Abstract
Extracellular matrix (ECM) plays essential signaling and structural roles required for the proper function of cardiac valves. Cardiac valves initially form as jelly-like cushions, which must adapt to withstand the increased circulation hemodynamics associated with fetal development and birth. This increased biomechanical stability of the developing valves is largely imparted by ECM proteins, which form a highly organized fibrous meshwork. Since heart valve defects contribute to most congenital heart diseases, understanding valve development will provide insight into the pathogenesis of various congenital valve anomalies. Thus, the goal of this study is to describe the spatiotemporal deposition of fibrous ECM proteins during cardiac valve development. Chick embryonic and fetal atrioventricular and semilunar valves were examined by light, confocal, and transmission electron microscopy (TEM). Our data demonstrate that fibrous ECM proteins are deposited when the leaflets are adopting an elongated and compacted phenotype. A general pattern of increased fibrotic ECM deposition was detected in valve tissues. Also, each ECM protein examined displayed a unique pattern of organization, suggesting that regulation of fibrous protein deposition is complex and likely involves both genetic and mechanical factors. In addition, the TEM study revealed the presence of membrane protrusions from valvular endocardium, indicating a potential mechanism for mechanical force transduction.
Keywords: cardiac valve development, spatiotemporal expression, collagen, tenascin, valve morphology, annulus fibrosus, membrane protrusion
Introduction
Cardiac valve formation is a complex process that includes three sequential processes: endocardial cushion formation, cushion elongation, and leaflet/cusp remodeling (Hinton et al., 2006; Combs & Yutzey, 2009). Endocardial cushions form as cells of the endocardium undergo an epithelial-to-mensenchymal transformation (EMT) in the primary heart tube with cells diving into gylcosaminoglycan-rich extracellular matrix (ECM) proteins that are secreted between the myocardial and endocardial layers of the early heart to form the atrioventricular (AV) and the outflow tract valve primordia (Eisenberg & Markwald, 1995; Schroeder et al., 2003; Person et al., 2005). The fate of the AV cushions is to fuse at the midline and contribute to the development of two of the AV valves (aortic leaflet of the mitral valve and the septal leaflet of the tricuspid valve) and the AV septal complex.
The development of the other two AV leaflets (mural leaflets of the tricuspid and mitral valves) initially occurs through the formation of “lateral” cushions located at the left and right side of the AV junction (Snarr et al., 2008). These prevalve cushions elongate via cell proliferation, migration, and enhanced ECM synthesis (Armstrong & Bischoff, 2004; Martinsen, 2005; Hinton et al., 2006). Valvular morphogenesis is a continual remodeling process whereby maturation of the cardiac valves is not completed until well into postnatal life. Fibrous ECM proteins provide the structural integrity required for valves to carry out their function of preventing regurgitation of blood in the heart. Mature cardiac valves are thin fibrous tissues that are composed of discrete, stratified layers of ECM: an elastin rich layer (atrialis in AV valves or ventricularis in semilunar valves), the proteoglycan rich spongiosa, and the collagen rich fibrosa (Hinton et al., 2006; Lincoln et al., 2006). Proper ECM organization, especially collagen fibril alignment, is essential for normal cardiac valve function. During heart development, the composition of the valve ECM undergoes important changes, including the reduction of glycosaminoglycan protein expression and accumulation of stress-resistant fibrous ECM proteins (Kruithof et al., 2007; Peacock et al., 2008). The studies cited above have described the initial events of valve formation and also mature cardiac valve structures. However, the spatiotemporal expression patterns of cardiac valve ECM proteins and the ultrastructure of valve tissue throughout embryonic development are not well understood.
Since chicken heart developmental processes share much in common to those of human, they are widely used for studying cardiac valve development, pathology, and physiology (Ward et al., 2005; Hinton et al., 2006; Butcher et al., 2007). In this study, we describe the distribution and organization of type I collagen (col1), type VI collagen (col6), and tenascin from Hamilton and Hamburger (HH) stages 25 through 44 chicken embryos. Critical stages were selected to present the major morphological and structural changes during embryonic cardiac valve development: endocardial cushion formation (HH 25), cushion elongation (HH 34 and 38), and leaflet/cusp remodeling (HH 42 and 44).
Col1, a heterotrimeric protein, is the major fibrillar collagen in cardiac valves and confers stiffness and strength to the valve leaflets (Lincoln et al., 2006; Kadler et al., 2007). Col6 forms a microfibrillar extracellular network that interacts with basement membranes, fibrillar collagens, and other ECM components as well as with receptors at the cell surface (van der Rest & Garrone, 1991; Brown & Timpl, 1995; Kadler et al., 2007). During cardiac valve development, col6 has a functional role in cushion tissue cell migration, proliferation, and septum-valve remodeling (Kitten et al., 1996; Klewer et al., 1998; Schroeder et al., 2003). Tenascin, a large elastic glycoprotein, mediates cell adhesion and migration and functions in resistance to mechanical strain (Oberhauser et al., 1998; Schellings et al., 2004).
Tenascin and collagens have been shown to be involved in a variety of connective tissue diseases in humans, including Marfan syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta, dominant cutis laxa, Stickler syndrome, and pseudoxanthoma elasticum (Burch et al., 1997; Nuytinck et al., 2000; Weis et al., 2000; Akishima et al., 2004; Anum et al., 2009; Fabbri et al., 2009; Lindeman et al., 2010). As these familial mutations are present at conception, it is likely that they have a profound and deleterious affect on how the cardiac valves are built. Additionally, aberrant col6 expression and function in cardiac valve extracellular matrix have been described in trisomy 21 (Down syndrome) fetuses with atrioventricular septal defects (Gittenbergerde Groot et al., 2003; Delom et al., 2009).
Although of great clinical relevance, a thorough evaluation of tenascin and collagen protein expression during cardiac morphogenesis has not been done. Thus, we have used correlative light, confocal, and transmission electron microscopy (TEM) analysis to determine the spatial and temporal expression and organization of col1, col6, and tenascin in the valves and septa of the heart at critical stages of development. These studies demonstrate the presence of aligned fibrous ECM proteins primarily in late stage valve tissues. Also, the TEM studies revealed the presence of membrane protrusions from the valvular endocardial cells, which may function as mediators of mechanical forces that regulate ECM organization during development.
This study of embryonic heart valve development can advance the current understanding of cardiac valve biology, which is essential to furthering our understanding of congenital heart diseases and the design of tissue-engineered cardiac valves.
Materials and Methods
Chicken Embryo and Heart Valve Collection
White leghorn chicken eggs (CBT Farms, Federalsburg, MD, USA) were incubated in a humidity-controlled 37°C incubator, and embryos were collected at HH stages 25, 34, 38, 42, and 44 corresponding to post-fertilization day (E) 5, 8, 12, 16, and 18. Hearts and valves were then dissected under a light microscope.
Light Microscopy
Embryonic hearts were dissected and fixed with 4% paraformaldehyde overnight at 4°C. After fixation, the hearts were embedded in paraffin and coronally sectioned at a thickness of 5 μm. Then the samples were deparaffinized and hydrated. Samples were mordanted in Bouin’s fixative for 1 h at 56°C and washed in running water. Then the samples were stained using Weigert’s iron hematoxylin working solution for 10 min, Biebrich scarlet-acid fuchsin for 2 min, phosphotungstic-phosphomolybdic acid for 12 min, and aniline blue solution for 5 min. Samples were then placed in 1% aqueous acetic acid for 5 min followed by dehydration. Finally, images were obtained using a Dako-Cytomation Automated Cell Imaging System.
Confocal Microscopy
Embryonic hearts were fixed, embedded, and sectioned as above. Sections were then permeablized in PBS-0.25% Triton X-100 for 20 min and blocked in 2% BSA/PBS overnight at 4°C. Primary antibodies for col1 (Chemicon International, Inc., Temecula, CA, USA), col6 (Developmental Studies Hybridoma Bank), and tenascin (Chemicon) were used at a concentration of 1:200 in 2% BSA/PBS overnight at 4°C. Alexa-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) were used at a concentration of 1:200 in 2% BSA/PBS overnight at 4°C. Nuclei were stained with DAPI (Molecular Probes®) at a concentration of 1:5,000 overnight at 4°C. Sections without primary antibody were used as negative control. Images were obtained using a Zeiss LSM 510 META confocal scanning laser microscope.
Transmission Electron Microscopy
For TEM, hearts were fixed in 2.5% glutaraldehyde in PBS (pH = 7.2) at room temperature for 24 h, rinsed in PBS, and post-fixed in 1% aqueous osmium tetroxide. Samples were dehydrated in a graded ethanol series, embedded in PolyBed 812 (Polysciences, Inc., Warrington, PA, USA), and sectioned at a thickness of 100 nm. Images were collected on a JEOL 200CX TEM.
Measurement of Collagen-Fiber Diameter
Collagen fiber diameter was measured by the method described previously with minor modifications (Schmults et al., 2004). Collagen fiber diameter of longitudinally cut fibers was measured directly on the photographs in millimeters by using Image-pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA), then adjusted for magnification to give a diameter in nanometers. Twenty fibers were measured per TEM image for a total of 80 fiber measurements from three different animals. Then the values of each fiber diameter were averaged, and the standard deviation was calculated.
Results
Collagen Distribution and Valve Morphology during Embryonic Development
Trichrome staining has been used to provide a general histological view of the valvular ECM composition during development. Collagenous proteins (blue staining) are faintly detectable in AV (Fig. 1A,B) and outflow tract cushions (Fig. 1C,D) during early valve development just prior to cushion fusion (HH 25). As valve cushions elongate into valve leaflets (HH 38), collagen staining is found in the atrialis (arrows, Fig. 2A) of the mural and aortic mitral valve leaflets. At this stage, mural and septal tricuspid valve leaflets contain a large population of cardiac myocytes (red staining, Fig. 3A). A thin layer of fibrous mesenchyme is observed on the atrial surface of the mural tricuspid leaflet (arrow, Fig. 3A). By HH 38, aortic leaflets have elongated, and collagen staining is present near the endocardial layer of the valve leaflets on both ventricular and fibrosal surfaces (arrows, Fig. 4A) as well as diffuse collagen staining throughout the rest of the leaflets.
Figure 1.

Collagen fiber staining in HH 25 chick embryonic cardiac cushions by Masson’s trichrome. Blue collagen fiber staining starts to appear (A) in the HH 25 AV cushion and (C) the outflow cushion. Higher magnification (40×) of (B) AV cushion and (D) outflow cushion shows collagen fibers scattered in between the mesenchyme (arrows). Nuclei: black; cytoplasm and muscle fibers: red; collagen fibers: blue; SC: superior cushion; IC: inferior cushion; LC: lateral cushion. Scale bar: (A, C) 100 μm; (B, D) 20 μm.
Figure 2.
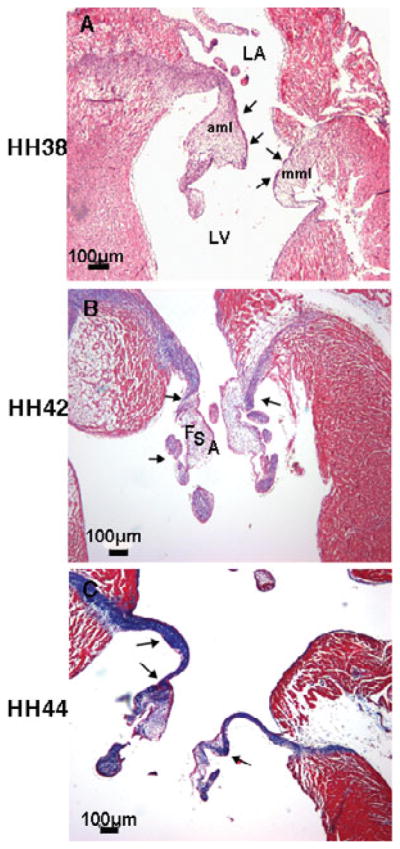
Collagen fiber staining in developing chick embryonic mitral valves by Masson’s trichrome. Increases and compartmentalization of collagen fiber (blue staining shown by arrows) were observed in developing mitral valve at (A) HH 38, (B) HH 42, and (C) HH 44. LV: left ventricle; LA: left atrium; mml: mural mitral leaflet; aml: aortic mitral leaflet; F: fibrosa; S: spongiosa; A: atrialis. Scale bar: 100 μm.
Figure 3.

Collagen fiber staining in developing chick embryonic tricuspid valves by Masson’s trichrome. The mural tricuspid leaflet stays muscularized (red staining) with a layer of collagen fiber (blue staining) on the atrialis side during development (arrows, A, B, C). Blue collagen fiber staining diffusely spread in the septal tricuspid leaflet during development (stars, A, B, C). RV: right ventricle; RA: right atrium; mtl: mural tricuspid leaflet; stl: septal tricuspid leaflet. Scale bar: 100 μm.
Figure 4.
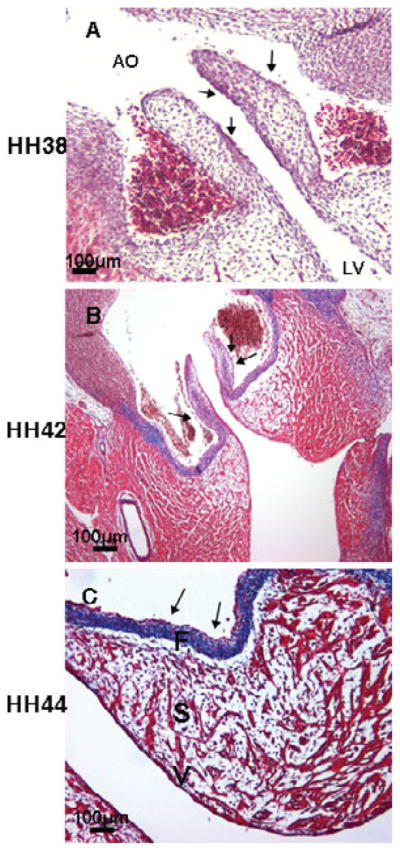
Collagen fiber staining in developing chick embryonic semilunar valves by Masson’s trichrome. Collagen staining was detectable in the fibrosa and ventricularis at HH 38 (arrows, A). By HH 42, collagen fiber staining was constrained in the distal part of the semilunar leaflets and the fibrosa (arrows, B). Condensed collagen fiber staining was observed only in the fibrosa at HH 44 (arrows, C). AO: aorta; F: fibrosa; S: spongiosa; V: ventricularis. Scale bar: 100 μm.
During mitral leaflet remodeling (HH 42 and 44), collagen staining is observed in the fibrosa and chordea tendineae (arrows, Fig. 2B) and expands to other layers in the proximal part of the mitral valves at HH 44 (arrows, Fig. 2C). At this stage, collagen staining is detected in the fibrosa and in all three layers in the distal part of the outflow leaflets at HH 42 (arrows, Fig. 4B), while clear stratification is observed in the HH 44 semilunar valve. Collagen staining becomes confined to the fibrosa (arrows, Fig. 4C). Valve tissue in mitral valves is more condensed in comparison to that found during the cushion elongation stages. Collagen fibers are diffusely present in the septal tricuspid leaflet during development (stars, Fig. 3A–C). The mural tricuspid leaflet, on the other hand, is continuous with the myocardium of the mural wall of the right ventricle. It contains only a thin layer of mesenchyme (arrows, Fig. 3B,C).
Another prominent observation from the HH 44 chick heart trichrome stain is the thick band of collagen extending from the orifice of the aortic mitral valve, through the interventricular septum (IVS), and into the septal leaflet of the tricuspid valve (arrows, Fig. 5A). This region is known as the annulus fibrosus, which develops from the AV cushion and becomes the continuity between the mitral and the tricuspid valves. Importantly, this area functions as an anchor of the aortic leaflet of the mitral valve and the septal tricuspid valve. Further analysis for specific fibrous proteins found in this region using confocal immunofluorescence microscopy detected the presence of col1 (green) and tenascin (red) in this region (arrows, Fig. 5B–D).
Figure 5.
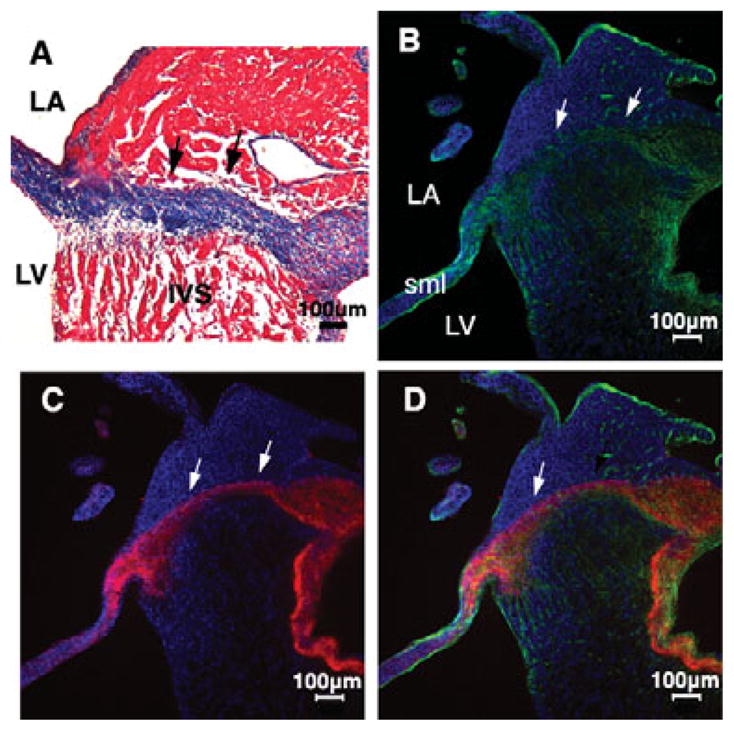
Annulus fibrosus was observed in the IVS of HH 44 embryonic chick heart by Masson’s trichrome and immunofluorescence staining. A specific collagen fiber band across IVS insulating the atrial myocardium from the ventrical myocardium was stained by Masson’s trichrome (A, black arrows). Confocal immunofluorescence against col1 (green, B) and tenascin (red, C) also showed the annulus fibrosus (white arrows). Nuclei (B–D, blue, Dapi). Merged image: D. Scale bar: 100 μm.
Organization and Deposition of Specific ECM Proteins in the Embryonic Mitral Valve
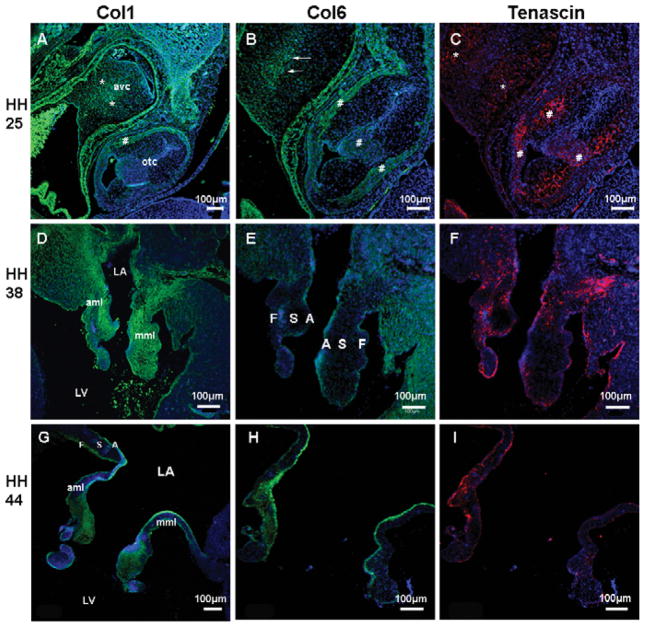
Confocal analysis was used to specifically examine the spatial and temporal expression patterns of the col1, col6, and tenascin proteins during valve development and to compare them with the studies above. At HH 25, just prior to fusion of the superior and inferior cushions, col1 and tenascin are diffusely expressed in the AV cushion with the most intense staining observed within cushion mesenchyme (stars, Fig. 6A,C). In contrast, col6 shows expression near the fusion line of the inferior and superior cushions at HH 25 (arrows, Fig. 6B). During cushion elongation (HH 38), col1 staining extends from the IVS and is expressed in the annulus fibrosa and in the atrialis of the aortic mitral leaflet, while in the mural mitral leaflet, col1 expression is widely dispersed in the annulus and the leaflet (Fig. 6D). Col6 and tenascin show expression in the annulus and spongiosa of both mural and aortic leaflets (Fig. 6E,F). During the leaflet remodeling (HH 44), col1 is localized to the atrialis, the fibrosa, and the distal part of spongiosa (Fig. 6G). Col6 is detected in the atrial aspect of both leaflets (Fig. 6H). Tenascin becomes restricted to the atrialis and fibrosa surfaces (Fig. 6I).
Figure 6.
Spatiotemporal expression of col1, col6, and tenascin in the cardiac cushions and developing mitral valve at HH 25, 38, and 44. Immunofluorescence staining was performed to detect col1 (green, A, D, G), col6 (green, B, E, H), and tenascin (red, C, F, I) expression. At cushion formation, col1 and tenascin were diffusely expressed in AV cushion mesenchyme (stars, A, C), while col6 showed more intense expression at the cushion fusion line (arrows, B). From the cushion elongation to the leaflet remodeling, expression pattern of col1 (D, G), col6 (E, H), and tenascin (F, I) in the mitral valve develop in a restricted manner. Nuclei (blue, Dapi). Avc: atrioventricular cushion; otc: outflow tract cushion. Scale bar: 100 μm.
Organization and Deposition of ECM Composition in Embryonic Tricuspid Valve
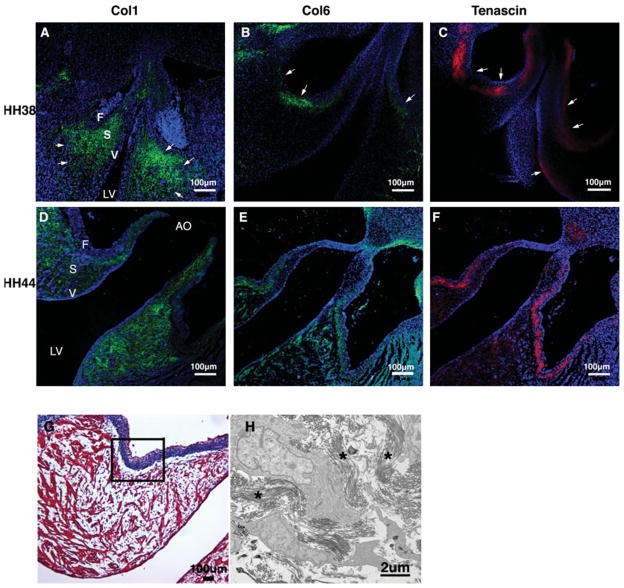
The progression of fibrous ECM protein expression during embryonic valve development of the tricuspid valve is shown in Figure 7. Expression of col1, col6, and tenascin in HH 25 cushions were as described above (Fig. 6A–C). At HH 38, col1 expression is widely distributed throughout the tricuspid valve leaflets and is more prominent in the annulus and mesenchymal/nonmyocardial aspect of the mural leaflet (Fig. 7A). Col6 is widely dispersed in the mural leaflet and is weakly detected in the septal tricuspid leaflet (Fig. 7B). Tenascin is observed in the distal tips of the mural and septal leaflets (Fig. 7C). During leaflet remodeling (HH 44), the three extracellular matrix proteins demonstrate a similar expression pattern in the mural leaflet in the nonmuscular part at the atrial side (arrows, Fig. 7D–F) and ramify into in the muscular part of the mural leaflet.
Figure 7.
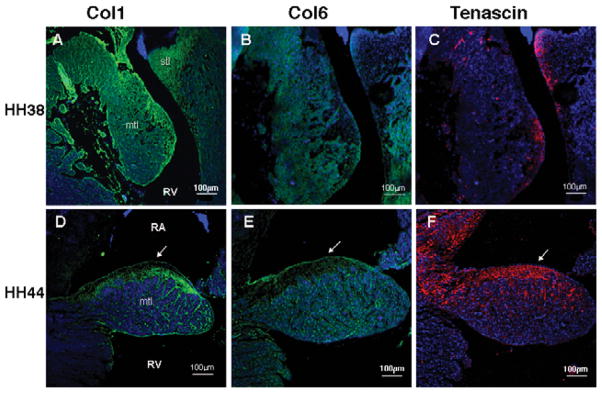
Expression pattern of col1, col6, and tenascin in developing tricuspid valve at HH 38 and 44. Immunofluorescence staining was performed to detect col1 (green, A, D), col6 (green, B, E), and tenascin (red, C, F) expression. From the cushion elongation to the leaflet remodeling, a fibrous matrix layer was formed above the muscular part of the mural tricuspid leaflet at the atrial aspect (arrows, D, E, F). Nuclei (blue, Dapi). Scale bar: 100 μm.
Organization and Deposition of ECM Composition in Embryonic Outflow Valve
During the early cushion stage (HH 25), col6 and tenascin are expressed in the outflow tract cushion and the myocardial wall (#, Fig. 6B,C). Col1 expression is detected in the myocardial wall (#, Fig. 6A). During cushion elongation (HH38), col1 expression is present in the arterial wall and extends into the spongiosa of the cusps (arrows, Fig. 8A). Col6 is expressed in the fibrosa layer (arrows, Fig. 8B). Tenascin expression overlaps col6 expression in the fibrosa and also is present in the ventricularis (arrows, Fig. 8C). During cusp remodeling (HH 44), col1 is localized to the ventricularis and the spongiosa (Fig. 8D), while tenascin expression is restricted to the annulus and the subendocardial layer of the fibrosa (Fig. 8F). Col6 is expressed in the fibrosa largely colocalized with tenascin and diffusely with the annulus and cusps (Fig. 8E). Noticeably, there is distinct blue collagen fiber staining in the fibrosa of the HH 44 semilunar valve (Fig. 8G), which overlays the col6 staining in the semilunar valve (Fig. 8E) but is reciprocal to the exclusive col1 expression in the spongiosa and the ventricularis of the semilunar valve by confocal immunofluorescence (Fig. 8D). TEM was performed to study collagen fiber organization in the fibrosa of the semilunar valve and shows abundant collagen fibers organized in the fibrosa at HH 44 (Fig. 8H).
Figure 8.
Differential expression of col1, col6, and tenascin in developing semilunar valve at HH 38 and 44. Immunofluorescence staining was performed to detect col1, col6, and tenascin expression. From the cushion elongation to the leaflet remodeling, col1 expression becomes confined to the spongiosa and the ventricularis (green, A, D), while expression of col6 (green, B, E) and tenascin (red, C, F) are more prominent in the subendocardial layer of the fibrosa. Further investigation of the fibrosa area (black frame, G) by TEM showed abundant collagen fibers in a well-organized manner at HH 44 (stars, H), which implies col1 is not the dominant collagen type forming collagen fibers in the fibrosa of semilunar valve. Nuclei (blue, Dapi). Scale bar: (A–G) 100 μm; (H) 2 μm.
Ultrastructure of Cardiac Mitral Valves during Embryonic Development
Collagen fibers are rarely observed in the mesenchyme of any valve leaflets before HH34 (Fig. 9A). At HH 38 and 40, short strands of collagen fibers are observed in close proximity to interstitial cells. In mitral valve leaflets, these collagen fibers are diffusely scattered throughout the extracellular matrix space (arrows, Fig. 9B,C). In the HH 44 mitral valve, abundant long strands of collagen fibers are deposited in a well-organized manner filling in the vast extracellular matrix space (arrows, Fig. 9D). Also, the average fiber diameter from various stages is 42.5 ± 6.82 nm, which matches the value of col1 fiber diameter reported by previous studies (Parry & Craig, 1984). Another finding from these TEM studies is the presence of endocardial membrane protrusions on the surface of valvular endothelial cells at all development stages (arrowheads, Fig. 10).
Figure 9.

Ultrastructure of chick mitral valve by TEM showed development of the extracellular collagen fiber organization. Collagen fibers were barely observed at HH 34 (A). There are short strands of collagen fiber scattering in the extracellular matrix at HH 38 (B, arrows). Long collagen fiber strands are formed in extracellular matrix space in well-organized manner at HH 40 (C, arrows) and HH 44 (D, arrows). Also, the fiber diameter is measured and averaged as described (42.5 ± 6.82 nm). Scale bar: (A–C) 2 μm; (D) 500 nm.
Figure 10.
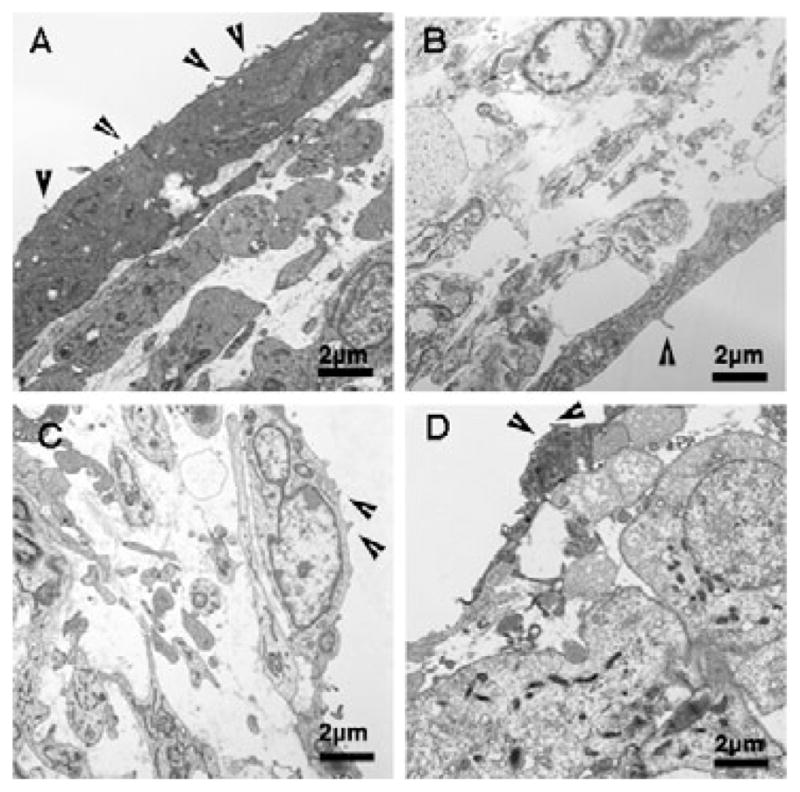
Ultrastructure of chick mitral valve by TEM showed membrane protrusions on the valvular endothelial cells. Membrane protrusions were observed aligning on the valvular endothelial cell surface throughout development: (A) HH 34, (B) HH 38, (C) HH 40, and (D) HH 44 (arrowheads). Scale bar: 2 μm.
Discussion
This study provides a detailed description of the timing and localization of col1, col6, and tenascin as early valve tissues mature from the soft, jelly-like endocardial cushions into tough, fibrous valve leaflets. The three investigated ECM fibrous proteins (col1, col6, and tenascin) are detectable in the endocardial cushions and throughout development become organized into stratified layers in the maturing valve leaflets. Different regulatory pathways are required for the regulation of regional deposition of each valvular ECM protein. For example, BMP2 induces Sox9 expression, which hierarchically induces aggrecan expression in the spongiosa of the valve, and fibroblast growth factor 4 induces tenascin expression through promotion of the transcriptional factor scleraxis in developing tendons (Lincoln et al., 2006). The delicate coordination of regional regulatory pathways implies a specific role for each ECM protein for proper valve function.
Collagen fibers and tenascin expressed in the fibrosa layer, which is at the side away from the blood flow, are the major structural components in the valves providing stiffness and strength to prevent regurgitation when the valve is closed. Proteoglycans in the spongiosa are suggested to absorb compressible forces on the leaflets and lubricate movements between the ventricularis/atrialis and the fibrosa layer. Elastin fibers in the atrialis/ventricularis layer, which is at the side facing the blood flow, confer elasticity to the valve, extending when the valve is open and recoiling when the valve is closed (Hinton et al., 2006). In contrast, col6 has been implied to play a regulatory role during valvulogenesis rather than to have a mechanical function. Regional col6 expression in chick endocardial cushions (arrows, Fig. 6B) and in mouse endocardial cushions (Klewer et al., 1998) is prominent on the cushion surface, where cushion fusion and EMT occur. This suggests col6 plays a role in cushion cell migration and differentiation. During late embryonic development, col6 expression is observed to largely colocalize with other ECM proteins, which is thought to function as a network to interact with various ECM proteins and maintain their proper organization. However, further study is required to prove the exact function of col6 during valvulogenesis.
The ECM proteins investigated in this study did not achieve their fully mature organization prior to hatching (HH 44). Therefore, leaflet ECM continues to mature during postnatal life, likely in response to increases in hemodynamic forces in the developing heart including blood pressure and shear stress. Our data show increasing collagen expression and collagen fiber formation during embryonic development. After birth, collagen fibrils become densely packed, and the fibrous collagen becomes the most abundant protein in the mature valves (Aikawa et al., 2006). Some evidence of ECM stratification was observed in our studies, specifically in the outflow valves at HH 44 (Fig. 4C). Indeed, studies of human semilunar cardiac valves showed that elastin content, collagen thickness, and orientation progressively increase with valve maturity from fetus to adult (Aikawa et al., 2006). In addition, during postnatal development, the valve leaflets continue to elongate, and the tricuspid valves undergo delamination and attain a stratified ECM (Kruithof et al., 2007). Moreover, the valve cells that were activated during embryonic stages undergo phenotypic changes at birth and gradually become quiescent (Aikawa et al., 2006). Normal valve development requires the spatiotemporal coordination of ECM expression and organization. Diseased valves usually exhibit increased ECM production and ECM disorganization (Kruithof et al., 2007). However, the mechanisms of regulating postnatal heart valve development as well as terminating this process are not well understood.
Although all endocardial cushions go through elongation and valve remodeling, each set of valves has a unique developmental pattern structurally and morphogenetically. The semilunar valves differ from AV valves in that the semilunar valves are cusp-shaped and do not have chordae tendineae. The morphogenesis of chicken tricuspid valves is also very different from the mitral valve and semilunar valve. During late embryonic valvulogenesis, the mitral and semilunar valves are thin, compacted, and have stratified extracellular matrix layers. In contrast, by HH 44 the mural tricuspid valve leaflet remains very muscular with a layer of mesenchyme and associated ECM proteins on the atrial aspect that is atypical of AV valves. The corresponding mural tricuspid valve leaflet of mammals develops into a fibrous leaflet by excavating from the myocardial wall, which is very different from that of avians (de Lange et al., 2004). In addition, previous studies indicated that the septal leaflet of the tricuspid valve is the only leaflet that develops by delaminating from the myocardial wall (de Lange et al., 2004; Gaussin et al., 2005). Together, it seems likely that the signaling pathways and regulatory factors in remodeling and patterning of heart valves are partly different among each set of valves during valvulogenesis.
It has been proposed that the orientation of ECM compartmentalization in mature heart valves is relative to blood flow direction (Rabkin et al., 2001; Lincoln et al., 2006). Correspondingly, our present study indicates that during embryonic development, collagen accumulates in the mitral and semilunar valves on the backside of their leaflets or fibrosa. Fluid force has been postulated as a key epigenetic factor in embryonic cardiogenesis. By manipulating blood flow in early mouse embryos, studies have demonstrated that hemodynamic force is essential for the development of the cardiovascular system (Lucitti et al., 2007). Another in vivo experiment using homozygous transgenic tie2::GFP zebrafish embryos showed a failure of valve formation under conditions of altered hemodynamics (Hove et al., 2003).
In addition, our TEM images show membrane protrusions along the valve endocardial cells from early to late embryonic valvulogenesis. Previous studies have described monocilia structures on the endocardial surface during early embryonic development (Van der Heiden et al., 2006). Cilia structures are coupled to calcium channels and are implicated as shear stress sensors created by blood flow (McGrath et al., 2003; Van der Heiden et al., 2006; Patwari & Lee, 2008). The membrane protrusions we observed did not appear to have a singular microtubule filament emanating from them, so they are not considered to be the monocillia described by Van der Heiden et al. (2006). Though the nature of these membrane protrusions remains to be determined, they are well situated to function as sensors of fluid flow. Together, the correlation between polarity of valve ECM and blood flow direction as well as the membrane protrusions found along heart valve endocardial cells support the concept that hemodynamics may play a regulatory role in instructing ECM organization during valvulogenesis.
Extensive studies have been carried out to understand valvulogenesis. But still, little is known about the dynamic patterning and organization of developing valve ECM. Our study is complementary to the current knowledge of valvulogenesis. A majority of congenital heart valve diseases appear to be genetically based and start to develop during embryogenesis (Garg et al., 2005; Nesta et al., 2005). Diseased heart valves exhibit disrupted ECM organization and valve cell distribution (Hinton et al., 2006). Therefore, characterization of the organization, histology, and morphology of normal heart valve development is important to advance the characterization of valve pathogenesis.
Determining the timing and mechanisms of ECM deposition in cardiac valves will benefit the long-term goal of developing new therapies for heart birth defects and pave the way for the in vitro production of replacement valvular and septal tissues. For tissue engineered heart valve design, developing methods to maintain, improve, or restore tissue function will be based on a thorough understanding of the native valve biology (Sacks et al., 2009).
Conclusions
The ECM is influential in maintaining cardiac valve structure and function, but its developmental pattern is not fully understood. Our study illustrates the deposition of select key ECM proteins, the pattern of ECM organization, the morphological development of valves, and the ultrastructure of chick atrioventricular and semilunar valves from the initiation of valve formation to the latest embryonic stages. The information from our study can be added to the current knowledge of cardiac valve development and shed light on the understanding of cardiac valve biology, pathology, and tissue-engineered valve construction.
Acknowledgments
This work was supported by funding from the National Institutes of Health (HL0860856, R.L.G.), National Science Foundation (FIBRE EF0526854 and EPS-0902795, R.A.N.), and the Foundation Leducq (Paris, France) Transatlantic Mitral Network of Excellence grant 07CVD04 (R.A.N.).
References
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- Akishima S, Sakurai J, Jikuya T. Stickler syndrome with rapidly progressive mitral valve regurgitation: Report of a case. Kyobu Geka. 2004;57(7):569–572. [PubMed] [Google Scholar]
- Anum EA, Hill LD, Pandya A, Strauss JF., 3rd Connective tissue and related disorders and preterm birth: Clues to genes contributing to prematurity. Placenta. 2009;30(3):207–215. doi: 10.1016/j.placenta.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ Res. 2004;95(5):459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Timpl R. The collagen superfamily. Int Arch Allergy Immunol. 1995;107(4):484–490. doi: 10.1159/000237090. [DOI] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17(1):104–108. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100(10):1503–1511. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart valve development: Regulatory networks in development and disease. Circ Res. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Männer J, Soufan AT, de Gierde Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95(6):645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Delom F, Burt E, Hoischen A, Veltman J, Groet J, Cotter FE, Nizetic D. Transchromosomic cell model of Down syndrome shows aberrant migration, adhesion and proteome response to extracellular matrix. Proteome Sci. 2009;7:31. doi: 10.1186/1477-5956-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Forni GL, Guerrini G, Borgna-Pignatti C. Pseudoxanthomaelasticum-like syndrome and thalassemia: An update. Dermatol Online J. 2009;15(7):7. [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97(3):219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenbergerde Groot AC, Bartram U, Oosthoek PW, Bartelings MM, Hogers B, Poelmann RE, Jongewaard IN, Klewer SE. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1109–1116. doi: 10.1002/ar.a.10126. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120(12):1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- Kitten GT, Kolker SJ, Krob SL, Klewer SE. Type VI collagen in the cardiac valves and connective tissue septa during heart development. Braz J Med Biol Res. 1996;29(9):1189–1193. [PubMed] [Google Scholar]
- Klewer SE, Krob SL, Kolker SJ, Kitten GT. Expression of type VI collagen in the developing mouse heart. Dev Dyn. 1998;211(3):248–255. doi: 10.1002/(SICI)1097-0177(199803)211:3<248::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kruithof BP, Krawitz SA, Gaussin V. Atrioventricular valve development during late embryonic and postnatal stages involves condensation and extracellular matrix remodeling. Dev Biol. 2007;302(1):208–217. doi: 10.1016/j.ydbio.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294(2):292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Lindeman JH, Ashcroft BA, Beenakker JW, van Es M, Koekkoek NB, Prins FA, Tielemans JF, Abdul-Hussien H, Bank RA, Oosterkamp TH. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci USA. 2010;107(2):862–865. doi: 10.1073/pnas.0910312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen BJ. Reference guide to the stages of chick heart embryology. Dev Dyn. 2005;233(4):1217–1237. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114(1):61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Nesta F, Leyne M, Yosefy C, Simpson C, Dai D, Marshall JE, Hung J, Slaugenhaupt SA, Levine RA. New locus for autosomal dominant mitral valve prolapse on chromosome 13: Clinical insights from genetic studies. Circulation. 2005;112(13):2022–2030. doi: 10.1161/CIRCULATIONAHA.104.516930. [DOI] [PubMed] [Google Scholar]
- Nuytinck L, Freund M, Lagae L, Pierard GE, Hermanns-Le T, De Paepe A. Classical Ehlers-Danlos syndrome caused by a mutation in type I collagen. Am J Hum Genet. 2000;66(4):1398–1402. doi: 10.1086/302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 1998;393(6681):181–185. doi: 10.1038/30270. [DOI] [PubMed] [Google Scholar]
- Parry DAD, Craig AS. Growth and development of collagen fibrils in connective tissue. In: Tuggeri A, Motta PM, editors. Ultrastructure of the Connective Tissue Matrix. The Hague: Martiners Nijhoff; 1984. pp. 34–64. [Google Scholar]
- Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008;103(3):234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock JD, Lu Y, Koch M, Kadler KE, Lincoln J. Temporal and spatial expression of collagens during murine atrioventricular heart valve development and maintenance. Dev Dyn. 2008;237(10):3051–3058. doi: 10.1002/dvdy.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Ann Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: Possible role during stress and remodeling. Cardiovasc Res. 2004;64(1):24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: Erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140(11):1373–1376. doi: 10.1001/archderm.140.11.1373. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: Coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81(7):392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenbergerde Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dynam. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5(13):2814–2823. [PubMed] [Google Scholar]
- Ward C, Stadt H, Hutson M, Kirby ML. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev Biol. 2005;284(1):72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Weis SM, Emery JL, Becker KD, McBride DJ, Jr, Omens JH, McCulloch AD. Myocardial mechanics and collagen sructure in the osteogenesis imperfecta murine (OIM) Circ Res. 2000;87:663–669. doi: 10.1161/01.res.87.8.663. [DOI] [PubMed] [Google Scholar]