Abstract
Congenital abnormalities of the kidneys and the urinary tract are the most common sonographically identified malformations in the prenatal period. Obstructive uropathies account for the majority of cases. The aim of prenatal diagnosis and management is to detect those anomalies having impact on the prognosis of the affected child and requiring early postnatal evaluation or treatment to minimize adverse outcomes.
In this paper, we summarize the embryology of kidneys and urinary tract, the normal sonographic appearance throughout pregnancy and the prenatal diagnosis of their congenital malformations.
Keywords: Prenatal ultrasound, CAKUT, antenatal hydronephrosis
Introduction
Congenital abnormalities of the genitourinary tract are the most common sonographically identified malformations, with an incidence of 1 to 4 in 1000 pregnancies (Grandjean et al., 1999). As such they represent 15-20% of all prenatally diagnosed congenital anomalies (Elder et al., 1997), obstructive uropathies accounting for the majority of cases. Prenatal diagnosis improves the outcome of the affected child because of early recognition and treatment of critical obstructions and urinary tract infections, preventing further renal damage and loss of renal function. With the introduction of modern ultrasound screening programs, about 60% of children having surgery for renal or urinary tract problems in their first five years of life are identified by prenatal ultrasound (Bhide et al., 2005).
This paper summarizes the embryology of the upper and lower urinary tract, the normal sonographic appearance throughout pregnancy and the prenatal diagnosis of their congenital malformations.
Embryology
The urinary tract develops from the third week of embryonic life from the intermediate mesoderm (kidneys and ureters) and from the urogenital sinus (bladder and urethra). Renal development passes through three stages: the pro- and mesonephros (both transitory), and the metanephros, which develops into the definitive kidney. The pronephros is a primitive excretion system which develops during the 4th week and involutes almost completely in the fifth week except for the most caudal part. Around the 25th day post-conception, the pronephric duct migrates caudally to become the mesonephric duct (Wolffian duct), which interacts with the surrounding mesenchyme and ignites the development of a set of 25 to 30 filtrating and excreting tubuli: the mesonephros. In the female all mesonephric tubules atrophy completely; in the male a few caudal tubules develop into the testicular efferent ducts. The permanent kidney develops from the interaction between three intermediate mesoderm structures of the sacral region: the ureteric bud- which is an epithelial diverticulum from the caudal part of the mesonephric duct, the metanephric blastema- the sacral part of the nephrogenic cord, and a glomerular capillary network. The ureteric bud outgrow is a critical element in the development of the kidney. Signals from the ureteric bud interact with the mesenchymal cells. In response, the mesenchymal cells differentiate into the different cell types of the glomerulus and the proximal tubule, the loop of Henle and the distal tubule. Consequently, mesenchymal cells excrete molecular signals that induce the ureteric bud to branch and interact with new mesenchymal zones to form a new set of glomeruli. This branching of the ureteric bud is essential in the development of the number of glomeruli or nephrons. At birth, each kidney contains about 1 000 000 nephrons. The structural development of these nephrons is completed around the 34th week of pregnancy, whereas the functional maturation continues for the first six months postnatally.
The upper urinary tract (bladder neck, ureter, pyelocaliceal system but also the most distal element of the nephron, the ductus colligens) is derived from the ureteric bud. The lower urinary tract develops from the urogenital sinus which is an endodermal derivate, segregated from the cloaca by the ingrowth of the urorectal septum. The bladder develops from the upper part of the urogenital sinus and the urethra from the lower part. The caudal common part of the mesonephric duct and the ureter is absorbed into the upper, postero-lateral wall of the urogenital sinus. After a cranio-caudal exchange of the position of the ureteral and mesonephric orifices, a triangular zone is created, called the bladder trigonum. In males, the internal sex organs are derived from the mesonephric duct. In females, the mesonephric duct atrophies to leave some embryonic remants such as the ductus longitudinalis epoöphori, epoöphoron and paroöphoron.
Ultrasound exploration of the normal urinary tract
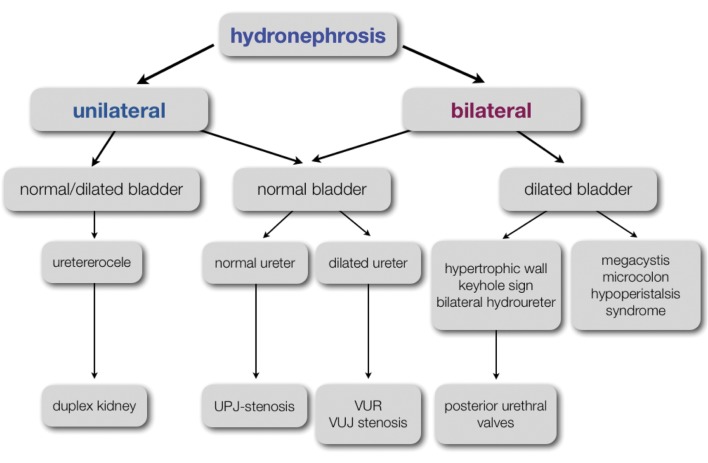
From about 9 to 12 weeks of pregnancy, the fetal kidneys and adrenal glands can be visualized at both sites of the lumbar spine. They are usually easy to identify because of their relatively hyperechogenic aspect in the first trimester (Fig. 1A). The visualization of the renal arteries by color Doppler can facilitate their identification. The sonographic cortico-medullary differentiation starts at 15 weeks and will become more clear with advancing gestational age. The outer, more hyperechogenic renal cortex can be clearly distinguished from the inner, more hypoechogenic medulla at the 20th week of gestation. Renal echogenicity will also decrease, lower than that of the liver and spleen from 17 weeks on (Fig. 1B). The antero-posterior diameter of the renal pelvis (APPD) should be less than 4 mm in the second trimester and less than 7mm in the third trimester, although cut-off values vary in literature and consensus is lacking (Nguyen et al., 2010). The fetal bladder should be visualized from 13 weeks on. Its identification is easy because of its pelvic location between the umbilical arteries (Fig. 1C). Fetal urinary production starts at 9 weeks of pregnancy and increases significantly beyond 16 weeks. At 20 weeks about 90% of amniotic fluid consists of fetal urine. Fetal urinary production is estimated to be around 7,3 ml/h at 24 weeks of pregnancy, increasing to 71,4 ml/h near term or about 300 ml/kg fetal weight/ day (Gilbert et al., 1993; Lee et al., 2007; Touboul et al., 2008; Peixoto et al., 2011). The contribution of lung fluids increases with advancing gestational age remaining far less important than urinary production (about 60-100 ml/kg fetal weight/day) (Gilbert et al., 1993, Underwood et al., 2005).
Fig. 1. Normal ultrasound image of the fetal kidneys and bladder. A. First trimester fetal kidneys at both sides of the lumbar spine. B. The fetal bladder between the umbilical arteries as seen in the first trimester of pregnancy. C. Decreased echogenicity and beginning of cortico-medullary differentiation in second trimester fetal kidneys.
The ultrasound examination of the normal urinary tract consists of the assessment of the presence, location and size of both kidneys and the evaluation of their structure and echogenicity. In addition, the presence, size and shape of the fetal bladder are examined, as well as development of the external genitalia and the amount of amniotic fluid. The amount of amniotic fluid may be estimated by eyeballing, or more objectively by measuring the deepest pocket or the amniotic fluid index. Reference curves for renal volume and for the amount of amniotic fluid have been published (Chitty et al., 2003; Tedesco et al., 2009; Machado et al., 2007).
Renal abnormalities
Abnormalities in number and location
Unilateral renal agenesis occurs in approximately 1/1,300 pregnancies (Cascio et al., 1999), the majority of which are probably cases of renal aplasia (Hiraoka et al., 2002). The difference between both cannot be made by ultrasound. Although unilateral renal agenesis is mostly isolated and sporadic, it might be part of a genetic syndrome, or occur in association with chromosomal or developmental defects (VACTERL association) and genital abnormalities. If associated anomalies are present, amniocentesis should be offered. On ultrasound examination the lumbar fossa is empty and the adrenal gland appears elongated (“lying down adrenal sign”). The amount of amniotic fluid will be normal. Recent follow up studies attribute an increased risk for chronic kidney disease in adulthood in patients with a solitary functioning kidney, especially in the presence of ipsilateral abnormalities of the kidney or urinary tract (Westland et al., 2011; Corbani et al., 2011). Clinical follow up is thus recommended.
Bilateral renal agenesis is a lethal condition with an incidence of 1/4,000 pregnancies (Potter, 1965). Ultrasound features are early anhydramnios from 16 weeks of gestation onwards, absence of bladder filling and empty lumbar fossae. Renal artery color Doppler and Magnetic Resonance Imaging (MRI) may be helpful in confirming the diagnosis.
Ectopic kidneys occur in about 1/1,000 pregnancies (Yuksel et al., 2004). They are usually smaller and may be malrotated. The pelvic location is the most common, but horseshoe kidneys, crossed (fused) ectopia and even intrathoracic kidneys have been described. Uncomplicated renal duplication should be considered as a normal variant. However, renal duplication is often associated with ureteral abnormalities and hydronephrosis.
Abnormalities in renal size, structure and echogenicity
Kidneys appear hyperechoic if they look brighter than the liver and spleen beyond 17 weeks of pregnancy. Hyperechoic kidneys are normal in premature baby’s and infants up to 6 months. However, they are an indicator of significant renal pathology in the pediatric population. Fetal hyperechoic kidneys present an etiological diversity with an outcome that is speficic for each of the conditions.
Non-hereditary, fetal hyperechoic kidneys can result from various causes such as an obstructive dysplasia, bilateral multicystic kidney disease, nephroblastomatosis, renal vein thrombosis, ischemia, infectious and metabolic diseases, nephrotic syndrome and aneuploidy. In case of an underlying genetic syndrome and in polycystic kidney disease, the recurrence rate is high. The presence of hyperechoic enlarged kidneys without associated malformations is most frequently associated with polycystic kidney disease (both autosomal recessive and dominant) (Chaumoitre et al., 2006). In case of bilateral, isolated hyperechogenic kidneys without family history or cysts, the diagnosis of the underlying etiology and the counseling of the parents on the long term prognosis may be challenging. Fetuses with very large kidneys and/or severe oligohydramnios are likely to have a poor outcome. With normal amniotic fluid volumes and moderately enlarged kidneys (< 4SD), the probability of survival without significant morbidity in infancy is high (14/17 survivors of whom 9 symptom-free) (Tsatsaris et al., 2002). The presence of associated malformations should raise the suspicion of an underlying genetic syndrome, frequently showing an autosomal recessive inheritance pattern (e.g. Bardet-Biedl, Meckel-Gruber, Beemer syndromes).
Detailed fetal ultrasound examination, fetal karyotyping, family history and ultrasound examination of the urinary system in parents are all important in the work up of hyperechoic enlarged kidneys.
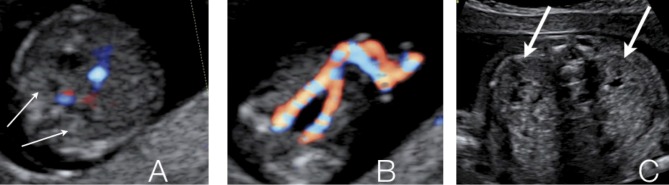
Autosomal recessive polycystic kidney disease (ARPKD) occurs with an incidence of 1 in 20,000 live births (Wilson, 2004). It is most frequently caused by a mutation in the PKHD-1 gene, but probably additional genes play a role. Disease expression may vary widely within affected families. The disease is characterized by cystic dilatation of the tubules, predominantly in the medulla. The outer cortex is spared. Additionally, patients have biliary dysgenesis and hepatic fibrosis. Ultrasound scans can be normal up to 20 weeks of pregnancy. Kidneys will be markedly enlarged (+4-15 SD) and hyperechoic without (or with reversed) corticomedullary differentiation and with a hypoechoic outer cortical rim (Brun et al., 2004) (Fig. 2). In the perinatal form, oligohydramnios as a consequence of renal failure results in lethal pulmonary hypoplasia. Children with the infantile and juvenile types develop chronic renal failure (with need for transplantation in their teens), hepatic fibrosis and portal hypertension. The recurrence rate is 25% and if the mutation is known, prenatal diagnosis can be offered.
Fig. 2. Ultrasound appearance of autosomal recessive polycystic kidney disease: bilateral markedly enlarged hyperechogenic kidneys without cortico-medullary differentiation.
Autosomal dominant polycystic kidney disease (ADPKD) has an incidence of 1 in 800 live births (Wilson, 2004). Inheritance is autosomal dominant with a 100% penetrance. Expression is variable and new mutations are frequent: the family history is positive in only half of cases (Brun et al., 2004). ADPKD is usually asymptomatic until the age of 30-40 years. It is rarely seen in fetal life. The disease is characterized by cysts in both renal cortex and medulla, in the liver, pancreas and spleen. On prenatal ultrasound, kidneys are usually moderately enlarged (+1-2 SD) with hyperechoic cortex and hypoechoic medulla (persisting corticomedullary differentiation) but other patterns are described, including absent or decreased corticomedullary differentiation or totally normal appearance (Brun et al., 2004). Cysts may be visible in the third trimester. The amniotic fluid volume is normal.
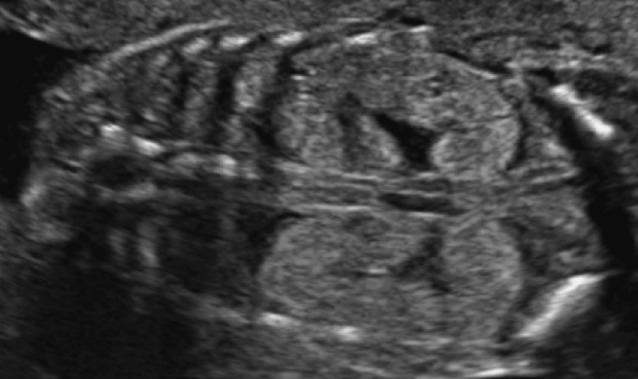
Multicystic kidney disease (MCKD) is a developmental disorder of the kidney, in which the normal renal parenchyma is replaced by multiple, non-communicating cysts of varying size. The renal outline is difficult to delineate and can be irregular (Fig. 3). The incidence is about 1/4,300 pregnancies (Schreuder et al., 2009). In 25-40% the contralateral kidney will also be abnormal, reflux being the most frequently associated anomaly (Schreuder et al., 2009). The natural course in utero is variable and the final result is a non-functional kidney. Bilateral multicystic kidney disease occurs in about 10-20% of cases and is a lethal condition.
Fig. 3. Typical ultrasound image of multicystic kidney disease: multiple, non-communicating cysts of varying size replace the normal renal parenchyma.
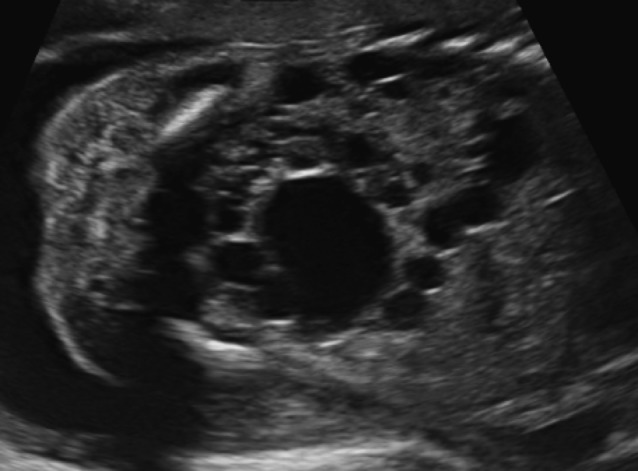
Obstructive cystic dysplasia is the most common cause of non-hereditary fetal renal cystic disease and hyperechoic kidneys. Ultrasound features are those of a lower or upper urinary tract obstruction in association with a hyperechoic appearance of the renal cortex and eventually the presence of cysts variable in size (Fig. 4). Renal size can also vary. Obstructive cystic dysplasia can be unilateral, bilateral or segmental but it is usually a progressive lesion. The amount of amniotic fluid is variable and renal function is usually impaired.
Fig. 4. A first trimester foetus with a megacystis (arrow) and renal dysplasia: small, hyperechogenic kidneys with dilated pelvis (thick arrows).
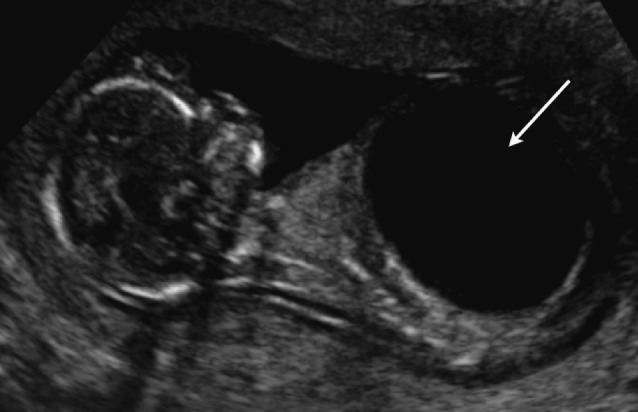
Simple renal cysts are rather uncommon in the fetus. They are usually solitary and localized in the upper pole of a normal kidney. There is no association with other malformations and the prognosis is good. A follow up ultrasound is usually recommended to exclude a more diffuse distribution or other cystic renal diseases.
Renal tumors are uncommon in fetal life. Mesoblastic nephroma, the most frequent tumor, is a benign, mostly large mesenchymal tumor, which appears as a solid or partially cystic mass, commonly associated with polyhydramnios. It has to be differentiated from Wilm’s tumors which have a good prognosis. Nephroblastomatosis is characterized by multiple benign nodular lesions and bilateral involvement.
Antenatal hydronephrosis
a. Definitions and grading
Pyelectasis is defined as a dilatation of the renal pelvis, whereas hydronephrosis consists of a dilatation of the renal pelvis and the calyces. Both are very common findings on prenatal ultrasound. Up to 10 resp. 15 mm the pelvic dilatation is classified as mild and moderate respectively. When the pelvic dilatation is more than 15 mm, hydronephrosis is called severe. The prenatal diagnosis of hydronephrosis favors early treatment of a critical obstruction and early recognition of urinary tract infections. This will prevent renal damage and loss of renal function and improves the outcome of the affected child.
The incidence of hydronephrosis varies between 0.6-4.5% in a non selected population (Blyth et al., 1993; Sairam et al., 2001; Gunn et al., 1995; Levi et al., 2003), differences being explained by the diversity of diagnostic criteria at various gestational ages. In about 20-40% the dilatation will be bilateral, and boys are affected twice as much as girls. In addition, hydronephrosis is reported as a part of more than 60 genetic syndromas and sporadic malformations.
The severity of renal pelvic dilatation can be assessed using different grading systems. Measuring the maximal antero-posterior diameter of the renal pelvis (APPD) on a transverse scan of the fetal abdomen is the generally accepted method. The grading system of the Society of Fetal Urology (SFU) classifies hydronephrosis in 4 degrees (grade 0-4) and takes into account the degree of pelvic dilatation, the number of calyces seen and the presence or severity of renal parenchymal thinning or atrophy (Fernbach et al., 1993). This grading system is less frequently used because it is relatively subjective.
An ideal threshold value for the renal pelvic diameter should enable to differentiate fetuses with physiological and transient hydronephrosis from those at risk for congenital anomalies of the genito-urinary tract. Unfortunately, as a consensus is lacking, thresholds vary between 4 and 10 mm in the second trimester, and between 7 and 10 mm in the third trimester. Using a cut-off value of 4 mm in the second trimester, results in a diagnosis of pyelectasis in 2% of pregnancies (Adra et al., 1995; Corteville et al., 1991). A 7 mm cut-off in the third trimester has a sensitivity of 100% and a false positive rate of 21% for the prediction of persisting postnatal congenital hydronephrosis and associated morbidity (Corteville et al., 1991), whereas a threshold of 10 mm has a lower sensitivity (82%) for only a small decrease in false positive rate (18%) (Anderson et al., 1995). Because even for mild degrees of pyelectasis a short and minimally invasive follow-up by postnatal ultrasound is recommended, a threshold of the APPD with higher sensitivity despite lower specificity is preferred.
b. Etiology
The etiology of antenatal hydronephrosis is very diverse (Fig. 5). The majority of cases are transient (48%) or physiological (15%). Only in a minority of cases, a significant underlying pathology of the urinary tract is found. Rare causes are an ectopic ureter, prune belly syndrome, urachal cysts and urethral atresia. (Woodward et al., 2002) The likelihood of an underlying abnormality correlates with the severity of the hydronephrosis. Transient or physiological hydronephrosis will mostly be mild, while uretero-pelvic junction (UPJ) stenosis almost always presents with severe hydronephrosis. A recent meta-analysis by Lee et al. demonstrated that every dilatation of the urinary tract regardless the degree, was associated with an overall increased risk (36%) of an underlying uropathy. The risk was depending on the severity of the hydronenphrosis, increasing from 12% in cases for mild hydronephrosis (APPD > 7 mm in the 2nd trimester and < 9 mm in the 3rd trimester), to 45% in cases of moderate hydronephrosis (APPD 7-9 mm in the 2nd trimester, 9-15 mm in the 3rd trimester) and up to 88% in cases of severe hydronephrosis (> 10 mm in the 2nd and > 15 mm in the 3rd trimester) (Lee et al., 2006; Coelho et al., 2007).
Fig. 5. The diverse etiology of fetal hydronephrosis (adapted from Woodward et al., 2002).
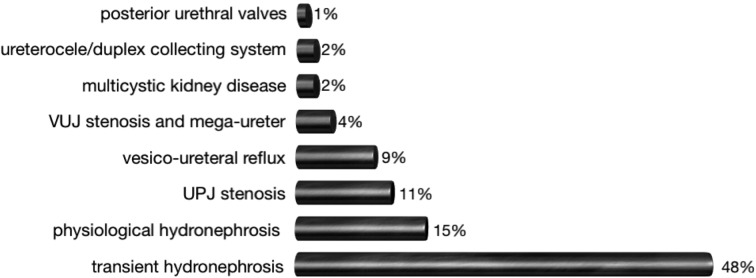
The majority (35-50%) of cases of mild antenatal hydronephrosis are transient dilatations. The etiology is unclear, possible causes are insufficient maturation of the pelvi-ureteral or vesico-ureteral junctions associated with increased urinary production or fetal ureteric folds. Physiological hydronephrosis can be secondary to a large, unobstructed renal pelvis and/or an extrarenal pelvis.
Uretero-pelvic junction (UPJ) stenosis occurs in about 1/500 live births (Liang et al., 2002). The male:female ratio is 3:1 and cases are usually sporadic. Bilateral renal involvement can be present in 10-40% of cases (most frequently vesico-ureteral reflux) and 10% of fetuses have extrarenal anomalies. The risk of aneuploidy seems to be slightly increased. The obstruction is either anatomical or more frequently, functional; the stenosis can be partial or complete. Causes are an intrinsic stenosis or the presence of valves (75%), an insertion anomaly of the ureter, peripelvic fibrosis or crossing vessels (20% are associated with an accessory renal artery). A severely dilated renal pelvis can rupture and evolve to perinephric urinoma and urinary ascites. The typical ultrasound presentation of UPJ-stenosis is a severe hydronephrosis without hydro-ureter and with a normal aspect of the bladder.
About 9-15% of fetuses with antenatal hydronephrosis will have vesico-ureteral reflux. The typical ultrasound presentation is that of an intermittent, variable degree of pelvic dilatation, with increase post-micturition or the impression of a persistent full bladder. There is a possible heritable predisposition because insufficiency of the vesico-ureteral valves is determined by the length of the terminal part of the ureter.
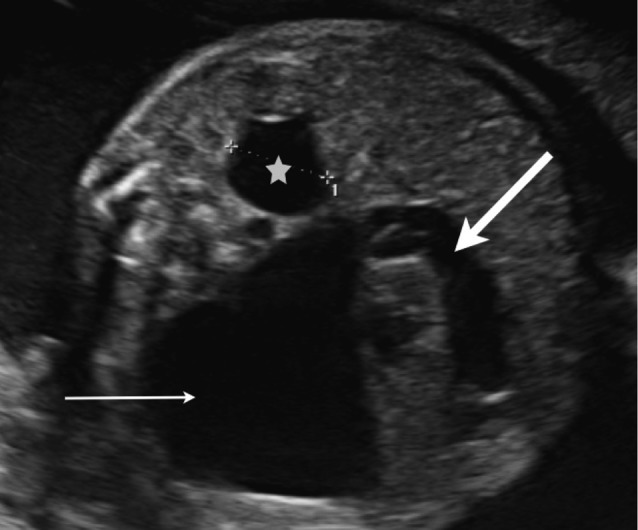
The prevalence of vesico-ureteral junction (VUJ) stenosis in the general population is about 1% (Hiraoka et al., 1999). The etiology is diverse. Possible causes are a primary mega-ureter, ureter stricture or atresia, a retrocaval ureter, vascular obstruction, valves, a diverticulum or a ureterocoele. Hydronephrosis and hydro-ureter without dilated bladder are suggestive of VUJ-stenosis, but the differential diagnosis with vesico-ureteral reflux can be difficult (Fig. 6).
Fig. 6. Severe hydro-ureteronephrosis due to vesico-ureteral junction stenosis: severely dilated renal pelvis (arrow) and dilated ureter (thick arrow). Mild pyelectasis in the contralateral kidney (*).
A ureterocoele is a cystic dilatation of the terminal part of the ureter within the bladder or urethra (Fig. 7). The incidence is about 1/5,000 neonates, and the male:female ratio is 1:3-5. In the majority of cases there is an association with a duplicated collecting system (80-90%) and ectopic ureter (75%), 10-20% of ureterocoeles are bilateral.
Fig. 7. The sonographic aspect of a ureterocoele: the terminal part of the ureter is dilated within the bladder (arrow).
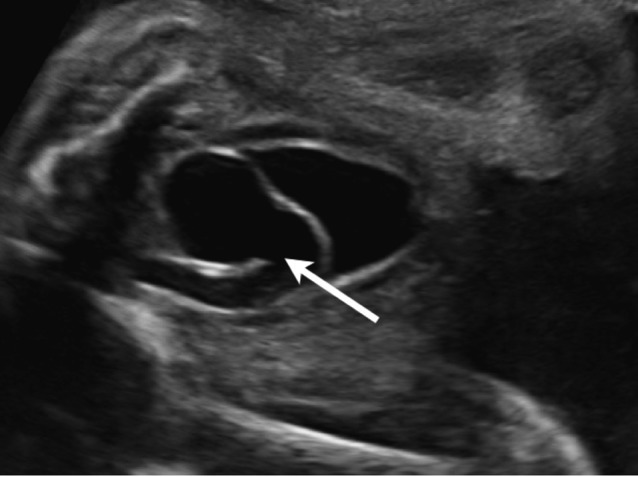
In a duplex kidney, the lower pole is often “refluxing”, while the upper pole can be obstructive due to the ectopic insertion of the ureter (median and inferior of the normal insertion site) or the ureterocoele. A large ureterocoele can obstruct the contralateral vesico-ureteral junction or the bladder neck, resulting in bilateral hydronephrosis.
Lower urinary tract obstruction occurs with an incidence of 2.2/10,000 live births (Morris et al., 2011). Posterior urethral valves (PUV) and urethral atresia are the most frequent underlying etiologies. Posterior urethral valves have an incidence of 1/5,000-8,000 neonates and they are the most important cause of severe, bilateral obstructive uropathy. The ultrasound features of this disease are a dilated proximal urethra (keyhole sign), a megabladder with a thick, hyperechogenic bladderwall and severe bilateral hydro-ureteronephrosis or signs of obstructive renal dysplasia (hyperechogenic appearance of the renal parenchyma with cortical cysts) (Fig. 4) occuring in a male fetus. Bilateral obstructive renal dysplasia will result in oligo-or anhydramnios, causing secondary pulmonary hypoplasia and limb deformities. Untreated, lower urinary tract obstruction has a high mortality (45%) and about 25-30% of the neonatal survivors will develop end stage renal failure (Biard et al., 2005). In addition to renal failure, children affected by LUTO suffer from bladder dysfunction, poor growth and male infertility in adolescence if they are uraemic (Freedman et al., 1999; Biard et al., 2005). Vesico-amniotic shunting to preserve renal function leads to a better perinatal survival but has not yet shown to ameliorate renal function on the long term (Biard et al., 2005, Morris et al., 2010). Fetal cystoscopy allows a more accurate diagnosis of the underlying etiology with the possibility to treat the PUV by hydro-or laserablation, however, when compared with vesico-amniotic shunting, no significant improvement in perinatal survival was found (Morris et al., 2011).
c. Differential diagnosis
The differential diagnosis of antenatal hydronephrosis is presented in a clinical algorithm (Fig. 8).
Fig. 8. Clinical algorithm for the differential diagnosis in fetal hydronephosis.
d. Prognosis
The degree of hydronephrosis indicates the likelihood of having a significant underlying pathology of the urinary tract and therefore determines the prognosis.
Mild pyelectasis is a benign condition with an excellent prognosis: the majority will resolve spontaneously in the prenatal period or within the first year of life (Sairam et al., 2001). Persistent cases may require postnatal intervention (Sairam et al., 2001; Persutte et al., 1997; Sidhu et al., 2006; Signorelli et al., 2005). In the study by Coelho et al. up to 18% of children with mild antenatal hydronephrosis had a significant uropathy and 7.8% had recurrent urinary tract infections compared to 1% in a non selected population (Coelho et al., 2007). Because mild pyelectasis may be indicative of obstructive uropathy or vesico-ureteral reflux, a close clinical surveillance for urinary tract infections and progression of hydronephrosis is advised, without exposure to invasive and repetitive diagnostic radiological examinations. Further prospective studies are needed to elucidate the clinical significance of mild pyelectasis and to standardize and optimize its management.
Mild pyelectasis is also a common finding in fetuses with Down syndrome, and as a consequence used as a marker in the screening for fetal aneuploidy. In a series of 375 fetuses with mild, isolated hydronephrosis the incidence of Down syndrome was 0.53% (Signorelli et al., 2005). However in case of isolated mild pyelectasis and in the absence of other sonographic features or maternal risk factors, the risk of aneuploidy is not increased (Coco et al., 2005; Chudleigh et al., 2001).
Fetuses with a prenatal diagnosis of moderate to severe hydronephrosis, will need additional postnatal invasive radiological examinations and an intensive follow up. Most authors agree that an antero-posterior diameter of the pelvis of more than 10 mm and certainly more than 15 mm is predictive of significant pathology of the urinary tract. More than 30% of fetuses with an APPD ≥ 10 mm in the third trimester, require surgical intervention and have an increased risk for urinary tract infections with the need for antibiotic profylaxis (Sairam et al., 2001; Broadley et al., 1999; Wollenberg et al., 2005). A 15mm threshold might be helpful to differentiate between those cases requiring surgery from those where conservative management will be sufficient (Coelho et al., 2007; Coplen et al., 2006). The predictive value for postnatal outcome for a third trimester scan is better than for a the second trimester ultrasound investigation (Ismaili et al., 2003).
The degree of antenatal hydronephrosis may change during the prenatal period, and the pre-and postnatal evolution of pyelectasis might be more predictive for outcome than the pyelectasis itself. The initial degree of pelvic dilatation is related to the prenatal evolution. A decrease in dilatation that sets off in the prenatal period is predictive of spontaneous resolution in the early neonatal period, whereas progression of hydronephrosis is directly related to a worse outcome. Even for cases resolving before birth, a short non-invasive postnatal follow-up to avoid a delay in medical or surgical treatment is recommended (Signorelli et al., 2005).
Besides the degree and the prenatal evolution of hydronephrosis, other relevant sonographic parameters determine its prognosis: uni- or bilateral renal involvement, features of renal dysplasia (hyperechoic renal parenchyma and cortical cysts), oligohydramnios and lunghypoplasia and the detection of associated malformations.
e. Management of antenatal hydronephrosis
The objective of prenatal ultrasound is to be as accurate as possible in describing the type of anomaly, to exclude associated malformations and to screen for parameters predictive of bad renal function. Therefore, karyotyping and further invasive testing may be indicated. Based on the gathered information, prognosis and perinatal management can be discussed with the parents.
In the presence of extra-renal malformations or soft markers, amniocentesis for karyotyping is indicated. In the presence of oligo- or anhydramnios and other signs predictive of deteriorated renal function (hyperechogenic renal parenchyma and cortical cysts), invasive testing to further evaluate renal function by means of β2-microglobulin or cystatin-C concentration in fetal serum or biochemistry of fetal urine can be performed. Urinary electrolytes and osmolality are indicators of fetal tubular function, whereas urinary β2-microglobulin represents the fetal glomerular function. While fetal serum cystatin C and β2-microglobulin concentrations may be useful predictors of postnatal renal function, the accuracy of fetal urine analysis is insufficient to predict poor postnatal renal function (Bökenkamp et al., 2001; Morris et al., 2007).
In cases of isolated hydronephrosis and absence of poor prognostic factors, ultrasound follow-up is scheduled on a regular basis to evaluate the evolution of the hydronephrosis and to assess the amount of amniotic fluid and fetal well-being. If the volume of the hydronephrotic kidney increases rapidly with additional compression of the surrounding organs or elevation of the diaphragm, prenatal aspiration or pyelo-amniotic shunting can be considered. In case of bilateral renal involvement with evolution to oligo- or anhydramnion, induction of labor should be considered after lung maturition is achieved. Earlier in pregnancy, fetal interventions for lower urinary tract obstruction improve perinatal survival but have not yet shown to ameliorate renal function on the long term (Morris et al., 2010).
Bladder anomalies
Anomalies of the bladder can be suspected when the bladder is enlarged or when the bladder is not visible during the entire ultrasound examination. Megacystis is defined as a bladder with longitudinal diameter > 7 mm in the first trimester (Sebire et al., 1996). In the second and third trimesters no clear definitions exist. It is rather a subjective impression of a persistently large bladder without apparent bladder cycle. The differential diagnosis of mega-cystis includes lower urinary tract obstruction, vesico-ureteral reflux, the megacystis-microcolon-hypoperistalsis syndrome- almost always occurring in female fetuses- and a pseudo-enlarged bladder in the female fetus in the third trimester of pregnancy. Because megacystis (longitudinal bladder diameter of 7-15 mm) in the first trimester of pregnancy is associated with a 25% risk of chromosomal defects, karyotyping is recommended (Liao et al., 2003).
When the bladder is repetitively not visible at all, the amount of amniotic fluid can help in making the differential diagnosis. In association with oligo-or anhydramnios, bilateral renal pathology should be suspected (e.g.bilateral multicystic kidney disease, severe bilateral UPJ-obstruction, bilateral renal agenesis or ARPKD). In severe intra-uterine growth restriction and twin-to-twin transfusion syndrome, the bladder can also be small. If the amniotic fluid volume is normal, a bladder anomaly should be considered. Bladder exstrophy is a very rare anomaly, occuring with a 2:1 male:female ratio. No bladder can be seen between the umbilical arteries, sometimes a soft tissue mass (bladder mucosa) is visible below the umbilicus. The genitalia are usually affected as well. Differential diagnosis should include cloacal extrophy and if an omphalocoele is present, the OEIS-complex (omphalocoele- exstrophy- imperforate anus) should be considered. Bladder rupture in the prenatal period is another very rare condition.
Conclusions
Prenatal diagnosis of renal and urinary tract malformations improves perinatal management and the prognosis of the affected child. The objective of prenatal ultrasound is to describe the type of anomaly as accurately as possible, to exclude associated malformations and to screen for parameters predictive of bad renal function, allowing for a multidisciplinary perinatal approach.
References
- Adra AM, Mejides AA, Dennaoui MS et, et al. Fetal pyelectasis: is it always physiologic? Am J Obstet Gynecol. 1995;173:1263–1266. doi: 10.1016/0002-9378(95)91367-x. [DOI] [PubMed] [Google Scholar]
- Anderson N, Clautice-Engle T, Allan R, et al. Detection of obstructive uropathy in the fetus: predictive value of sonographic measurements of renal pelvic diameter at various gestational ages. AJR Am J Roentgenol. 1995;164:719–723. doi: 10.2214/ajr.164.3.7863901. [DOI] [PubMed] [Google Scholar]
- Bhide A, Sairam S, Farrugia MK, et al. The sensitivity of antenatal ultrasound for predicting renal tract surgery in early childhood. Ultrasound Obstet Gynecol. 2005;25:489–492. doi: 10.1002/uog.1875. [DOI] [PubMed] [Google Scholar]
- Biard JM, Johnson MP, Carr MC MC, et al. Long-term outcomes in children treated by prenatal vesico-amniotic shunting for lower urinary tract obstruction. Obstet Gynecol. 2005;106:503–508. doi: 10.1097/01.AOG.0000171117.38929.eb. [DOI] [PubMed] [Google Scholar]
- Blyth B, Snyder HM, Duckett JW. Antenatal diagnosis and subsequent management of hydronephrosis. J Urol. 1993;149:693–698. doi: 10.1016/s0022-5347(17)36185-2. [DOI] [PubMed] [Google Scholar]
- kenkamp A, Dieterich C, Dressler F. Fetal serum concentrations of cystatin C and beta-2 microglobulin as predictors of postnatal kidney function. Am J Obstet Gynecol. 2001;185:468–475. doi: 10.1067/mob.2001.115283. [DOI] [PubMed] [Google Scholar]
- Broadley P, McHugo J, Morgan I, et al. The 4 year outcome following the demonstration of bilateral renal pelvic dilatation on prenatal renal ultrasound. Br J Radiol. 1999;72:265–270. doi: 10.1259/bjr.72.855.10396216. [DOI] [PubMed] [Google Scholar]
- Brun M, Maugey-Laulom B, Eurin D, et al. Prenatal sonographic patterns in autosomal dominant polycystic kidney disease: a multicenter study. Ultrasound Obstet Gynecol. 2004;24:55–61. doi: 10.1002/uog.1098. [DOI] [PubMed] [Google Scholar]
- Cascio S, Paran S, Puri P. Associated urological anomalies in children with unilateral renal agenesis. J Urol. 1999;162:1081–1083. doi: 10.1016/S0022-5347(01)68074-1. [DOI] [PubMed] [Google Scholar]
- Chaumoitre K, Brun M, Cassart M, et al. Differential diagnosis of fetal hyperechogenic cystic kidneys unrelated to renal tract anomalies: a multicenter study. Ultrasound Obstet Gynecol. 2006;28:911–917. doi: 10.1002/uog.3856. [DOI] [PubMed] [Google Scholar]
- Chitty LS, Altman DG. Charts of fetal size: kidney and renal pelvis measurements. Prenat Diagn. 2003;23:891–897. doi: 10.1002/pd.693. [DOI] [PubMed] [Google Scholar]
- Chudleigh PM, Chitty LS, Pembrey M, et al. The association of aneuploidy and mild fetal pyelectasis in an unselected population: the results of a multicenter study. Ultrasound Obstet Gynecol. 2001;17:197–202. doi: 10.1046/j.1469-0705.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Coco C, Jeanty P. Isolated fetal pyelectasis and chromosomal abnormalities. Am J Obstet Gynecol. 2005;193:732–738. doi: 10.1016/j.ajog.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Coelho GM, Bouzada MCF, Pereira AK, et al. Outcome of isolated antenatal hydronephrosis: a prospective cohort study. Pediatr Nephrol. 2007;22:1727–1734. doi: 10.1007/s00467-007-0539-6. [DOI] [PubMed] [Google Scholar]
- Coplen DE, Austin PF, Yan Y, et al. The magnitude of fetal renal pelvic dilatation can identify obstructive postnatal hydronephrosis, and direct postnatal evaluation and managemen. J Urol. 2006;176:724–727. doi: 10.1016/j.juro.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Corbani V, Ghiggeri GM, Sanna-Cherchi S. Congenital solitary functioning kidneys: which ones warrant follow-up into adult life? Nephrol Dial Transplant. 2011;26:1458–1460. doi: 10.1093/ndt/gfr145. [DOI] [PubMed] [Google Scholar]
- Corteville JE, Gray DL, Crane JP. Congenital hydronephrosis: correlation of fetal ultrasonographic findings with infant outcome. Am J Obstet Gynecol. 1991;165:384–488. doi: 10.1016/0002-9378(91)90099-d. [DOI] [PubMed] [Google Scholar]
- Davies F, Coles GA, Harper PS, et al. Polycystic kidney disease re-evaluated: a population- based study. Q J Med. 1991;79:477–485. [PubMed] [Google Scholar]
- Elder JS. Antenatal hydronephrosis. Fetal and neonatal management. Pediatr Clin North Am. 1997;44 doi: 10.1016/s0031-3955(05)70558-7. [DOI] [PubMed] [Google Scholar]
- Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society of Fetal Urology. Pediatr Radiol. 1993;23:478–480. doi: 10.1007/BF02012459. [DOI] [PubMed] [Google Scholar]
- Freedman AL, Johnson MP, Smith CA, et al. Long-term outcome in children after antenatal intervention for obstructive uropathies. Lancet. 1999;354:374–377. doi: 10.1016/S0140-6736(98)11006-1. [DOI] [PubMed] [Google Scholar]
- Gilbert GM, Brace RA. Amniotic fluid volume changes throughout pregnancy. Semin Perinatol. 1993;17:150–157. [PubMed] [Google Scholar]
- Grandjean H, Larroque D, Levi S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus Study. Am J Obstet Gynecol. 1999;181:446–454. doi: 10.1016/s0002-9378(99)70577-6. [DOI] [PubMed] [Google Scholar]
- Gunn TR, Mora JD, Pease P. Antenatal diagnosis of urinary tract abnormalities by ultrasonography after 28 weeks’gestation: incidence and outcome. Am J Obstet Gynecol. 1995;172:479–486. doi: 10.1016/0002-9378(95)90560-x. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Hori C, Tsukahara H. Vesicoureteral reflux in male and female neonates as detected by voiding ultrasonography. Kidney Int. 1999;55:1486–1490. doi: 10.1046/j.1523-1755.1999.00380.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Tsukahara H, Ohshima Y, et al. Renal aplasia is the predominant cause of congenital solitary kidneys. Kidney International. 2002;61:1840–1844. doi: 10.1046/j.1523-1755.2002.00322.x. [DOI] [PubMed] [Google Scholar]
- Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics. 2001;108:E7. doi: 10.1542/peds.108.1.e7. [DOI] [PubMed] [Google Scholar]
- Ismaili K, Hall M, Donner C, et al. Reults of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003;188:242–246. doi: 10.1067/mob.2003.81. [DOI] [PubMed] [Google Scholar]
- Lee RS, Cendron M, Klnnamon DD, et al. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006;118:586–593. doi: 10.1542/peds.2006-0120. [DOI] [PubMed] [Google Scholar]
- Levi S. Mass screening for fetal malformations: the Eurofetus study. Ultrasound Obstet Gynecol. 2003;22:555–558. doi: 10.1002/uog.935. [DOI] [PubMed] [Google Scholar]
- Liang CC, Cheng PJ, Lin CJ, et al. Outcome of prenatally diagnosed fetal hydronephrosis. J Reprod Med. 2002;47:27–32. [PubMed] [Google Scholar]
- Liao AW, Sebire NJ, Geerts L, et al. Megacystis at 10-14 weeks of gestation: chromosomal defects and outcome according to bladder length. Ultrasound Obstet Gynecol. 2003;21:338–341. doi: 10.1002/uog.81. [DOI] [PubMed] [Google Scholar]
- Machado MR, Cecatti JG, Krupa F, et al. Curve of amniotic fluid index measurements in low risk pregnancy. Acta Obset Gynecol Scand. 2007;86:37–41. doi: 10.1080/00016340600994976. [DOI] [PubMed] [Google Scholar]
- Morris RK, Jones E, Kilby MD. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenat Diagn. 2007;27:900–911. doi: 10.1002/pd.1810. [DOI] [PubMed] [Google Scholar]
- Morris RK, Malin GL, Khan KS. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG. 2010;117:382–390. doi: 10.1111/j.1471-0528.2010.02500.x. [DOI] [PubMed] [Google Scholar]
- Morris RK, Tonks A, Kilby MD, et al. Fetal lower urinary tract obstruction: an epidemiological population based study for outcome. Reprod Sci. 2011;18:366A. [Google Scholar]
- Morris RK, Ruano R, Kilby MD. The effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol. 2011;37:626–637. doi: 10.1002/uog.8981. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Herndon CDA, Cooper C, et al. The society for fetal urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010;6:212–231. doi: 10.1016/j.jpurol.2010.02.205. [DOI] [PubMed] [Google Scholar]
- Filho FM, Sa RA, Velarde LGC, et al. Normal range for fetal urine production rate by 3D-ultrasound in Brazilian population. Arch Gynecol Obstet. 2011;283:497–500. doi: 10.1007/s00404-010-1397-1. [DOI] [PubMed] [Google Scholar]
- Persutte WH, Koyle M, Lenke RR, et al. Mild pyelectasis ascertained with prenatal ultrasonography is pediatrically significant. Ultrasound Obstet Gynecol. 1997;10:12–18. doi: 10.1046/j.1469-0705.1997.10010012.x. [DOI] [PubMed] [Google Scholar]
- Potter EL. Bilateral absence of ureters and kidneys: a report of 50 cases. Obstet Gynecol. 1965;25:1–12. doi: 10.1016/s0029-7844(02)02394-3. [DOI] [PubMed] [Google Scholar]
- Sairam S, Habib A, Sasson S, et al. Natural history of fetal hydronephrosis diagnosed on mid-trimester ultrasound. Ultrasound Obstet Gynecol. 2001;17:191–196. doi: 10.1046/j.1469-0705.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- Schreuder MF, Westland R, Van Wijk AE. Unilateral multicystic dysplastic kidney: a meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant. 2009;24:1810–1818. doi: 10.1093/ndt/gfn777. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Von Kaisenberg C, Rubio C, et al. Fetal megacystis at 10-14 weeks of gestation. Ultrasound Obstet Gynecol . 1996;8:387–390. doi: 10.1046/j.1469-0705.1997.08060387.x. [DOI] [PubMed] [Google Scholar]
- Sidhu G, Beyene J, Rosenblum ND. Outcome of isolated antenatal hydronephrosis: a systematc review and meta-analysis. Pediatr Nephrol. 2006;21:218–224. doi: 10.1007/s00467-005-2100-9. [DOI] [PubMed] [Google Scholar]
- Signorelli M, Cerri V, Taddei F, et al. Prenatal diagnosis and management of mild fetal pyelectasis: implications for neonatal outcome and follow-up. Eur J Obstet Gynecol Reprod Biol. 2005;118:154–159. doi: 10.1016/j.ejogrb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Tedesco GD, De Silva Bussamra LC, Araujo EJ EJ, et al. Reference range of fetal renal volume by three-dimensional ultrasonography using the VOCAL method. Fetal Diagn Ther. 2009;25:385–391. doi: 10.1159/000236151. [DOI] [PubMed] [Google Scholar]
- Tsatsaris V, Gagnadoux MF, Aubry MC, et al. Prenatal diagnosis of bilateral isolated fetal hyperechogenic kidneys. Is it possible to predict long term outcome? BJOG. 2002;109:1388–1393. doi: 10.1046/j.1471-0528.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- Touboul C, Boulvain M, Picone O, et al. Normal fetal urine production rate estimated with 3-dimensional ultrasonography using the rotational technique (virtual organ computer-aided analysis) Am J Obstet Gynecol. 2008;199:57e1–5. doi: 10.1016/j.ajog.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: nog just fetal urine anymore. J Perinatol. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- Westland R, Schreuder MF, Bökenkamp A et, et al. Renal injury in children with a solitary functioning kidney- the KIMONO study. Nephrol Dial Transplant. 2011;26:1533–1541. doi: 10.1093/ndt/gfq844. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystic kidney disease. N Eng J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Neuhaus TJ, Willi UV, et al. Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol. 2005;25:483–488. doi: 10.1002/uog.1879. [DOI] [PubMed] [Google Scholar]
- Woodward M, Frank D, et al. Postnatal management of antenatal hydronephrosis. BJU International. 2002;89:149. doi: 10.1046/j.1464-4096.2001.woodward.2578.x. [DOI] [PubMed] [Google Scholar]
- Yuksel A, Batukan C, et al. Sonographic findings of fetuses with an empty renal fossa and normal amniotic fluid volume. Fetal Diagn Ther. 2004;19:525–532. doi: 10.1159/000080166. [DOI] [PubMed] [Google Scholar]