Abstract
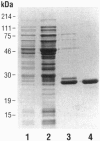
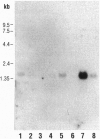
An enzyme that reduces methionine sulfoxide [Met(O)] residues in proteins [peptide Met(O) reductase (MsrA), EC 1.8.4.6; originally identified in Escherichia coli] was purified from bovine liver, and the cDNA encoding this enzyme was cloned and sequenced. The mammalian homologue of E. coli msrA (also called pmsR) cDNA encodes a protein of 255 amino acids with a calculated molecular mass of 25,846 Da. This protein has 61% identity with the E. coli MsrA throughout a region encompassing a 199-amino acid overlap. The protein has been overexpressed in E. coli and purified to homogeneity. The mammalian recombinant MsrA can use as substrate, proteins containing Met(O) as well as other organic compounds that contain an alkyl sulfoxide group such as N-acetylMet(O), Met(O), and dimethyl sulfoxide. Northern analysis of rat tissue extracts showed that rat msrA mRNA is present in a variety of organs with the highest level found in kidney. This is consistent with the observation that kidney extracts also contained the highest level of enzyme activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. R., Weinbaum G., Weissbach L., Weissbach H., Brot N. Enzymatic reduction of oxidized alpha-1-proteinase inhibitor restores biological activity. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7483–7486. doi: 10.1073/pnas.78.12.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders M. W., Ratnayake J. H., Hanna P. E., Fuchs J. A. Thioredoxin-dependent sulfoxide reduction by rat renal cytosol. Drug Metab Dispos. 1981 Jul-Aug;9(4):307–310. [PubMed] [Google Scholar]
- Brot N., Weissbach H. Biochemistry of methionine sulfoxide residues in proteins. Biofactors. 1991 Jun;3(2):91–96. [PubMed] [Google Scholar]
- Brot N., Weissbach L., Werth J., Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Werth J., Koster D., Weissbach H. Reduction of N-acetyl methionine sulfoxide: a simple assay for peptide methionine sulfoxide reductase. Anal Biochem. 1982 May 15;122(2):291–294. doi: 10.1016/0003-2697(82)90283-4. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun J. R., Menn J. J. Sulfoxide reduction in relation to organophosphorus insecticide detoxification. Science. 1976 Jan 16;191(4223):187–188. doi: 10.1126/science.1246606. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douch P. G., Buchanan L. L. Some properties of the sulphoxidases and sulphoxide reductases of the cestode Moniezia expansa, the nematode Ascaris suum and mouse liver. Xenobiotica. 1979 Nov;9(11):675–679. doi: 10.3109/00498257909042335. [DOI] [PubMed] [Google Scholar]
- Fliss H., Vasanthakumar G., Schiffmann E., Weissbach H., Brot N. Enzymatic reduction of oxidized chemotactic peptide N-formyl-L-methionyl-sulfoxide-L-leucyl-L-phenylalanine. Biochem Biophys Res Commun. 1982 Nov 16;109(1):194–201. doi: 10.1016/0006-291x(82)91584-4. [DOI] [PubMed] [Google Scholar]
- Fukazawa H., Tomisawa H., Ichihara S., Tateishi M. Purification and properties of methyl sulfoxide reductases from rat kidney. Arch Biochem Biophys. 1987 Aug 1;256(2):480–489. doi: 10.1016/0003-9861(87)90605-9. [DOI] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Miceli M. V., Liles M. R., Newsome D. A. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994 Sep;214(1):242–249. doi: 10.1006/excr.1994.1254. [DOI] [PubMed] [Google Scholar]
- Moskovitz J., Rahman M. A., Strassman J., Yancey S. O., Kushner S. R., Brot N., Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995 Feb;177(3):502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. A., Brot N., Weissbach H. High level expression and purification of peptide methionine sulfoxide reductase in Escherichia coli. Cell Mol Biol. 1992 Aug;38(5):529–542. [PubMed] [Google Scholar]
- Rahman M. A., Nelson H., Weissbach H., Brot N. Cloning, sequencing, and expression of the Escherichia coli peptide methionine sulfoxide reductase gene. J Biol Chem. 1992 Aug 5;267(22):15549–15551. [PubMed] [Google Scholar]
- Spector A., Scotto R., Weissbach H., Brot N. Lens methionine sulfoxide reductase. Biochem Biophys Res Commun. 1982 Sep 16;108(1):429–434. doi: 10.1016/0006-291x(82)91884-8. [DOI] [PubMed] [Google Scholar]
- Sánchez J., Nikolau B. J., Stumpf P. K. Reduction of N-acetyl methionine sulfoxide in plants. Plant Physiol. 1983 Nov;73(3):619–623. doi: 10.1104/pp.73.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wong P. S., Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]