Abstract
Therapy for advanced non-small-cell lung cancer has developed significantly with new awareness of histologic subtype as an important factor in guiding treatment and the development of targeted agents for molecular subgroups harboring critical mutations that spur on cancer growth. In this comprehensive review, we look back at developments in targeted therapy for advanced non-small-cell lung cancer, reviewing in detail efforts, both successful and in some cases less so, to target EGFR, VEGF and ALK. This review provides an overview of where the field stands at present and the areas we feel are most likely to provide challenges and potential successes in the next 5 years including immune checkpoint inhibition, epigenetic therapy and driver mutation targeting.
Keywords: ALK, crizotinib, EGFR, epigenetic, erlotinib, immune checkpoint, non-small-cell lung cancer, PD1, targeted therapy, VEGF
Lung cancer is the most common cause of cancer death, with approximately 1.4 million deaths worldwide annually and more deaths in the USA attributable to lung cancer than the next three most common cancers combined [1,101]. Non-small-cell lung cancer (NSCLC) now accounts for more than 85% of lung cancers in western countries, with 20–30% of NSCLC occurring in never smokers [2,102]. The rise in NSCLC incidence in never or light smokers has coincided with the discovery that a proportion of NSCLC, in particular those occurring in never or light smokers, are driven by oncogenic mutations that, when selectively inhibited, can lead to dramatic tumor regression and prolonged survival. This finding has revolutionized the field of NSCLC research, and in conjunction with studies demonstrating that histologic subtype influences response to chemotherapy, has led to increasingly targeted efforts to personalize medicine. This article will review the background of personalized medicine in lung cancer, examining attempts to discover and target molecular pathways that drive NSCLC growth while providing a comprehensive review of recent developments in targeted therapy, and looking forward to an exciting and challenging time as novel therapeutics, including immune checkpoint inhibition and epigenetic therapy, are examined.
The past
For the first 70 years after its emergence as a major health problem in the early 20th century, NSCLC was a surgical disease, with pneumonectomy and in later years lobectomy being curative for a small number of patients with localized disease, while no effective treatment was available for the majority of patients who presented with advanced disease [3]. From the early days of cytotoxic chemotherapy, numerous agents were studied in NSCLC without survival advantages until the advent of platinum-based doublet chemotherapy in the early 1980s [4]. During the subsequent two decades, research focused on adding novel cytotoxic drugs to a platinum backbone; however, differences in survival between chemotherapy doublets were only occasionally apparent and often not reproduced [5–8]. This led to platinum plus ‘another’ drug (e.g., etoposide, vinorelbine or paclitaxel) being the first-line standard of care chemotherapy for advanced NSCLC for approximately 25 years, with choice of the second drug largely based on toxicity profile and individual preference.
Many research efforts during this time focused on dose intensification of chemotherapy or addition of a third cytotoxic agent to a platinum doublet; however, the benefits of either approach were inconsistent [9,10]. Important developments in molecular biology were also occurring during this time, in particular, the elucidation of cellular pathways in tumor cells driving tumor growth and metastasis and these discoveries would lead to rapidly accelerated targeted agent development, particularly the development of small-molecule TKIs and targeted monoclonal antibodies. By the start of the 21st century, a general consensus was that a plateau in the development of cytotoxic chemotherapy had been reached and new directions were needed [9,10].
EGF receptor
The EGF receptor (EGFR) is overexpressed in 50–80% of NSCLC, while increased gene copy number is noted in up to 60% of tumors [11–13]. EGFR plays an integral role in tumor growth as a component of two principal cellular pathways that drive tumor growth and spread, the PI3K/AKT/mTOR pathway and the RAS/RAF/MEK/MAPK pathway; therefore, it was an obvious target for drug development [14].
Agents targeting EGFR in advanced NSCLC
Tyrosine kinase inhibitors
EGFR TKIs are small molecules that selectively bind the tyrosine kinase region of the intracellular domain of EGFR, preventing adenosine triphosphate binding and EGFR autophosphorylation, thus inhibiting EGFR signal transduction [15]. Early studies of the EGFR TKIs, gefitinib and erlotinib, suggested that certain clinical characteristics correlated with responsiveness to these agents; these included east Asian ethnicity, adenocarcinoma histology, female gender, and light or nonsmoking history [16].
Two early randomized studies of gefitinib in unselected advanced NSCLC patients, the INTACT 1 and 2 studies, compared first-line platinum doublet chemotherapy combined with gefitinib versus chemotherapy alone [17,18]. No benefit was shown for the addition of gefitinib in either study. The INTEREST study compared docetaxel chemotherapy with gefitinib in 1466 pretreated NSCLC patients; this study achieved its primary end point of noninferiority of gefitinib versus docetaxel [19]. Conversely, a smaller Japanese study in 489 pretreated patients failed to demonstate noninferiority of gefitinib when compared with docetaxel, while a Korean study with the primary end point of progression-free survival (PFS) showed significantly prolonged PFS associated with gefitinib [20,21]. Subsequently, the ISEL study randomized 1692 patients with advanced NSCLC refractory or intolerant to chemotherapy to gefitinib or placebo [22]. The primary end point of median overall survival (OS) did not show a significant benefit for gefitinib. Despite the results of a preplanned subgroup analysis enriched for never smokers and Asian patients that did show improved survival in these groups, the ISEL study caused the US FDA to rescind its approval of gefitinib as second-line therapy for patients with advanced NSCLC. In contrast, the BR.21 study, which compared erlotinib with placebo in 731 pretreated patients, demonstrated a survival advantage for erlotinib over placebo, leading to the use of erlotinib rather than gefitinib in the second-line setting in North America [23].
These and other data suggested that further development of EGFR TKIs would benefit from the development of a biomarker to predict responsiveness. The presence of activating mutations of EGFR in tumor was a promising candidate when first examined in the IPASS study, which randomized 1217 Asian patients with advanced lung adenocarcinoma to first-line gefitinib or carboplatin/paclitaxel [24]. The patient population for this study selected patients with clinical characteristics (light/nonsmoker, adenocarcinoma) thought to confer sensitivity to EGFR TKIs. This study demonstrated an improvement in PFS for gefitinib, with subgroup analysis suggesting this benefit was principally in the 60% of patients with activating mutations in EGFR, in particular, exon 19 deletions, exon 21 mutation (L858R) or exon 18 mutation (G719X). Subsequent analysis of this study did not demonstrate an OS advantage for gefitinib in the study population as a whole or in EGFR mutation-positive patients; however, two-thirds of this subgroup received post-study treatment with an EGFR TKI [25]. By contrast, patients without an EGFR mutation had significantly shorter PFS with gefitinib compared with chemotherapy, while OS was not significantly different.
Two Japanese studies comparing first-line gefitinib with platinum doublet chemotherapy in EGFR mutant patients have confirmed a significant PFS benefit for gefitinib; however, these again did not show an OS benefit, probably due to post-study crossover [26,27]. Based on these data, gefitinib has achieved regulatory approval in Europe for the initial treatment of patients with advanced NSCLC harboring activating mutations of EGFR [103].
Erlotinib has been compared with chemotherapy in the first-line setting in two recently published studies conducted in China and Europe. The Chinese OPTIMAL trial randomized 154 EGFR-mutant patients to erlotinib or carboplatin/gemcitabine [28]. PFS was significantly increased by 4 months in the erlotinib arm; however, the high rate of crossover − 76% of the patients received postprotocol EGFR TKI – meant that no OS advantage was demonstrated [29]. The European EURTAC study randomized 174 patients to erlotinib or platinum doublet chemotherapy [30]. Median PFS was significantly prolonged by almost 5 months for erlotinib versus chemotherapy; however, no difference was seen in OS [104]. Most recently, the LUX-Lung 3 trial, which compared the irreversible EGFR/HER2 TKI afatinib versus cisplatin/pemetrexed as first-line therapy for EGFR-mutant patients, demonstrated a 4.2-month improvement in PFS in the intention-to-treat population and a 6.7-month PFS increase in those patients with the most common sensitizing mutations of EGFR [31].
The toxicity profile of EGFR TKIs such as gefitinib, erlotinib and afatinib is significantly different to chemotherapy, with much less myelosuppression, nausea and neurotoxicity; however, more frequent rash and diarrhea. In most cases, EGFR TKIs appear to be better tolerated by patients than chemotherapy. Taken collectively, these studies confirm the role of EGFR mutation testing in guiding the management of advanced NSCLC patients and represent the first predictive molecular marker to be discovered in the disease. Advanced NSCLC patients with tumors harboring sensitizing mutations in EGFR are likely to have a much higher response rate, prolonged PFS, and may have prolonged OS when treated with EGFR TKIs instead of chemotherapy in the first-line setting, which has led the National Comprehensive Cancer Network to recommend routine testing for EGFR mutations in nonsquamous NSCLC and the use of first-line erlotinib for EGFR-mutant advanced NSCLC in recent guidelines [105]. In the second and subsequent line setting, erlotinib has been shown to be superior to placebo and EGFR TKIs are a reasonable alternative treatment option to single-agent chemotherapy, particularly for nonsquamous NSCLC.
Cetuximab
Cetuximab is a monoclonal antibody targeting EGFR that has been approved for the treatment of advanced colorectal cancer and advanced head and neck cancer. The Phase III FLEX study randomized 1125 patients with newly diagnosed advanced NSCLC to platinum doublet chemotherapy with or without cetuximab, which was administered both concurrently and as a weekly maintenance therapy after six cycles of chemotherapy [32]. The cetuximab-containing arm of this study showed approximately a 6-week survival advantage over chemotherapy alone. This benefit appeared to be present for all major analyzed histologic and clinical subgroups, and the objective response rate was also higher for the cetuximab-containing arm (36 vs 29%). Importantly, however, significantly increased serious toxicities in the cetuximab-containing arm included rash, febrile neutropenia, diarrhea and infusion reactions. Subsequent analysis suggested that development of an acneiform rash in the first 3 weeks of treatment was associated with a better response to treatment and median survival (15 vs 8.8 months; p < 0.0001) and this has been suggested as a possible clinical marker for continuing the drug [33]. Analysis of tumor specimens from this study also suggested that high EGFR immunohistochemical expression may predict benefit from cetuximab when compared with low expression [34]. Another Phase III study, BMS-099, which evaluated the addition of cetuximab to carboplatin/paclitaxel, demonstrated a 1.3-month difference in survival favoring cetuximab that did not reach statistical significance; however, this was similar numerically to that seen in the FLEX study [35]. An ongoing Southwest Oncology Group Phase III study is evaluating the addition of cetuximab to platinum doublet chemotherapy combined with the VEGF antibody, bevacizumab (ClinicalTrials.gov identifier: NCT00946712) [106]. To date, cetuximab has not achieved regulatory approval in the USA or Europe for the treatment of advanced NSCLC, while the 6-week survival benefit and toxicity seen in FLEX allied to the high cost of the drug have stirred debate on its role in the management of advanced NSCLC [36].
VEGF
High levels of VEGF expression are associated with a poor prognosis in NSCLC and are a potent stimulant of tumor angiogenesis and growth [37]. This finding has driven the investigation of VEGF-targeted agents in advanced NSCLC.
Bevacizumab
The recombinant humanized monoclonal antibody, bevacizumab, binds VEGF, thereby blocking the VEGF pathway. The first-line Phase III ECOG study, E4599, randomized 878 patients with advanced nonsquamous NSCLC to carboplatin/paclitaxel for six cycles with or without concurrent bevacizumab followed by maintenance bevacizumab until disease progression [38]. Patients with squamous carcinoma, history of hemoptysis, therapeutic anticoagulation or brain metastases were excluded due to bleeding events in an earlier phase study that led to the Phase III studies [39]. Patients who received carboplatin/paclitaxel/bevacizumab in the Phase III study had a 2-month improvement in median OS compared with chemotherapy alone, response rate was more than doubled (35 vs 15%) and, importantly, 1-year survival (51 vs 44%) and 2-year survival (23 vs 15%) were significantly better in patients who received bevacizumab. While bevacizumab was well tolerated overall, seven deaths did occur due to hemoptysis or hematemesis, and grade 3–4 hypertension was more common in the bevacizumab-containing arm (7 vs 0.7%).
The Phase III AVAiL trial evaluated two different doses of bevacizumab (7.5 and 15 mg/kg) in a similar design and study population to E4599; however, it differed in choice of platinum doublet, and cisplatin/gemcitabine was used in place of carboplatin/paclitaxel [40]. This study met its primary end point, which was to prolong PFS for both doses of bevacizumab while response rate was also significantly increased. However, subsequent updated analysis failed to show an OS benefit for the addition of bevacizumab to cisplatin/gemcitabine [41].
Data from the AVAPERL study that examined the addition of maintenance pemetrexed to bevacizumab after induction cisplatin/pemetrexed/bevacizumab showed a 3.6-month improvement in PFS for the addition of maintenance pemetrexed; however, OS data are not yet mature [107]. An ongoing Phase III study is analyzing combination chemotherapy with dual-antibody blockade using cetuximab and bevacizumab (ClinicalTrials.gov identifier: NCT00946712) [106]. The ATLAS study compared maintenance bevacizumab versus the combination of bevacizumab/erlotinib after platinum doublet chemotherapy with bevacizumab in 768 first-line patients [42]. While there was a 1-month improvement in PFS, no OS benefit for the addition of erlotinib was shown. Most recently, the POINTBREAK study randomized 939 nonsquamous patients to first-line carboplatin/paclitaxel/bevacizumab (CTB) followed by maintenance bevazicumab versus carboplatin/pemetrexed/bevacizumab (CPB) followed by maintenance bevacizumab/pemetrexed [108]. This study failed to achieve its primary end point of prolonged OS for the pemetrexed/bevacizumab-containing arm and, while there was a marginal improvement in PFS of 2 weeks, response rates were similar between the two groups. Toxicity was different in the two arms with more anemia, thrombocytopenia and fatigue in the pemetrexed arm, and increased rates of febrile neutropenia, peripheral neuropathy and alopecia in the paclitaxel arm. Of note, in this era of rising drug expenditures, the cost of carboplatin/pemetrexed/bevacizumab is approximately US$13,000 per cycle, almost twice that of carboplatin/paclitaxel/bevacizumab.
VEGF TKIs & other agents targeting tumor vasculature
Several small-molecule TKIs targeted at VEGF and other growth pathways have been studied in advanced NSCLC; however, results to date have been disappointing, with several agents increasing PFS; however, no durable OS advantage has been demonstrated to date.
Cediranib, a TKI that blocks multiple VEGF receptors, was studied in a large Phase II study in advanced NSCLC (including squamous and nonsquamous patients), with 296 patients being randomized to carboplatin/paclitaxel chemotherapy with or without cediranib [43]. While the cediranib-containing arm showed a higher response rate than chemotherapy alone, it was also associated with significantly higher rates of hypertension, hand–foot syndrome and gastrointestinal upset, and treatment was implicated in 13% of deaths in the cediranib arm. This led investigators to conclude that the combination was not tolerable at the investigated cediranib 30 mg dose. Survival was not significantly increased in the cediranib arm.
Similarly sorafenib, a multitargeted TKI that inhibits VEGF, showed a 2-week improvement in median PFS; however, no difference in OS compared with placebo was seen when added to cisplatin/gemcitabine chemotherapy [44]. In the second and subsequent-line setting, sunitinib failed to show a survival benefit when compared with erlotinib [45].
Vandetanib, a TKI that inhibits EGFR, VEGF and RET-dependent signaling, showed prolonged PFS combined with docetaxel in a Phase III study but no OS advantage, while in the ZEAL study, combination with pemetrexed failed to prolong PFS or OS [46,47]. In a Phase III study of EGFR TKI-pretreated patients vandetanib again failed to prolong survival as a single agent when compared with placebo [48].
Motesanib, the selective inhibitor of VEGF receptors 1, 2 and 3, PDGF receptor and Kit did not prolong OS when added to first-line carboplatin/paclitaxel in nonsquamous NSCLC [49].
Aflibercept, a recombinant human fusion protein targeted at VEGF, recently failed to demonstrate a survival advantage in a 913-patient Phase III study when added to docetaxel for platinum-pretreated nonsquamous NSCLC [50]. The vascular-disrupting agent, ASA404, which targets established vasculature in tumors, did not improve survival when added to carboplatin/paclitaxel in the frontline setting [51].
Collectively, Phase III studies of small molecules targeting tumor angiogenesis or vasculature in NSCLC (Table 2) have enrolled more than 8000 patients over the past decade without demonstrating an improvement in OS for any of the agents studied. These failures highlight the need for the development of efficacy biomarkers at an earlier stage in drug development.
Table 2.
Selected randomized studies of VEGF inhibition in advanced non-small-cell lung cancer.
| Study (year) | Patient population | Study arms | PFS | OS | Ref. |
|---|---|---|---|---|---|
| Bev | |||||
| E4599 USA (2006) | n = 878; first-line nonsquamous NSCLC, no brain metastases, hemoptysis or therapeutic anticoagulation | Carbo/paclitaxel × 6 cycles ± concurrent Bev followed by maintenance | Carbo/paclitaxel + Bev − 6.2 months; Carbo/paclitaxel − 4.5 months; p< 0.001; S | Carbo/paclitaxel + Bev − 12.3 months; Carbo/paclitaxel − 10.3 months; p< 0.001; S | [38] |
| AVAiL Europe (2009) | n = 1043; first-line nonsquamous NSCLC, no brain metastases, hemoptysis or therapeutic anticoagulation | Cis/Gem × 6 cycles ± concurrent Bev followed by maintenance | Cis/Gem – Bev 6.5 m and 6.7 months; Cis/Gem − 6.1 months; p = 0.003, p = 0.03;NS | Cis/Gem + Bev −13.6 m and 13.4 months; Cis/Gem − 13.1 months; p = 0.42, p = 0.761; NS | [41] |
| AVAPERL Europe Preliminary report (2011) | n = 253; first-line nonsquamous NSCLC, no brain metastases, hemoptysis or therapeutic anticoagulation | Cis/Pem/Bev − 4 cycles followed by maintenance Bev ± Pern | Maintenance Pem/Bev− 10.2 m Maintenance Bev− 6.6 months; p< 0.001; S | Not yet mature | [104] |
| ATLAS USA Preliminary report (2010) | n = 768; first-line nonsquamous NSCLC, no brain metastases, hemoptysis or therapeutic anticoagulation | Platinum doublet/Bev × 4 cycles followed by maintenance Bev ± erlotinib | Maintenance Bev + placebo − 3.7 months; Maintenance Bev + erlotinib − 4.8 months; p = 0.0012; S | Bev + placebo − 13.3 months; Bev + erlotinib − 14.4 months; p = 0.56; NS | [42] |
| POINTBREAK USA Preliminary report (2012) | n = 939; first-line nonsquamous NSCLC, no brain metastases, hemoptysis or therapeutic anticoagulation | Carbo/Pem/Bev ×4→ maintenance Pem/Bevvs Carbo/taxol/Bev → maintenance Bev | Carbo/Pem/Bev − 6 months; Carbo/taxol/Bev − 5.6 months; p = 0.012; S | Carbo/Pem/Bev − 12.8 months; Carbo/taxol/Bev− 13.4 months; p = 0.949; NS | [108] |
| VEGF-targeted TKls | |||||
| BR24 Americas/Europe/Australasia (2009) | n = 296; first-line NSCLC, unselected | Carbo/paclitaxel × 6–8 cycles ± cediranib p.o. daily | Carbo/paclitaxel + cediranib − 5.6 months; Carbo/paclitaxel − 5 months; p = 0.13; NS | Carbo/paclitaxel + cediranib − 10.5 months; Carbo/paclitaxel − 10.1 months; p = 0.11; NS | [43] |
| NexUS Europe (2012) | n = 772; first-line NSCLC (squamous patients excluded from analysis) | Cis/Gem × 6 cycles ± sorafenib | Cis/Gem + sorafenib − 6 months; Cis/Gem − 5.5 months; p = 0.008 | Cis/Gem + sorafenib − 12.4 months; Cis/Gem − 12.5 months; p = 0.40; NS | [44] |
| SUN 1087 Europe/Asia (2012) | n = 960; second or subsequent line NSCLC, unselected | Erlotinib ± sunitinib | Erlotinib + sunitinib − 3.6 months; erlotinib − 2 months; p = 0.0023; S | Erlotinib + sunitinib − 9.0 months; erlotinib − 8.5 months; p = 0.14; NS | [45] |
| ZODIAC USA/Asia/Europe (2010) | n = 1391; second-line NSCLC | Docetaxel ±vandetanib | Docetaxel + vandetanib − 4 months; docetaxel − 3.2 months; p< 0.0001; S | Docetaxel + vandetanib − 10.3 months; docetaxel −9.9 months; p = 0.371; NS | [46] |
| ZEAL Europe/Australia (2011) | n = 434; second line, unselected | Pern ± vandetanib | Pern + vandetanib − 4.4 months; Pern − 3 months; p = 0.108; NS | Pern + vandetanib − 10.5 months; Pern − 9.2 months; p = 0.219; NS | [47] |
| ZEPHYR Asia (2012) | n = 924; second-line or subsequent line NSCLC, unselected | Vandetanib vs placebo | Vandetanib− 1.9 months; placebo − 1.8 months; p< 0.001; S | Vandetanib − 8.5 months; Placebo − 1.8 months; p = 0.527; NS | [48] |
| MONET1 Europe/Asia/USA (2012) | n = 1090; first-line nonsquamous | Carbo/paclitaxel ± motesanib | Carbo/paclitaxel + motesanib− 5.6 months; Carbo/paclitaxel − 5.4 months; p< 0.001; S | Carbo/paclitaxel + motesanib− 13 months; Carbo/paclitaxel − 11 months; p = 0.14; NS | [49] |
| Other VEGFIvasculature-targeted agents | |||||
| VITAL Europe/Americas (2012) | n = 913; second-line nonsquamous | Docetaxel ± aflibercept | Docetaxel + aflibercept − 5.2 months; docetaxel − 4.1 months; p = 0.0035; S | Docetaxel + aflibercept− 10.1 months; docetaxel − 10.4 months; p = 0.9; NS | [50] |
| ATTRACT-1 USA/Asia/Europe (2011) | n= 1299; first-line NSCLC; unselected | Carbo/paclitaxel ± ASA404 | Carbo/paclitaxel + ASA404− 5.5 months; Carbo/paclitaxel − 5.5m; p = 0.727; NS | Carbo/paclitaxel + ASA404 − 13.4 months; Carbo/paclitaxel − 12.7 months; p = 0.535; NS | [51s] |
Bev: Bevacizumab; Carbo: Carboplatin; Cis: Cisplatin; Gem: Gemcitabine; NS: No statistically significant difference: NSCLC: Non-small-cell lung cancer: OS: Overall survival: p.o.: Perorem: Pern: Pemetrexed: PFS: Progression-free survival: S: Statistically significant difference:.
ALK translocations & crizotinib
The EML4–ALK fusion oncogene, which arises from an inversion on the short arm of chromosome 2 joining exons 1–13 of EML4 to exons 20–29 of ALK, was first reported in NSCLC in 2007 [52]. Fusion of ALK with other partners occurs but is rare [53]. Subsequent studies confirmed the presence of ALK fusion genes in 2–7% of NSCLC, arising more commonly in nonsmokers and almost exclusively in tumors of nonsquamous histology [54].
Crizotinib is a potent oral ATP-competitive inhibitor of both ALK and the c-Met/HGF receptor tyrosine kinase [55]. In a Phase I dose–escalation study of 82 predominantly pretreated NSCLC patients with tumors harboring the ALK translocation, an overall response rate of 57% was seen and the estimated 6-month PFS was 72% [56]. Updated results from this study confirmed a median PFS of 9.7 months and estimated 12-month OS of 74.8% [57].
These results led to the accelerated approval of crizotinib for the treatment of advanced NSCLC harboring ALK translocations by the FDA in August 2011 [109].
Preliminary results from PROFILE 1005, a large, single-arm Phase II study of crizotinib in pretreated advanced NSCLC harboring the ALK fusion gene, reported a response rate of 53% and a median PFS of 8.5 months [58]. Two international Phase III registration trials of crizotinib have recently reported PFS results in ALK rearranged advanced NSCLC.
In the second-line setting, PROFILE 1007 compared crizotinib with either docetaxel or pemetrexed and randomized 347 patients in 20 countries [59]. The median PFS for crizotinib was 7.7 versus 3.0 months with chemotherapy (p < 0.0001), while the response rate was 65.3% with crizotinib versus 19.3% for chemotherapy (p < 0.0001). Quality of life was superior for crizotinib-treated patients based on patient-reported outcomes regarding time to deterioration in lung cancer symptoms, which showed a median of 5.6 months with crizotinib versus 1.4 months with chemotherapy (p < 0.0001). Overall survival from this study is yet to mature but is likely to be confounded by high rates of post-progression crossover from chemotherapy to crizotinib.
In the first-line setting, PROFILE 1014 is an international Phase III study comparing crizotinib with cisplatin/pemetrexed or carboplatin/pemetrexed in ALK-positive nonsquamous advanced NSCLC (ClinicalTrials.gov identifier: NCT01154140) [106].
Despite these very encouraging results, the majority of patients develop resistance to crizotinib within a year of commencing treatment and several secondary resistance mutations have been described [60]. Recent data suggest that an even rarer molecular subtype of NSCLC harboring a translocation in ROS1 may also respond to crizotinib [61].
The present: treating advanced NSCLC in 2013
The management of advanced NSCLC has developed significantly over the past 5 years.
Advanced NSCLC without a targetable driver mutation
The importance of histology in guiding the choice of systemic therapy was shown in studies by Scagliotti et al. of platinum doublet chemotherapy incorporating pemetrexed compared with non pemetrexed-containing chemotherapy [62]. High immunohistochemical expression of thymidylate synthase in squamous NSCLC may provide the basis for the reduced sensitivity of these tumors to pemetrexed-based regimens; however, this has yet to be prospectively validated [63]. For advanced nonsquamous NSCLC not harboring an EGFR mutation or ALK translocation, platinum/pemetrexed is now a first-line standard of care providing an approximate 2-month survival advantage over non pemetrexed-containing platinum doublet chemotherapy. The role of maintenance therapy with single-agent pemetrexed after initial platinum doublet chemotherapy has been clarified to a degree by the recently reported PARAMOUNT study, which showed a 22% reduction in the risk of death in favor of maintenance pemetrexed versus placebo for patients with response or stable disease after initial treatment with platinum/pemetrexed [64]. Switch maintenance, that is, immediate use of pemetrexed for patients with stable disease or response to a different platinum doublet has also been show to prolonged survival [65]. The POINTBREAK study failed to demonstrate a survival advantage for carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab versus carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab alone [108]. ECOG 5508, which is testing pemetrexed/bevacizumab versus bevacizumab alone after carboplatin/paclitaxel/bevacizumab induction, should further clarify the switch maintenance question (ClinicalTrials.gov identifier: NCT01107626) [106]. The role of cetuximab combined with chemotherapy is as yet unclear given the relatively significant toxicity and moderate survival benefit demonstrated in studies to date, it is currently not approved in Europe or North America for the treatment of NSCLC. For both squamous and nonsquamous patients, the SATURN study demonstrated that maintenance erlotinib prolonged OS by approximately 5 weeks when compared with placebo [66]. Molecular analyses from this study suggest that, while patients with NSCLC harboring an EGFR mutation derived the greatest PFS benefit from maintenance erlotinib, wild-type EGFR patients also benefited and the presence of KRAS mutations was the only marker prognostic for reduced PFS [67].
Overall, the relatively high number of patients in recent maintenance studies who did not receive standard second-line chemotherapy and the initial use of four instead of six cycles of platinum doublet chemotherapy has led to continued debate among experts regarding the maintenance approach [68]. Current guidelines reflect the recent rapid pace of progress in the understanding of NSCLC and are flexible in recommending platinum doublet plus bevacizumab as first-line therapy for mutation-negative patients until further trial data become available [105].
Advanced NSCLC with a sensitizing EGFR mutation or ALK translocation
It is currently recommended that all patients with nonsquamous NSCLC have their tumors tested for EGFR mutations or ALK translocations, and planned updates to guidelines will also suggest mutation testing in squamous patients who are nonsmokers [105]. If a sensitizing mutation of EGFR is found to be present, first-line treatment with oral erlotinib (or gefitinib if approved) is recommended and provides a PFS benefit (and may prolong OS) over first-line platinum doublet chemotherapy. For patients with an EGFR mutation that have not received first- or subsequent-line EGFRTKI, then erlotinib or gefitinib is recommended upon disease progression. For patients with ALK translocations, the recommendations are for first-line crizotinib or for it to be given on disease progression if not received previously.
Despite the efficacy of EGFR TKIs and crizotinib for tumors harboring sensitizing EGFR mutations or ALK translocations, almost all patients ultimately develop disease progression on these agents, usually due to the development of resistance mutations. Currently, the standard treatment for these patients reverts to chemotherapy similar to that for mutation-negative patients; however, this may change as agents targeting resistance are developed and pathways of resistance are further elucidated.
Expert commentary & five-year view
While significant progress has been made to decodethe NSCLC genome, in particular demonstrating that approximately 50% of the tumors harbor critical driver mutations that if targeted successfully may lead to rapid tumor regressions and prolonged survival, developments in squamous NSCLC and lung tumors that lack apparent driver mutations have lagged behind [69]. Promising developments in immunotherapy, mutation targeting and epigenetic therapy are discussed in this section, providing a glimpse of where therapy for advanced NSCLC may be by 2018.
Immune checkpoint inhibition in NSCLC
CTLA-4 is the prototypical immune checkpoint, a transmembrane receptor whose expression is induced by T-cell activation leading to downregulation of T-cell responses and consequent suppression of the innate response to foreign tumor neoantigens [70]. Ipilimumab is a fully human IgG1 monoclonal antibody that binds to CTLA-4, blocking its interaction with its ligand on antigen-presenting cells and thus preventing suppression of antitumor immunity [71]. In NSCLC, a Phase II study of 204 chemotherapy-naive patients assigned patients to carboplatin/paclitaxel chemotherapy with placebo or combined with concurrent or phased ipilimumab [72]. The primary end point was PFS by immune response (irPFS). The phased ipilimumab regimen of two cycles of chemotherapy followed by four cycles of chemotherapy with ipilimumab demonstrated improved irPFS compared with the control arm of chemotherapy plus placebo (5.7 vs 4.6 months; p = 0.05) and a trend toward improved OS, which did not meet statistical significance (12.2 vs 8.3 months; p = 0.23). Interestingly, in subgroup analysis, the effect of phased ipilimumab appeared to be confined to squamous histology patients leading to a Phase III trial currently being conducted in this cohort, a group for whom recent progress has been limited (ClinicalTrials.gov identifier: NCT01285609) [106].
Another immune checkpoint, programmed death-1 (PD-1) is a coinhibitory molecule expressed on the surface of activated T cells, antigen-specific T cells after chronic antigen exposure, B cells and myeloid cells. Its ligand, programmed death ligand-1 (PD-L1), can be expressed in human tumors and has been associated with a poor prognosis [73–75]. Data from a large Phase I study of anti-PD-1 antibody (nivolumab) in 296 patients with advanced melanoma, renal cell carcinoma or NSCLC were recently reported and results in a heavily pretreated NSCLC cohort of 76 patients were particularly encouraging [76]. Tumor response and prolonged stabilization of disease was seen in 18% of the lung cancer patients with 26% of the patients being progression free 6 months after starting therapy while in the squamous subset, six out of 18 patients responded to therapy. Expression of PD-L1 on tumor cells as a putative marker of response, however, requires further evaluation in larger studies. Phase III studies of this agent are ongoing or planned, including in combination with chemotherapy in NSCLC, while other PD-1-targeted agents are also in clinical development in various tumor types including NSCLC (ClinicalTrials.gov identifier: NCT01721772 [106]) [77].
Recently reported studies of anti-PD-L1 antibody have also shown promising activity and good tolerability in advanced NSCLC, with 31% of heavily pretreated patients on a Phase I study progression free at 6 months [78].
These developments represent a significant change in the traditional perception of NSCLC as a nonimmunogenic tumor and we believe that these agents will add to the treatment paradigm for this disease within the next 5 years.
Epigenetic therapy in NSCLC
Aberrant epigenetic regulation of gene expression has been implicated in both tumor growth and chemoresistance [79]. DNA promoter hypermethylation and chromatin deacetylation are two of the most important proposed mechanisms of epigenetic tumorigenesis [80]. The reversal of epigenetic alterations is one of the most promising areas of cancer research, with the potential to modify the underlying biology of tumors and their response to cytotoxic and other therapies.
Azacytidine is a cytidine analog that inhibits DNA methyl-transferase activity, causing loss of DNA methylation that has been approved for the treatment of myelodysplastic syndrome [81]. Histone deacetylase inhibitors such as etinostat also affect gene expression, and combination therapy with azacytidine may synergistically reactivate silenced gene sensitizing tumors to therapy [82]. In resected early-stage NSCLC, gene methylation has been associated with a significantly worse prognosis [83]. Recently reported data from a Phase I study of combination epigenetic therapy with azacytidine and etinostat in 45 heavily pretreated advanced NSCLC patients demonstrated good tolerance and promising signals of efficacy, with two patients having prolonged responses of 8 and 14 months, respectively [84]. In addition, ten patients had stable disease for more than 3 months, with two of these patients having stable disease for 14 and 18 months. Interestingly, patients who received epigenetic therapy appeared to have unexpectedly good responses to post-study treatment, with four out of 19 patients having a major response to the immediate subsequent therapy and two long-term survivors of more than 3 years who received only one post-study therapy. The authors have also developed a target gene methylation signature that may predict response to therapy; however, this requires validation in future studies.
Driver mutations & resistance
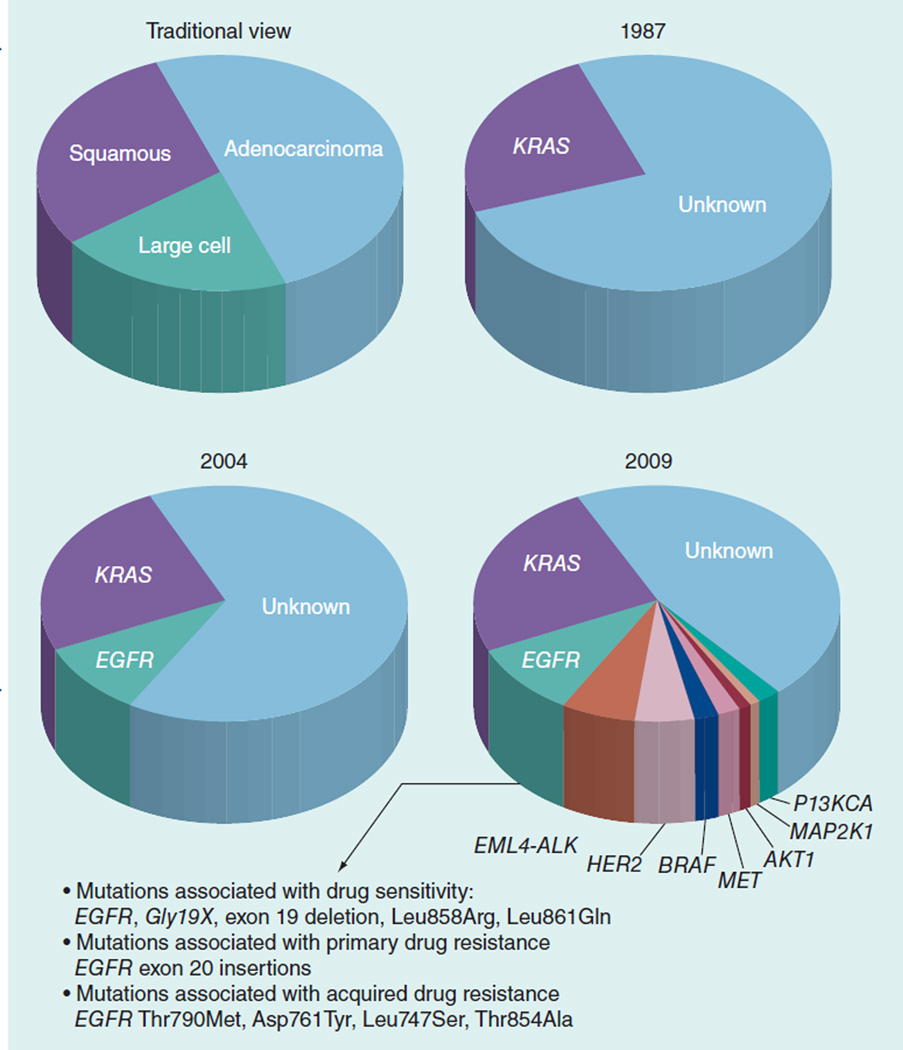
As discussed, more than 50% of lung adenocarcinoma tumors have identifiable mutations in critical oncogenes that potentiate tumor proliferation and spread (Figure 1) [69]. While approved agents, erlotinib and crizotinib, targeting the EGFR and the ALK fusion gene, respectively, are available, these mutations account for less than 20% of lung adenocarcinoma and drug resistance develops within 1 year of commencing treatment for the majority of patients. There is an urgent need for investigation of currently available and novel agents targeting KRAS-mutant NSCLC and rarer mutations including PIK3CA, NRAS, HER2 and KIF5B–RET. Despite the rarity of some of these mutations, due to the overall high incidence of lung cancer, effective agents have the potential to benefit thousands of patients. For example, while RET kinase fusions are present in only 1% of the lung cancer patients; this would represent more than 10,000 patients annually worldwide [85]. While the RET-targeting agent, vandetanib, and the HER2-targeting agent, trastuzumab, have been studied in unselected NSCLC populations, it is likely that the potential efficacy in molecularly selected subgroups may have been missed, with recent evidence suggesting that HER2-mutant NSCLC may be targeted with currently available therapies [86].
Figure 1.
New driver mutations in non-small-cell lung cancer.
BRAF mutations stimulate the MAPK pathway in NSCLC and are present in 1–5% of NSCLC tumors [87,88]. Unlike EGFR and ALK molecular aberrations, BRAF mutations appear to occur more frequently in current or former smokers with lung adenocarcinoma, and while the V600E subset are associated with a poor prognosis, other more common NSCLC BRAF mutations do not appear to be prognostic [88]. Several agents that target mutant BRAF or its pathways are currently under investigation in solid tumors, including advanced NSCLC with early-phase results pending (ClinicalTrials.gov identifiers: NCT01086267, NCT00888134, NCT01248247 and NCT01336634 [106]).
Mutations in KRAS are the most common lung cancer-driver mutations occurring in approximately 25% of lung adenocarcinomas, and almost exclusively in current or former smokers [89]. Ongoing studies aimed at targeting pathways activated in KRAS-mutant NSCLC include those involving the MEK-and MET-mediated signaling (ClinicalTrials.gov identifiers: NCT01395758 and NCT01362296 [106]).
The MET oncogene is amplified in 21% of NSCLC in Caucasians and drives tumor cell proliferation and metastasis [90,91]. MET amplification is also a frequent mechanism of resistance to EGFR tyrosine kinase inhibition in EGFR-mutant patients [92]. Spigel et al. conducted a randomized, 137-patient Phase II study of erlotinib combined with a MET-targeted monoclonal antibody (onartuzumab [MetMAb]) versus erlotinib alone in second- or third-line advanced NSCLC [93]. Patients with high MET expression by immunohistochemistry appeared to benefit from the addition of MetMAb to erlotinib in both PFS (erlotinib/MetMAb; 2.9 vs 1.5 months erlotinib alone; p = 0.04) and by almost 9 months in OS (erlotinib/MetMAb; 12.6 vs 3.8 months erlotinib; p = 0.002). This finding has led to a Phase III study comparing these combinations in the MET-positive advanced NSCLC population, while studies of a MET-directed TKI (tivantinib) are also in progress (ClinicalTrials.gov identifiers: NCT01456325, NCT01519804, NCT01496742 and NCT01395758 [106]) [94].
Efforts to target resistance mutations such as T790M, the most common mechanism of resistance to EGFR TKIs in EGFR-mutant NSCLC, are in progress along with the development of irreversible TKIs such as afatinib, which may have activity in combination with cetuximab in EGFR-mutant NSCLC resistant to first-generation EGFR TKIs [95,96].
As the field develops, we hope that by 2018 many, if not all, of these driver mutations will have therapeutic agents approved or in late-stage development along with effective strategies to overcome inherent and acquired resistance.
Table 1.
Selected randomized studies of EGF receptor targeted agents in advanced non-small-cell lung cancer.
| Study (year) | Patient population | Study arms | PFS/TTTF | OS | Ref. |
|---|---|---|---|---|---|
| EGFR tyrosine kinase inhibitors | |||||
| INTACT 1, mainly Europe/USA (2004) | n = 1093; first line – unselected for clinical characteristics or mutation status | Cis/Gemcitabine × 6 cycles ± gefitinib 500 or 250 mg | Gefitinib 500 mg − 5.5 months; gefitinib 250 mg − 5.8 months; placebo − 6.0 months; p = 0.7633; NS | Gefitinib 500 mg − 9.9 months; gefitinib 250 mg − 9.9 months; placebo − 10.9 months; p = 0.4560; NS | [17] |
| INTACT 2, mainly USA (2004) | n = 1037; first line – unselected | Carbo/paclitaxel × 6 cycles ± gefitinib 500 or 250 mg | Gefitinib 500 mg − 4.6 months; gefitinib 250 mg − 5.3 months; placebo − 5.0 months; p =0.0562; NS | Gefitinib 500 mg − 8.7 months; gefitinib 250 mg − 9.8 months; placebo − 9.9 months; p = 0.64;NS | [18] |
| INTEREST Europe/Asia/USA (2008) | n = 1466; ≥ second line – unselected | Gefitinib vs docetaxel | Gefitinib − 2.2 months; docetaxel − 2.7 months; p = 0.47; NS | Gefitinib − 7.6 months; docetaxel − 8 months; gefitinib was noninferior | [19] |
| V-15-32 Japan (2008) | n = 489; ≥ second line – unselected, although 32% never smoker | Gefitinib vs docetaxel | Gefitinib − 2 months; docetaxel − 2 months; p = 0.335; NS | Gefitinib 11.5 months; docetaxel 14 months; gefitinib noninferiority not demonstrated | [20] |
| ISTANA Korea (2010) | n = 161; second line – unselected, although 41% never smoker | Gefitinib vs docetaxel | Gefitinib − 3.3 months; docetaxel − 3.4 months; p = 0.0441 ;S | Gefitinib 14.1 months; docetaxel − 12.2 months; p = 0.437; NS | [21] |
| BR.21 Americas/Europe/Australia (2005) | n = 731; ≥second line and not a candidate for further chemotherapy – unselected | Erlotinib vs placebo | Erlotinib − 2.2 months; placebo − 1.8 months; p< 0.001; S | Erlotinib − 6.7 months; placebo − 4.7 months; p< 0.001; S | [23] |
| ISEL Asia (2009) | n = 1129; ≥ second line; unselected | Gefitinib vs placebo | Gefitinib − 3.0 months; placebo − 2.6 months; p = 0.0006; S | Gefitinib − 5.6 months; placebo − 5.1 months; p = 0.087; NS | [22] |
| IPASS Asia (2009) | n = 1217; first line; all adenocarcinoma and light/nonsmoker; 60% EGFR mutant | Gefitinib vs Carbo/paclitaxel | In the EGFR-mutant subgroup; gefitinib − 9.5 months; Carbo/paclitaxel − 6.3 months; HR:0.48;S | Gefitinib− 18.8 months; Carbo/paclitaxel − 17.4 months; p = 0.109; NS | [25] |
| WJOG 172 Japan (2010) | n = 177; first line; EGFR mutant | Gefitinib vs cisplatin/docetaxel | Gefitinib − 9.2 months; Cis/docetaxel − 6.3 months; p< 0.0001; S | Gefitinib − 36 months; Cis/docetaxel − 39 months; HR: 1.185; 95% CI: 0.767−1.829; NS | [26] |
| NEJSG Japan (2010) | n = 230; first line; EGFR mutant | Gefitinib vs Carbo/paclitaxel | Gefitinib 10.8 months Carbo/paclitaxel − 5.4 months; p< 0.001; S | Gefitinib − 27.7 months; Carbo/paclitaxel − 26.6 months; p = 0.483; NS | [27] |
| OPTIMAL China (2011) | n = 154; first line; EGFR mutant | Erlotinib vs Carbo/gemcitabine | Erlotinib− 13.1 months; Carbo/gemcitabine − 4.6 months; p< 0.0001; S | No significant difference in OS | [28,29] |
| EURTAC Europe (2012) | n = 174; first line; EGFR mutant | Erlotinib vs platinum doublet | Erlotinib − 9.7 months; chemotherapy − 5.2 months; p< 0.0001 | Erlotinib − 22.9 months; chemotherapy − 20.8 months; NS | [30] |
| LUX-Lung 3 Asia (72%)/Europe/North America (2012) | n = 345; first line; EGFR mutant | Afatinib vs cisplatin/pemetrexed | Afatinib − 11.1 months; Cis/pemetrexed − 6.9 months; p = 0.0004 | OS not yet mature | [31] |
| FLEX Europe (2009) | n = 1125; first line; EGFR-expressing | Cis/Vin × 6 cycles ± cetuximab followed by maintenance weekly cetuximab (in the cetuximab arm only) | Cis/Vin/cetuximab − 4.8 months; Cis/Vin alone −4.8 months; p = 0.39; NS | Cis/Vin/cetuximab − 11.3 months; Cis/Vin alone − 10.1 months; p = 0.169; S | [31] |
| BMS099 USA (2010) | n = 676; first line; unselected | Carbo/paclitaxel × 6 ± cetuximab followed by maintenance cetuximab | Carbo/paclitaxel/cetuximab − 4.4 months; Carbo/paclitaxel − 4.2 months; p = 0.236; NS | Carbo/paclitaxel/cetuximab − 9.7 months; Carbo/paclitaxel − 8.4 months; p = 0.169; NS | [34] |
Carbo: Carboplatin; Cis: Cisplatin; EGFR: EGF receptor; HR: Hazard ratio; n: Number of subjects enrolled; NS: No statistically significant difference; OS: Overall survival; PFS: Progression-free survival; RR: Objective response rate; S: Statistically significant difference; TTTF: Time to treatment failure; Vin: Vinorelbine.
Table 3.
Studies of crizotinib in advanced ALK-positive non-small-cell lung cancer.
| Study (year) | Phase | Study arm(s) | ORR and PFS | OS | Ref. |
|---|---|---|---|---|---|
| Initial dose–escalation/expansion study (2010); n = 143 ALK-positive NSCLC patients; 84% pretreated | I | Crizotinib 250 mg b.i.d. p.o. daily | ORR: 60.8% (95% CI: 52.3– 68.9); PFS: 9.7 months (95% CI: 7.7–12.8) | OS at 12 months: 74.8% (95% CI: 66.4–81) | [56] |
| PROFILE 1005(2012); n = 255; 85% pretreated | II | Single-arm crizotinib 250 mg b.i.d. p.o. daily | ORR: 53% (95% CI: 47–60); PFS: 8.5 months (95% CI: 6.2–9.9) | OS: not yet mature | [58] |
| PROFILE 1007(2013); n = 347; second line | III | Crizotinib vs docetaxel or pemetrexed | ORR: crizotinib 65.3% vs 19.3% chemotherapy; p < 0.0001 PFS: crizotinib 7.7 vs 3.0 months chemotherapy; p < 0.001 | OS: not yet mature | [59] |
| PROFILE 1014; first-line advanced ALK-positive nonsquamous NSCLC (ClinicalTrials.gov identifier: NCT01154140) | III | Crizotinib vs cisplatin/pemetrexed or carboplatin/pemetrexed | Not available as yet | Not available as yet | [106] |
b.i.d.: Two-times a day: NSCLC: Non-small-cell lung cancer: ORR: Overall response rate: OS: Overall survival: PFS: Progression-free survival: p.o.: Per orem.
Table 4.
Selected ongoing and planned studies of novel targeted agents in non-small-cell lung cancer.
| Agent(s) | Phase | ClinicalTrials.gov identifir† |
Patient population and status |
|---|---|---|---|
| Anti-PD-1 | |||
| Nivolumab + first-line platinum doublet | I | NCT01454102 | First-line NSCLC; recruiting |
| Nivolumab vs docetaxel | III | NCT01642004 | Second-line NSCLC; recruiting |
| Nivolumab | II | NCT01721759 | Third-line squamous; recruiting |
| MK-3475 | I | NCT01295827 | Solid tumors including NSCLC; recruiting |
| Epigenetic therapy | |||
| 5-azacytidine + etinostat | II | NCT01207726 | Adjuvant therapy for resected stage 1 NSCLC; recruiting |
| Driver mutations/amplifications | |||
| Selumetinib (MEK inhibitor) + erlotinib | II | NCT01229150 | KRAS-mutant NSCLC; recruiting |
| Erlotinib + tivantinib vs chemotherapy | II | NCT01395758 | KRAS-mutant NSCLC; recruiting |
| Onartuzumab + erlotinib | III | NCT01456325 | Met-positive NSCLC; recruiting |
| Dabrafenib | II | NCT01336634 | BRAF-mutant NSCLC; recruiting |
NSCLC: Non-small-cell lung cancer; PD-1: Programmed death-1.
Data taken from [106].
Key issues.
The key to the future of advanced non-small-cell lung cancer therapy will be discovering rational molecular targets and testing new agents in those subgroups most likely to benefit. EGF receptor tyrosine kinase inhibitors and crizotinib represent notable successes using this strategy.
Driver mutations that spur cancer growth in the majority of lung adenocarcinoma have been discovered, and agents targeting these mutations are in preclinical and clinical development. Squamous tumors have proven more difficult to target to date; however, developments with immune checkpoint inhibition and epigenetic therapy are promising for this subgroup.
While bevacizumab prolongs survival when added to chemotherapy, studies using other agents have been disappointing and a biomarker of response is needed to guide antiangiogenic therapy.
Resistance to targeted agents is a major problem, and strategies aimed at overcoming resistance are likely to include next-generation tyrosine kinase inhibitors and combination therapy targeting multiple resistance pathways.
Footnotes
Financial & competing interests disclosure
DS Ettinger is an advisor for Gilead, Roche/Genentech, Boehringer-Ingelheim, Biodesix and Eli Lilly & Co. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Yano T, Haro A, Shikada Y, Maruyama R, Maehara Y. Non-small cell lung cancer in never smokers as a representative ‘non-smoking-associated lung cancer’: epidemiology, clinical features. Int. J. Clin. Oncol. 2011;16(4):287–293. doi: 10.1007/s10147-010-0160-8. [DOI] [PubMed] [Google Scholar]
- 3.Overholt RH. Primary carcinoma of the lung: early diagnosis and treatment by pneumonectomy. N. Engl. J. Med. 1936;214:93–100. [Google Scholar]
- 4.Longeval E, Klastersky J. Combination chemotherapy with cispladn and etoposide in bronchogenic squamous cell carcinoma and adenocarcinoma. A study by the EORTC Lung Cancer Working Party (Belgium) Cancer. 1982;50(12):2751–2756. doi: 10.1002/1097-0142(19821215)50:12<2751::aid-cncr2820501210>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Ruckdeschel JC, Finkelstein DM, Ettinger DS, et al. A randomized trial of the four most active regimens for metastatic non-small-cell lung cancer. J. Clin. Oncol. 1986;4(1):14–22. doi: 10.1200/JCO.1986.4.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cispladn versus etoposide with cispladn: results of an Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2000;18(3):623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 7.Kelly K, Crowley J, Bunn PA, Jr., et al. Randomized Phase III trial of paclitaxel plus carbopladn versus vinorelbine plus cispladn in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J. Clin. Oncol. 2001;19(13):3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 8. Schiller JH, Harrington D, Belani CP, et al. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. •• Seminal paper that demonstrated that chemotherapy for non-small-cell lung cancer had reached a plateau.
- 9.Paccagnella A, Oniga F, Bearz A, et al. Adding gemckabine to paclitaxel/carboplatin combination increases survival in advanced non-small-cell lung cancer: results of a Phase II-III study. J. Clin. Oncol. 2006;24(4):681–687. doi: 10.1200/JCO.2005.03.2722. [DOI] [PubMed] [Google Scholar]
- 10.Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA. 2004;292(4):470–484. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 11.Rusch V, Klimstra D, Venkatraman E, Pisters PW, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor α is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin. Cancer Res. 1997;3(4):515–522. [PubMed] [Google Scholar]
- 12.Fujino S, Enokibori T, Tezuka N, et al. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer. Eur. J. Cancer. 1996;32A(12):2070–2074. doi: 10.1016/s0959-8049(96)00243-2. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Varella-Garcia M, Bunn PA, Jr., et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003;21(20):3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 14.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318(2):124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a Phase I trial. J. Clin. Oncol. 2002;20(9):2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 16.Ho C, Murray N, Laskin J, Melosky B, Anderson H, Bebb G. Asian ethnicity and adenocarcinoma histology continues to predict response to gefitinib in patients treated for advanced non-small cell carcinoma of the lung in North America. Lung Cancer. 2005;49(2):225–231. doi: 10.1016/j.lungcan.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Giaccone G, Herbst RS, Manegold C, et al. Gefidnib in combination with gemckabine and cisplatin in advanced non-small-cell lung cancer: a Phase III trial – INTACT 1. J. Clin. Oncol. 2004;22(5):777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Herbst RS, Giaccone G, Schiller JH, et al. Gefidnib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a Phase III trial–INTACT 2. J. Clin. Oncol. 2004;22(5):785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 19.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised Phase III trial. Lancet. 2008;372(9652):1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama R, Nisldwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefidnib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J. Clin. Oncol. 2008;26(26):4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefidnib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin. Cancer Res. 2010;16(4):1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 22. Thatcher N, Chang A, Parikh P, et al. Gefidnib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. • This paper led to approval being rescinded by the US FDA for gefitinib in pretreated patients.
- 23.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlodnib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 24.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 25. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a Phase III, randomized, open-label, first-line study of gefitinib versus carbopladn/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. •• Highlights the relevance of EGF receptor (EGFR) mutations in sensitivity to EGFR TKIs.
- 26.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group. Gefitinib versus cispladn plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 27.Maemondo M, Inoue A, Kobayasld K, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated. EGFR. N. Engl. J. Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 28. Zhou C, Wu YL, Chen G, et al. Erlodnib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. •• Prospectively confirmed EGFR mutation status as a predictive factor.
- 29.Sun Y, Wu YL, Zhou CC, et al. Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer. 2013;79(2):143–150. doi: 10.1016/j.lungcan.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 30. Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlodnib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. •• First study in Caucasian EGFR mutant patients.
- 31.Chih-Hsin Yang J, Schuler MH, Yama-moto N, et al. Lux-Lung 3: A randomized, open-label, Phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations; Chicago, IL, USA. Presented at: 2012 ASCO Annual Meeting; 2012. Jun, pp. 1–5. [Google Scholar]
- 32.Pirker R, Pereira JR, Szczesna A, et al. FLEX Study. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised Phase III trial. Lancet. 2009;373(9674):1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 33.Gatzemeier U, von Pawel J, Vynnychenko I, et al. First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX Phase 3 study. Lancet Oncol. 2011;12(1):30–37. doi: 10.1016/S1470-2045(10)70278-3. [DOI] [PubMed] [Google Scholar]
- 34.Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the Phase 3 FLEX study. Lancet Oncol. 2012;13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 35.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carbopl-atin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter Phase III trial BMS099. J. Clin. Oncol. 2010;28(6):911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 36.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J. Natl Cancer Inst. 2009;101(15):1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J. Thorac. Oncol. 2009;4(9):1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 38. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. • Led to the approval of bevacizumab.
- 39.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized Phase II trial comparing bevacizumab plus carbopladn and paclitaxel with carbopladn and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22(11):2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cispladn plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 41.Reck M, von Pawel J, Zatloukal P, et al. BO17704 Study Group. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised Phase III trial (AVAiL) Ann. Oncol. 2010;21(9):1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabbinavar FF, Miller VA, Johnson BE, et al. Overall survival in ATLAS, a Phase IIIb trial comparing bevacizumab therapy with or without erlodnib after completion of chemotherapy with bevacizumab for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer; Chicago, IL, USA. Presented at: 2010 ASCO Annual Meeting; Jun, 2012. pp. 4–8. [Google Scholar]
- 43.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carbopladn and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J. Clin. Oncol. 2010;28(1):49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 44.Paz-Ares LG, Biesma B, Heigener D, et al. NSCLC [Non-Small-Cell Lung Cancer] Research Experience Utilizing Sorafenib (NExUS) Investigators Study Group Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2012;30(25):3084–3092. doi: 10.1200/JCO.2011.39.7646. [DOI] [PubMed] [Google Scholar]
- 45.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlodnib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a Phase III trial. J. Clin. Oncol. 2012;30(17):2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 46.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, Phase 3 trial. Lancet Oncol. 2010;11(7):619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind Phase III trial. J. Clin. Oncol. 2011;29(8):1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 48.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind Phase III trial (ZEPHYR) J. Clin. Oncol. 2012;30(10):1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 49.Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind Phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J. Clin. Oncol. 2012;30(23):2829–2836. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 50.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled Phase III trial. J. Clin. Oncol. 2012;30(29):3640–3647. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- 51.Lara PN, Jr., Douillard JY, Nakagawa K, et al. Randomized Phase III placebo-controlled trial of carbopladn and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29(22):2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 52.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi K, Choi YL, Togashi Y, et al. KIF5B–ALK, a novel fusion oncokmase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin. Cancer Res. 2009;15(9):3143–3149. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 54.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK . J. Clin. Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 56. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. • Led to the approval of crizotinib.
- 57.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a Phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer. Lung Cancer. 2012;77(2):365–370. doi: 10.1016/j.lungcan.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Shaw AT. Phase 3 randomized study of crizotinib versus pemetrexed or docetaxel chemotherapy in advanced, ALK-positive NSCLC (PROFILE 1007); Vienna, Austria. Presented at: 2012 ESMO Annual Meeting; 2012. Sep 30, (Abstract LBA1) [Google Scholar]
- 60.Voena C, Chiarle R. The battle against ALK resistance: successes and setbacks. Expert Opin. Investig Drugs. 2012;21(12):1751–1754. doi: 10.1517/13543784.2012.717930. [DOI] [PubMed] [Google Scholar]
- 61.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cispladn plus gemcitabine with cispladn plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 63.Ceppi P, Rapa I, Lo Iacono M, et al. Expression and pharmacological inhibition of thymidylate synthase and Src kinase in nonsmall cell lung cancer. Int. J. Cancer. 2012;130(8):1777–1786. doi: 10.1002/ijc.26188. [DOI] [PubMed] [Google Scholar]
- 64. Gridelli C, de Marinis F, Di Maio M, et al. Maintenance treatment of advanced non-small-cell lung cancer: results of an International Expert Panel meeting of the Italian association of thoracic oncology. Lung Cancer. 2012;76(3):269–279. doi: 10.1016/j.lungcan.2011.12.011. •• Provided prospective evidence for continuation maintenance.
- 65.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, Phase 3 study. Lancet. 2009;374(9699):1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 66.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. SATURN investigators. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled Phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 67.Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29(31):4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 68.Edelman MJ, Le Chevalier T, Soria JC. Maintenance therapy and advanced non-small-cell lung cancer: a skeptic’s view. J. Thorac. Oncol. 2012;7(9):1331–1336. doi: 10.1097/JTO.0b013e3182629e37. [DOI] [PubMed] [Google Scholar]
- 69.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat. Med. 2012;18(3):349–351. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 70.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolchok J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann. Oncol. 2012;23(Suppl. 8):15–21. doi: 10.1093/annonc/mds258. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase II study. J. Clin. Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Schwartz JC, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20(3):337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 74.Dong H, Strome SE, Salomao DR, et al. Turnor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 75.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 76. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. •• Most promising agent currently in development for advanced non-small-cell lung cancer.
- 77.Patnaik A, Mishra SS, Senapati SB, Patra SK, Tripathy K, Burma S. Primary intraosseous malignant peripheral nerve sheath tumor of spine with a giant paraspinal and retrospinal subcutaneous extension. Surg Neurol. Int. 2012;3:157. doi: 10.4103/2152-7806.105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of and-PD-L1 antibody in patients with advanced cancer. N. Engl J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethyladon. N Engl. J. Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 81.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodys-plastic syndrome: a study of the cancer and leukemia group B. J. Clin. Oncol. 2002;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 82.Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999;21(1):103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 83.Brock MV, Hooker CM, Ota-Machida E, et al. DNA methyladon markers and early recurrence in stage I lung cancer. N. Engl J. Med. 2008;358(11):1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 84.Juergens RA, Wrangle J, Vendetti FP, et al. Combination epigenedc therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1(7):598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012;18(3):378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 86.Kelly RJ, Carter C, Giaccone G. Personalizing therapy in an epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small-cell lung cancer using PF-00299804 and trastuzumab. J. Clin. Oncol. 2010;28(28):e507–e510. doi: 10.1200/JCO.2010.29.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin. Oncol. 2011;29(26):3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 88.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J. Clin. Oncol. 2011;29(15):2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann. Oncol. 2011;22(12):2616–2624. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beau-Faller M, Ruppert AM, Voegeli AC, et al. MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naive cohort. J. Thorac. Oncol. 2008;3(4):331–339. doi: 10.1097/JTO.0b013e318168d9d4. [DOI] [PubMed] [Google Scholar]
- 91.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer. 2012;12(2):89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 92.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl Acad. Sci. USA. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized Phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC; Chicago, IL, USA. Presented at: 2011 ASCO Annual Meeting; 2011. Jun, pp. 3–7. [Google Scholar]
- 94.Spigel DR, Edelman MJ, Mok T, et al. Treatment rationale study design for the metlung trial: a randomized, double-blind Phase III study of onartuzumab (MetMAb) in combination with erlotinib versus erlotinib alone in patients who have received standard chemotherapy for stage iiib or iv met-positive non-small-cell lung cancer. Clin. Lung Cancer. 2012;13(6):500–504. doi: 10.1016/j.cllc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Ayoola A, Barochia A, Belani K, Belani CP. Primary and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: an update. Cancer Invest. 2012;30(5):433–446. doi: 10.3109/07357907.2012.666691. [DOI] [PubMed] [Google Scholar]
- 96.Janjigian YY, Groen HJ, Horn L, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib; Chicago, IL, USA. Presented at: 2012 ASCO Annual Meeting; 2012. Jun, pp. 1–5. [Google Scholar]
Websites
- 101.WHO. Worldwide estimate of lung cancer incidence and mortality. http://globocan.iarc.fr.
- 102.National Cancer Institute: SEER. http://seer.cancer.gov.
- 103.European Medicines Agency. Iressa. www.emea.europa.eu/humandocs/Humans/EPAR/iressa/iressa.htm.
- 104.ESMO 2012 late breaking abstracts. www.onkologiportalen.dk/fmfiles/re7237004/Herceptin/Aktuelt/okto-ber2012/abstracts-1.pdf.
- 105.National Comprehensive Cancer Network. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 106.ClinicalTrials.gov. http://clinicaltrials.gov.
- 107.Medscape. www.medscape.org/viewarticle/754969_transcript.
- 108.Global Resource for Advancing Cancer Research. http://cancergrace.org/lung/2012/09/07/4-key-points-from-pointbreak-trial.
- 109.Xalkori®. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202570s002lbl.pdf.