Abstract
Individuals with Down syndrome (DS) acquire Alzheimer’s-like dementia (AD) and associated neuropathology earlier and at significantly greater rates than age-matched normosomic individuals. However, biological mechanisms have not been discovered and there is currently limited therapy for either DS- or AD-related dementia. Segmental trisomy 16 (Ts65Dn) mice provide a useful model for many of the degenerative changes which occur with age in DS including cognitive deficits, neuroinflammation, and degeneration of basal forebrain cholinergic neurons. Loss of noradrenergic locus coeruleus (LC) neurons is an early event in AD and in DS, and may contribute to the neuropathology. We report that Ts65Dn mice exhibit progressive loss of norepinephrine (NE) phenotype in LC neurons. In order to determine whether LC degeneration contributes to memory loss and neurodegeneration in Ts65Dn mice, we administered the noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4; 2 doses of 50 mg/kg, i.p.) to Ts65Dn mice at four months of age, prior to working memory loss. At eight months of age, Ts65Dn mice treated with DSP-4 exhibited an 80% reduction in hippocampal NE, coupled with a marked increase in hippocampal neuroinflammation. Noradrenergic depletion also resulted in accelerated cholinergic neuron degeneration and a further impairment of memory function in Ts65Dn mice. In contrast, DSP-4 had minimal effects on normosomic littermates, suggesting a disease-modulated vulnerability to NE loss in the DS mouse model. These data suggest that noradrenergic degeneration may play a role in the progressive memory loss, neuroinflammation, and cholinergic loss occurring in DS individuals, providing a possible therapeutic avenue for future clinical studies.
Keywords: Alzheimer’s disease, cholinergic neurons, cytokines, Down syndrome, hippocampus, memory, neuroinflammation, neurotoxins, noradrenergic
INTRODUCTION
Individuals with Down syndrome (DS) develop neuropathological hallmarks of Alzheimer’s disease (AD), including amyloid plaques, neurofibrillary tangles, neuroinflammation, and basal forebrain cholinergic neuron (BFCN) degeneration early in life [1]. Cholinergic degeneration is thought to be a major contributor to the cognitive impairment occurring in AD as well as DS [1]. The noradrenergic (NE) neurons of the nucleus locus coeruleus (LC) provide noradrenergic input throughout the neuraxis, modulate certain aspects of cognition, and degenerate in multiple neurodegenerative diseases, including Parkinson’s disease, AD, and DS, leading to reduced NE levels in target regions [2]. LC neuronal loss is an early event in AD, noted even in patients with mild cognitive impairment [3]. The loss of LC neurons correlates with dementia severity, as well as amyloid plaques and neurofibrillary tangles [4].
The impact of LC neuron loss in AD is unclear, in part because noradrenergic input plays a complex role in memory. While noradrenergic depletion by the selective neurotoxin N-(2-chloroethyl)-N-ethylbromo-benzylamine (DSP-4) does not negatively affect spatial memory function in intact rodents, it potentiates memory deficits in conjunction with cholinergic deficits [5, 6]. Activation of adrenergic receptors on astrocytes and microglia lowers expression of pro-inflammatory cytokines [7]. Conversely, NE depletion by DSP-4 increases inflammatory response to amyloid-β delivery [8], leading to enhanced neuropathology and memory loss in transgenic AD mouse models [9]. However, specific biological mechanisms for these effects have not been explored.
Ts65Dn mice contain a partial trisomy of murine chromosome 16, in a region syntenic to human chromosome 21 [10]. These mice recapitulate several of the degenerative hallmarks in DS individuals, including increased amyloid-β levels [11], neuroinflammation [12, 13], and age-dependent degeneration of cholinergic neurons [14]. Ts65Dn mice exhibit progressive age-related memory dysfunction in working and reference memory tasks starting at 6 months of age [15, 16], mimicking cognitive impairment in DS individuals with aging [17].
Ts65Dn mice exhibit a deficient response to noradrenergic activity [18], and recently morphologic changes in the LC have been shown in Ts65Dn mice [19]. However, whether LC changes are a late manifestation of Ts65Dn pathology or an early contributor to inflammatory and neurodegenerative changes has yet to be determined. In addition, although loss of LC neurons has been shown in the Ts65Dn mice, these findings were not correlated to other pathology in this model, and the role of LC loss in memory and neuropathology has yet to be determined. Therefore, the purpose of this study was 2-fold: 1) to further elucidate the temporal and spatial extent of LC NE neuron degeneration during aging in Ts65Dn mice, and 2) to investigate effects of the NE neurotoxin DSP-4 on cognitive performance and brain morphology in the same model.
MATERIALS AND METHODS
Animal care
Ts65Dn mice, partially trisomic for a segment of murine chromosome 16 [20] and their normosomic (NS) littermates were obtained from Jackson Laboratories (Bar Harbor, Maine). Because this background strain (C3H) carries the retinal degeneration allele (rd), all mice were screened and were found free of retinal degeneration at Jackson Laboratories. All mice were single housed, received food and water ad libitum, and were maintained on a 12-hour light/dark cycle. All experimental procedures were approved by the institutional animal care and use committee (IACUC) of the Medical University of South Carolina.
DSP-4 delivery
N-(2-chloro ethyl)-N-ethyl-bromo-benzyl amine (DSP-4) is an alkylating agent that acts at adrenergic synapses to specifically deplete NE levels [21]. Intraperitoneal (i.p.) injections of 40–50 mg/kg DSP-4 produce retrograde degeneration of noradrenergic terminals that result in an 80% reduction in cortical NE levels and substantial reduction in tyrosine hydroxylase (TH)-immunoreactive LC neuronal cell bodies [2, 22]. Ts65Dn mice and their normosomic littermates were randomly assigned to either 0.9% saline (NS Sal, n = 8; Ts65Dn Sal, n = 6) or DSP-4 (NS DSP-4, n = 8; Ts65Dn DSP-4, n = 5) injections at four months of age. Injections of 50 mg/kg DSP-4 (or 0.9% Sal) were given i.p. to the mice on two occasions separated by a period of seven days. This injection paradigm produces a degeneration of NE terminals that is maintained for several months [22].
Water radial arm maze testing
Following activity testing, mice were trained on a “win-stay” water-escape radial-arm maze (WRAM). This task tests spatial memory function in a manner similar to the Morris water maze, with the added benefits of allowing for more rapid testing and more quantifiable results (errors). In addition, it removes the opportunity for thigmotaxic behavior, which can confound interpretation of the outcome and has been documented in Ts65Dn mice [23, 24]. The apparatus had eight arms (each 38.1 cm long and 12.7 cm wide) and was filled with water (kept at 25°C) made opaque with black non-toxic paint. The maze had an escape platform hidden 1 cm below the water at the end of one of the eight arms. This task has been modified from a task described previously [25], and has been used in mouse models for AD to evaluate spatial memory function. As Ts65Dn mice are smaller than their NS littermates, and are more susceptible to water temperature and fatigue [26], we reduced testing to 12 trials and expanded to a third day of testing. The goal arm containing the platform was kept consistent through all trials of testing (a win-stay paradigm), while the start arm was varied for each trial. For the first nine trials of day 1, the visible and hidden platforms were alternated, while in the last three trials the platform remained hidden. On days 2 and 3, all trials were run using the hidden platform. Incorrect arm entries were counted as errors, and were measured over a 60 s time frame. An arm entry was counted when the tip of a mouse’s snout reached a mark delineated on the outside of the arm (11 cm into the arm). Consistent with previous studies, inactive mice were assigned one error for every 20 s a mouse failed to make an arm selection, though no errors were assigned in this study. For statistical evaluation, errors were averaged into three trial blocks, with four blocks per day. These trial blocks were analyzed statistically by a repeated-measures ANOVA using Statview (SAS Institute, Cary, NC).
Spontaneous locomotion
Ts65Dn and NS mice were tested to evaluate the effects of DSP-4-treatment on motor activity. Spontaneous activity (total distance traveled) was assessed in a Digiscan Animal Activity Monitor system for 1 h (Omnitech Electronics Columbus, OH), as described previously [27]. Each activity unit contains 16-photo beams positioned 5 cm apart, 8 on the x-axis, and 8 on the y-axis. The Digiscan analyzer is interfaced with an IBM XT computer using ILAM software (Coulbourn Instruments, Lehigh Valley, PA). Total activity is recorded as total centimeters traveled over the testing intervals described for each experiment. Center time measures the proportion of time spent in the center of the arena (a percentage of total time). On the day of testing, the mice were transferred from the animal colony into the laboratory in groups of six and tested in a darkened environment.
Tissue preparation
The brain was hemi-sected, with the right side post-fixed in 4% PF, while the left side was dissected for biochemical analysis of frontal cortex, cerebellum, and hippocampus. The hippocampus was further divided into dorsal and ventral regions. All dissected regions were weighed, frozen individually on dry ice, and kept at −80°C. Post-fixed brain regions were kept in 4% PF for 48 h, after which they were transferred to 30% sucrose in 0.1 M phosphate buffer for at least 48 h before sectioning. The brain tissue was sectioned coronally at a thickness of 45 µm on a Microm cryostat (Thermo Fisher Scientific Inc., Waltham, MA).
High-performance liquid chromatography coupled with electrochemical detection
Brain tissue levels of NE, serotonin, and dopamine and their metabolites were determined using a previously described protocol [28]. In brief, the mobile phase consisted of a citrate/acetate buffer solution (pH 4.0–4.1) containing 0.3 mM 1-octane sulfonic acid, 0.67 mM citric acid monohydrate, 0.1 M sodium acetate, and 0.13 mM EDTA with 7% methanol delivered at a flow rate of 2 ml/min. Samples were injected into a Keystone ODS-Hypersil column (C18; particle size: 3 µm; 100 × 4.6 mm), and detected using an ESA Coulochem III system with dual colorimetric detectors (Model 5011 dual analytical cell). The oxidation potential was set at +0.4 V, and the second reducing electrode was set at −0.25 V for detection. Compounds were quantified based on peak heights and retention times relative to known standard concentrations of each compound.
Immunohistochemistry
All immunohistochemistry was conducted as previously reported utilizing the avidin-biotin method followed by nickel-enhanced diaminobenzidine [29] development [15]. Using 45 µm coronal sections, histochemical analysis of the LC was performed for tyrosine hydroxylase (TH), the hippocampus was immunostained for the pan-microglial marker CD45 and the calcium binding protein Calbindin D-28k, and the basal forebrain was stained for the high-affinity NGF receptor TrkA, a marker of BFCNs [15, 29]. Free-floating sections were rinsed with 0.01 M tris buffered saline (TBS), incubated in a solution containing 0.3% hydrogen peroxide and 20% methanol in TBS to inhibit residual endogenous peroxidase, and blocked for 1 h in 10% normal goat serum (NGS) and 0.3% Triton X-100. Sections were incubated for 48 h at 4°C in primary antibody (rabbit anti-TrkA, 1 : 10,000, generously provided by Dr. L. Reichardt; rabbit anti-TH, 1 : 2000, Pel-Freeze Biologicals, Rogers, AR; rat anti-CD45, 1 : 1000, Serotec, Oxford, UK). Next, sections were incubated for 1 h with secondary antibody (TrkA and TH: biotinylated anti-rabbit secondary antibody, 1 : 200; CD45: biotinylated anti-rat secondary antibody, 1 : 200; Vector Laboratories, Burlingame, CA) followed by incubation with an avidin–biotin complex (ABC Elite; Vector) before development in DAB. Immunohistochemistry for Calbindin D-28k in the hippocampus was performed according to the peroxidase antiperoxidase method described previously [13], using primary rabbit anti-Calbindin D-28k (1 : 1000, Chemicon International, Billerica, MA), biotinylated goat anti-rabbit secondary (1 : 200, Covance, Madison, WI), and incubation with rabbit peroxidase-antiperoxidase (1 : 200, Covance) before DAB development. Sections were mounted on subbed slides, dehydrated with increasing gradients of ethanol, cleared with two incubations of xylene, and coverslipped with Permount (Fisher, Mansfield, TX). Controls included sections without primary antibody or secondary antibody. All sections for each antibody were processed together to avoid batch-to-batch differences. Antibody penetration throughout the full section depth was determined by stepwise focusing through the z-plane of several sections.
Stereology and image analysis
Stereology of LC and MSN
Quantitative estimates of the total number of TH-positive neurons in the locus coeruleus (LC) and TrkA-positive neurons in the medial septal nucleus (MSN) were performed using the optical fractionator method. This is an unbiased, stereological cell counting method that is not affected by the volume of reference (LC) or size of the counted elements (TH-positive neurons). After randomly selecting the initial section for each brain region, every 3rd subsequent section was immunostained for either TH (LC, 7–9 sections, both hemispheres) or TrkA (MSN, 8–9 sections), providing for a systematic random design according to standard procedures [30]. The optical fractionator method (StereoInvestigator software; MicroBrightfield, Colchester, VT) was used for unbiased neuronal cell counting. For the LC, Disector counting frames were set at 75 × 75 µm, while the grid size was 125 × 125 µm, yielding a sampling frame area of 0.36, while for the MSN a 50×50 µm Disector counting frame and a 100 × 100 grid size (sampling frame area = 0.25) were used, according to our previously published stereology methods [30]. A 3 µm guard zone was employed to prevent the counting of artifacts in the tissue section. The landmarks for outlining the LC and MSN were defined according to the Franklin and Paxinos atlas [31]; the MSN was separated from the ventral diagonal band by the anterior commissure. Neurons were counted using a 60× objective lens (1.4 numerical aperture).
BFCN cell area
Cell area and volume measurements for TrkA-positive neurons were performed on every 3rd serial section stained for TrkA, using the nucleator probe within StereoInvestigator [32]. The nucleator uses a series of six rays that extend out from a point marked at the nucleus. Each intersection of the rays with the cell boundary is located and marked, and together they provide an estimation of cell area. Neurons were randomly selected via the optical fractionator program, with at least 10 neurons sampled per section and at least 60 neurons sampled per brain by an independent and blinded investigator, with neurons measured using a 60× objective lens (1.4 numerical aperture).
Density measurements
Staining intensity of TH immunoreactivity in the LC and of Calbindin D-28k and CD45 immunoreactivity in the cornu ammonis 1 and 2 regions of the hippocampus (CA1/CA2) was assessed as described previously in order to examine whether TH expression was also reduced following the lesions [13]. In brief, staining intensity was determined using NIH Image software to measure a gray scale value within the range of 0 to 256, where 0 represents white and 256 black. Images were captured with a Nikon Eclipse E-600 microscope. Staining density was obtained when background staining was subtracted from mean staining intensities on every 6th section through the hippocampus using the same systemic random design described above for cell counts.
Quantitative real-time PCR
Total RNA was extracted from hippocampal tissue using 1 ml Trizol (Invitrogen, Carlsbad, CA). After mixing in chloroform (0.2 ml), the samples were centrifuged for 15 min at 14,000× g. The aqueous phase was transferred and mixed with isopropyl alcohol. RNA was then isolated using RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized by reverse transcription of 1 µg RNA using a high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). Real-time quantitative RT-PCR was performed on an ABI 7000 (Applied Biosystems, Foster City, CA) by monitoring the increase in FAM reporter dye fluorescence resulting from Taqman probe binding to DNA. For a 20 µl PCR reaction, 100 ng DNA was added to Gene Expression Master Mix and either IL-1β or BDNF Taqman Gene Expression Assay (Applied Biosystems, Assay ID: Mm01336189_m1 or Mm01334042_m1, respectively). The reaction was run using the default setting profile (95°C, 15 s; 60°C, 1 m; 40 cycles). All PCR reactions were performed in duplicate. Gene expression changes were quantified by the ΔΔCt method, which calculates relative fold changes normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Assay ID: Mm99999915_g1).
Statistics
All data were analyzed by one-way analysis of variance [33] and Fisher’s post hoc analysis unless otherwise noted. The data for the win-stay WRAM were analyzed using a 1-between (group) × 1-within (days) repeated-measures analysis of variance (Statview Version 4.0, JMP, Cary, NC). To determine maze acquisition, the error differences between day 1 and day 2 were analyzed using a one-way t-test (hypothesized mean = 0), where a statistical difference from this mean was operationally defined as learning. For “overnight remembering”, block differences between block 1 (on days 2 and 3) and block 4 (on days 1 and 2) were analyzed by ANOVA. Significance was set at a p value of less than 0.05 for all statistical analysis. For correlation studies, the Pearson correlation coefficient was utilized, providing a measure of the strength of linear dependence between two variables.
RESULTS
Study 1: age-related alterations in inflammatory and noradrenergic markers
Age-related LC degeneration in Ts65Dn mice
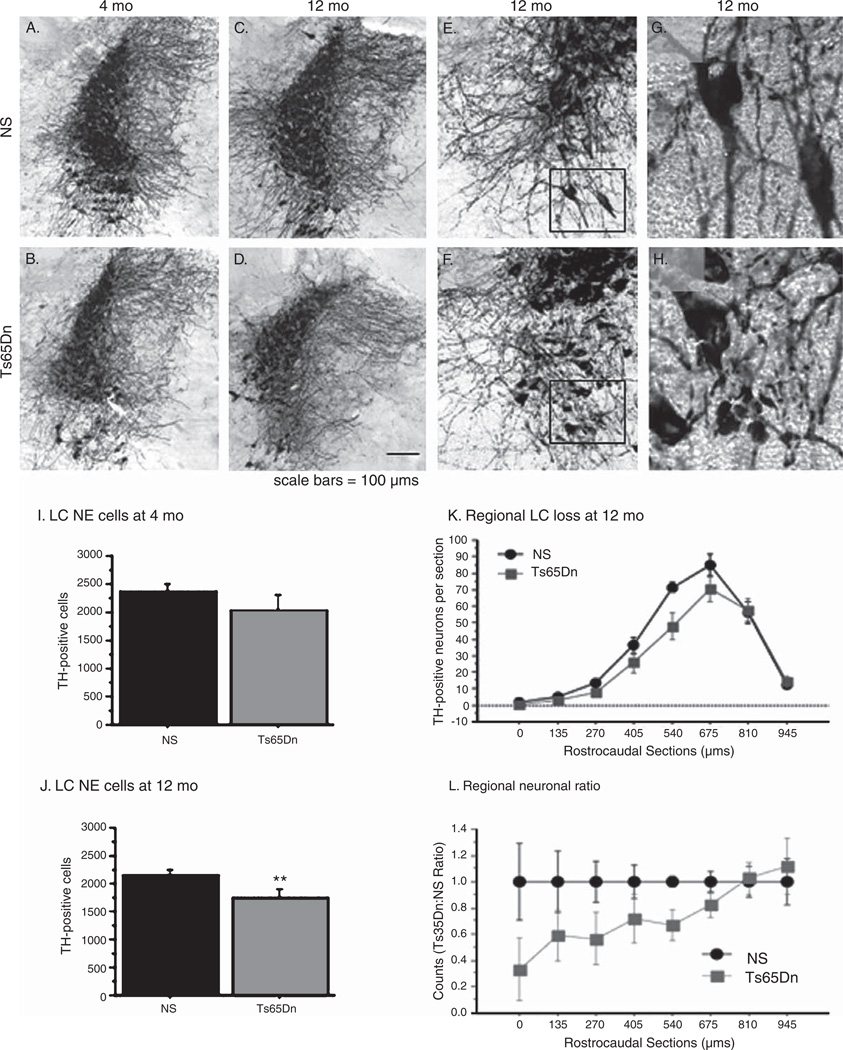
LC exhibits NE neuronal degeneration in several neurodegenerative diseases, including AD, Parkinson’s disease, and DS [34]. Since the Ts65Dn mice recapitulate other neurodegenerative changes seen in DS, we sought to evaluate the effects of aging on the central NE system in these mice. At 4 months of age (Fig. 1A and B), there was no observable loss in the number of TH-positive LC neurons in Ts65Dn mice, though when compared to the densely packed neuronal distribution found in normosomic littermates (Fig. 1A), reductions in neuronal size and fiber density were observed in Ts65Dn mice (Fig. 1B). At 12 months of age, we observed significantly greater degenerative changes in the TH-positive neurons in Ts65Dn mice, including regional loss of TH-positive neurons and reduced fiber density (Fig. 1D, F, H) compared with NS mice (Fig. 1C, E, G). As shown in higher magnification (Fig. 1E–H), LC NE neurons underwent significant shrinkage and loss of processes between 4 and 12 months of age in Ts65Dn but not in NS mice, particularly in the ventral portion of the nucleus, and remaining neurites at the latter time point exhibited numerous axonal swellings (see Fig. 1H) suggesting further degeneration of this system with aging, as previously described in the literature [35, 36]. Unbiased stereologic cell counts of TH-positive neurons in the LC were performed on 4 and 12 month-old mice in order to confirm observed degenerative alterations. At four months of age, there were no significant differences in TH-positive neurons between genotypes [Fig. 1I, F(1,8) = 1.2; p > 0.05], while at 12 months of age, there was a 20% decrease in the total number of TH-positive neurons in the LC in Ts65Dn mice [Fig. 1J, F(1,17) = 7.096; p < 0.05] compared to NS mice.
Fig. 1.
Locus Coeruleus neurons degenerate in Ts65Dn mice with aging. A–H) TH immunostaining of the LC demonstrated that at 4 mo NS mice (A) showed only mildly stronger staining than in Ts65Dn mice (B), while in 12 mo animals, Ts65Dn mice (D, F, H) exhibited altered neuronal morphology, axonal swellings, and fewer axonal processes relative to NS mice (C, E, G). I–J) A reduction in TH-positive neurons was present at 12 mo (J) but not at 4 mo (I) of age in Ts65Dn mice as evidenced by stereological counting. K, L) An analysis of the rostrocaudal distribution of TH neurons in the LC indicated that neuron reductions in Ts65Dn mice occurred primarily in rostral LC (mean ± SEM **p < 0.01; n = 5 per group for 4 mo, n = 9–10 per group for 12 mo). L = ratio of Ts65Dn versus normosomic mice at each portion of the LC, rostral to caudal.
We also examined whether the neuronal loss in Ts65Dn mice with age was focal in origin or consistent throughout the rostro-caudal extension of LC. Loss of LC noradrenergic neurons in AD is primarily localized to the rostral neurons that project to the forebrain regions of the cerebral cortex and the hippocampus [37]. To examine whether a similar pattern of degeneration was present in Ts65Dn mice, we plotted the neuronal numbers counted during our stereologic estimates (performed on every third section using a systematic random design) of 12-month old mice along the rostro-caudal plane. As Fig. 1K and L illustrates, the alterations in TH-positive neuronal number were limited to the more rostral regions of the LC, while the most caudal regions of the LC did not show a reduction in neuronal number. This distribution of neuronal loss is similar to that seen in AD and DS, and serves to provide greater support for the efficacy of Ts65Dn as a model for degenerative changes in DS.
Reduction in hippocampal morphology in aged Ts65Dn mice
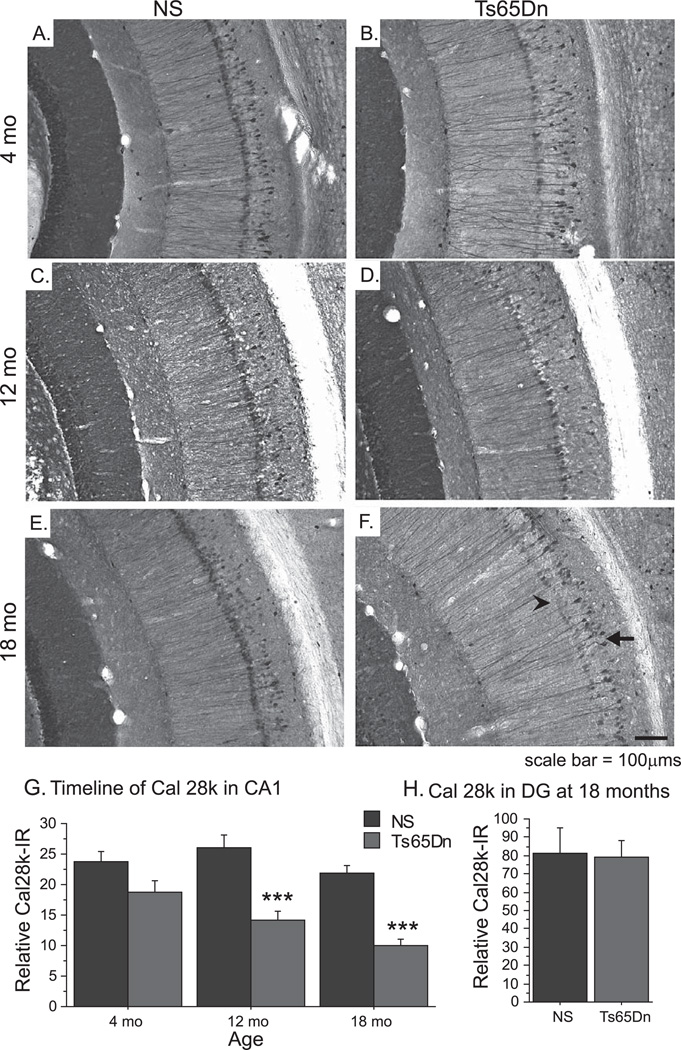
We also examined age-related alterations in the expression of Cal 28k morphology in the hippocampus, another marker that degenerates in AD, and has shown alterations in Ts65Dn mice [13]. Figure 2 illustrates Cal 28k-immunoreactivity (Cal 28k-IR) in the ventral hippocampus at three age points: 4 months, 12 months, and 18 months of age. While the CA 1 region of young NS mice (Fig. 2A) demonstrated robust Cal 28k-IR in the pyramidal neurons and their apical dendrites and in interneurons of the stratum oriens, the hippocampus of 4 mo Ts65Dn mice exhibited fewer apical dendrites and weaker expression of this marker in the soma of pyramidal neurons (Fig. 2B), although densitometric analysis did not reveal significant overall alterations at this age. While the NS hippocampus maintained a similar staining pattern throughout aging (Fig. 2C and E), Cal 28k-IR decreased progressively with age in Ts65Dn mice (Fig. 2D and F). These changes were most apparent in the soma and apical dendrites of pyramidal neurons at 18 months (2F, arrow), while Cal 28k staining in the neurons of the stratum oriens appeared less affected (2F, arrowhead). Densitometric measurements revealed that significant decreases in Cal 28k-IR were present at 12 and 18-months of age in Ts65Dn mice compared to NS mice [Fig. 2G, F(1,17) = 16.095; p < 0.001) and F(1,6) = 50.367; p < 0.001, respectively]. Evaluating densitometry as a function of age indicated that the reduction in Cal 28k-IR was progressive over time only in Ts65Dn mice, as Ts65Dn mice showed reduced Cal 28k-IR across time [One-way ANOVA of all Ts65Dn ages: F(2,14) = 8.090; p < 0.01] while NS mice did not [F(2,19) = 0.68; p < 0.1]; Loss of Cal 28k-positive staining was restricted to the CA1 region of the hippocampus, since density measurements in the dentate gyrus did not reveal alterations between NS and Ts65Dn mice even at 18 months of age (Fig. 2H).
Fig. 2.
Altered Calbindin D-28k morphology with age in Ts65Dn mice. A–F) Cal 28k immunostaining of the hippocampus in 4 mo (A, B), 12 mo (C, D), and 18 mo (E, F) mice demonstrates the effects of aging on hippocampal morphology in NS (A, C, E) and Ts65Dn (B, D, F) mice. G) Densitometric analysis revealed that overall Cal 28k-IR in CA1 pyramidal neurons of the hippocampus was reduced with age in Ts65Dn mice, while density in NS mice remained stable. H) Cal 28-IR in the dentate gyrus was not altered at 18 mo (mean ± SEM; +p = 0.08; ***p < 0.001; n = 5 per group for 4 mo, n = 9–10 for 12 mo, n = 4 for 18 mo).
The parallel degenerative alterations observed in LC-TH and hippocampal Cal 28k immunostaining during normal aging in Ts65Dn mice set the stage for a second study, in which LC neurons were exposed to a selective NE neurotoxin, DSP-4, in order to examine if the LC loss observed in Ts65Dn mice plays a role for degenerative alterations observed elsewhere in the brain.
Study 2: Effects of NE degeneration in Ts65Dn mice
Spatial memory function is disrupted following NE depletion
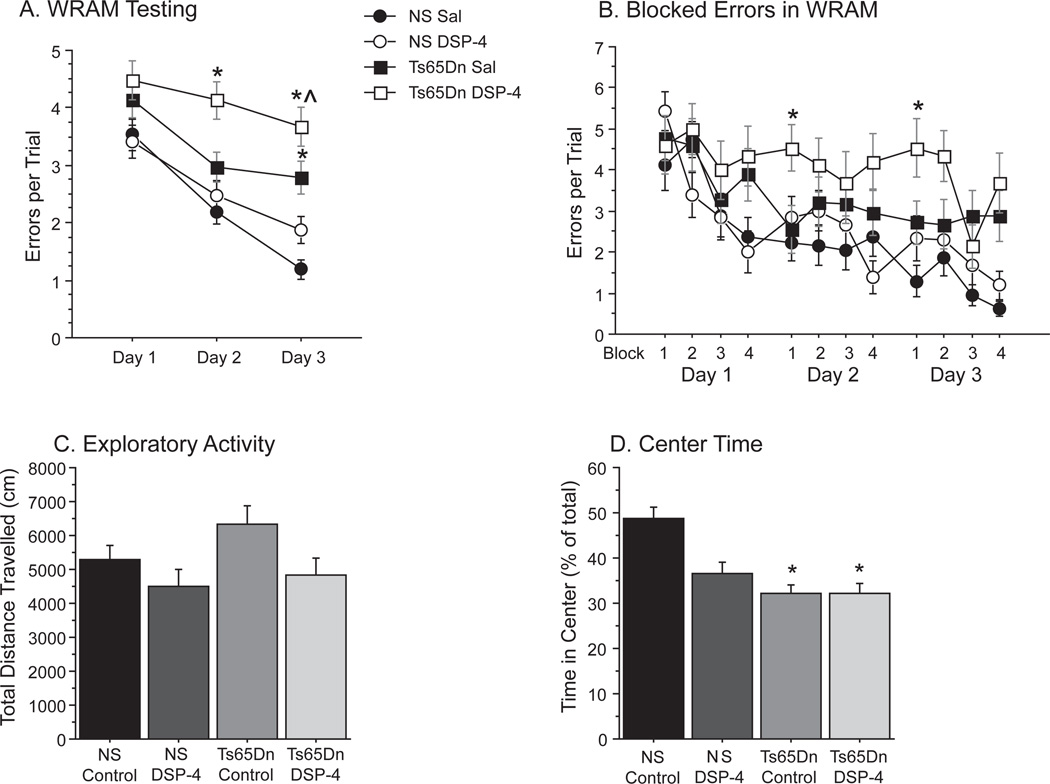
In order to evaluate whether degeneration of the LC neurons influences the degenerative changes exhibited in the Ts65Dn mouse, we subjected 4-month-old Ts65Dn mice to a neurotoxin (DSP-4, 2 doses of 50 mg/kg i.p., 7 days apart) which specifically targets noradrenergic terminals [38]. Four months following toxin delivery, the mice underwent spatial memory testing. Mice were subjected to a win-stay version of the WRAM that consisted of 12 trials per day over the course of 3 days (Fig. 3A). A group-wise comparison of performance across days revealed an overall group effect of errors [Repeated measures ANOVA: F(3,19) = 11.202; p < 0.001], as NS Sal mice committed fewer errors than both Ts65Dn Sal (p < 0.01) and Ts65Dn DSP-4 (p < 0.001) mice. Ts65Dn Sal mice performed better than Ts65Dn DSP-4 across days of testing (p < 0.05). Ts65Dn DSP-4 mice exhibited the greatest performance disparity on the second day of testing [Day 2: F(3,19) = 5.976; p < 0.01], where they committed significantly more errors than all other groups, including the Ts65Dn Sal mice (p < 0.05). Learning was operationally defined as the reduction of errors across days. Ts65Dn Sal mice committed fewer errors on the second day of testing compared to day 1 (one group t-test: t5 = −6.025; p < 0.01), indicating a measure of learning in the task, and began to diverge from the NS Sal group only on the third day of testing. Conversely, Ts65Dn DSP-4 mice failed to improve their performance throughout testing, as their error rate on day three was not significantly different than day one for the same group (t5 = −2.15; p > 0.05). The lack of learning of this task seen in the Ts65Dn DSP-4 mice strongly suggests that chronic NE depletion exacerbates the learning and memory problems previously observed at this age in Ts65Dn mice.
Fig. 3.
NE depletion further disrupted spatial memory function and activity in Ts65Dn mice. A) Performance in the WRAM is indicated by errors per trial across days (mean ± SEM, *p < 0.05 relative to NS Sal, ^p < 0.05 relative to Ts65Dn Sal). B) Analysis of errors across blocks (made up of three trials) demonstrate performance within each day of testing, and indicate that DSP-4 treatment alters “overnight remembering” on the first block of testing of days 2 and 3. C) Total activity was measured over a one-hour session (distance traveled, cm). D) Time spent in the center region, as a percentage of total time, showing that Ts65Dn mice spent less time in the center relative to NS mice (mean±SEM, *p < 0.05 relative to NS Sal; NS Sal n = 8; NS DSP-4 n = 8; Ts65Dn Sal n = 6; Ts65Dn DSP-4 n = 5).
In addition to measuring overall error rate across days, spatial testing in this manner can assess overnight retention by comparing performance on the first block of testing on day 2 with the last block of testing on day 1 (as well as between days 2 and 3). As Fig. 3B illustrates, NS Sal and Ts Sal mice each improved their performance on block 1 of days 2 and 3 compared to block 4 of the previous day, while NS DSP-4 and Ts65Dn DSP-4 mice both showed higher error rates, suggesting that the DSP-4 toxin affected retention from one day to the next [F(3,19) = 3.76; p < 0.05]. Indeed, a two-way ANOVA (genotype × treatment) evaluating overnight remembering confirmed that treatment had a significant influence on next day errors whereas genotype did not [Treatment effect: F(1,19) = 8.4; p < 0.01, Genotype effect: F(1,19) = 1.2; p > 0.05], as DSP-4 mice performed significantly worse than saline treated controls, regardless of genotype. Interestingly, NS DSP-4 mice appeared to show greater impairments in overnight retention than Ts65Dn DSP-4 mice, though this is most likely due to the fact that Ts65Dn DSP-4 mice were already performing at chance levels (chance ≈ 5 errors per trial). These results suggest that NE depletion specifically affected memory consolidation, or retention, in these mice across days of testing.
Spontaneous locomotion was altered following DSP-4 lesions
In addition to assessing spatial memory function, we also exposed the mice in all groups to one hour in a spontaneous locomotion box four months following DSP-4 toxin (see methods and Fig. 3C–D). Overall activity, as measured by total distanced traveled over the testing period (Fig. 3C), showed marginal increases in Ts65Dn Sal activity [F(3,23) = 2.586; p = 0.077], though an evaluation of treatment and genotype effects uncovered a reduction in exploratory activity following DSP-4 treatment, regardless of genotype [2 × 2 ANOVA: Treatment effects:F(1,23) = 5.333; p < 0.05]. We also evaluated the time spent in the center of the chamber (Fig. 3D), since “center time” can serve as an indicator of exploratory activity (as opposed to overall activity), and is affected by familiarity and/or reduced anxiety [39]. There were significant effects of center time [F(3,23) = 3.083; p < 0.05], as Ts65Dn Sal (p < 0.01) and Ts65Dn DSP-4 (p < 0.05) both spent less time moving across the center of the chamber than NS Sal mice, and NS DSP-4 mice showed a trend towards reduced center time as well. While both Ts65Dn groups exhibited statistically significant reduced center time compared to the NS saline group, they were not statistically significant from the NS DSP-4 group (p > 0.05), suggesting that there were effects on center time of the DSP-4 toxin also in NS mice.
Chronic suppression of NE levels following DSP-4 toxicity
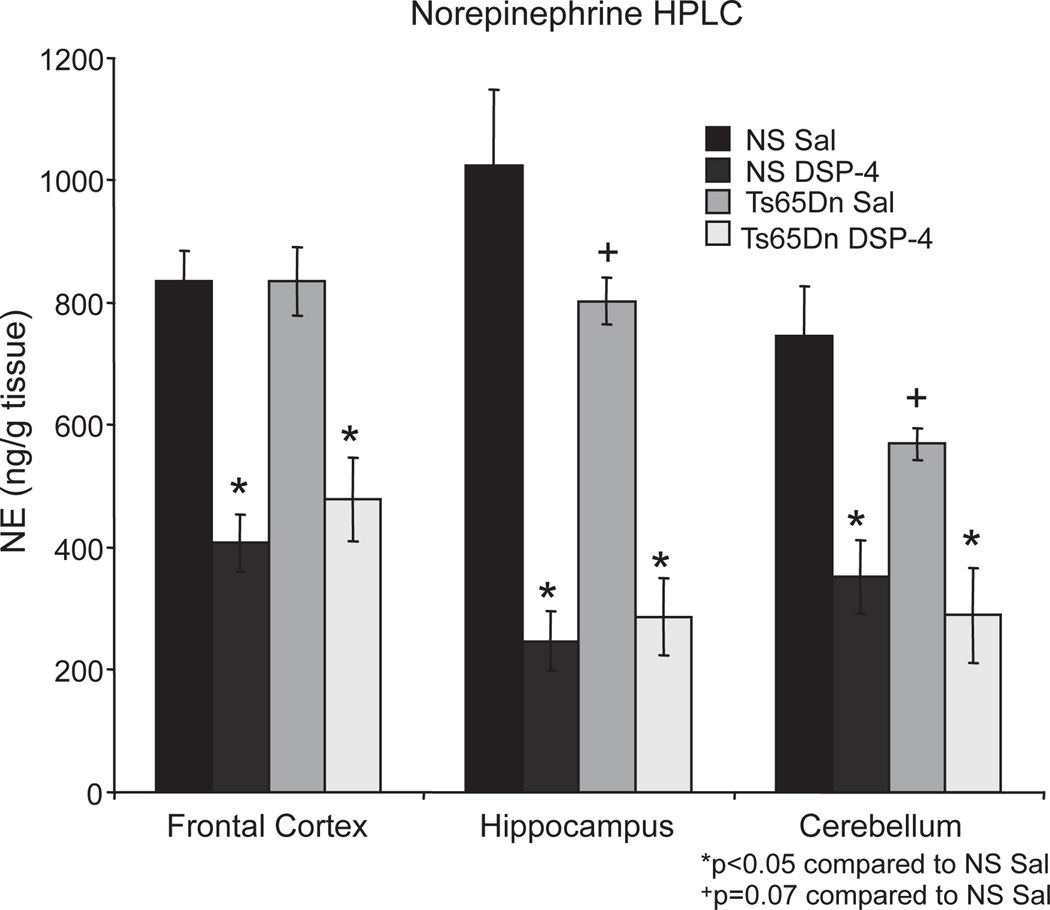
Following testing, the mice were sacrificed and NE levels in the frontal cortex, hippocampus, and cerebellum were assessed using high performance liquid chromatography with electrochemical detection (HPLC-EC), according to our standard protocols [28]. At eight-months of age, DSP-4-treated mice regardless of genotype exhibited significant reductions in NE levels throughout the brain (Fig. 4). NS and Ts65Dn mice that received DSP-4 treatment (NS DSP-4 and Ts65Dn DSP-4, respectively) showed 50% reductions in the frontal cortex and cerebellum [FC: F(3,23) = 19.4; p < 0.001; cerebellum: F(3,23) = 9.911; p < 0.001, respectively], while hippocampal (Hpc) NE levels were reduced by nearly 80% [Hpc: F(3,23) = 21.8; p < 0.001] compared to their saline-treated controls (NS Sal and Ts65Dn Sal). Interestingly, at eight months-of-age there was a 20% reduction in NE levels in Ts65Dn Sal mice in the hippocampus relative to their age-matched controls, though this reduction failed to reach significance (Fisher’s post-hoc: p > 0.05). These reductions are consistent with our findings regarding LC cell degeneration occurring at 12 months-of-age in Ts65Dn mice (Fig. 1) as well as with documented reductions in hippocampal NE levels in Ts65Dn mice by 18 months-of-age [19], and these changes may indicate early NE dysregulation. Studies have indicated that DSP-4 may sometimes have effects on serotonergic terminals in rodents [38]. However, HPLC-EC analyses revealed no significant reductions in serotonin levels in the frontal cortex, hippocampus, or cerebellum in DSP-4-treated animals in the present study [FC: F(3,23) = 0.871; p > 0.05, Hpc: F(3,23) = 0.386; p > 0.05; cerebellum:F(3,23) = 0.257; p > 0.05], nor were any changes observed in dopamine, DOPAC or 5-hydroxyindoleacetic acid levels (data not shown).
Fig. 4.
DSP-4 administration resulted in a long-term NE depletion. HPLC analysis of monamines showed reduced NE levels in DSP-4 treated mice regardless of genotype, with 50% reductions in the frontal cortex and cerebellum and a 75% loss in the hippocampus. DSP-4 did not alter levels of other monoamines, such as serotonin (5-HT), dopamine, or their metabolites (data not shown).
Chronic LC degeneration following DSP-4 administration
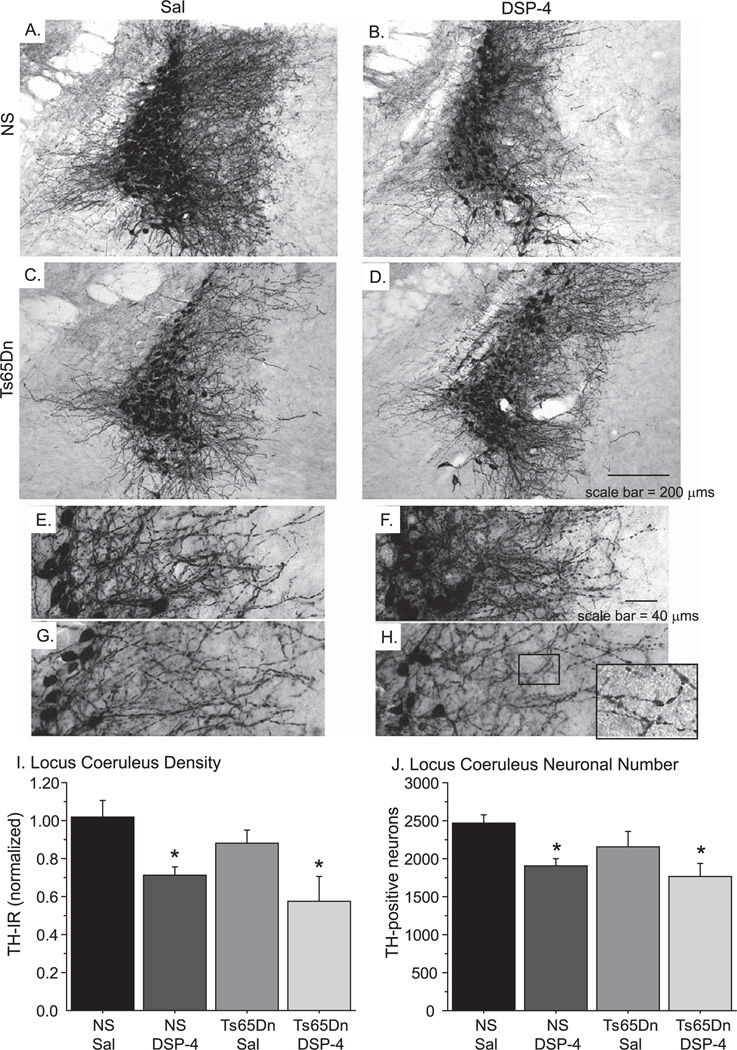
We also examined the effects of DSP-4 treatment on TH immunoreactive cell bodies in the LC. Histology of the LC indicated that DSP-4 had long-lasting effects on both TH-positive neuronal bodies and axons in the LC (Fig. 5). While the LC of NS Sal mice presented robust TH-positive neuronal and neurite staining (Fig. 5A, E), NS DSP-4 mice exhibited significant reductions in TH immunoreactivity throughout the LC (Fig. 5B, F). These reductions were also apparent between Ts65Dn Sal (Fig. 5C, G) and Ts65Dn DSP-4 (Fig. 5D, H) mice. High magnification micrographs are shown in Fig. 5E–H to demonstrate the difference in neurite density, as well as neuritic degeneration observed in Ts65Dn mice with DSP-4 lesions (inset in Fig. 5H). In order to examine effects of the neurotoxin on neurites in the LC region, densitometric measurements were utilized and showed 30–40% reductions in overall TH-immunoreactivity in the LC in DSP-4-treated animals [Fig. 5I, F(3,23) = 5.365; p < 0.01], with a significant reduction in the density of TH-immunoreactive neurites as well as cell body shrinkage observed in the Ts65Dn DSP-4 group (Fig. 5D, H). An overall ANOVA analysis revealed that significant group differences existed for unbiased stereologic cell counts of LC TH-immunoreactivity neurons [Fig. 5J, F(3,20) = 4.721, p < 0.05]. Fisher’s post-hoc analysis confirmed that NS DSP-4 and Ts65Dn DSP-4 groups each exhibited a significant 20–25% reduction in TH-positive neurons compared to NS Sal mice (post-hoc: p < 0.01 and p < 0.01, respectively).
Fig. 5.
DSP-4 administration induced LC degeneration. A–H) TH immunostaining of NS Sal (A, E) and Ts65Dn Sal (C, G) mice revealed that saline did not disrupt noradrenergic neurons of the LC, while NS DSP-4 (B, F) and Ts65Dn DSP-4 (D, H) mice showed significant reductions in TH-positive nerve fibers and LC neurons. E–H represents a higher magnification of images shown in A–D, and the inset in H shows axonal swellings in the Ts65Dn DSP-4 group, representing neurite degeneration frequently observed in this group. I, J) Densitometry confirmed that TH-immunoreactivity was significantly reduced following DSP-4 injection (I), while stereologic cell counts showed a 20% loss in LC neurons (J; mean ± SEM; *p < 0.05).
DSP-4 lesions led to elevated neuroinflammation in Ts65Dn mice
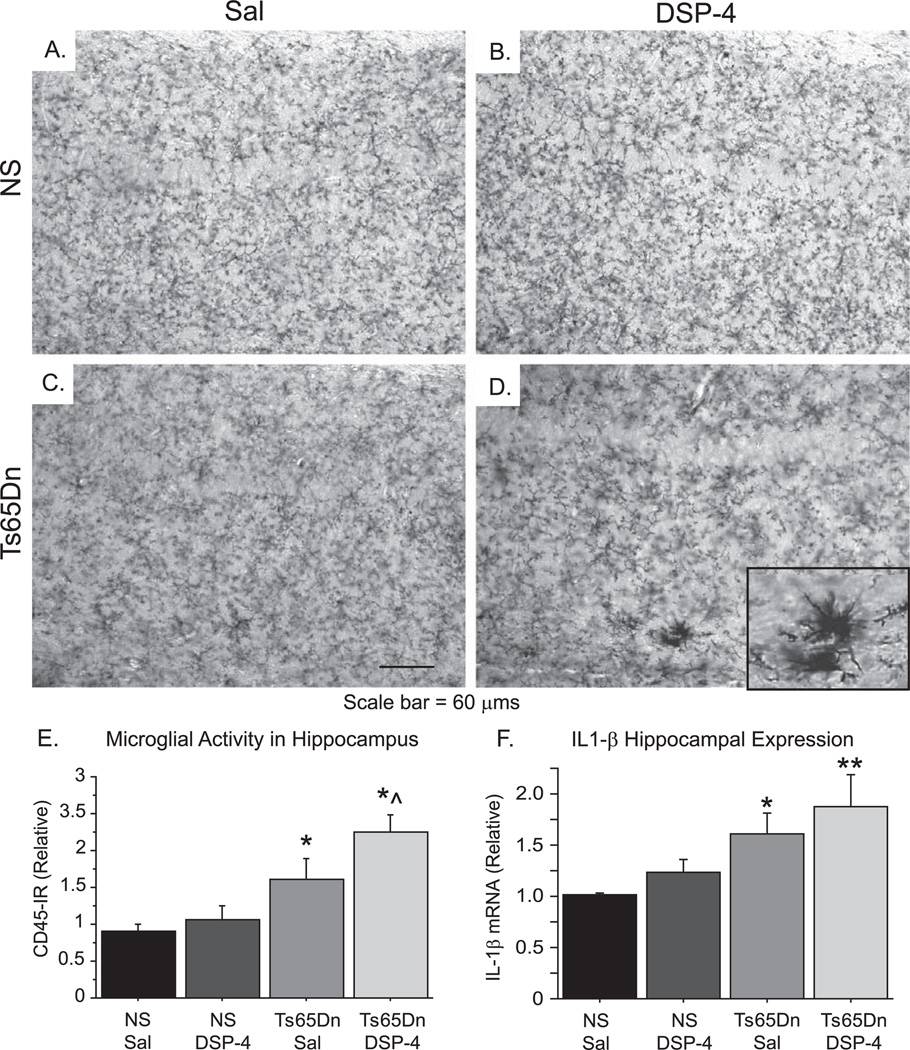
NE has been shown to act on microglia to modulate activation and neuroinflammatory signaling [7]. We examined microglial pathology in the hippocampus using the pan-microglial marker CD45 (Fig. 6). DSP-4 appeared to have little effect on inflammatory morphology in normosomic mice, as the hippocampus of both NS Sal and NS DSP-4 mice (Fig. 6A and B, respectively) showed microglia primarily in the “resting” state, with thin, weakly stained processes and undefined cell bodies. The hippocampus of Ts65Dn Sal mice exhibited increased microglial activation compared to NS Sal mice (Fig. 6C), with several microglia showing thickened processes and notable cell bodies, and the Ts65Dn DSP-4 hippocampus had even more robust inflammatory staining, greatly enlarged microglia, and the formation of microglial aggregates, particularly in the CA1 region of the hippocampus (Fig. 6D, see insert). An overall ANOVA of CD45-ir density in the hippocampus of the different groups revealed a significant overall group effect [Fig. 6E, F(3,18) = 7.289; p < 0.01]. Follow-up analysis using Fisher’s post-hoc analysis confirmed a significant increase in density of the CD45-immunoreactivity in Ts65Dn DSP-4 mice compared to NS Sal (p < 0.001), as well as compared to Ts65Dn Sal mice (p < 0.05). These data suggest that disrupting NE input to the hippocampus potentiates inflammatory morphology in Ts65Dn mice.
Fig. 6.
Microglial activation was increased in Ts65Dn mice treated with DSP-4. A–D) Microglial immunostaining in the hippocampus with CD45, a pan-microglial marker, showed that NE depletion in NS mice did not induce enhanced microglial activity (NS Sal, A; NS DSP-4, B), while alterations in microglial morphology in Ts65Dn Sal mice (C) were aggravated by DSP-4 (Ts65Dn DSP-4, D). E) Densitometry revealed a significant increase in microglial immunoreactivity in the hippocampus of Ts65Dn DSP-4 mice compared to Ts65Dn Sal mice (mean ± SEM; *p < 0.05 and **p < 0.01 compared to NS Sal; ^p < 0.05 compared to Ts65Dn Sal). F) IL-1β mRNA analysis by quantitative RT-PCR in the hippocampus showed a step-wise increase in the levels of this pro-inflammatory cytokine in Ts65Dn treatment groups with or without DSP-4 lesions.
Gene expression alterations in NE depleted mice
NE signaling has previously been shown to alter gene expression, so we assessed markers of inflammatory and neurotrophic gene expression in the hippocampus. Expression of IL-1β mRNA, an important pro-inflammatory cytokine that can be modulated by NE signaling, exhibited significant group differences [Fig. 6F; F(3,13) = 3.861; p < 0.05], and Fisher’s post-hoc analysis revealed that both Ts65Dn Sal and Ts65Dn DSP-4 mice exhibited significant elevations in hippocampal IL-1β mRNA expression compared to NS Sal mice (p < 0.05 and p < 0.01, respectively). The elevated IL-1β mRNA expression following DSP-4 lesions in Ts65Dn mice, but not in the NS mice, provides additional proof that neuroinflammation may be triggered in the hippocampus as a result of the NE denervation in Ts65Dn this Down syndrome mouse model. We also evaluated mRNA expression of brain-derived neurotrophic factor (BDNF), a neurotrophin heavily regulated by NE input into the hippocampus, as demonstrated by us and others [40]. BDNF mRNA expression (Fig. 7E) in the hippocampus showed the opposite group-wise trend [F(3,19) = 3.088; p < 0.05], with Fisher’s post-hoc anaylsis demonstrating that Ts65Dn Sal mice and Ts65Dn DSP-4 mice each exhibiting significant reductions (p < 0.05 and p < 0.01, respectively) compared with the NS Sal treatment group, suggesting a relationship between the neuropathology caused by the segmental trisomy, NE denervation, and expression of growth factors in the NE target region, the hippocampus. Since the expression of Cal 28k is dependent on BDNF, the reduction in BDNF levels may provide a substrate for the continuous loss of Cal 28k expression in Ts65Dn mice with or without NE denervation.
Fig. 7.
Cal 28k was affected by DSP-4 administration in Ts65Dn mice. A–C) Cal 28k immunostaining of NS Sal (A), Ts65Dn Sal (B), and Ts65Dn DSP-4 (C) showed that the distribution of Cal 28k was altered in the CA1 region of Ts65Dn DSP-mice, with greater loss in pyramidal neurons (arrowhead) than in interneurons of stratum oriens (arrow). D) Densitometry confirmed decreased Cal 28k-IR in the Ts65Dn DSP-4 group (mean ± SEM, *p < 0.05 relative to NS Sal). E) Hippocampal gene expression of BDNF mRNA was reduced in the Ts65Dn saline and DSP-4 groups compared to NS groups (mean ± SEM; *p < 0.05).
Cal 28k morphology is altered following NE terminal destruction
We also evaluated Cal 28k morphology in the hippocampus following NE depletion. As was seen in Ts65Dn mice at twelve months of age (Fig. 2F), the hippocampus of Ts65Dn Sal mice at eight months of age exhibited reduced Cal 28k-IR in the soma and apical dendrites of pyramidal neurons (Fig. 7B) compared to NS Sal mice (Fig. 7A). While there were no observable differences in Cal 28k morphology between NS Sal and NS DSP-4, Ts65Dn DSP-4 mice exhibited a more pronounced reduction of Cal 28k-IR in pyramidal neurons (Fig. 7C) relative to other groups, only showing remaining staining of interneurons in the stratum oriens. Densitometric analysis using an overall ANOVA confirmed alterations in total Cal 28k staining in the CA1 [Fig. 7D; F(3,20) = 4.650; p < 0.01, as Cal 28k levels in Ts65Dn DSP-4 mice were significantly reduced relative to NS Sal mice (Fisher’s posthoc, p < 0.01). The changes apparent in Cal 28k in Ts65Dn DSP-4 shared similarities with the morphology of aged Ts65Dn mice (Fig. 2H), indicating that the DSP-4 induced NE denervation may accelerate age-related neuropathology occurring in Ts65Dn mice.
NE Depletion potentiates cholinergic degeneration in Ts65Dn mice
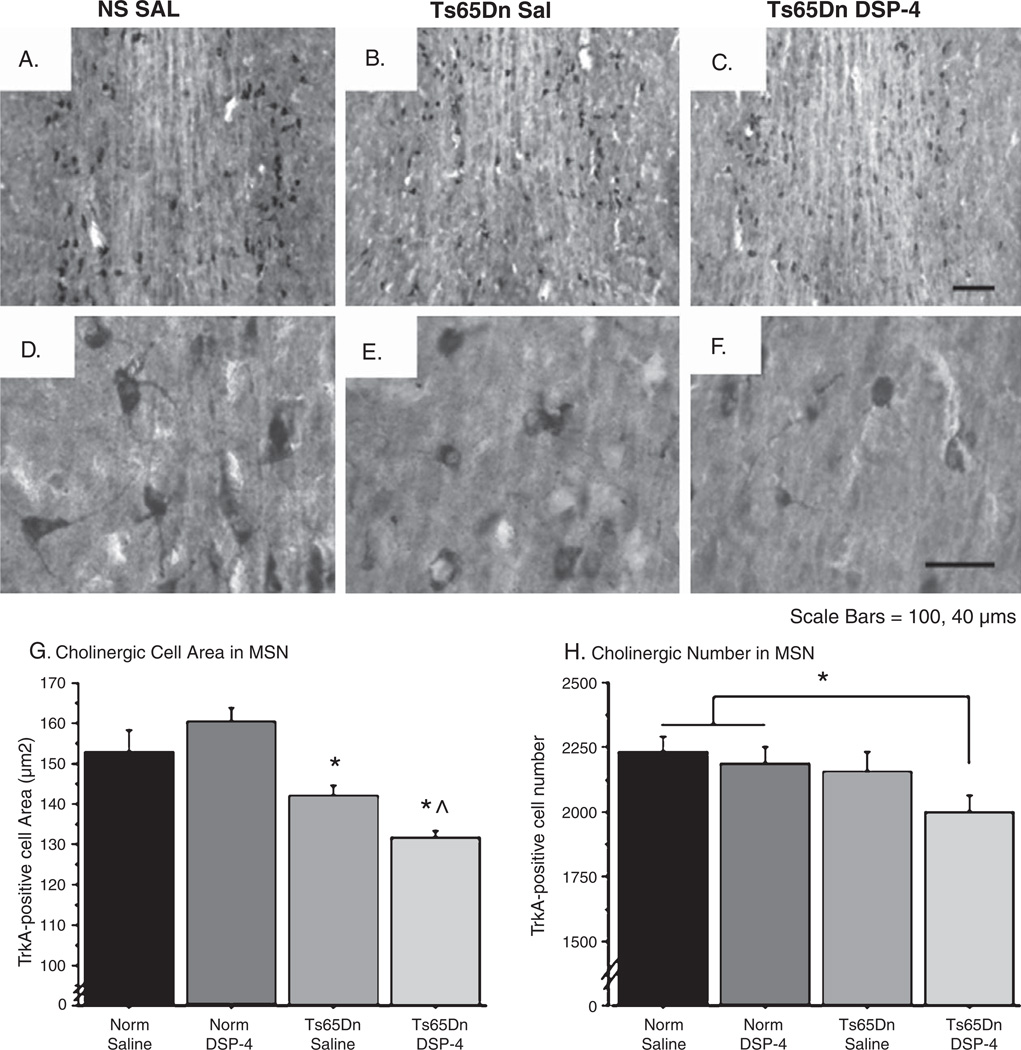
Ts65Dn mice exhibit age-associated degeneration of BFCNs, with significant reductions in neuronal number by 10–12 months of age (see Discussion). We next determined whether noradrenergic depletion could exacerbate degenerative pathology in these mice. Immunohistochemistry of the high-affinity NGF receptor TrkA, a reliable marker of BFCNs, demonstrated robust neuronal staining throughout the medial septal nucleus (MSN) in NS controls (Fig. 8A). Ts65Dn Sal mice exhibited no observable reduction in neuronal number at this age (Fig. 8B), though on close examination demonstrated altered neuronal morphology; reduced somal staining density, fewer observable processes, and atrophic cell bodies (Fig. 8E) in BFCNs when compared to NS Sal (8D). There were no observable morphological differences between NS Sal and NS DSP-4 mice, as evidenced by both cell counts and cell size measurements (Fig. 8G and H). In contrast, Ts65Dn DSP-4 mice contained fewer TrkA-positive neurons in the MSN (Fig. 8C), and the degenerative alterations evident in Ts65Dn Sal were exaggerated following the DSP-4 lesion, since mostly atrophic cell bodies without observable neurites were present in this group (Fig. 8F). Neuronal area measurements using the Nucleator program (Microbrightfield, MBF Bioscience, Stereologer software, Williston, VT) and an overall one-way ANOVA analysis revealed significant group differences [Fig. 8G, F(3,17) = 14.408; p < 0.001]. Consistent with previous studies [15], Fisher’s post-hoc analysis confirmed that Ts65Dn Sal mice had significantly smaller neurons than NS Sal mice (p < 0.05), and DSP-4-treated Ts65Dn mice exhibited further cell body atrophy, with a 15% reduction compared with NS groups, regardless of treatment, and significantly smaller average cell body size compared to the Ts65Dn Sal mice (p < 0.05). BFCN atrophy is an important measure of neuronal dysfunction, especially for BFCN neurons, has been shown in response to retrograde degeneration [41], and indicates phenotypic impairments while proceeding actual cell death. One-way ANOVA analysis of unbiased stereological cell counts of the MSN revealed no significant neuronal losses between groups [Fig. 8H; F(3,17) = 2.268; p > 0.1], though a 10% reduction in BFCNs in Ts65Dn DSP-4 was observed. As NS Sal and NS DSP-4 mice exhibited no statistical differences, these data were collapsed in subsequent analyses, revealing significant overall group differences when NS groups were compared with Ts65Dn saline or DSP-4 groups [F(2,18) = 3.322; p < 0.05]. Furthermore, when comparing Ts65Dn DSP-4 mice with both NS groups, Ts65Dn DSP-4 mice had significantly fewer MSN TrkA-positive neurons [Fisher’s post-hoc: p < 0.01]. These data suggest that the DSP-4-induced NE denervation, along with the resultant increase in neuroinflammation, potentiates the neurodegeneration of the cholinergic system in Ts65Dn mice.
Fig. 8.
Reduced cell size and number of BFCNs in Ts65Dn mice after DSP-4 administration. A–F) TrkA-positive cholinergic neurons in the MSN are shown in NS Sal (A, D), Ts65Dn Sal (B, E), and Ts65Dn DSP-4 (C, F) at 20× and 40×, respectively. Cell body atrophy was observed in Trk-A I.R. neurons in Ts65Dn mice (E), which was further exacerbated by the DSP-4 lesion (F). Also note that TrkA-I.R. cells in both Ts65Dn gropus exhibit fewer processes and less robust labeling with the TrkA antibody overall. G) Cell size measurements confirmed that Ts65Dn DSP-4 average cell area was significantly lower than in all other groups, including Ts65Dn Sal mice. H) Stereology of TrkA-positive neurons in the MSN depicted a significant reduction in the number of neurons in Ts65Dn DSP-4 mice compared to NS mice (mean ± SEM, *p < 0.05 relative to NS Sal, ^p < 0.05 relative to Ts65Dn Sal).
Correlation studies
In order to determine the relationship between NE denervation, memory performance, and hippocampal and basal forebrain markers, correlation studies were undertaken. The Pearson correlation coefficient, which is mainly sensitive to a linear relationship between two variables, was employed. As expected, there was a strong correlation between the loss of LC neurons in DSP-4 treated mice of both genotypes and NE levels in the hippocampus (r = 0.536; p < 0.01). We also found significant correlations between TH-positive cell counts as well as TH-immunoreactivity in the LC and CD45 expression in the hippocampus (r = −0.523; p < 0.05), suggesting that the degree of microglial activation in the hippocampus may be dependent upon the degree of LC innervation. Similar to previous findings from our group, the radial arm maze errors (Day 2) negatively correlated with BFCN cell loss (r = −0.534; p < 0.05), and BFCN cell loss also correlated negatively with CD45 expression in the hippocampus (r = −0.523; p < 0.05), indicating that microglial activation and BFCN loss may be related. In addition, the BFCN cell size and NE levels in the prefrontal cortex were significantly correlated (p < 0.01), suggesting that the DSP-4 lesion directly affected the well-being of cholinergic neurons. Finally, there was a negative correlation between Cal 28k immunodensity in the hippocampus and Day 2 (r = −0.604; p < 0.01) and Day 3 errors (r = −0.473; p < 0.05), suggesting that hippocampal Cal 28k levels may affect the behavioral component of our studies. When each of the four groups was examined for correlations separately, some interesting patterns emerged. The NS saline group demonstrated few correlative significant measures (only one correlation was significant; BFCN cell counts versus Cal 28k density in the hippocampus (r = 0.996; p < 0.001)). The Ts65Dn saline group exhibited several interesting relationships, namely a significant correlation between hippocampal NE levels and BFCN cell size (r = −0.829; p < 0.05), hippocampal NE levels and Cal 28k density (r = 0.826; p < 0.05), TH staining density and day 2 errors (r = 0.847; p < 0.05), as well as TH stereological cell counts in the LC and hippocampal CD45 density (r = −0.831; p < 0.05). Collectively, these data again suggest that Ts65Dn mice are more sensitive to fluctuations in NE levels and LC-NE neuron degeneration than NS mice are. In the DSP-4 lesioned groups, BFCN stereological cell counts correlated significantly with TH density in the hippocampus in the NS-DSP-4 group (r = 0.942; p < 0.01), and LC TH cell counts in the Ts65Dn-DSP-4 group (r = −0.934; p < 0.05), suggesting a strong correlation between the NE lesion and cholinergic atrophy and cell loss in the basal forebrain. Although correlations can suggest possible mechanistic relationships, statistical dependence is not sufficient to demonstrate the presence of such a relationship. Further studies will be needed to fully determine the role of NE innervation upon hippocampal inflammatory markers, BFCN cell loss, and memory deterioration in Ts65Dn mice.
DISCUSSION
The current study provides further evidence that the noradrenergic system degenerates in Ts65Dn mice with age, and that alterations to this system may contribute to the memory loss and neuropathology that develops in this mouse model. Histological analysis of the LC showed a significant reduction in the number of LC TH-positive neurons in Ts65Dn mice with age compared to NS mice, and depletion of NE levels in Ts65Dn mice via DSP-4 resulted in aggravated memory dysfunction in a spatial navigation task, particularly in terms of retention between days of testing. Morphological assessment demonstrated that reduced NE levels and a corresponding loss in LC NE neurons significantly correlated with resultant increased levels of neuroinflammation in the hippocampus. In addition, NE depletion led to enhanced degeneration of BFCNs and hippocampal Cal 28k-positive neurons, suggesting that NE loss might be involved in the observed neuropathology in Ts65Dn mice.
Ts65Dn mice showed alterations in LC morphology at an early age, with significant cell losses at 12 months of age. These findings are consistent with previous studies that show that LC NE neuronal loss occurs after 3 months-of-age in Ts65Dn mice [19]. These LC NE changes in Ts65Dn mice are interesting due to the lack of LC pathology noted in some other rodent models of AD. LC neuronal loss has been noted in aged female mice doubly transgenic for AβPP and PS1 mutations (dtg AβPP/PS1) [42], but not in mice containing AβPP or PS1 transgenes alone [43]. Ts65Dn mice exhibit elevated AβPP and amyloid-β levels [44, 45], but several other genes triplicated may contribute to LC damage. In particular, the overexpression of Cu/Zn superoxide dismutase (SOD-1) leads to increased oxidative stress [46]. Noradrenergic neurons are highly sensitive to oxidative stress, and oxidative stress levels are increased in DS individuals [47] and in Ts65Dn mice [30], proposing a possible mechanism for the LC loss. Similar to our findings in Ts65Dn mice, the rostral neurons of the LC exhibit selective degeneration in AD and DS [34], suggesting that LC dysfunction may originate via retrograde degeneration from damage to noradrenergic terminals in the hippocampus and the cortex. However, this mechanism will have to be explored in future studies. We found that NE levels in the hippocampus of Ts65Dn mice at eight months of age were 20% lower compared to NS Sal mice, supporting evidence showing LC NE degeneration as early as 6 months in Ts65Dn mice [19]. Therefore, Ts65Dn mice may prove to be a useful tool to examine the neurotransmitter alterations that occur during early AD.
NE is important for attention and arousal [48]. However, NE depletion by DSP-4 results in few memory deficits in healthy animals, but when DSP-4 is given in conjunction with cholinergic toxicity, memory deficits show further impairments [5]. We found that Ts65Dn DSP-4 mice had significantly more errors than Ts65Dn Sal mice, and exhibited no significant improvement across days of testing, suggesting that damage to the noradrenergic system exacerbated memory deficits in Ts65Dn mice. Even though DSP-4 rarely causes memory deficits in intact animals, impairments in long-term retention have been noted [49]. While the study in question evaluated much longer time intervals (16 days), the pattern of normal acquisition but poor recollection may be similar to the DSP-4 induced “overnight forgetting” that was observed in the present study. Overnight retention is often considered an indication of memory consolidation [50], so the detrimental effects of DSP-4 may indicate that NE depletion disrupted these processes.
The disruptive effects on memory seen in Ts65Dn mice treated with DSP-4 may result in part through disruption of cholinergic signaling. This was supported by concurrent exacerbation of BFCN neuronal atrophy in Ts65Dn mice treated with DSP-4 in the current study, and a significant correlation between both hippocampal NE levels and LC cell counts and BFCN atrophy and cell loss in Ts65Dn DSP-4 lesioned mice. Ts65Dn mice exhibit reductions in BFCN size at four months of age [15, 16], and neuronal loss by 12 months of age [13, 14]. Previous studies in AβPP23 mice have indicated that NE depletion reduces the number of cholinergic terminals [22]. The data presented here supports this conclusion, and further suggests that the loss of LC neurons not only can influence cholinergic signaling, but can contribute to degeneration of cholinergic neurons, providing a possible mechanism for the progressive nature of LC-NE and BFCN neuronal loss in AD and DS in humans.
Due to the modulatory role NE plays in proinflammatory cytokine and neurotrophic factor expression, it is postulated that the loss of LC neurons in AD may sensitize forebrain neurons to degenerative changes [2]. Previous work evaluating the effects of DSP-4 in AβPP transgenic mice have shown similar effects, including increased glial activation and enhanced deposition of amyloid plaques [9, 22, 51]. In particular, DSP-4 treated mice had potentiated expression of cytokines such as IL-1β following amyloid-β injection [8]. IL-1β levels in the temporal lobe are elevated prior to AD-like pathology in DS [52], proposing a central role for this pro-inflammatory cytokine in propelling neuropathological pathways. Ts65Dn saline-treated mice expressed higher levels of IL-1β than NS saline-treated mice, suggesting that microglial activation and IL-1β overproduction may be involved in the neuropathology observed here, as has been suggested for other models and for DS and AD in humans. Since Ts65Dn mice do not exhibit amyloid plaques, contrary to transgenic AD models, the focus in the current manuscript was on LC NE effects on cholinergic and hippocampal neuronal integrity in this mouse model, and not on production of amyloid per se. Since all the tissue was utilized for analysis of pro-inflammatory cytokines and growth factors (Figs 6 and 7), we did not have sufficient tissue to carry out amyloid or AβPP studies, which will be the focus of future studies.
Cal 28k, a calcium binding protein involved in neuronal excitability [53], undergoes reduced expression in the basal forebrain and hippocampus both with normal aging and AD [54]. Our laboratory has previously shown significant reduction in Cal 28k expression in the hippocampus of Ts65Dn mice at 10 months of age, alleviated with anti-inflammatory or antioxidant treatment [13, 30]. Reduced Cal 28k herein was observed with normal aging, but also following DSP-4 treatment in Ts65Dn mice, and correlations suggested that NE depletion accelerated loss of hippocampal Cal 28k expression in Ts65Dn mice. The Cal 28k IR loss in Ts65Dn mice may be linked to increased neuroinflammation observed in the hippocampus of Ts65Dn DSP-4 mice, since we found significant correlations between CD45 immunoreactivity and Cal 28k immunostaining in the hippocampus, providing an interesting approach for continued studies.
NE neurons also support the production of neurotrophic factors, especially BDNF. Here, we show reduced BDNF expression in the hippocampus of Ts65Dn mice. Our laboratory has previously shown that BDNF protein levels in the frontal cortex of Ts65Dn mice correlates with memory function [55], indicating the potential importance of BDNF for the behavioral paradigms tested here. BDNF is produced in LC neurons and undergoes anterograde and retrograde transport [56, 57]. While LC neurons require BDNF, they also provide BDNF to other brain regions [58, 59], and DSP-4 administration blunts BDNF expression following stimulation [60]. Together, these studies combined with our findings suggest that the reduced levels of BDNF observed with aging and in AD and Parkinson’s disease [61] may, at least in part, stem from disruptions in noradrenergic signaling. The decreased BDNF expression in Ts65Dn mice may contribute to neuropathology observed after DSP-4 lesions, since BFCNs and Cal 28k neurons both depend on BDNF for survival and function [62]. Recent studies support this, as BDNF blockade prevents the protective effect of NE on neurons following amyloid-β-induced toxicity in vitro [63]. Together, these studies suggest that BDNF provides necessary neurotrophic support for susceptible neuronal populations in AD and DS, and that the DSP-4 lesion reduced BDNF production, particularly in Ts65Dn mice.
Combined with other recent studies, which found that treatment with the NE precursor L-threo-DOPS prevents neuroinflammation brought on by DSP-4 in an AβPP transgenic mouse model [64] and facilitates contextual memory in Ts65Dn mice [19], there is strong evidence for LC degeneration driving neuroinflammatory changes and contributing to cognitive deficits. Future studies should evaluate whether pharmacologic modulation of NE, such as with α-2 antagonists, can attenuate the degenerative changes seen in DS, using the Ts65Dn mice as an exploratory pharmacological model. The data in this study provide further evidence for the usefulness of the Ts65Dn mouse as a model for neuronal degeneration in DS and AD brains, and supports the hypothesis that impaired NE signaling contributes to neurodegenerative changes in AD.
ACKNOWLEDGMENTS
This work was made possible by a grant from the National Institutes on Aging (AG012122). The authors would like to thank Ms. Claudia Umphlet and Mr. Alfred Moore for expert technical assistance.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=645).
REFERENCES
- 1.Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 2.Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, Hauser DL. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–262. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Abe K, Horiuchi M, Yoshimura K. Potentiation by DSP-4 of EEG slowing and memory impairment in basal forebrain-lesioned rats. Eur J Pharmacol. 1997;321:149–155. doi: 10.1016/s0014-2999(96)00934-x. [DOI] [PubMed] [Google Scholar]
- 6.Chopin P, Colpaert FC, Marien M. Effects of acute and subchronic administration of dexefaroxan, an alpha(2)-adrenoceptor antagonist, on memory performance in young adult and aged rodents. J Pharmacol Exp Ther. 2002;301:187–196. doi: 10.1124/jpet.301.1.187. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 8.Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinin S, Gavrilyuk V, Polak PE, Vasser R, Zhao J, Heneka MT, Feinstein DL. Noradrenaline deficiency in brain increases beta-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol Aging. 2007;28:1206–1214. doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Galdzicki Z, Siarey RJ. Understanding mental retardation in Down’s syndrome using trisomy 16 mouse models. Genes Brain Behav. 2003;2:167–178. doi: 10.1034/j.1601-183x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 11.Hunter CL, Isacson O, Nelson M, Bimonte-Nelson H, Seo H, Lin L, Ford K, Kindy MS, Granholm AC. Regional alterations in amyloid precursor protein and nerve growth factor across age in a mouse model of Down’s syndrome. Neurosci Res. 2003;45:437–445. doi: 10.1016/s0168-0102(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 12.Contestabile A, Fila T, Bartesaghi R, Contestabile A, Ciani E. Choline acetyltransferase activity at different ages in brain of Ts65Dn mice, an animal model for Down’s syndrome and related neurodegenerative diseases. J Neurochem. 2006;97:515–526. doi: 10.1111/j.1471-4159.2006.03769.x. [DOI] [PubMed] [Google Scholar]
- 13.Hunter CL, Bachman D, Granholm AC. Minocycline prevents cholinergic loss in a mouse model of Down’s syndrome. Ann Neurol. 2004;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci U S A. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- 16.Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 17.Nelson L, Johnson JK, Freedman M, Lott I, Groot J, Chang M, Milgram NW, Head E. Learning and memory as a function of age in Down syndrome: a study using animal-based tasks. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:443–453. doi: 10.1016/j.pnpbp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Dierssen M, Vallina IF, Baamonde C, Garcia-Calatayud S, Lumbreras MA, Florez J. Alterations of central noradrenergic transmission in Ts65Dn mouse, a model for Down syndrome. Brain Res. 1997;749:238–244. doi: 10.1016/s0006-8993(96)01173-0. [DOI] [PubMed] [Google Scholar]
- 19.Salehi A, Faizi M, Colas D, Valletta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner SL, Aisen P, Shamloo M, Mobley WC. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med. 2009;1 doi: 10.1126/scitranslmed.3000258. 7ra17. [DOI] [PubMed] [Google Scholar]
- 20.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 21.Dudley MW, Howard BD, Cho AK. The interaction of the beta-haloethyl benzylamines, xylamine, and DSP-4 with catecholaminergic neurons. Annu Rev Pharmacol Toxicol. 1990;30:387–403. doi: 10.1146/annurev.pa.30.040190.002131. [DOI] [PubMed] [Google Scholar]
- 22.Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss WD, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A, Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- 24.Costa AC, Walsh K, Davisson MT. Motor dysfunction in a mouse model for Down syndrome. Physiol Behav. 1999;68:211–220. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stasko MR, Costa AC. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- 29.Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockrow J, Prakasam A, Huang P, Bimonte-Nelson H, Sambamurti K, Granholm AC. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Exp Neurol. 2009;216:278–289. doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 32.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The newstereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 33.Casanova MF, Walker LC, Whitehouse PJ, Price DL. Abnormalities of the nucleus basalis in Down’s syndrome. Ann Neurol. 1985;18:310–313. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- 34.German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- 35.Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, Pareyson D. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61:631–636. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- 36.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- 38.Fornai F, Bassi L, Torracca MT, Alessandri MG, Scalori V, Corsini GU. Region- and neurotransmitter-dependent species and strain differences in DSP-4-induced monoamine depletion in rodents. Neurodegeneration. 1996;5:241–249. doi: 10.1006/neur.1996.0032. [DOI] [PubMed] [Google Scholar]
- 39.Crawley JN. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. 2nd edition. Hoboken, NJ: John Wiley & Sons, Inc.; 2007. [Google Scholar]
- 40.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofroniew MV, Pearson RC, Eckenstein F, Cuello AC, Powell TP. Retrograde changes in cholinergic neurons in the basal forebrain of the rat following cortical damage. Brain Res. 1983;289:370–374. doi: 10.1016/0006-8993(83)90045-8. [DOI] [PubMed] [Google Scholar]
- 42.O’Neil JN, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, Manaye KF. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34:102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szapacs ME, Numis AL, Andrews AM. Late onset loss of hippocampal 5-HT and NE is accompanied by increases in BDNF protein expression in mice co-expressing mutant APP and PS1. Neurobiol Dis. 2004;16:572–580. doi: 10.1016/j.nbd.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer’s disease in Ts65Dn mice: effects of estrogen. Neurobiol Aging. 2004;25:873–884. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Bar-Peled O, Korkotian E, Segal M, Groner Y. Constitutive overexpression of Cu/Zn superoxide dismutase exacerbates kainic acid-induced apoptosis of transgenic- Cu/Zn superoxide dismutase neurons. Proc Natl Acad Sci U S A. 1996;93:8530–8535. doi: 10.1073/pnas.93.16.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallardo FV, Degan P, d’Ischia M, Kelly FJ, Zatterale A, Calzone R, Castello G, Fernandez-Delgado R, Dunster C, Lloret A, Manini P, Pisanti MA, Vuttariello E, Pagano G. Multiple evidence for an early age pro-oxidant state in Down Syndrome patients. Biogerontology. 2006;7:211–220. doi: 10.1007/s10522-006-9002-5. [DOI] [PubMed] [Google Scholar]
- 48.Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- 49.Decker MW, McGaugh JL. Effects of concurrent manipulations of cholinergic and noradrenergic function on learning and retention in mice. Brain Res. 1989;477:29–37. doi: 10.1016/0006-8993(89)91391-7. [DOI] [PubMed] [Google Scholar]
- 50.Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Pugh PL, Vidgeon-Hart MP, Ashmeade T, Culbert AA, Seymour Z, Perren MJ, Joyce F, Bate ST, Babin A, Virley DJ, Richardson JC, Upton N, Sunter D. Repeated administration of the noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) modulates neuroinflammation and amyloid plaque load in mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. J Neuroinflammation. 2007;4:8. doi: 10.1186/1742-2094-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonnenberg JL, Frantz GD, Lee S, Heick A, Chu C, Tobin AJ, Christakos S. Calcium binding protein (calbindin-D28k) and glutamate decarboxylase gene expression after kindling induced seizures. Brain Res Mol Brain Res. 1991;9:179–190. doi: 10.1016/0169-328x(91)90001-e. [DOI] [PubMed] [Google Scholar]
- 54.Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:249–259. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- 55.Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 56.Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobreviela T, Pagcatipunan M, Kroin JS, Mufson EJ. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: relationship to BDNF mRNA expressing neurons. J Comp Neurol. 1996;375:417–444. doi: 10.1002/(SICI)1096-9861(19961118)375:3<417::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 58.Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA. Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci. 2000;20:274–282. doi: 10.1523/JNEUROSCI.20-01-00274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willis L, Quintero EM, Nelson M, Granholm A. Regulation of trophic factor expression by innervating target regions in intraocular double transplants. Cell Transplant. 2005;14:21–29. [PubMed] [Google Scholar]
- 60.Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119:721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- 61.Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 62.Knusel B, Beck KD, Winslow JW, Rosenthal A, Burton LE, Widmer HR, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The alpha2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology. 2006;31:1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]