecificity, GATA3 is a potential tool for characterization of carcinomas and selected mesenchymal and neuroectodermal tumors. Table 3 outlines potential diagnostic applications of GATA3 showing contrasting pairs of expectedly positive and negative tumors.
Table 3.
Potential discriminatory value of GATA3 immunohistochemistry in tumor diagnosis –examples of contrasting pairs of tumors with potential morphologic mimicry.
| Positive tumors | Negative tumors |
|---|---|
| Metastatic lobular carcinoma of the breast |
Gastric/intestinal signet ring cell carcinoma |
| Metastatic ductal carcinoma | Pulmonary/gastrointestinal/ovarian carcinoma |
| Urothelial carcinoma | Prostate carcinoma |
| Squamous carcinoma of skin origin | Squamous carcinoma of pulmonary origin |
| Malignant mesothelioma | Pulmonary adenocarcinoma |
| Pancreatic adenocarcinoma | Gastrointestinal adenocarcinoma Cholangiocarcinoma |
| Chromophobe renal carcinoma | Clear cell renal carcinoma |
| Endodermal sinus tumor Choriocarcinoma |
Embryonal carcinoma, Seminoma |
| Paraganglioma | Neuroendocrine carcinoma |
Acknowledgments
Source of Funding: This work was supported as a part of NCI’s intramural research program.
Footnotes
Conflicts of Interest: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222:42–49. doi: 10.1002/jcp.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng R, Blobel G. GATA Transcription Factors and Cancer. Genes Cancer. 2010;1:1178–1188. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 4.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oosterwegel M, Timmerman J, Leiden J, et al. Expression of GATA-3 during lymphocyte differentiation and mouse embryogenesis. Dev Immunol. 1992;3(1):1–11. doi: 10.1155/1992/27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellheyer K, Krahl D. Expression pattern of GATA-3 in embryonic and fetal human skin suggests a role in epidermal and follicular morphogenesis. J Cutan Pathol. 2010;37:357–361. doi: 10.1111/j.1600-0560.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 8. [Google Scholar]
- 9.Home P, Ray S, Dutta D, et al. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. J Biol Chem. 2009 Oct 16;284(42):28729–37. doi: 10.1074/jbc.M109.016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ralston A, Cox BJ, Nishioka N, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137:395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 11.Song H, Suehiro J, Kanki Y, et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J Biol Chem. 2009;284:29109–24. doi: 10.1074/jbc.M109.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Esch H, Devriendt K. Transcription factor GATA3 and the human HDR syndrome. Cell Mol Life Sci. 2001;58:1296–1300. doi: 10.1007/PL00000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimino-Mathews A, Subhawong AP, Illei PB, et al. GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. Hum Pathol. 2013 Jan 31; doi: 10.1016/j.humpath.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez RS, Wang UJ, Kraus T, et al. GATA-3 expression in male and female breast cancers: comparison of clinicopathologic parameters and prognostic relevance. Hum Pathol. 2012 Dec 21; doi: 10.1016/j.humpath.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Yoon NK, Maresh EL, Shen D, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol. 2010;41:1794–1801. doi: 10.1016/j.humpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Shi J, Wilkerson ML, et al. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64. doi: 10.1309/AJCP5UAFMSA9ZQBZ. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–680. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto H, Izumi K, Yao JL, et al. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol. 2012;43:2033–2040. doi: 10.1016/j.humpath.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472–1476. doi: 10.1097/PAS.0b013e318260cde7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruver AM, Amin MB, Luthringer DJ, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Ach Pathol Lab Med. 2012;13:1339–1346. doi: 10.5858/arpa.2011-0575-OA. [DOI] [PubMed] [Google Scholar]
- 21.Esheba GE, Longacre TA, Atkins KA, et al. Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am J Surg Pathol. 2009;33:347–353. doi: 10.1097/PAS.0b013e3181908e24. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen M. A simple method for generating multitissue blocks without special equipment. Appl Immunohistochem Mol Morphol. 2012;20:410–412. doi: 10.1097/PAI.0b013e318245c82f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulbinas A, Berberat PO, Dambrauskas Z, et al. Aberrant gata-3 expression in human pancreatic cancer. J Histochem Cytochem. 2006;54:161–169. doi: 10.1369/jhc.5A6626.2005. [DOI] [PubMed] [Google Scholar]
- 24.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–52. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Störkel S, Steart PV, Drenckhahn D, et al. The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56:237–45. doi: 10.1007/BF02890022. [DOI] [PubMed] [Google Scholar]
- 26.Störkel S, Pannen B, Thoenes W, et al. Intercalated cells as a probable source for the development of renal oncocytoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:185–9. doi: 10.1007/BF02890016. [DOI] [PubMed] [Google Scholar]
- 27.Eble JN, Hull MT. Morphologic features of renal oncocytoma: a light and electron microscopic study. Hum Pathol. 1984;15:1054–61. doi: 10.1016/s0046-8177(84)80249-x. [DOI] [PubMed] [Google Scholar]
- 28.Shen SS, Krishna B, Chirala R, et al. Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol. 2005;18:933–40. doi: 10.1038/modpathol.3800373. [DOI] [PubMed] [Google Scholar]
- 29.Ulbright T. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005;18(Suppl 2):WS61–79. doi: 10.1038/modpathol.3800310. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka D, Wang BY, Sun CCJ. A study of Phox2B and Gata3 expression in tumors of autonomic nervous system derivation. Abstract. Mod Pathol. 2013;26(Suppl 2):136. [Google Scholar]
- 31.Daigeler A, Klein-Hitpass L, Stricker I, et al. Malignant fibrous histiocytoma-pleomorphic sarcoma, NOS gene expression, histology, and clinical course. A pilot study. Langenbecks Arch Surg. 2010;395:261–275. doi: 10.1007/s00423-009-0465-0. [DOI] [PubMed] [Google Scholar]