Abstract
Objectives
Although beer and liquor have been associated with risk of incident gout, wine has not. Yet anecdotally, wine is thought to trigger gout attacks. Further, how much alcohol intake is needed to increase the risk of gout attack is not known. We examined the quantity and type of alcohol consumed on risk of recurrent gout attacks.
Methods
We conducted a prospective internet-based case-crossover study in the United States among participants with gout and who had at least one attack during the one year of follow-up. We evaluated the association of alcohol intake over the prior 24 hours as well as the type of alcoholic beverage with risk of recurrent gout attack, adjusting for potential time-varying confounders.
Results
This study included 724 participants with gout (78% men, mean age 54 years). There was a significant dose-response relationship between amount of alcohol consumption and risk of recurrent gout attacks (p<0.001 for trend). The risk of recurrent gout attack was 1.36 (95% CI: 1.00 to 1.88) and 1.51 (95% CI: 1.09 to 2.09) times higher for >1–2 and >2–4 alcoholic beverages, respectively, compared with no alcohol consumption in the prior 24 hours. Consuming wine, beer, or liquor, was each associated with an increased risk of gout attack.
Conclusions
Episodic alcohol consumption, regardless of type of alcoholic beverage, was associated with an increased risk of recurrent gout attacks, including potentially with moderate amounts. Persons with gout should limit alcohol intake of all types to reduce the risk of recurrent gout attacks.
Keywords: gout attacks, alcohol, triggers, internet, case-crossover
INTRODUCTION
Gout, a crystal-induced arthritis associated with hyperuricemia,1 is presently the most common inflammatory arthritis, affecting 8.3 million US adults.2 Recurrent attacks constitute the main clinical burden of gout. Despite available urate-lowering therapies, the risk of recurrent gout attacks remains high, with the risk of having at least one attack in a year being 69%.3 Strategies to prevent not only disease onset but also recurrent attacks are needed, given the rising incidence and prevalence of gout.2,4–6
Alcohol has long been recognized anecdotally as a potential risk factor for recurrent gout attacks. However, most studies to date have focused on alcohol consumption in relation to the risk of initial occurrence of gout.7–9 In a large prospective cohort study, total alcohol consumption was strongly associated with an increased risk of incident gout.8 Additionally, the risk of incident gout varied by type of beverage consumed, with an increased risk observed for beer and liquor, but not wine.8 However, patients often report wine as a trigger for recurrent gout attacks, and historic depictions of gout often included wine, although this may have partly been related to lead contamination in the Roman era. We have previously reported that overall alcohol consumption increased the risk of recurrent gout attacks; however, due to insufficient cases at the time, we were unable to evaluate whether moderate intakes of alcohol and whether specific type of alcoholic beverage were associated with an increased risk of recurrent gout attack.10 Further, gout treatment guidelines vary regarding recommendations about quantity and type of alcohol intake.11–13 Clarification of the risk for recurrent gout attacks imparted by specific types of alcoholic beverages would have practical clinical implications for management of patients with established gout.
To address this knowledge gap, we analyzed 724 gout subjects that were prospectively recruited from across the US in an internet-based study. We used a case-crossover study design to quantify the risk of gout attack in relation to amount of alcohol consumption, particularly moderate intakes, and evaluated whether the effect on recurrent gout attacks varied by consumption of specific type of alcoholic beverage.
METHODS
Study Design
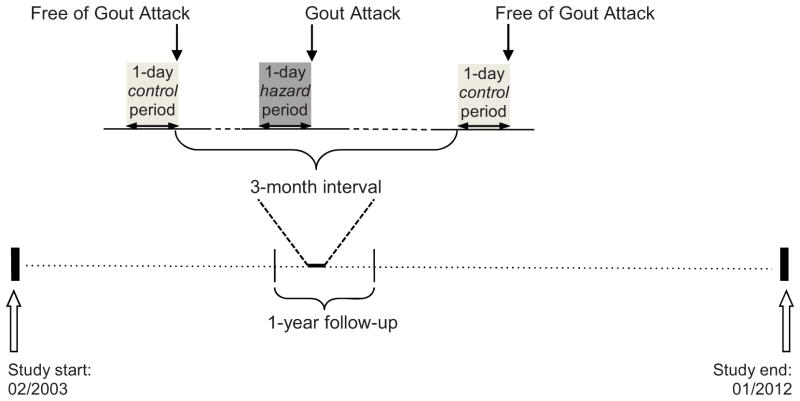
The Boston University online gout study is an internet-based case-crossover study conducted over the period of 2003–2012 to examine a set of putative risk factors for recurrent gout attacks. The details of the study have been described previously.10,14,15 In brief, we constructed a website (https://dcc2.bumc.bu.edu/GOUT) on an independent secure server within the Boston University Medical Center domain. Recruitment occurred primarily by means of an advertisement on Google® linked to the search term “gout”. Individuals were directed to the study website when they clicked on this link. The study design and timing of exposure assessments are illustrated in Figure 1. With this study design, each subject serves as his or her own control. This self-matching eliminates confounding by factors that are constant within an individual but differ between study subjects (e.g., sex, race, socioeconomic status).
Figure 1.
Case-crossover study design and timing of exposure measurements in relation to gout attacks
Gout attacks could occur at any time during the 1-year follow-up period in each subject. Hazard period refers to the 1-day period prior to a gout attack. Up to four control periods were selected from the intercritical period every 3 months during the 1 year of follow-up. Exposure to alcohol and other time-varying factors (potential confounders) were compared between hazard and control periods. The current study spanned between February 2003 and January 2012.
Study Sample
The study website provided information about the study, and for interested potential participants, administered a screening questionnaire that collected sociodemograhic information, gout-related data (e.g., features, duration, medications used, number of gout attacks in the prior 12 months), comorbidities, and other medication use. Eligible subjects were those who reported a gout attack within the previous year, were age 18 years or older, were residents of the US, provided informed consent, and agreed to release medical records. We reviewed the medical records and/or checklist completed by their physician of the components of the American College of Rheumatology (ACR) Preliminary Classification Criteria for Gout.16 Two rheumatologists (DJH, TN) reviewed all medical records and checklists to determine whether subjects met a diagnosis of gout according to the ACR criteria, using similar methods of confirmation as used in the Health Professional Follow-Up Study.8 This study was approved by the institutional review board of Boston University Medical Center.
Ascertainment of gout attacks
For each gout attack that occurred during the one-year follow-up period, we collected the onset date of the attack, anatomical location of the attack, clinical symptoms and signs (maximal pain within 24 hours, redness, swelling), medications used to treat the attack (e.g., colchicine, NSAIDs, systemic or intra-articular glucocorticoids), and whether a healthcare professional was seen for attack management. This method of identifying gout attacks is in keeping with approaches used in gout trials17–19 and the provisional definition of flare in patients with established gout that includes only patient-reported elements.20 We additionally restricted our gout attack definitions to those that were treated with at least one gout-related medication typically used to treat attacks (listed above), those with first metatarsophalangeal involvement, those with maximal pain within 24 hours, those with redness, and those with a combination of these features (i.e., those with at least 2, 3, or all 4 features).
Ascertainment of risk factors
Subjects were queried about the frequency and quantity of a set of putative risk factors (e.g., dietary factors, medication use, physical activity, geography) during the 24-hours prior to that gout attack (hazard-period).15,21 The same questions were also asked over a 24-hour period when they were attack-free (control period) at study entry (for those subjects who entered the study during an intercritical period), and at 3, 6, 9, and 12 (for those subjects who entered the study at the time of a gout attack) months of follow-up. (Figure 1)
Standardized questions regarding alcohol intake included the number of servings of wine, beer (including light beer, ciders, and malt beverages), or liquor (either straight or in a mixed drink) consumed during the prior 24-hour period for control and hazard periods. Explanation and pictorial depiction of standard serving sizes (i.e., a 12-ounce bottle or can of beer; a 5-ounce glass of wine; and 1 to 1.5 ounces of liquor)22 were provided with color images. Information on potential confounders, such as diuretic use, food and beverage intake from which purine consumption could be calculated,15 and gout-related medication were also collected during the control and hazard periods.
Statistical Analysis
The total amount of alcohol intake (grams/day) was estimated based on number of servings reported in a 24-hour period as ([0.57 * # of cocktails (liquor)/day] + [0.44 * # of bottles/cans of beer/day] + [0.40 * # of glasses of wine/day])*28.35.23 This latter term represents 28.35 grams of alcohol per fluid ounce. One typical drink is approximately 15 grams of alcohol.24 We divided total amount of alcohol consumption in the hazard and control periods into seven categories: no alcohol consumption, >0–1 drink, >1–2, >2–4, >4–6, >6–8, and more than 8 drinks. Moderate alcohol intake is considered to be no more than 2 drinks per day for men and no more than 1 drink per day for women.22 We grouped the daily consumption of each specific alcoholic beverage into the following categories based on their distribution: for wine, no wine consumption, >0–1, >1–2, and >2 servings; for beer and for liquor, no consumption, >0–2, >2–4, >4–6, and >6 servings.
We examined the relation of total alcohol intake over 24 hours to the risk of recurrent gout attacks using conditional logistic regression, which takes into account the matching of each subject’s own hazard and control periods.25 In multivariable regression models, we adjusted for diuretic use, purine intake, gout-related medication use (allopurinol, colchicine, NSAIDs, other urate-lowering therapies), and water intake. To better depict the dose-response relation between alcohol consumption and risk of gout attacks, we used quadratic spline regression to smooth the dose-risk curve.26 We then evaluated the association of alcohol intake with risk of gout attacks according to subgroups defined by sex, age (<55 vs. ≥55) and BMI (<30 vs. ≥30). We also evaluated the joint effects of purine intake (< 850mg (median value for 24-hour intake) vs. ≥850mg), diuretic use, allopurinol use, colchicine use, and NSAID use with alcohol intake in the prior 24 hours. Finally, we assessed the independent effect of each specific type of alcoholic beverage with conditional logistic regression adjusting for potential confounders listed above as well as consumption of the other types of alcoholic beverages.
RESULTS
There were 724 participants (mean age 54 years) who completed both hazard and control period questionnaires over a consecutive 12-month period between February 2003 and January 2012. As shown in Table 1, the majority of participants was male (78%), obese (mean BMI 32 kg/m2), and White (89%). Participants were recruited from 49 states and the District of Columbia. Of these participants, 614 (85%) met the ACR Preliminary Classification Criteria for Gout. Approximately 48% were on urate-lowering therapy (allopurinol: 44%; other: 4%); 25% used colchicine for prophylaxis and/or gout attacks while 38% used NSAIDs for prophylaxis and/or gout attacks.
Table 1.
Baseline characteristics of participants in the internet-based case-crossover study of gout, 2003–2012
| Participant Characteristic | N=724 |
|---|---|
| Age, yrs [mean (SD), range] | 54.5 (12.5), 21–88 |
| BMI, kg/m2 [mean (SD), range] | 32.1 (6.9), 14.7–69.9 |
| Male [n (%)] | 568 (78.5) |
| Disease duration [mean years (SD), range] | 8.0 (9.3), 1–55 |
| White [n (%)] | 642 (88.7) |
| Completed college [%] | 58.1 |
| Household income ≥ $50,000 [%] | 58.6 |
| Mean number of alcoholic beverages per 24-hour period (calculated from 3380 24-hour hazard and control periods) | 1.2 |
During the one-year follow-up period, there were 1,434 gout attacks, primarily occurring in the lower extremity (92%), particularly in the first metatarsophalangeal joint, and had features of maximal pain within 24 hours or redness (89%). Eighty-nine percent of these gout attacks were treated with colchicine, NSAIDs, systemic or intra-articular glucocorticoids, or a combination thereof.
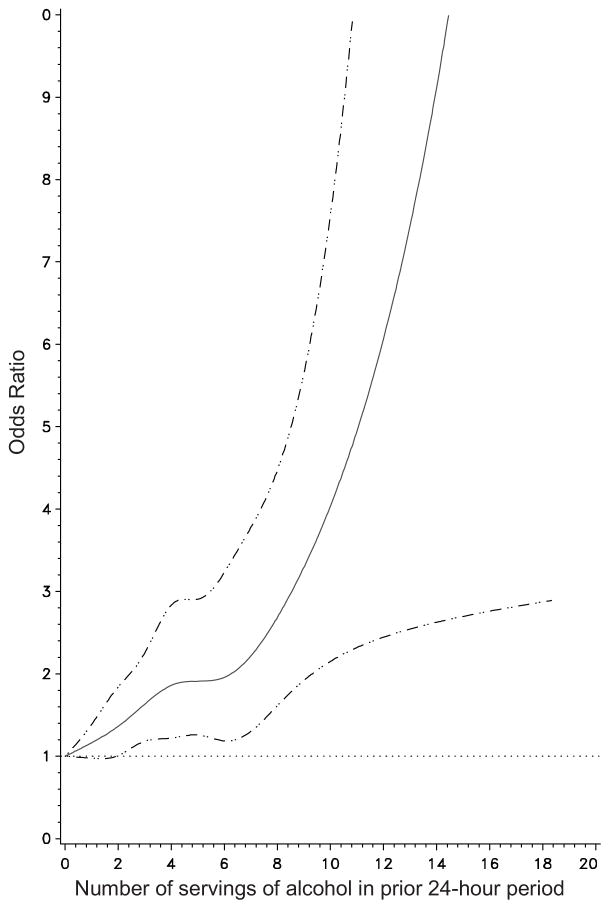
Approximately 44% of subjects reported any alcohol intake during hazard, control, or both periods. The mean number of standard servings of alcohol was 1.0 during a control period and 1.4 during a hazard period. The risk of recurrent gout attacks increased as the amount of alcohol consumption increased (Figure 2). While having up to one drink in a 24-hour period did not increase the risk of attack significantly (OR=1.13, 95% CI 0.80–1.58), consuming >1–2 drinks in a 24-hour period was associated with 36% higher risk of recurrent attack (OR=1.36, 95% CI 1.00–1.88) compared with those with no alcohol intake in that period, indicating that a moderate amount of alcohol intake within a 24-hour period may increase the risk of recurrent gout attacks (Table 2).
Figure 2.
Effect of alcohol consumption on risk of recurrent gout attack
Spline regression depicting the relation of total alcohol intake to the risk for recurrent gout attack.
Table 2.
Total alcohol intake over the prior 24-hour period and the risk of recurrent gout attacks
| Number of servings of alcohol over the prior 24-hour period | Number of hazard periods (N=1434) | Number of control periods (N=1946) | Crude OR | Adjusted OR* (95% CI) |
|---|---|---|---|---|
| 0 | 856 | 1222 | 1.0 | 1.0 (referent) |
| >0–1 | 93 | 145 | 1.12 | 1.13 (0.80–1.58) |
| >1–2 | 121 | 185 | 1.26 | 1.36 (1.00–1.88) |
| >2–4 | 178 | 223 | 1.60 | 1.51 (1.09–2.09) |
| >4–6 | 94 | 105 | 2.13 | 1.87 (1.19–2.93) |
| >6–8 | 48 | 40 | 2.65 | 2.33 (1.28–4.24) |
| >8 | 44 | 26 | 3.90 | 3.13 (1.63–6.02) |
| P for linear trend: | <0.001 |
adjusted for purine intake, allopurinol or other ULT, NSAID, colchicine, diuretic use, and water intake in prior 24-hour period
When we limited our analyses to only those subjects who fulfilled the ACR Preliminary Classification Criteria for Gout (N=614), the results did not change substantially, with the multivariable adjusted ORs (95% CI) being 1.09 (0.76–1.55), 1.36 (0.96–1.93), 1.50 (1.07–2.18), 2.05 (1.26–3.35), 2.50 (1.33–4.71), 3.40 (1.63–7.09) for >0–1, >1–2, >2–4, >4–6, >6–8, and >8 servings, respectively, compared with no alcohol intake in the prior 24 hours. When we used more stringent definitions of gout attack, the results were also similar. For example, requiring at least 2 of the following features: first metatarsophalangeal involvement, maximal pain within 24 hours, redness, use of a typical gout attack treatment (N=687), the corresponding multivariable adjusted ORs were 1.10 (0.78–1.56), 1.38 (0.99–1.92), 1.43 (1.03–1.99), 2.05 (1.30–3.24), 2.42 (1.32–4.42), and 3.42 (1.76–6.67), respectively.
Participants were required to complete their control period questionnaires once every 3 months. It was possible that control periods may have over-represented certain days of the week; for example when internet access may have been more accessible, such as in the office. We therefore performed additional analyses according to weekday versus weekend reporting. The effect estimates of alcohol consumption for weekdays were similar to those for the weekend; the adjusted ORs of recurrent gout attacks for >1–2 drinks in the prior 24 hours were 1.48 for weekdays and 1.30 for weekends.
Moderate alcohol consumption (i.e., up to 2 drinks/day for men and up to 1 drink/day for women) was associated with a 41% increased risk of recurrent gout attacks for men (adjusted OR=1.41, 95% CI 1.00–2.01), but not for women (adjusted OR=1.06, 95% CI 0.49–2.30) compared with those who did not drink any alcohol in the prior 24-hour period, although there were too few women to precisely estimate this effect (p=0.4 for interaction by gender).
The combined effects of alcohol intake with concurrent intake of purines and use of gout-related medications are shown in Table 3. Increasing numbers of servings of alcohol in combination with either high purine consumption or diuretic use were associated with higher risk of recurrent gout attacks. In contrast, use of allopurinol mitigated the effects of alcohol intake, as did colchicine although to a lesser extent. NSAID use did not modify the effect of alcohol intake.
Table 3.
Combined effects of alcohol intake and other time-varying risk factors (purine intake, diuretic use, allopurinol use, colchicine use, NSAID use) on risk of gout attack
| Exposure to risk factor in prior 24 hours | Number of alcohol servings in prior 24 hours | Adjusted** OR (95% CI) |
|---|---|---|
| Purine intake: | ||
| <850mg* | 0 | 1.0 (ref) |
| <850mg | >0–1 | 0.88 (0.54–1.45) |
| <850mg | >1–2 | 1.50 (1.01–2.23) |
| <850mg | >2 | 1.83 (1.24–2.68) |
| ≥850mg | 0 | 2.35 (1.88–2.93) |
| ≥850mg | >0–1 | 3.16 (2.00–4.99) |
| ≥850mg | >1–2 | 2.65 (1.66–4.24) |
| ≥850mg | >2 | 4.17 (2.95–5.89) |
|
| ||
| Diuretic use: | ||
| No | 0 | 1.0 (ref) |
| No | >0–1 | 1.26 (0.85–1.86) |
| No | >1–2 | 1.38 (0.96–1.97) |
| No | >2 | 1.61 (1.17–2.20) |
| Yes | 0 | 2.40 (0.59–3.62) |
| Yes | >0–1 | 2.12 (1.02–4.42) |
| Yes | >1–2 | 3.44 (1.70–6.93) |
| Yes | >2 | 5.82 (2.94–11.53) |
|
| ||
| Allopurinol use: | ||
| No | 0 | 1.0 (ref) |
| No | >0–1 | 1.04 (0.70–1.55) |
| No | >1–2 | 1.58 (1.08–2.31) |
| No | >2 | 1.74 (1.26–2.41) |
| Yes | 0 | 0.45 (0.33–0.62) |
| Yes | >0–1 | 0.61 (0.32–1.17) |
| Yes | >1–2 | 0.43 (0.24–0.79) |
| Yes | >2 | 0.70 (0.42–1.17) |
|
| ||
| Colchicine use: | ||
| No | 0 | 1.0 (ref) |
| No | >0–1 | 1.17 (0.81–1.68) |
| No | >1–2 | 1.37 (0.97–1.93) |
| No | >2 | 1.70 (1.24–2.32) |
| Yes | 0 | 0.82 (0.55–1.20) |
| Yes | >0–1 | 0.59 (0.24–1.47) |
| Yes | >1–2 | 1.03 (0.45–2.40) |
| Yes | >2 | 1.18 (0.63–2.19) |
|
| ||
| NSAID use: | ||
| No | 0 | 1.0 (ref) |
| No | >0–1 | 1.06 (0.72–1.56) |
| No | >1–2 | 1.44 (1.00–2.06) |
| No | >2 | 1.72 (1.25–2.37) |
| Yes | 0 | 1.36 (1.03–1.80) |
| Yes | >0–1 | 1.62 (0.84–3.16) |
| Yes | >1–2 | 1.45 (0.80–2.62) |
| Yes | >2 | 2.03 (1.29–3.19) |
median value of purine intake in prior 24-hours
mutually adjusted for each other as well as other urate-lowering therapies and water intake
As shown in Table 4, each type of alcoholic beverage intake was associated with an increased risk of recurrent gout attacks. Consuming >1 to 2 servings of wine over the prior 24 hours significantly increased the risk of recurrent gout attack (adjusted OR 2.38, 95% CI: 1.57–3.62). For beer, having up to two servings and >2–4 servings were associated with a non-significant 29% and statistically significant 75% higher risk for recurrent gout attack, respectively, compared with no such intake. There was also an increased risk of recurrent gout attacks with increasing amounts of liquor consumption, with those consuming >2 to 4 servings of such beverages having 1.67 times higher risk of an attack compared with no such intake in the prior 24-hour period.
Table 4.
Specific alcoholic beverage intake over the prior 24-hour period and risk of recurrent gout attacks
| Number of servings of specific alcoholic beverages over the prior 24-hour period | Number of hazard periods (N=1434) | Number of control periods (N=1946) | Adjusted OR* | Adjusted OR** (95% CI) |
|---|---|---|---|---|
| Wine: | ||||
| 0 | 1194 | 1664 | 1.0 | 1.0 |
| >0–1 | 102 | 133 | 1.26 | 1.25 (0.87–1.80) |
| >1–2 | 89 | 80 | 2.34 | 2.38 (1.57–3.62) |
| >2 | 49 | 69 | 1.35 | 1.41 (0.86–2.32) |
| P for linear trend | <0.001 | <0.001 | ||
| Beer: | ||||
| 0 | 1124 | 1601 | 1.0 | 1.0 |
| >0–2 | 92 | 129 | 1.28 | 1.29 (0.91–1.83) |
| >2–4 | 99 | 114 | 1.73 | 1.75 (1.19–2.59) |
| >4–6 | 52 | 49 | 2.56 | 2.60 (1.40–4.81) |
| >6 | 67 | 53 | 2.40 | 2.32 (1.25–4.31) |
| P for linear trend | <0.001 | 0.001 | ||
| Hard liquor: | ||||
| 0 | 1199 | 1673 | 1.0 | 1.0 |
| >0–2 | 68 | 113 | 0.97 | 0.92 (0.62–1.37) |
| >2–4 | 60 | 57 | 1.66 | 1.67 (1.00–2.78) |
| >4–6 | 75 | 86 | 1.63 | 1.56 (0.95–2.57) |
| >6 | 31 | 17 | 2.97 | 2.79 (1.26–6.16) |
| P for linear trend | 0.002 | 0.005 | ||
adjusted for purine intake, allopurinol or other ULT, NSAID, colchicine, and diuretic use, in prior 24-hour period
additionally mutually adjusted for other types of alcohol intake
Similar findings were observed when analyses were limited to those participants who reported only drinking one type of alcoholic beverage during the course of the study. Compared with no intake of each specific type of alcoholic beverage during the prior 24 hours, the adjusted ORs for a recurrent gout attack were 3.96 (95% CI 1.84–8.52), 3.63 (95% CI 1.92–6.87), and 4.44 (95% CI 1.17–16.91) for consumption of up to 2 servings of wine, beer, and liquor, respectively.
DISCUSSION
While alcohol has long been thought to trigger gout attacks anecdotally, the results from our study confirm that alcohol intake, potentially even moderate amounts, increases the risk of recurrent gout attacks in a short time following consumption. Further, all types of alcoholic beverages, whether it was wine, beer, or liquor, were associated, to varying degrees, with an increased risk for recurrent gout attacks. These effects were stronger in the presence of high purine intake and diuretic use, while mitigated to varying degrees by allopurinol and colchicine use; NSAIDs did not modify the effects of alcohol intake on risk of recurrent gout attacks.
Ethanol ingestion can increase serum urate through both decreased urate excretion and increased urate production. Reduced renal urate excretion can occur because of lactic acidemia associated with acute excessive alcohol intake, as well as the acidemia associated with fasting that is often concomitant with such intake.27,28 Metabolism of ethanol also accelerates adenosine triphosphate degradation into uric acid precursors.28–30 While alcohol has definitively been associated with hyperuricemia,31–33 and variably associated with incident gout,7–9 the findings of our study support the importance of alcohol, regardless of type, as a trigger in established gout.
Why might wine not increase the risk for incident gout in an observational cohort, yet appear to increase the risk of recurrent gout attacks? One might expect the effects of ethanol to be similar regardless of the type of alcoholic beverage. Indeed, all types of alcohol can lead to increased urate levels due to a variety of mechanisms, including ethanol content, thereby increasing the risk of gout attacks. However, one may expect a greater effect of beer on hyperuricemia than other types of alcohol because it not only contains ethanol, but also has high levels of guanosine, a purine that is highly absorbable.27,34 On the other hand, persons who drink wine often have a healthier lifestyle than those who drink beer or spirits. For instance, wine drinkers tend to buy healthier foods and follow healthier diets than beer drinkers.35–38 Thus the lack of association between wine and incident gout from an observational study may be related to residual confounding from other healthy lifestyle factors. By using a case-crossover study design to assess the triggering effects of alcohol consumption, we minimize such “healthy lifestyle factors” that vary greatly between persons but are relatively consistent within an individual.
Additionally, risk factors for triggering recurrent gout attacks among persons with established gout may not be the same as those for incident gout among persons who are free of gout. Persons with established gout may have altered renal handling compared with those who do not have gout (i.e., at risk for incident gout), and therefore risk factors may affect the two groups differently. Further, the short-term effects of a risk factor may differ from its long-term effects. An example of such a paradoxical phenomenon is the well-known increased flare risk during urate-lowering therapy initiation, whereas over the long-term, such therapy reduces the risk of flares.
Several characteristics of this study are worth noting. The case-crossover study is an ideal design to assess the acute effect of triggers. Since each participant serves as his/her own control, this study design eliminates the effects of time-invariant confounding factors between persons.39 Recruitment of a large number of participants from all over the US through the internet highlights a novel aspect of this study. Finally, the online design enabled participants to enter data in real-time, thereby minimizing the potential for recall bias.
Our study has some limitations as well. First, although we collected information on major potential time-varying confounders and adjusted for them in the analyses, residual confounding bias may remain. Second, because it is widely assumed that alcohol may trigger gout attacks, recall bias and differential reporting is a possibility. We attempted to minimize these biases by collecting information on a broad range of potential exposures, capturing data in real-time, and ensuring the study participants were not primed regarding study hypotheses. Third, as with many epidemiologic studies, dietary intake was not independently verified. Fourth, allowing some flexibility for participants to choose which day of the week, albeit within a fixed time window, to complete a control period questionnaire can potentially introduce bias. Nevertheless, when we performed additional analyses stratified according to weekday versus weekend reporting, results did not vary materially. Finally, like other epidemiologic studies of gout8 and what is common in clinical practice, most of our participants did not have a crystal-proven diagnosis of gout. However, the majority in our study met ACR Preliminary Classification Criteria for gout and/or had a physician-diagnosis of gout, and the clinical characteristics of participants in our study are similar to what would be expected of gout patients.
In summary, the present study supports the role of episodic alcohol intake in triggering gout attacks, even for moderate amounts and regardless of type of alcohol. Thus, in addition to the general medical management of their gout, persons with established gout should consider limiting all types of alcohol intake as another preventive strategy to reduce their risk for recurrent gout attacks.
Clinical Significance.
Episodic intake of any type of alcohol, whether it is beer, wine, or liquor, can increase risk of gout attacks
Increasing amounts of alcohol intake of any type, even at moderate levels, can increase risk of gout attacks.
Clinicians and patients with gout should therefore consider limiting the consumption of all types of alcohol, not just beer.
Acknowledgments
This work was supported by the following grants: T. Neogi’s support included NIAMS K23 AR055127, Arthritis Foundation Arthritis Investigator Award, American College of Rheumatology Research Education Fund Junior Career Development Award in Geriatric Medicine (T. Franklin Williams Scholars Program); Y. Zhang’s support included NIAMS AR47785, American College of Rheumatology Research Education Foundation Health Professional Investigator Award.
Footnotes
Boston University Medical Center Institutional Review Board approval protocol number: H-22804
Conflict of interest statement: All of the work is original, all authors meet criteria for authorship, including acceptance of responsibility for scientific content of the manuscript.
There are no potential conflicts of interest for all authors.
All authors had access to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–52. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 3.Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J Rheumatol. 2006;33:104–9. [PubMed] [Google Scholar]
- 4.Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? Journal of Rheumatology. 2002;29:2403–6. [PubMed] [Google Scholar]
- 5.Chen SY, Chen CL, Shen ML, Kamatani N. Trends in the manifestations of gout in Taiwan. Rheumatology (Oxford) 2003;42:1529–33. doi: 10.1093/rheumatology/keg422. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–81. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38:628–32. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Woods R, Chaisson CE, et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119:800 e13–8. doi: 10.1016/j.amjmed.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford) 2007;46:1372–4. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna D, Fitzgerald JD, Khanna PP, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012. 2012;64:1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Chaisson CE, McAlindon T, et al. The online case-crossover study is a novel approach to study triggers for recurrent disease flares. J Clin Epidemiol. 2007;60:50–5. doi: 10.1016/j.jclinepi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Chen C, Choi H, et al. Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis. 2012;71:1448–53. doi: 10.1136/annrheumdis-2011-201215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 17.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–8. doi: 10.1002/art.27327. [DOI] [PubMed] [Google Scholar]
- 18.Sundy JS, Baraf HS, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711–20. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 19.So A, De Meulemeester M, Pikhlak A, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–76. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 20.Gaffo AL, Schumacher HR, Saag KG, et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum. 2012;64:1508–17. doi: 10.1002/art.33483. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Chen C, Hunter DJ, Chaisson CE, Choi H, Neogi T. Cherry consumption and risk of recurrent gout attacks. Arthritis and Rheumatism. 2010;62:S567. doi: 10.1002/art.34677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Kreger BE, Dorgan JF, Splansky GL, Cupples LA, Ellison RC. Alcohol consumption and risk of breast cancer: the Framingham Study revisited. Am J Epidemiol. 1999;149:93–101. doi: 10.1093/oxfordjournals.aje.a009791. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Moderate alcohol consumption and the risk of breast cancer. N Engl J Med. 1987;316:1174–80. doi: 10.1056/NEJM198705073161902. [DOI] [PubMed] [Google Scholar]
- 25.Stokes ME, Davis CS, Koch GG. Chapter 10: Conditional Logistic Regression. In: Stokes ME, Davis CS, Koch GG, editors. Categorical Data Analysis Using the SAS System. 2. Cary: SAS Institute, Inc; 2000. pp. 271–322. [Google Scholar]
- 26.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Eastmond CJ, Garton M, Robins S, Riddoch S. The effects of alcoholic beverages on urate metabolism in gout sufferers. Br J Rheumatol. 1995;34:756–9. doi: 10.1093/rheumatology/34.8.756. [DOI] [PubMed] [Google Scholar]
- 28.Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol. 2002;29:1350–5. [PubMed] [Google Scholar]
- 29.Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. 1982;307:1598–602. doi: 10.1056/NEJM198212233072602. [DOI] [PubMed] [Google Scholar]
- 30.Puig JG, Fox IH. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. 1984;74:936–41. doi: 10.1172/JCI111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HK, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004;51:1023–9. doi: 10.1002/art.20821. [DOI] [PubMed] [Google Scholar]
- 32.Gaffo AL, Roseman JM, Jacobs DR, Jr, et al. Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis. 2010;69:1965–70. doi: 10.1136/ard.2010.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu KH, See LC, Huang YC, Yang CH, Sun JH. Dietary factors associated with hyperuricemia in adults. Semin Arthritis Rheum. 2008;37:243–50. doi: 10.1016/j.semarthrit.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Gibson T, Rodgers AV, Simmonds HA, Toseland P. Beer drinking and its effect on uric acid. Br J Rheumatol. 1984;23:203–9. doi: 10.1093/rheumatology/23.3.203. [DOI] [PubMed] [Google Scholar]
- 35.Johansen D, Friis K, Skovenborg E, Gronbaek M. Food buying habits of people who buy wine or beer: cross sectional study. Bmj. 2006;332:519–22. doi: 10.1136/bmj.38694.568981.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjonneland A, Gronbaek M, Stripp C, Overvad K. Wine intake and diet in a random sample of 48763 Danish men and women. Am J Clin Nutr. 1999;69:49–54. doi: 10.1093/ajcn/69.1.49. [DOI] [PubMed] [Google Scholar]
- 37.Barefoot JC, Gronbaek M, Feaganes JR, McPherson RS, Williams RB, Siegler IC. Alcoholic beverage preference, diet, and health habits in the UNC Alumni Heart Study. Am J Clin Nutr. 2002;76:466–72. doi: 10.1093/ajcn/76.2.466. [DOI] [PubMed] [Google Scholar]
- 38.McCann SE, Sempos C, Freudenheim JL, et al. Alcoholic beverage preference and characteristics of drinkers and nondrinkers in western New York (United States) Nutrition, metabolism, and cardiovascular diseases : NMCD. 2003;13:2–11. doi: 10.1016/s0939-4753(03)80162-x. [DOI] [PubMed] [Google Scholar]
- 39.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]