Abstract
Objective: to explore the potential of 3D Power Doppler Angiography (3D PDA) to evaluate the cerebral circulation in normal and growth restricted fetuses (IUGR).
Study design: in a pilot study, we enrolled 51 appropriate for gestational age (AGA) pregnancies and 17 singleton pregnancies presenting IUGR, all between 22 and 38 weeks of gestation. Using 3D power Doppler ultrasound, a volume acquisition of the fetal brain was performed. Two regions of interest (ROI) were defined within the fetal brain. Zone 1 is anterior to the cavum septi pellucidi (CSP). Zone 2 is defined by a rectangle obtained tracing a contour between the temporal bones as wide as the CSP, corresponding to the area of the middle cerebral artery. The Flow Index (FI), the Vascularization Index (VI), the Vascularization and Flow Index (VFI) were determined in both areas in both IUGR and AGA fetuses by a single operator. IUGR fetuses were divided into three groups: Group 1, with normal pulsatility index (PI) of umbilical artery (UA), middle cerebral artery (MCA) and ductus venosus (DV); Group 2, IUGR fetuses with abnormal UA PI, normal MCA PI, normal DV PI; in Group 3, IUGR fetuses with abnormal UA PI, MCA PI and DV PI.
Results: FI and VFI values of zone 1 were increased in Group 1.Values of VFI in zone 2 were increased in Group 2.
Conclusions: Our findings are in line with recent studies in growth-restricted fetuses suggesting that the anterior cerebral artery shows Doppler signs of vasodilatation before these are observed in the MCA, demonstrating the “frontal brain sparing effect”.
Keywords: Growth restricted fetus, three dimensional power Doppler angiography, brain sparing effect
Introduction
The evaluation of fetal brain blood flow can be considered very important because deficits in the perfusion of this territory may lead to inadequate development of the central nervous system and even jeopardize fetal vitality (Fouron et al., 2005; Geva et al., 2006; Geva et al., 2006).
Fetal intrauterine growth restriction (IUGR) associated with placental insufficiency can present well-recognized perinatal and long-term consequences (Fouron et al., 2005). Some authors demonstrated that neurodevelopment dysfunction in IUGR infants involves general cognitive competence, suggesting dysfunction in the frontal lobe networking, limbic system and hippocampus and changes in the morphology of neural structures such as the retinal optical nerve (Geva et al., 2006; Geva et al., 2006).
The presence of neurological damage originating in different brain areas is associated with an unpaired blood supply (Scherjon et al., 2000). The “brain sparing effect” (blood flow centralization process) can be considered as an adaptive response that preserves brain oxygen supply in the presence of chronic hypoxia (Marsal et al., 2002). This process is identified clinically by a reduced Doppler pulsatility index (PI) in the middle cerebral artery (MCA) (Baschat et al., 2000; Baschat and Harman, 2001). However, vasodilatation of the MCA might have a poor sensitivity to detect fetuses in the initial stages of increased brain perfusion.
Longitudinal studies on Doppler evaluation of different brain arteries in the presence of growth restriction suggest that MCA PI is reduced in a later stage than other brain vessels, such as the anterior cerebral artery (Dubiel et al., 2002; Figueroa-Diesel et al., 2007).
The standard technique used to assess fetal blood flow is usually the bi-dimensional Doppler. In contrast to this conventional method, which analyzes the frequency shift of blood velocity information, power Doppler sonography uses the amplitude component of the signals received to represent the number of moving blood cells (Pairleitner et al., 1999). In fact power Doppler is useful in situations of low-velocity blood flow (Rubin et al., 1994) because it allows the detection of minimal alterations in blood flow (Rubin et al., 1995). Moreover, power Doppler does not show aliasing effect and the colour map is independent of the insonation angle (Yu et al., 2003).
The introduction of 3D power Doppler (3D-PD) and the vascularization histogram allowed to quantify the vascularization and blood flow to the placenta and several fetal organs (Merce et al., 2004; Merce et al., 2005).
The use of 3D-PD is useful in the evaluation of fetal brain vessels because of their small caliber.
Aim of the study
The aim of the present study is to explore the possible use of 3D Power Doppler Angiography (3D-PDA) using VOCAL software (GE Healthcare, USA) in the assessment of different cerebral regions in normal and growth restricted fetuses (IUGR). This is a pilot study, that means a small experiment designed to test the method and gather information prior to a larger study.
Methods
Between January 2010 and April 2011, 17 singleton pregnancies with intrauterine growth restriction (IUGR) and 51 appropriate for gestational age pregnancies (AGA) were included. Pregnancies with maternal complications, fetal malformations or chromosomal defects, or conceived after assisted reproduction, were excluded. Gestational age at the enrollment varied between 22 and 38 weeks, based on first trimester ultrasound dating of pregnancy. In all cases the growth potential of each fetus was confirmed after birth.
All ultrasound examinations were performed using General Electric E 8 (General Electric Corp., Milkwaukee, WI, USA) with a 5-MHz trans-abdominal probe equipped with automatic volume measurements, colour, pulsed and power Doppler options.
IUGR was defined as an ultrasound-estimated fetal weight below the 10th centile (P10) for gestational age according to the Hadlock 4 equation for fetal weight estimation (Hadlock et al., 1985), using biparietal diameter, head circumference, abdominal circumference and femur length (Baschat et al., 2001).
Pulsed wave Doppler flow analysis of the umbilical artery was obtained from a free-floating central section of the cord at an angle close to 0°.
The MCA is sampled at the proximal end of the vessel close to the circle of Willis with a near 0° angle of insonation. Each uterine artery can be assessed using the colour Doppler flow to identify the crossing over with the internal iliac artery and vein just before it enters the uterus. Three subsequent blood velocity waveforms for each vessel were analyzed for PI according to Gosling et al. (Gosling et al., 1971). For each of these three vessels, an abnormal PI was defined as a deviation from the mean by 20% (Hernandez-Andrade et al., 2002). The results were checked against previously published reference ranges (Mari and Deter, 1992; Hofstaetter, Dubiel et al. 1996; Gudmundsson and Maršál, 1988).
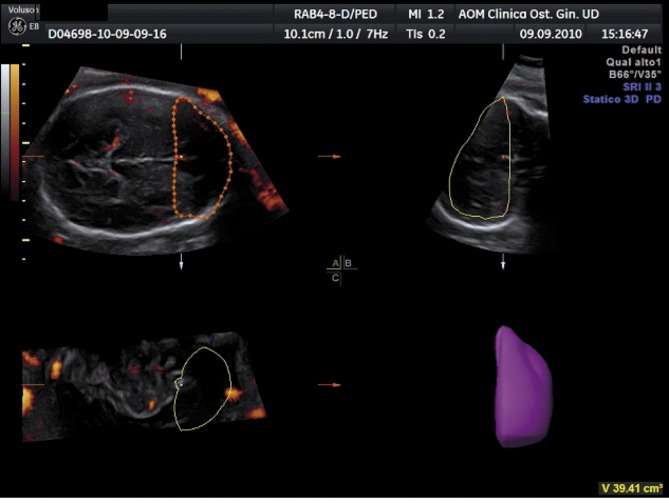
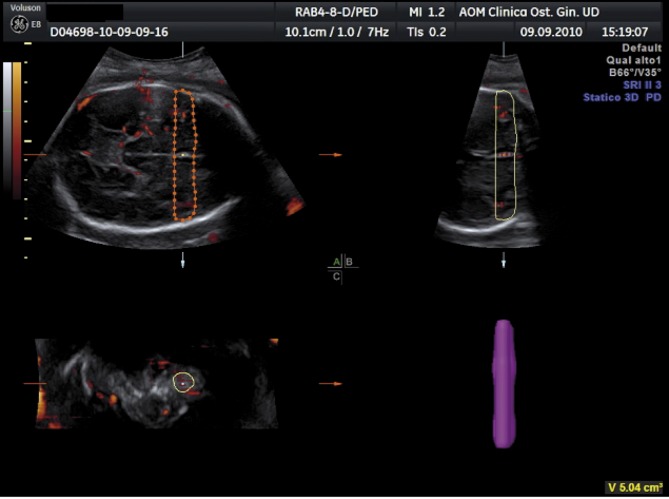
3D-PDA images of the fetal brain were acquired during fetal rest, and using the same presets for each acquisition. The angle of acquisition was set at 35°, the pulsed repetition frequency (PRF) of the power Doppler at 0,9. Power Doppler signals from the fetal brain were recorded in the biparietal plane including landmarks like the thalami, the third ventricle, the cavum septi pellucidi (CSP), the tentorial hiatus, and a symmetrical display of the calvaria (Fig. 1).
Fig. 1.
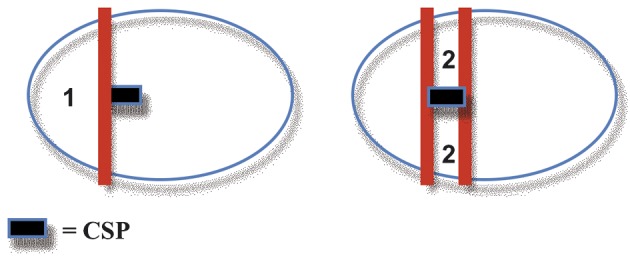
After displaying three simultaneous perpendicular planes on the monitor (axial, sagittal and coronal) the size of the region of interest (ROI) was adapted manually to create the 2 zones of the fetal brain to be analyzed. These 2 zones of the fetal brain were defined by using anatomy landmarks to realize a high good reproducibility of this method among different operators. The first zone (zone 1) was obtained by tracing the contour of the anterior part of the fetal brain up to the perpendicular line crossing the anterior delineation of the CSP (Fig. 2). The second one (zone 2) is defined by a rectangle reaching from both temporal bones with the width of CSP included (Fig. 3).
Fig. 2. 3D-PDA of Zone 1 “Frontal Zone”.
Fig. 3. 3D-PDA of Zone 2 “Temporal Zone”.
The volume of the investigated zones and the blood flow indices were calculated using VOCAL™ software. A rotation step for each contour plane was selected with a 30° degree angle chosen arbitrarily. This procedure of rotating the reference plane was done until a full rotation of 180 degrees was achieved. The fetal brain volumes were calculated after all contours traced (6 steps). Then the Vocal Histogram switch was activated for the automatic calculation of the 3D-PDA vascular indices (VI = vascularization index, FI = flow index, VFI = vascularization and flow index). The VI identifies the number of coloured voxels in the ROI, which is an estimate of the number of vessels within that tissue. The FI is the average colour value of all the colour voxels and represents both the average blood flow intensity. The VFI is the average colour value of all the gray and colour voxels and represents both blood flow and vascularization.
For further analysis IUGR fetuses were categorized into one of the following three groups, based on the UA PI, the MCA PI and the DV PI. In Group 1, IUGR is associated with normal UA PI, normal MCA PI and normal DV PI; Group 2 represents IUGR fetuses with abnormal UA PI (> 2 SD), normal MCA PI and normal DV PI; in Group 3, fetuses with IUGR have an abnormal UA PI (> 2 SD), an abnormal MCA PI (< 2 SD) and an abnormal DV PI (> 2 SD).
The results of 3D-PDA analysis of zone 1 and zone 2 were correlated with pregnancy outcome parameters at birth, such as gestational age (GA) at delivery, caesarean section (CS) rate, preeclampsia, birthweight, APGAR score, neonatal intensive care unit (NICU) admission, in-utero mortality, neonatal mortality, perinatal mortality. Differences between AGA and growth-restricted foetuses were evaluated using Student’s test and differences within different IUGR groups were evaluated by ANOVA test. P < 0,05 was considered significant. The study was approved by the local Ethics Committee and written consent was obtained from all participants.
Results
Table 1 shows the perinatal outcomes and characteristics of the population studied. 17 IUGR fetuses and 51 appropriate-for-gestational age (AGA) fetuses matched by gestational age were evaluated. Gestational age (GA) at delivery was similar between Group 1, Group 2 and Group 3. Caesarean Section rate was statistically not significantly different in Group 1, Group 2 and in Group 3 compared with the Control Group. Five cases were associated with pre-eclampsia equally divided among the 4 different groups. The mean birthweight was lower in Group 1, Group 2 and 3 if compared with Controls. APGAR scores at 1 and 5 min were comparable. There was one case of intra-uterine fetal death in Group 1.
Table 1. Perinatal outcomes of the study population (Mean (range or standard deviation) or %.
| Control Group | Group 1 | Group 2 | Group 3 | |
| (AGA n = 51) | (n = 5) | (n = 3) | (n = 9) | |
| Gestational Age (GA) at delivery | 39ws + 1d | 35ws + 0 | 34ws + 5d | 34ws + 4d |
| (37 + 1-41 + 3) | (33 + 1-37 + 2) | (32 + 5-36 + 3) | (32 + 0-36 + 2) | |
| Caesarean Section rate % | 33% (17/51) | 40% (2/5) | 33% (1/3) | 55% (5/9) |
| Pre-eclampsia % | 2% (1/51) | 20% (1/5) | 25% (1/4) | 33% (3/9) |
| Birth weight (mean) | 3342 gr | 1916* gr | 2020* gr | 1767* gr |
| (503) | (99) | (65) | (170) | |
| Apgar score (1/5 min) | 8/9 | 7/8 | 8/8 | 6/9 |
| (1.39/1.92) | (0.89/0.81) | (0.57/0.57) | (1.99/0.48) | |
| NICU admission | 4% (2/51) | 20% (1/5) | 33% (1/3) | 33% (3/9) |
| In utero mortality | 0 | 20% (1/5) | 0 | 0 |
| Neonatal mortality | 0 | 0 | 0 | 0 |
| Perinatal mortality | 0 | 0 | 0 | 0 |
* P < 0,01 vs Control Group (ANOVA).
Table 2 shows the values of the vascular parameters (VI, FI and VFI) and the volume of the sampled brain in zone 1 for the Control Group and for the IUGR groups at different hemodynamic stages (Group 1, Group 2 and Group 3).
Table 2. Three dimensional Power Doppler Angiography parameters values in Zone 1 in the Control Group and Foetuses with Intrauterine Growth Restrictions at different hemodynamic stages (Group 1, Group 2 and Group 3).
| Vascularity Index | Flow Index | Vascular Flow Index | Volume | |
| Control Group | 2.27 (0.3) | 31.96 (7) | 0.73 (0.3) | 32.92 (6.27) |
| Group 1 | 5.53* (2.1) | 24.12 (5.8) | 1.35* (0.6) | 17.25 (4.6) |
| Group 2 | 1.48 (0.25) | 26.6 (5.9) | 0.55 (0.025) | 20.87 (5.6) |
| Group 3 | 2.16 (0.2) | 31.21(6.9) | 0.75 (0.05) | 29 (6.1) |
Mean value and standard deviation of 3DPDA in each Group. Group 1, normal umbilical artery (UA) pulsatily index (PI) and normal middle cerebral artery (MCA) PI; Group 2, abnormal umbilical artery (UA) pulsatily index (PI) (mean > 2 SD) and normal middle cerebral artery (MCA) PI; Group 3 abnormal umbilical artery (UA) pulsatily index (PI) (mean > 2 SD) and abnormal middle cerebral artery (MCA) PI (PI < 2 SD) and pathological ductus venous (DV) PI (mean > 2 SD). AGA, appropriate for gestational age. * P < 0,05 vs Controls (Student’s t-test) and p < 0,05 vs Group 2 and Group 3 (ANOVA)
VI and VFI values were significantly increased in fetuses with IUGR and normal Doppler flow indices of the UA, MCA and DV compared to Control Group (AGA).
Table 3 shows the results of the VI, FI and VFI and the volume of the sampled brain in zone 2 in the Control Group and in the fetuses with IUGR at different hemodynamic stages.
Table 3. Three dimensional Power Doppler Angiography vascular parameters values in Zone 2 in the Control Group and Foetuses with Intrauterine Growth Restrictions at different hemodynamic stages (Group 1, Group 2 and Group 3).
| Vascularity Index | Flow Index | Vascular Flow Index | Volume | |
| Control Group | 3.38 (0.6) | 27.67 (5.8) | 1.15 (0.3) | 5.13 (1.2) |
| Group 1 | *0.95 (0.3) | 29.56(7.6) | *0.21 (0.1) | 2.81 (0.7) |
| Group 2 | 4.95 (1.2) | 30.29 (7.7) | 2.10 (0.2)* | 7.10 (2.3)* |
| Group 3 | 5.17 (1.2) | 34.09 (6.3) | 3.5 (0.2)** | 5.21 (1.5) |
Mean value and standard deviation of 3DPDA in each Group. Group 1, normal umbilical artery (UA) pulsatily index (PI) and normal middle cerebral artery (MCA) PI ; Group 2 , abnormal umbilical artery (UA) pulsatily index (PI) (mean > 2 SD) and normal middle cerebral artery (MCA) PI ; Group 3 abnormal umbilical artery (UA) pulsatily index (PI) (mean > 2 SD) and abnormal middle cerebral artery (MCA) PI (PI < 2 SD) and pathological ductus venous (DV) PI (mean > 2 SD).
AGA, appropriate for gestational age.
*P < 0,05 vs Controls (Student’s t-test) and p < 0,05 vs Group 1 and Group 3 (ANOVA).
** P < 0,05 vs Controls (Student’s t-test) and p < 0,05 vs Group 1 and Group 2 (ANOVA)
VI and VFI values were significantly decreased in fetuses with IUGR and normal values of fetal arterial and venous Doppler (Group 1) compared to Control Group. The VFI both in the Group 2 and 3 were very significantly increased compared to Control Group. Besides, the volume of the sampled brain (zone 2) is significantly increased in the fetuses with an abnormal UA PI and a normal MCA PI.
Discussion
The Vascularity Index (VI) and Vascular Flow Index (VFI) obtained by three-dimensional Power Doppler Angiography of the frontal zone of the fetal brain (zone 1), sprinkled mainly by the anterior cerebral artery, demonstrate the “frontal brain sparing effect” in fetuses with IUGR presenting with normal Doppler flow indices of umbilical and middle cerebral arteries. On the other hand, these vascular parameters were decreased in zone 2, suggesting a vascular redistribution during brain sparing effect according to a regional increase in bloody supply to the frontal region. This shift may indicate that in very early stages of IUGR general cognitive functions, such as impulse control, language, memory, problem solving and socialization may be preferentially preserved suggesting a hierarchical order in the protection of the brain functions (Fuster, 2002).
Our preliminary findings are in line with recent studies in growth-restricted fetuses, suggesting that the anterior cerebral artery shows Doppler signs of vasodilatation before these are observed in the MCA (Dubiel et al., 2002; Figueroa-Diesel et al., 2007; Hernandez-Andrade et al., 2008).
Our data might suggest that an abnormal MCA might actually indicate the starting point after which the hemodynamic protection of the frontal area start to decline. On the other hand, the data on the Vascular Flow Index in zone 2 in growth restricted fetuses with abnormal UA and MCA PI revealed a concordance between three-dimensional Power Doppler Angiography and bidimensional Doppler flow analysis.
Most current clinical protocols for fetal growth restriction are based on the assumptions that the onset of a brain-sparing effect is indicated by a reduced MCA PI, representing a protective hemodynamic response in the entire fetal brain. The results obtained by power Doppler Angiography (3D-PDA) of this pilot study show a different pattern of vascular blood distribution in the brain of IUGR fetuses in relation to the bidimensional Doppler findings.
According to these results, 3D sonography and power Doppler angiography can be considered as new techniques offering to study additional vascular parameters of the fetal brain allowing for the evaluation of early non invasive “brain sparing markers” in IUGR fetuses.
Furthermore, construction of reference charts and an intra- inter- observer variability study of vascular indices of fetal brain circulation obtained in 3D-PDA mode in normal pregnancies will be planned.
The clinical significance of the observations reported in the present study remains to be established by larger prospective studies with long term postnatal neurological follow-up.
References
- Baschat AA, Gembrush U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571–577. doi: 10.1046/j.0960-7692.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- Baschat AA, Gembruch U, Reiss I. Relationship between arterial and venous Doppler and perinatal out come in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16:407–413. doi: 10.1046/j.1469-0705.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- Baschat AA, Harman CR. Antenatal assessment of the growth restricted fetus. Curr Opin Obstet Gynecol. 2001;13:161–168. doi: 10.1097/00001703-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Dubiel M, Gunnarsson GO, Gundmusson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultraound Obstet Gynecol. 20:117–121. doi: 10.1046/j.1469-0705.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- Figueroa-Diesel H, Hernandez-Andrade E, Rojas R. Doppler changes in the main fetal brain arteries at different stages hemodynamic adaption in severe intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007;30:297–302. doi: 10.1002/uog.4084. [DOI] [PubMed] [Google Scholar]
- Fouron JC, Gosselin J, Raboisson MJ. The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am J Obstet Gynecol. 2005;192:497–503. doi: 10.1016/j.ajog.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y. Neuropsycological out come in children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118 doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y. Memory functions of children born with asymmetric intrauterine growth restriction. 2006;(1117):186–194. doi: 10.1016/j.brainres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Gosling RG, Dunbar G, King DH, et al. The quantitative analysis of occlusive peripheral arterial disease by a nonintrusive ultrasound technique. Angiology. 1971;22:52–55. doi: 10.1177/000331977102200109. [DOI] [PubMed] [Google Scholar]
- Gudmundsson S, Maršál K. Umbilical and uteroplacental blood flow velocity waveform in normal pregnancy – A cross-sectional study. Acta Obstet Gynecol Scand. 1988;67:347–354. [PubMed] [Google Scholar]
- Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements – A prospective study. AJOG. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Andrade E, Brodszki J, Lingman G, et al. Uterine artery score and perinatal outcome. Ultrasound Obstet Gynecol. 2002;19:438–442. doi: 10.1046/j.1469-0705.2002.00665.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Andrade E, Figueroa-Diesel H, Jansson T. Changes in regional fetal cerebral blood perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet Gynecol. 2008;32:71–76. doi: 10.1002/uog.5377. [DOI] [PubMed] [Google Scholar]
- Hofstaetter C, Dubiel M, Gudmundsson S, Maršál K. Uterine artery color Doppler assisted velocimetry and perinatal outcome. Acta Obstet Gynecol Scand. 1996;75:612–619. doi: 10.3109/00016349609054684. [DOI] [PubMed] [Google Scholar]
- Mari G, Deter RL. Middle cerebral artery flow velocity waveforms in normal and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1992;166:1262–1270. doi: 10.1016/s0002-9378(11)90620-6. [DOI] [PubMed] [Google Scholar]
- Marsal K. Intrauterine growth restriction. Curr Opin Obstet Gynecol. 2002;14:127–135. doi: 10.1097/00001703-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Merce LT, Barco MJ, Bau S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. J Perinat Med. 2004;32:228–233. doi: 10.1515/JPM.2004.043. [DOI] [PubMed] [Google Scholar]
- Merce LT, Barco MJ, Bau S, Kupesic S, Kurjak A. Assessment of placental vascularization by three-dimensional power Doppler “vascular biopsy” in normal pregnancies. 771. 2005;46:765. [PubMed] [Google Scholar]
- Onwudiwe N, Yu CK, Poon LC, Spiliopoulos I, Nicolaides KH. Prediction of pre-eclampsia by a combination of maternal history, uterine artery Doppler and mean arterial pressure. Ultrasound Obstet Gynecol. 2008;32:877–883. doi: 10.1002/uog.6124. [DOI] [PubMed] [Google Scholar]
- Pairleitner H, Steiner H, Hasenoehrl G, Staudach A. Three-dimensional power Doppler sonography: Imaging and quantifying blood flow and vascularization. Ultrasound Obstet Gynecol. 1999;14:139–143. doi: 10.1046/j.1469-0705.1999.14020139.x. [DOI] [PubMed] [Google Scholar]
- Rubin JM, Adler RS, Fowlkes JB, Spratt S, Pallister JE, Chen JF, Carson PL. Fractional moving blood volume: Estimation with power Doppler US. Radiology. 1995;197:183–190. doi: 10.1148/radiology.197.1.7568820. [DOI] [PubMed] [Google Scholar]
- Rubin JM, Bude RO, Carson PL, Bree RL, Adler RS. Power Doppler US: A potentially useful alternative to mean frequency-based color Doppler. Radiology. 1994;190:853–856. doi: 10.1148/radiology.190.3.8115639. [DOI] [PubMed] [Google Scholar]
- Scherjon S, Briet J, Oosting H, Kok J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very pretermi infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics. 2000;105:385. doi: 10.1542/peds.105.2.385. [DOI] [PubMed] [Google Scholar]
- Yu CH, Chang CH, Ko HC, Chen WC, Chang FM. Assessment of placental fractional moving blood volume using quantitative three- dimensional power Doppler ultrasound. Ultrasound Med Biol. 2003;29:19–23. doi: 10.1016/s0301-5629(02)00695-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Merialdi M, Platt LD. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;202:522–528. doi: 10.1016/j.ajog.2009.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]