Abstract
Objective: The aim of this study was to develop birth weight references for twins. Mean birth weights of individual twins are lower than those of singletons, hence singleton birth weight curves may not be suitable to assess twin birth weights.
Study design: Twin birth weight curves were developed according to gestational age, gender, parity and mode of conception. The curves are based on population-based data of 40,494 twins born in Flanders, Belgium between 1987 and 2007.
Results: A different growth potential was found comparing the birth weights of twins and singletons. Twins deviate from the singleton curve from 30 weeks gestational age on.
Conclusion: Our study underlines that singleton birth weight curves differ from twin birth weight curves. We developed specific twin birth weight curves can be used in clinical practice in order to follow growth patterns of twins in utero.
Keywords: Standards, twin birth weight, birth weight curves, twin specific birth weight curves
Introduction
Birth weight is an important determinant of neonatal outcome. Neonates with a birth weight below the 10th percentile are diagnosed as growth retarded and these children are at a higher risk for neonatal morbidity and mortality (Devlieger et al., 2000; Gielen et al., 2008b). Twins are eight times more likely to have a low birth weight and ten times more likely to have a very low birth weight compared to singletons (Min et al., 2000). Amongst others shared placental masses and peripheral umbilical cord insertions, which are more common in twin pregnancies, are responsible for birth weight disadvantages in twins (Blickstein et al., 2006; Gielen et al., 2008a). In singletons the highest growth peak sets between 36 and 38 weeks gestational age, for twins this peak is situated between 32 and 36 weeks (Gielen et al., 2008b; Min et al., 2000; Loos et al., 2005). Notwithstanding different growth potential in singletons and twins (Gielen et al., 2008b; Loos et al.,2005), twin specific birth weight charts in Western countries are still lacking while birth weights of singletons were mapped already in 1963 (Devlieger et al., 2000). Several North – American studies made standards and references for twin birth weight, specifically for twins born in the USA (Min et al., 2000). In Belgium, we found only one previous study concerning twin birth weight curves (Gielen et al., 2007). Worldwide there are large ethnic differences in relation to the distribution of birth weights and the average birth weight (Hur et al., 2005; Goedhart et al., 2008). There are two main groups of determinants affecting birth weight, i.e. constitutional determinants and lifestyle-related determinants (e.g. smoking behavior of the mother, maternal body mass index). The constitutional determinants, such as maternal height, may contribute to the natural cause of (ethnic) heterogeneity in birth weight (Goedhart et al., 2008).
There is a growing interest for twin-specific birth weight curves for gestational age and mode of conception, in that twin pregnancies are nowadays more frequent (twin epidemic in assisted reproduction). In Belgium, about 1.8% of all pregnancies are twin pregnancies. Approximately 63% of all twins is the result of a spontaneous conception (Cammu et al., 2008). Just over half (57.4%) of multiple births in Flanders has a birth weight less than 2500 grams and a similar percentage (57.2%) of twins are born before 37 weeks gestational age (Cammu, et al. 2008). The purpose of this study was to create birth weight references for a twin population. These birth weight curves could effectively be used in clinical practice by gynecologists, neonatologists, pediatricians and researchers studying the perinatal morbidity and mortality (Devlieger et al., 2000; Roberts et al., 1999). To encourage and promote the use of these curves in clinical practice we created a user friendly percentile map.
Methods
The population-based birth register from the Study Centre for Perinatal Epidemiology (SPE) was used for our analysis. From 1986 on, each birth of a singleton or multiple in Flanders (Northern part of Belgium) and the University Hospital of Brussels, is registered in this database. The official and SPE-encoded form is completed by the midwife or obstetrician who supervised the birth and is subsequently returned to the SPE. As such, approximately 60,000 births are added each year. All data in this database refer to live- and stillborn children with a birth weight of 500 grams or more. Data regarding the certainty of gestational age is also included. Gestational age is confirmed with ultrasound and is recorded in weeks. The SPE-database uses full weeks of pregnancy, e.g. 35 weeks and 6 days is considered as 35 weeks. The birth weight is not rounded. A number of measures are taken in order to obtain optimal registration. For example, each participating hospital receives guidelines on how complete the obstetric and perinatal file on an annual basis. Furthermore, by using a fault detection program, the quality of data is checked for internal inconsistencies, accuracy and completeness. When data are incomplete, the specific maternity wards receive additional questionnaires or they are contacted in order to receive the missing data (Cammu et al., 2009; Dhont et al., 1999). The minimum epidemiological requirements for standards of birth weights by gestational age are described in the article by Devlieger et al.: “... the population should be unselected (not only a hospital population), preferably within a defined geographical unit, a population without major ethnic diversity” (Devlieger et al., 2000). If the standards meet these requirements, which is the case in SPE-data, they assume quality and reliability.
Study sample
Inclusion criteria for this study are twin pregnancies, a birth weight greater than 500 grams and a gestational age from 24 to 40 weeks. Exclusion criteria were present malformations and stillbirth, in that the abnormal birth weight of malformed fetuses or stillbirths may indicate an early death with a birth weight not corresponding to the gestational age. Other studies developing birth weight curves generally use the same inclusion and exclusion criteria (Roberts and Lancaster, 1999; Devlieger et al., 2000; Min et al., 2000; Kato, 2004; Gielen et al., 2007; Gielen et al., 2008b). The included variables in our analysis were: parity, mode of conception (naturally, ovulation induction and IVF-ICSI), gestational age, mode of delivery, birth weight and sex. Unfortunately there were no data available about zygosity and chorionicity.
Between 1987 (the first full year of registration) and 2007, 42.629 individual twins (not paired) were initially identified. Of these, 703 were excluded because of fetal deaths and 1256 for malformations. Because we developed percentiles from 24 to 40 weeks, 63 twins who were born before 24 weeks and 82 twins who were born later than 40 weeks were excluded. There are no missing values detected, albeit 31 outliers. The result hereby is that the study sample is filtered by 0.08% extreme outliers. An outlier is generally defined as an extreme high or low value, in this research we considered outliers as values three times the standard deviation away from the mean. The outliers were excluded, in that they were most likely the result of incorrect registration of a too high or too low gestational age and less the result of entering the wrong birth weight (Cammu et al., 2009). The remaining twins (n = 40.494) made up the final study sample. For mode of conception in twins, a separate subgroup was required because only data from the past 10 years (1998-2007) were available for analysis. After considering the in- and exclusion criteria, the final study sample for mode of conception consists of 21.071 individual twins.
Data-analysis
The developed birth weight curves are based on cross-sectional birth weight data. Birth weight has a positive linear relationship with gestational age. The distribution of birth weight has a unimodal structure and skewed to the left by extremely preterm births, coupled with extremely low birth weights. A retrospective non-interventional research design is used to investigate the influence of gestational age, parity, sex and mode of conception on birth weight.
The statistical analysis of the perinatal data was performed by the statistic software package SPSS (version 13). Curves were created for gestational week 24 to week 40. For these gestational ages, a sufficient number (≥ 100) of twins were available for calculation of percentiles per week. The percentiles were based on the actual value of the birth weights of twins. A note hereby is that birth weight is rounded off with 5 to 10 grams in some maternity wards, it was not possible to account for this in our analysis (Cammu et al., 2009). Another remark is that there may be an introduction of bias because the SPE only include neonates with a birth weight greater or equal to 500 grams. The surviving neonates weighing less than 500 grams are as such not included in the birth weight curves (Devlieger et al., 2000).
The data for this research couldn’t distinguish the first and second born of the same twin.
Accordingly it was not possible to create birth weight percentiles to compare the birth weights for the first and second born in one twin.
Attention is paid to influencing factors (confounders) by ANOVA and independent sample T-test, according to the literature. The variables available at the SPE are maternal age and mode of delivery. The adjustment for ethnicity was not possible because there was not enough data on ethnicity for the complete period studied. The independent sample t-test was used to determine significant birth weight differences according to maternal age and mode of delivery.
Results
Our results show a polynomial relationship between birth weight and gestational age. Our multivariate analyses shows that mode of conception has no impact on birth weight differences. Parity, gender and mode of delivery do have an influence on the birth weight.
Birth weight curve for the general twin population
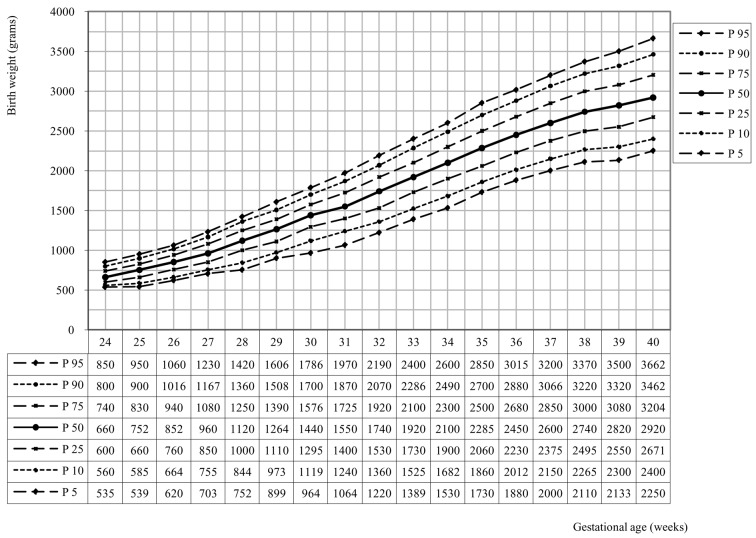
As shown in figure 1, the relationship between birth weight and gestational age is obvious, in that the average birth weight increases with 141 grams per week up to 40 weeks. At 32 weeks there is a visible maximum weight increase of 190 grams.
Fig. 1. Birth weight curve for all twins (n = 40.494).
Table 1 shows the characteristics of the population, table 2 indicates the average twin birth weight per gestational age.
Table I. Characteristics of the twins.
| Mean | Standard Deviation | Range | |
| Maternal age | 30,0 years | 5,0 | 14-51 |
| Birth weight | 2396 grams | 562 | 500-4750 |
| Median | Min | Max | |
| Parity | 2 | 1 | 16 |
| Percentage | |||
| Gender distribution: | |||
| * Male | 50,4% | ||
| * Female | 49,6% | ||
| Induction | 24,2 % | ||
| Mode of delivery: | |||
| * vaginally | 57.2% | ||
| * planned caesarean sections | 27.9% | ||
| * secondary caesarean sections | 14.9% | ||
Table II. Average twin birth weight per gestational age.
| Gestational age (weeks) | Number of twins | Average birth weight (grams) | Standard Deviation (grams) | Median (grams) |
| 24 | 119 | 672 | 94 | 660 |
| 25 | 154 | 751 | 122 | 752 |
| 26 | 233 | 852 | 134 | 852 |
| 27 | 264 | 967 | 171 | 960 |
| 28 | 343 | 1120 | 204 | 1120 |
| 29 | 445 | 1251 | 218 | 1264 |
| 30 | 594 | 1419 | 243 | 1440 |
| 31 | 747 | 1548 | 268 | 1550 |
| 32 | 1260 | 1725 | 293 | 1740 |
| 33 | 1734 | 1908 | 305 | 1920 |
| 34 | 3043 | 2090 | 326 | 2100 |
| 35 | 4239 | 2281 | 346 | 2285 |
| 36 | 7192 | 2453 | 351 | 2450 |
| 37 | 9157 | 2608 | 372 | 2600 |
| 38 | 7342 | 2740 | 384 | 2740 |
| 39 | 2532 | 2814 | 413 | 2820 |
| 40 | 1096 | 2935 | 428 | 2920 |
| 494 |
In multivariate analyses we examined the impact of several possible confounders.
Influence of mode of conception on twin birth weight
In this study we did not find a birth weight difference between twins after medical assisted conception and natural conception. After classification for mode of conception, the number of twins was only sufficient for gestational ages 31 to 39 weeks in order to generate percentile curves.
Birth weight curve by parity and gender
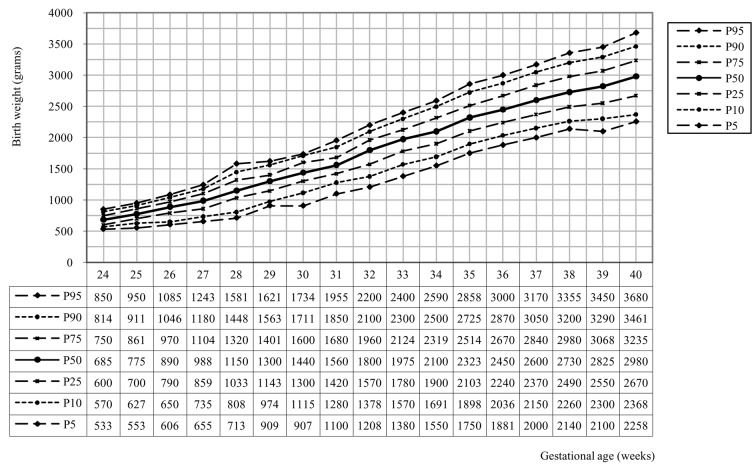
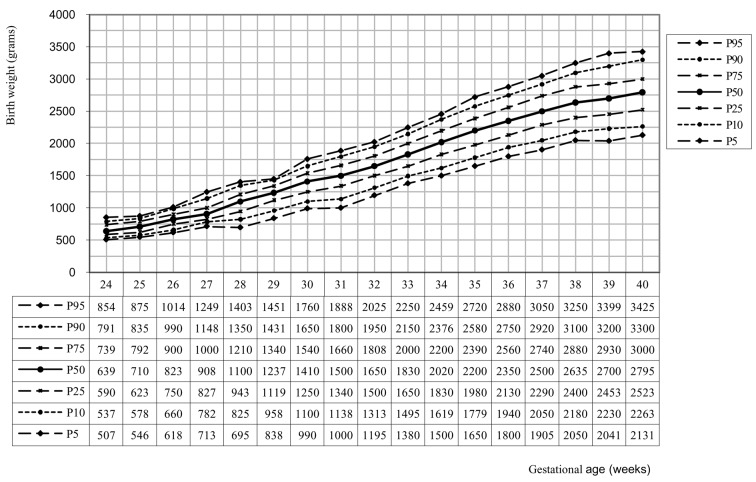
We also developed twin birth weight curves along parity and gender (see figure 2 and 3). The percentiles from these curves are representative from 29 weeks pregnancy on (28 weeks in primparae with boys). In primiparae and multiparae boys weigh more than girls. For both girls and boys, children in primiparae weigh less than children in multiparae.
Fig. 2. Birth weight curve for primiparae + boys (n = 9 719).
Fig. 3. Birth weight curve for primiparae + girls (n = 9 649).
The influence of mode of delivery on twin birth weight
In this study we have calculated the influence of mode of delivery. The independent sample t-test shows that the birth weight difference in the total group of vaginal deliveries and caesarean sections is significantly different. The average twin birth weight among vaginal deliveries is 89 grams higher than the average twin birth weight in caesarean sections (p < 0.001). Of all twins in Flanders, 43.4% is born by caesarean section. According to the SPE data, the group of the caesarean sections of twins 65% is born by a planned caesarean section and 35% is born by a secondary caesarean section. We noticed a significant difference in mean birth weight of 92 grams in favor of planned caesarean sections.
The independent sample T-test was used to calculate the difference in gestational age between secondary caesarean sections and planned ones. The gestational age in secondary caesarean sections was (in full weeks) 35.2 weeks, in planned ones 35.8 weeks. Clinically, the difference is a half week (p < 0.001).
It is of importance to evaluate the usefulness of twin – specific birth weight curves by comparing them with the previous used singleton birth weight curves.
Singletons versus twins
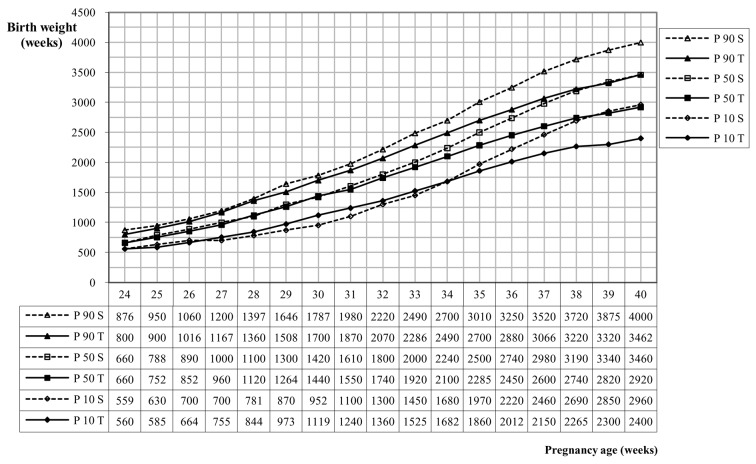
Figure 4 shows a comparison of the singleton-curve (Devlieger et al., 2000) and the new twin birth weight curve. This figure depicts a more sigmoid - shaped curve in singletons, while the curves of the twins are more straightforward. As for the P90 and the P50, twins and singletons grow similar until about 30 weeks of gestation. From this point on, the percentiles of the individual twins deviate from singletons with lower birth weights for twins. For the P10 the birth weight of the singletons are lower from 27 to 33 weeks of gestation in comparison with twins.
Fig. 4. Comparative birth weight curve SPE singletons (S) (n = 429 070) versus SPE twins (T) (n = 40 494).
Discussion
Based on cross-sectional data, this study examined retrospectively how birth weight of twins is influenced by gestational age, parity, gender, mode of conception, maternal age and mode of delivery.
Our results indicate that birth weight curves of singletons differ from birth weight curves of twins. There is an important divergence between both curves from 30 weeks gestational age on. We can cautiously notice that twin birth weight curves are more suitable to assess twin birth weights because of the differences between the birth weight curves of twins and singletons.
Variables influencing birth weight of twins: maternal age and mode of delivery
Maternal age and mode of delivery have an impact on the birth weight of twins. As described in the literature by Delbaere (Delbaere et al., 2008) and Wang (Wang et al., 2005), mean twin birth weight is paradoxically higher in elderly primiparae between 35 and 40 years compared to younger mothers, which is not the case in singletons (Delbaere et al., 2008).
After calculating the influence of mode of delivery, we found that birth weights in the total group of vaginal deliveries and caesarean sections is significantly different (p < 0.001).
The reason for caesarean sections differ fundamentally between twins and singletons. For twins, the main reasons for caesarean section are twins themselves and twins have more risk of abnormal positions (mainly breech) resulting in caesarean section.
Preterm birth in twins is usually the result of spontaneous preterm labor, premature rupture of membranes without labor and cesarean delivery (Hediger et al., 2005). This might explain the birth weight difference between spontaneous and non-spontaneous twin deliveries, with lower birth weights after spontaneous labor.
Influence of mode of conception on twin birth weight
Our results show no difference in birth weight according mode of conception. This is an important finding because, in Belgium, approximately 37% of all twins is the result of a medical assisted conception.
Comparison of our new – developed curve with other curves
The first birth weight standard for twins, used for comparison, belongs to Gielen et al. (2007). By using the SPE database, it was possible to include a larger sample in this study (n = 40.494 twins, compared to 8.454 twins in Gielen). This is particularly important for the lower gestational ages (with smaller proportions of twins). Our curves at gestational age of 24 weeks is based on a sample size of 119 twins; in the study of Gielen et al. (2007), no sample sizes were reported for different gestational ages, but we estimate the sample size at gestational age 24 weeks to be 25 twins. The advantage of the research of Gielen is the inclusion of data on chorionicity and zygosity. Furthermore, twins in our analysis are not paired, as such the two members of a twin are not linked to each other. As a consequence, it is not possible to calculate the birth weight difference within a twin and no separate analysis could be done for heterogeneous (dizygotic) twins. By these major differences between the two databases, it was challenging to compare these curves. First, Gielen et al. (2007) developed their curves until 42 weeks. Since no sample sizes are reported, it is difficult to assess whether the percentiles are representative. Secondly, the percentiles of the curves in this study are created with the true values of the birth weights and are not made fluent. Finally, they divided their curves according to chorionicity which complicates comparison. Another remark is the smaller distribution of the percentiles from Gielen (Gielen et al., 2007). These differences may be due to the use of a smaller samples resulting in less representative percentiles per pregnancy week or the use of a computer program to smooth their curves.
Finally, our birth weight curve has been compared with a Japanese twin birth weight curve (Kato, 2004). We assessed a divergence between the Japanese curve and ours from 32 weeks gestational age on. From this point on, the Japanese twins weigh approximately 150 to 200 grams less than the Flemish twins. The average birth weights and their distribution in Caucasian countries are higher than in East Asian countries for both boys and girls (Hur et al., 2005). For this reason Asian birth weight curves cannot be used for our population. This finding puts further emphasis on the importance of population-based birth weight curves in a Western population specifically (Hur et al., 2005; Goedhart et al., 2008).
Comparison of the SPE-singletons - curve versus the SPE-twins-curve
In singletons the highest growth peak is between 36 and 38 weeks, whereas in twins this peak is between 32 and 36 weeks (Min et al., 2000; Loos et al., 2005; Gielen et al., 2008b). It is not clear why this acceleration starts earlier in twin pregnancy. However between 30 and 32 weeks gestational age appears to be a significant cut-off value for twin neonatal morbidity and mortality. Neonates born after 32 weeks have a lower incidence of morbidity and mortality compared with neonates who are born very preterm (before 32 weeks) (Cammu et al., 2009). The percentiles of twins and singletons diverge significantly from 34 weeks gestational age on, as has been reported by other researchers previously (Roberts and Lancaster, 1999; Gielen et al., 2008b). As such, it is recommended to observe the fetal weight of the twins in practice, in order to notice changes in the growth pattern accurately (Blickstein, 2004). The findings of Gielen et al. show that neonatal mortality is associated with lower birth weights, shorter gestational age and more discrepancy in the growth of twins. Neonatal mortality is particularly important when the birth weight is located below the P3 (Gielen et al., 2008b).
Twin birth weight curves according to gestational age are important in clinical practice, in that they allow more accurate identification of growth retarded twins at birth. Birth weight curves for singletons are unsuitable to predict and assess the birth weight of twins. For this reason it is important to use twin specific birth weight curves in order to detect twins with an increased risk of neonatal morbidity and mortality. These curves should be used together with other obstetric and ultrasound data.
Strengths of the SPE-database are its population-based character and the considerable sample size. This is particularly of interest when analyzing the birth weight of twins – making up only a subset of the general population – and at less current gestational ages. The developed curves however don’t consider discongruent growth within a twin because the study uses data of non paired twins, which is an important limitation of the twin birth weight curves.
Further research is required for the validation and implementation of twin birth weight curves into clinical practice. Only when this condition is satisfied, twins at risk of perinatal morbidity and mortality can be identified. Twin specific birth weight curves are important for obstetricians to make predictions of the birth weights of twins. Pediatricians can use these curves to situate the birth weight of the individual twins by gender in relation to other twins. It is therefore crucial to disseminate this knowledge among gynecologists, pediatricians, midwives and other stakeholders and encourage the use of the twin-specific birth weight for gestational age references.
References
- Blickstein I. Is it normal for multiples to be smaller than singletons? Best Pract Res Clin Obstet Gynaecol. 2004;18:613–623. doi: 10.1016/j.bpobgyn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Blickstein I, Mincha S, Goldman RD. The Nortwestern twin chorionicity study: testing the ‘placental crowding’ hypothesis. J Perinat Med. 2006;34:158–161. doi: 10.1515/JPM.2006.028. [DOI] [PubMed] [Google Scholar]
- Cammu H, Martens G, Martens E. Perinatale activiteiten in Vlaanderen 2008. SPE; 2009. [Google Scholar]
- Delbaere I, Verstraelen H, Goetgeluk S. Perinatal outcome of twin pregnancies in women of advanced age. Human Reproduction. 2008;23:2145–2150. doi: 10.1093/humrep/den134. [DOI] [PubMed] [Google Scholar]
- Devlieger H, Martens G, Bekaert A. Standaarden van geboortegewicht-voor-zwangerschapsduur voor de Vlaamse boreling. Tijdschr Geneesk. 2000;56:1–14. [Google Scholar]
- Dhont M, De Sutter P, Ruyssinck G. Perinatal outcome of pregnancies after assisted reproduction: A case-control study. Am J Obstet Gynacol. 1999;181:688–695. doi: 10.1016/s0002-9378(99)70514-4. [DOI] [PubMed] [Google Scholar]
- Gielen M, Lindsey PJ, Derom C. Modeling Genetic and Environmental Factors to Increase Heritability and Ease the Identification of Candidate Genes for Birth Weight: A Twin Study. Behav Genet. 2008;38:44–54. doi: 10.1007/s10519-007-9170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, Lindsey PJ, Derom C. Twin-Specific Intrauterine ‘Growth’ Charts Based on Cross-Sectonal Birthweight Data. Twin Res Hum Genet. 2008;11:224–235. doi: 10.1375/twin.11.2.224. [DOI] [PubMed] [Google Scholar]
- Gielen M, Lindsey PJ, Derom C. Twin Birth Weight Standards. Neonatology. 2007;92:164–173. doi: 10.1159/000102055. [DOI] [PubMed] [Google Scholar]
- Goedhart G, van Eijsden M, van der Wal M. Ethnic differences in term birthweight: the role of constitutional and environmental factors. Paediatr Perinat Epidemiol. 2008;22:360–368. doi: 10.1111/j.1365-3016.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Luke B, Gonzales-Quintero VH. Fetal growth rates and the very preterm delivery of twins. Am J Obstet Gynecol. 2005;193:1498–1507. doi: 10.1016/j.ajog.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hur Y, Luciano M, Martin NG. A Comparison of Twin Birthweight Data From Australia, the Netherlands, the United States, Japan, and South Korea: Are Genetic and Environmental Variations in Birthweight Similar in Caucasians and East Asians? Twin Res Hum Genet. 2005;8:638–648. doi: 10.1375/183242705774860169. [DOI] [PubMed] [Google Scholar]
- Kato N. Reference birthweight range for multiple birth neonates in Japan. BMC Pregnancy and Childbirth. 2004;4:1–9. doi: 10.1186/1471-2393-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RFL, Derom C, Derom R. Determinants of birthweight and intrauterine growth in liveborn twins. Paediatr Perinat Epidemiol. 2005;19(Suppl 1):15–22. doi: 10.1111/j.1365-3016.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Min SJ, Luke B, Gillespie B. Birth weight references for twins. Am J Obstet Gynecol. 2000;182:1250–1257. doi: 10.1067/mob.2000.104923. [DOI] [PubMed] [Google Scholar]
- Roberts CL, Lancaster P. National birthweight percentiles by gestational age for twins born in Australia. J Paediatr Child Health. 1999;35:278–282. doi: 10.1046/j.1440-1754.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Wang YA, Sullivan EA, Black D. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83:1650–1658. doi: 10.1016/j.fertnstert.2004.12.033. [DOI] [PubMed] [Google Scholar]