Abstract
Background
Little is known about the effect of guidelines that recommend shared decision making on physician practice patterns. The objective of this study was to determine the association between physicians’ perceived effect of guidelines on clinical practice and self-reported prostate-specific antigen (PSA) screening patterns.
Methods
This was a cross-sectional study using a nationally representative sample of 3914 primary care physicians participating in the 1998–1999 Community Tracking Study Physician Survey. Responses to a case vignette that asked physicians what proportion of asymptomatic 60-year-old white men they would screen with a PSA were divided into 3 distinct groups: consistent PSA screeners (screen all), variable screeners (screen 1%–99%), and consistent nonscreeners (screen none). Logistic regression was used to determine the association between PSA screening patterns and physician-reported effect of guidelines (no effect v. any magnitude effect).
Results
Only 27% of physicians were variable PSA screeners; the rest were consistent screeners (60%) and consistent nonscreeners (13%). Only 8% of physicians perceived guidelines to have no effect on their practice. After adjustment for demographic and practice characteristics, variable screeners were more likely to report any magnitude effect of guidelines on their practice when compared with physicians in the other 2 groups (adjusted odds ratio 1.73; 95% confidence interval = 1.25–2.38; P = 0.001).
Conclusions
Physicians who perceive an effect of guidelines on their practice are almost twice as likely to exhibit screening PSA practice variability, whereas physicians who do not perceive an effect of guidelines on their practice are more likely to be consistent PSA screeners or consistent PSA nonscreeners.
Keywords: prostate-specific antigen, mass screening, guidelines, physicians’ practice patterns
Clinical practice guidelines are “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”1 Clinical practice guidelines have been shown to influence practice in settings where the guidelines have clear recommendations for or against a particular intervention or process.2–6 In these settings, clinical guidelines may reduce variation in health care quality and improve equity in health care.
However, the effect of guidelines that advocate shared decision making on physician practice patterns is unknown. Shared decision making is the process by which physicians and patients share information with each other, take steps to participate in the decision-making process, and agree on a course of action.7 Prostate cancer–screening guidelines advocate shared decision making. Prostate cancer is the most common cancer in US men, but the utility of screening for prostate cancer with a prostate-specific antigen (PSA) test is controversial.8 Although there are 2 large randomized clinical trials currently in progress to determine the utility of PSA screening to date,9,10 it is unknown whether screening reduces mortality from prostate cancer. Therefore, beginning in 1996 and 1997, the guidelines from the American Cancer Society,11 American College of Physicians,12 and the US Preventive Services Task Force13 recommended shared decision about PSA screening (see the appendix).
A previous physician focus group study demonstrated that physicians who routinely screen with a PSA were more likely to report that clinical practice guidelines were not a factor in their screening decisions.14 We hypothesized that physicians who report a strong effect of guidelines on clinical practice are more likely to be variable PSA screeners because PSA screening guidelines call for incorporating patient preferences and values in decision making. As considerable time, effort, and resources are devoted to developing and implementing guidelines, knowing the effect of guidelines that promote shared decision making on physician practice patterns has important implications on future efforts to create and implement guidelines.
METHODS
This study was approved by the Institutional Review Board at the University of Pennsylvania. We used cross-sectional survey data from the 1998–1999 (Round Two) Community Tracking Study (CTS) Physician Survey.15,16 The CTS Physician Survey is a biannual longitudinal telephone survey of non–federally employed physicians at 60 sites (51 metropolitan US areas and 9 nonmetropolitan US areas) and of a supplemental national sample of physicians conducted by the Center for Studying Health System Change, which is sponsored by the Robert Wood Johnson Foundation. Data for Round Two were collected just after the concept of shared decision making was introduced in the guidelines in 1996 and 1997 in response to the widespread interest in and rapid uptake of PSA screening for prostate cancer.17
The aim of the CTS Physician Survey is to track changes in the health care system and the effects of these changes on the delivery of care by physicians. Participants of the CTS Physician Survey are physicians who provide direct care to patients at least 20 h per week in an office-based or hospital practice. It excludes residents and fellows. Details of the survey are available at www.hschange.org/index.cgi?data=98. The total number of completed interviews for the 1998–1999 survey was 12,280, for a response rate of 60.9%.
The CTS Physician Survey contains information on physician demographics, medical education, specialty, board certification, practice setting, number of years in practice, practice ownership, practice revenue, source of practice revenue, and provision of charity care. In addition, the survey asks about the perceived effect of clinical practice guidelines on practice. The 1998–1999 round of the CTS Physician Survey also measured PSA screening practice style using a case vignette.
Selection of Study Subjects
Of the 12,280 total responders in the 1998–1999 CTS Physician Survey, 7556 were primary care physicians. For this study, we excluded primary care physicians practicing pediatrics, obstetrics and gynecology, and subspecialties (n = 3642) because they are less likely to provide care for the reference patient described in the case vignette: an adult male patient presenting for prostate cancer screening. The final analytic sample consists of 3914 primary care physicians in family practice, internal medicine, and general practice.
Data Collection
Data for the 60 sites were collected by the Center for Studying Health System Change using stratified random sampling with probability proportional to population size. The supplemental sample was selected with stratified random sampling and was included to increase the precision of the national estimates. The sample frame was developed by combining lists of physicians from the American Medical Association and the American Osteopathic Association. Primary care physicians were oversampled in the site sample. The CTS Physician Survey was conducted using a telephone interview. Use of the data was made available through a restricted data use agreement between the principal investigator and the Inter-university Consortium for Political and Social Research at the University of Michigan.
Dependent Variable
The dependent variable is the physician responses to the PSA screening case vignette, which reads as follows:
What about PSA (Prostate-specific Antigen) screening in an asymptomatic 60 year old white man who has no family history of prostate cancer and a normal digital rectal exam? For what percentage of such patients would you recommend a PSA test? Consider all your patients with similar clinical descriptions.
Responses ranged from 0% to 100%. Responses were collapsed to create 3 categories: consistent screeners, consistent nonscreeners, and variable screeners, to represent those who would screen all (100%), none (0%), and some (1%–99%) of the patients represented in the case vignette, respectively. Each of these 3 variables were dichotomized to compare the level to all other physicians, thereby creating three 0/1 variables.
Independent Variables
The independent variable in this study is the physicians’ perceived effect of guidelines on their practice derived from the following question:
How large an effect does your use of formal, written practice guidelines such as those generated by physician organizations, insurance companies or HMOs [health maintenance organizations] or government agencies, have on your practice of medicine?
Each response was based on a 6-point scale with anchors at no effect and very large effect. For this analysis, we dichotomized the independent variable into no effect (reference) versus any magnitude effect.
Covariates
The multivariate models adjust for physician age; gender; race; Latino ethnicity; practice specialty; board certification status; foreign medical graduate status; practice setting; number of years in practice; salaried status; income in 1997; Medicare, Medicaid, and managed care as a source of practice revenue; and provision of any charity care.
Statistical Analysis
All statistical analyses were conducted using Stata/SE version 8.2.18 Descriptive statistics were used to examine the demographic and practice characteristics of consistent screeners, consistent nonscreeners, and variable screeners and their responses to the case vignette. An unadjusted and a multivariate logistic regression model were estimated for each of the 3 groups of physicians, consistent screeners, consistent nonscreeners, and variable screeners, that compared the perceived effect of guidelines in each of the screening group to the other 2 screening groups, yielding a total of 6 regression models. Multivariate models adjusted for physician and practice characteristics. All logistic regression models were estimated taking into account the CTS Physician Survey’s complex design. Given the fixed sample size for the current study of 3914 primary care physicians who participated in the 1998–1999 CTS Physician Survey and completed the PSA screening vignette, using a 2-sided statistical test, with an α set at .05 and a minimum detectable difference of 10% probability of being a consistent screener among those who declare no effect versus those who declare any effect of guidelines, this study had 93% power.
RESULTS
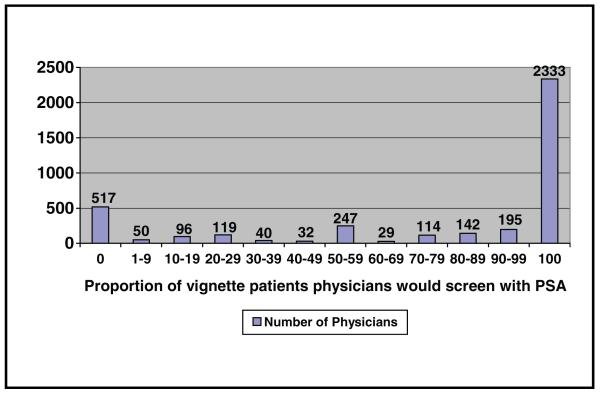
Figure 1 shows the frequency of physician responses to the case vignette. The majority (60%) of physicians reported they would recommend screening to all asymptomatic 60-year-old white men (consistent screeners), whereas only 13% reported they would not recommend screening to any such patients (consistent nonscreeners). The remaining 27% of physicians reported that they would recommend screening to 1% to 99% of such patients (variable screeners).
Figure 1. Distribution of physician responses to case vignette.
Only 319 (8%) of the physicians perceived guidelines to have no effect on practice, whereas the remaining 3591 (92%) physicians perceived at least some effect of the guidelines. Of these, 13% reported a very small, 27% a small, 35% a moderate, 14% a large, and 4% a very large effect of guidelines on practice.
Table 1 presents the physician and practice characteristics of physicians who were classified as consistent nonscreeners, variable screeners, and consistent screeners. P values are presented for the relationship between the 3 categories of screeners and the independent variable and are based on the np trend statistic. Compared with the remainder of physicians, physicians who were variable PSA screeners were least likely to be white (P = 0.003), most likely to practice internal medicine (P = 0.05), and most likely to be solo practioners (P < 0.0001).
Table 1.
Characteristics of Primary Care Physicians Who Are Consistent Nonscreeners, Variable Screeners, and Consistent Prostate-Specific Antigen Screeners
| Characteristic of Physicians | Consistent Nonscreeners (n = 517) |
Variable Screeners (n = 1064) |
Consistent Screeners (n = 2333) |
P Valuea |
|---|---|---|---|---|
| Age, x̄ (s) | 45.2 (9.8) | 46.3 (10.4) | 48.8 (11.1) | < 0.0001 |
| Male, n (%) | 351 (67.9) | 782 (73.5) | 1837 (78.7) | < 0.0001 |
| Race, no. (%) | 0.003 | |||
| White | 377 (72.9) | 764 (71.8) | 1774 (76.0) | |
| Black | 24 (4.6) | 54 (5.1) | 99 (4.2) | |
| Asian | 79 (15.3) | 184 (17.3) | 288 (12.3) | |
| Native | 3 (0.5) | 3 (0.3) | 12 (0.5) | |
| Other | 8 (1.6) | 11 (1.0) | 28 (1.2) | |
| Hispanic/Latino ethnicity, n (%) | 26 (5.0) | 48 (4.5) | 132 (5.7) | 0.30 |
| Specialty, n (%) | 0.05 | |||
| Family practice | 207 (40.0) | 382 (35.9) | 828 (35.5) | |
| Internal medicine | 289 (55.9) | 635 (59.7) | 1378 (59.1) | |
| General practice | 21 (4.6) | 47 (4.4) | 127 (5.4) | |
| Board certified, no. (%) | 450 (87.6) | 863 (81.7) | 1844 (79.7) | < 0.0001 |
| Foreign medical graduate, n (%) | 105 (20.3) | 267 (25.1) | 553 (23.7) | 0.32 |
| Type of practice, n (%) | < 0.0001 | |||
| Solo, 2, or group physician practice | 215 (41.6) | 611 (67.4) | 1481 (63.5) | |
| Hospital or medical school | 48 (9.3) | 74 (7.0) | 166 (7.1) | |
| HMO | 155 (30.0) | 226 (21.2) | 395 (16.9) | |
| Other | 99 (19.4) | 153 (14.4) | 291 (12.5) | |
| No. of years in practice, x̄ (s) | 13.1 (10.2) | 14.1 (10.2) | 17.0 (11.3) | < 0.0001 |
| Salaried, n (%) (n = 2876) | 349 (67.5) | 626 (58.8) | 1172 (50.2) | 0.02 |
| Annual net income in 1997, x̄ (s) | 123,011 (58,567) | 125,561 (58,966) | 139,464 (65,631) | < 0.0001 |
| Source of practice revenue, x̄ (s) | ||||
| Medicare | 33.2 (21.4) | 35.9 (20.7) | 34.9 (21.3) | 0.38 |
| Medicaid | 15.4 (15.3) | 14.9 (15.4) | 10.3 (13.5) | < 0.0001 |
| Managed care | 51.4 (30.1) | 45.1 (27.4) | 48.2 (27.6) | 0.66 |
| No charity care provided in previous month, n (%) | 169 (32.7) | 280 (26.3) | 746 (32.0) | 0.28 |
| How large an effect does your use of formal, written practice guidelines such as those generated by physician organizations, insurance companies or HMOs, or government agencies have on your practice of medicine? n (%) |
||||
| No effect | 38 (7.4) | 65 (6.1) | 216 (9.2) | |
| Very small effect | 55 (10.6) | 120 (11.3) | 315 (13.5) | |
| Small effect | 112 (21.7) | 300 (28.3) | 630 (27.0) | |
| Moderate effect | 216 (41.8) | 390 (36.8) | 757 (32.5) | |
| Large effect | 68 (13.2) | 149 (14.0) | 324 (13.9) | |
| Very large effect | 28 (5.4) | 37 (3.5) | 90 (3.9) |
Note: HMO = health maintenance organization. In some cases, percentages do notaddup to 100% because of rounding.
P values are based on np trend.
Table 2 shows the results of unadjusted and multivariate logistic regression models for the association between the perception of any effect of guidelines on practice and PSA screening pattern. After adjustment for demographic and practice characteristics, physicians who were variable PSA screeners were more likely to report any magnitude effect of guidelines on their practice when compared with physicians in the other 2 groups (adjusted odds ratio [AOR] 1.73; 95% confidence interval [CI] = 1.25–2.38; P = 0.001). Given the heterogeneity in the comparison groups, we compared variable screeners to each of the other 2 groups in separate analyses. When variable screeners were compared with consistent screeners only (omitting the consistent nonscreeners), the AOR was 1.83 (95% CI = 1.32–2.54; P = 0.001; not shown in the table). A comparison with consistent nonscreeners did not yield statistically significant results (not shown in the table). Table 2 also shows that in multivariate models physicans who consistently screened their patients with a PSA test were significantly less likely to report any magnitude effect of guidelines on their clinical practice when compared with physicians in the other 2 groups (AOR = 0.61; 95% CI = 0.47–0.79; P < 0.0001). Physicians who consistently did not screen their patients did not signficantly differ in the reported effect of guidelines when compared with the other 2 groups of physicians (AOR = 1.16; 95% CI = 0.79–1.71; P = 0.43).
Table 2.
Association Between the Perception of Any Effect of Guidelines on Practice and Being a Consistent Nonscreener, Variable Screener, and Consistent Screener of Prostate-Specific Antigen
| How Large an Effect Does Your Use of Formal, Written Practice Guidelines Such as Those Generated by Physician Organizations, Insurance Companies or HMOs, or Government Agencies Have on Your Practice of Medicine? |
Unadjusted Model |
Multivariate Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Consistent Nonscreener (n = 517) | 1.38 | 0.94-2.02 | 0.10 | 1.16 | 0.79-1.71 | 0.43 |
| Variable Screener (n = 1,061) | 1.76 | 1.27-2.42 | 0.001 | 1.73 | 1.25-2.38 | 0.001 |
| Consistent Screener (n = 2,332) | 0.56 | 0.42-0.74 | 0.0001 | 0.61 | 0.47-0.79 | 0.0001 |
Note: HMO = health maintenance organization; OR = odds ratio; CI = confidence interval. Each model compares physicians with a specific screening pattern against the other 2 groups of physicians. Models are adjusted for physician age; sex; race; Latino ethnicity; specialty; board certification status; foreign graduate status; practice type; number of years in practice; salaried status; income earned in 1997; proportion of Medicare, Medicaid, and managed care as a source of revenue; and charity care provided in the previous month.
Table 3 shows the results of the unadjusted and multivariate logistic regression models for the association between being a variable PSA screener (compared with all other physicians) and physician and practice characteristics as well as the perception that guidelines have any effect on practice (v. no effect). In both unadjusted and multivariate models, an income of $200,000 to $299,999, providing any charity care in the previous month, Medicaid as a source of practice revenue, and the perception that guidelines had an effect on practice were directly associated with being a variable screener. In addition, in multivariate models, nonwhite physicians were more likely to be a variable screeners, and Latino physicians were less likely to be variable screeners.
Table 3.
Unadjusted and Multivariate Logistic Regression Results for the Association between Variable Screeners and Physician Demographic Characteristics, Practice Characteristics, and Perceived Effect of Guidelines
| Characteristic of Physicians Who Are Variable PSA Screeners |
Unadjusted Model |
Multivariate Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 0.98 | 0.97–0.99 | 0.003 | 0.99 | 0.94–1.02 | 0.25 |
| Female | 1.28 | 0.89–1.84 | 0.18 | 1.07 | 0.80–1.42 | 0.65 |
| Nonwhite race (compared with white) | 1.09 | 1.01–1.18 | 0.25 | 1.09 | 1.02–1.17 | 0.01 |
| Hispanic (compared with not Hispanic) | 0.90 | 0.68–1.19 | 0.45 | 0.72 | 0.53–0.97 | 0.03 |
| Specialty | ||||||
| Family practice (reference) | — | — | — | — | — | — |
| Internal medicine | 0.95 | 0.79–1.15 | 0.65 | 0.91 | 0.78–1.07 | 0.25 |
| General practice | 0.72 | 0.40–1.30 | 0.27 | 0.84 | 0.49–1.46 | 0.54 |
| Board certification | 1.05 | 0.78–1.43 | 0.73 | 1.01 | 0.76–1.33 | 0.96 |
| Foreign medical graduate | 1.12 | 0.78–1.60 | 0.64 | 0.89 | 0.59–1.34 | 0.58 |
| Type of practice (%) | ||||||
| Solo, 2, or group physician practice (reference) | — | — | — | — | — | — |
| HMO | 0.95 | 0.64–1.40 | 0.78 | 1.16 | 0.77–1.73 | 0.47 |
| Hospital or medical school | 1.18 | 0.94–1.48 | 0.14 | 1.09 | 0.88–1.35 | 0.41 |
| Other | 1.03 | 0.83–1.29 | 0.79 | 0.87 | 0.66–1.16 | 0.35 |
| Number of years in practice | 0.98 | 0.97–0.99 | < 0.0001 | 1.00 | 0.97–1.04 | 0.92 |
| Salaried | 1.08 | 0.98–1.19 | 0.125 | 1.12 | 0.98–1.28 | 0.09 |
| Annual income in 1997 | ||||||
| $0–$99,999 (reference) | — | — | — | — | — | — |
| $100,000–$199,999 | 0.75 | 0.52–1.09 | 0.14 | 0.76 | 0.52–1.12 | 0.16 |
| $200,000–$299,999 | 0.49 | 0.30–0.82 | 0.007 | 0.51 | 0.31–0.85 | 0.01 |
| ≥$300,000 | 0.69 | 0.29–1.64 | 0.39 | 0.75 | 0.31–1.83 | 0.52 |
| Provide any charity care in previous month | 1.31 | 1.08–1.59 | 0.008 | 1.23 | 1.01–1.51 | 0.04 |
| Source of practice revenue | ||||||
| % Medicare (s) | 1.00 | 1.00–1.01 | 0.18 | 1.00 | 1.00–1.01 | 0.04 |
| % Medicaid (s) | 1.02 | 1.10–1.02 | < 0.0001 | 1.01 | 1.01–1.02 | < 0.0001 |
| % Managed care (s) | 0.99 | 0.99–1.00 | < 0.0001 | 0.99 | 0.99–1.00 | < 0.0001 |
| How large an effect does your use of formal, written practice guidelines … have on your practice of medicine? |
||||||
| Any effect versus no effect | 1.76 | 1.27–2.42 | 0.001 | 1.73 | 1.25–2.38 | 0.001 |
Note: PSA = prostate-specific antigen; OR = odds ratio; CI = confidence interval; HMO = health maintenance organization. A consistent screener is defined as screening with PSA at least 80% of patients represented by vignette. All P values are 2 tailed.
DISCUSSION
One of the 1st clinical practice guidelines to be widely used was created in 1938 by the American Academy of Pediatrics to provide parameters for the immunization of children.19 Clinical practice guidelines have since become commonplace, and as of 2007, there were 2249 clinical practice guidelines in the National Guideline Clearinghouse, the national repository of evidence-based guidelines.20 Considerable time, effort, and resources are devoted to developing and implementing guidelines.21 Thus, knowledge of how physicians perceive and interpret guidelines is important for providing high-quality care. To our knowledge, this one is the 1st study to use nationally representative physician survey data to examine physician PSA screening patterns and their perceived effect of guidelines on clinical practice.
Our research shows several important findings. First, the majority of physicians (60%) reported that they consistently recommend PSA screening to all their asymptomatic 60-year-old patients. This finding is consistent with previous research that has shown that many, if not most, physicians order screening PSAs at least occasionally.22–34 Thus, it is not surprising that 75% of men older than 50 years in the United States have previously had a PSA test.35
Second, although guidelines recommend shared decision making, only 27% of physicians are variable PSA screeners. Thus, the majority of physicians have a consistent screening strategy, indicating that they may be less responsive to patient values and preferences. From this perspective, the message of shared decision making appears to have had only a limited impact on clinical practice. Research on patients supports this inference. In a cross-sectional analysis of data from the 2000 National Health Interview Survey, approximately one third of men reported their physician did not discuss advantages and disadvantages of prostate cancer screening before offering testing.36 Two additional studies suggest the problem is even more concerning: one fourth of men who have undergone PSA testing were unaware they had been tested.37,38 These findings add to the concern that a significant proportion of men are not being given the opportunity to make an informed decision about prostate cancer screening and that the prostate cancer screening guideline recommendation of shared decion making is not being implemented. Much research has been conducted on guideline implemenation. A recent literature review of the facilitators of guideline implementation found that among the 70 successful facilitators identified in the literature, 7 categories emerged: 1) data feedback, 2) reminders or checklists, 3) peer review and in-person feedback, 4) direct supervision, 5) in-service or other educational interventions, 6) mandates, and 7) monetary incentives.39 Multifaceted interventions targeting different barriers to change are likely to be required to effectively change physician PSA screening behavior.40–43
Third, although only a small minority (8%) of physicians report that guidelines have no effect on their clinical practice, those physicians are much less likely to be variable PSA screeners. Our findings are similar to prior focus group research14 that shows that routine PSA screeners were less likely to be familiar with the guidelines about PSA screening compared with routine nonscreeners. In fact, in that same study, routine screeners were frequently unable to describe the recommendations of any specific organization, were unaware of the controversy about PSA screening, and believed that population-based screening was universally endorsed. In addition, most routine screeners in that study said that clinical guidelines were not a factor in their screening decisions and that, instead, their practices were based on their clinical experience. Less is known about variable screeners, but one hypothesis is that physicians who are variable screeners interpret the current guidelines in a way that recognizes that screening decisions should be individualized. Based on the findings of this study, clinical guidelines that recommend individualized, informed, shared decision making appear to have some impact on clinical practice: Physicians who report guidelines have an effect on clinical practice are more likely to have PSA screening practice patterns consistent with shared decision making.
Fourth, the current research demonstrates that physicians who are consistent PSA screeners differ from those who are consistent nonscreeners and variable screeners in other ways. Compared with all the remainder of physicians, consistent PSA screeners are more likely to be older, male, white, and in practice longer and to have a higher income and are less likely to be board certified, salaried, and have the lowest proportion of Medicaid as a source of revenue. Conversely, consistent PSA nonscreeners had personal and practice characteristics that were the opposite of consistent screeners. It is possible that the demographic profile of the physicians who are routine screeners represents a group of physicians who are paternalistic, whereby patient input is not sought and thus practice variation is reduced. Cooper and others14 previously demonstrated that consistent screeners and consistent nonscreeners vary in substantive ways. The major factor influencing PSA practice patterns for consistent screeners was professional and personal experience that supported PSA screening and patient expectations to be screened, whereas the major factor influencing consistent nonscreeners was the lack of definitive evidence of the benefit of PSA screening.
Our study has several limitations. First, the question about screening guidelines in the CTS Physician Survey was not specific to PSA screening, and the data did not allow us to evaluate which clinical practice guidelines are responsible for the perceived effect of guidelines on practice, thereby creating the potential for misclassification bias. Physicians receive guidelines from multiple organizations through different media, and they assimilate the contents of these to largely varying degrees depending on the source. In fact, physicians may experience “guideline fatigue” and not adopt a clinical practice guideline at all.44 A potential solution is to convene a multisociety task force composed of members of all the relevant organizations to design a single, uniform set of clinical practice guidelines about a topic, as was done with the case of colorectal cancer screening.45 Although design and approval of these guidelines are more time and labor intensive, such guidelines have the potential to be much more widely and consistently implemented.
Second, the literature shows that there may be other important drivers of PSA screening that we did not have data for and thus could not adjust for, for example, concerns about medical-legal risk.46 Third, the dependent variable, PSA screening, was measured using a single isolated variable: a case vignette. Although the case vignette allowed us to control for patient factors and isolate the physician factors associated with PSA screening decision making, a broader assessment of a range of clinical scenarios would strengthen our results. However, several studies have supported the validity of case vignettes in measuring actual physician behavior as responses to case vignettes are correlated with actual clinical behavior.47–50 Fourth, there is the potential for nonresponse bias, given that the response rate for the 1998–1999 CTS Physician Survey was 61%, a response rate that is not unusually low for physician surveys. Finally, the inferences drawn from this cross-sectional study are limited because this study cannot prove causality between the effect of guidelines and the PSA screening behavior of physicians.
Despite these limitations, this is one of the largest studies to examine the relationship between physician attitudes, guidelines, and PSA screening patterns. These results can inform health care policy makers who seek to improve the quality of cancer screening decisions and develop effective clinical guidelines.
ACKNOWLEDGMENT
Dr. Guerra acknowledges the National Cancer Institute (grant K01 CA-097925) and the Robert Wood Johnson Foundation Amos Medical Faculty Development Program (grant 051895) for their grant support as well as the Center for Studying Health System Change for granting the authors the use of the Community Tracking Study Physician Survey restricted data for the analyses described in this article. Dr. Pagán acknowledges the financial support of the Department of Defense (grant W81XWH-06-1-0334).
APPENDIX
| Organization | Year | Recommendation from Guidelines |
|---|---|---|
| American Cancer Society11 | 1997 | “The ACS recommends that both the PSA test and the digital rectal exam be offered annually, beginning at age 50, to men who have a life expectancy of at least 10 years and to younger men who have a high risk. Information should be provided to patients about the risks and benefits of screening.” |
| American College of Physicians12 | 1997 | “Rather than screening all men for prostate cancer as a matter of routine, physicians should describe the potential benefits and known harms of screening, diagnosis, and treatment; listen to the patient’s concerns; and then individualize the decision to screen.” |
| US Preventive Services Task Force13 | 1996 | “Routine screening for prostate cancer with DRE, serum tumor markers (e.g., PSA), or Transrectal Ultrasound is not recommended (“D” recommendation). Patients who request screening should be given objective information about the potential benefits and harms of early detection and treatment.” |
Note: PSA = prostate-specific antigen; DRE = digital rectal examination.
Footnotes
The results of this work were previously presented at the 29th annual meeting of the Society of General Internal Medicine, 29 April 2006, Los Angeles, California.
Contributor Information
Carmen E. Guerra, Division of General Internal Medicine, University of Pennsylvania School of Medicine, Philadelphia; Leonard Davis Institute of Health Economics, University of Pennsylvania School of Medicine, Philadelphia; Abramson Cancer Center, University of Pennsylvania, Philadelphia.
Phyllis A. Gimotty, Department of Biostatistics, University of Pennsylvania School of Medicine, Philadelphia.
Judy A. Shea, Division of General Internal Medicine, University of Pennsylvania School of Medicine, Philadelphia; Leonard Davis Institute of Health Economics, University of Pennsylvania School of Medicine, Philadelphia.
José A. Pagán, Department of Economics and Finance and the Institute for Population Health Policy, University of Texas–Pan American, Edinburg, Texas.
J. Sanford Schwartz, Division of General Internal Medicine, University of Pennsylvania School of Medicine, Philadelphia; Leonard Davis Institute of Health Economics, University of Pennsylvania School of Medicine, Philadelphia; Abramson Cancer Center, University of Pennsylvania, Philadelphia.
Katrina Armstrong, Division of General Internal Medicine, University of Pennsylvania School of Medicine, Philadelphia; Leonard Davis Institute of Health Economics, University of Pennsylvania School of Medicine, Philadelphia; Abramson Cancer Center, University of Pennsylvania, Philadelphia.
REFERENCES
- 1.Field M, Lohr K, editors. Clinical Practice Guidelines: Directions for a New Program. National Academy Press; Washington (DC): 1990. [PubMed] [Google Scholar]
- 2.Weingarten SR, Riedinger MS, Conner L, et al. Practice guidelines and reminders to reduce duration of hospital stay for patients with chest pain. Ann Intern Med. 1994;120(4):257–63. doi: 10.7326/0003-4819-120-4-199402150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Grimshaw J, Russell I. Effect of clinical guidelines on medical practice: a systematic review of rigourous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 4.Grimshaw J, Freemantle N, Wallace S, et al. Developing and implementing clinical practice guidelines. Qual Health Care. 1995;4:55–64. doi: 10.1136/qshc.4.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachtel TJ, O’sullivan P. Practice guidelines to reduce testing in the hospital. J Gen Intern Med. 1990;5:335–41. doi: 10.1007/BF02600402. [DOI] [PubMed] [Google Scholar]
- 6.Rhew D, Reidinger M. A prospective, multicenter study of a pneumonia practice guideline. Chest. 1998;114(1):115–9. doi: 10.1378/chest.114.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan SL, Harris RP, Woolf SH. Shared Decision-Making Workgroup of the U.S. Preventive Services Task Force. Shared decision making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Schroder FH. Detection of prostate cancer: the impact of the European Randomized Study of Screening for Prostate Cancer (ERSPC) Can J Urol. 2005;12(Suppl 1):2–6. [PubMed] [Google Scholar]
- 10.Taylor KL, Shelby R, Gelmann E, McGuire C. Quality of life and trial adherence among participants in the prostate, lung, colorectal, and ovarian cancer screening trial. J Natl Cancer Inst. 2004;96(14):1083–94. doi: 10.1093/jnci/djh194. [DOI] [PubMed] [Google Scholar]
- 11.Von Eschenbach A, Ho R, Murphy GP, Cunningham N, Lins N. American Cancer Society Guideline for the early detection of prostate cancer update 1997. CA Cancer J Clin. 1997;47(5):261–4. doi: 10.3322/canjclin.47.5.261. [DOI] [PubMed] [Google Scholar]
- 12.American College of Physicians Clinical guideline, part III: screening for prostate cancer. Ann Intern Med. 1997;126(6):480–4. [PubMed] [Google Scholar]
- 13.US Preventive Services Task Force . Screening for Prostate Cancer: Guide to Clinical Preventive Services. 2nd ed. Williams & Wilkins; Baltimore (MD): 1996. [Google Scholar]
- 14.Cooper C, Merritt T, Ross L, John L, Jorgensen C. To screen or not to screen, when clinical guidelines disagree: primary care physicians’ use of the PSA test. Prev Med. 2004;38:182–91. doi: 10.1016/j.ypmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf CE, Kemper P, Kohn LT, Pickering JD. Site Definition and Sample Design for the Community Tracking Study. Center for Studying Health System Change; Washington (DC): 1996. [Google Scholar]
- 16.Version ICPSR. Inter-university Consortium for Political and Social Research; Ann Arbor (MI): 2004. Community Tracking Study Physician Survey: 1998-1999 Program. [Google Scholar]
- 17.Mulley A. Supporting the patient’s role in decision making. J Occup Med. 1990;32:1227–8. doi: 10.1097/00043764-199012000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Stata/SE Statistical Software Release 8.2. Stata Corporation; College Station (TX): 2004. [Google Scholar]
- 19.American Academy of Pediatrics . Policy Reference Guide of the American Academy of Pediatrics. American Academy of Pediatrics; Elk Grove Village (IL): 1997. [Google Scholar]
- 20.National Guideline Clearinghouse [Accessed 13 November 2007]; Available from: URL: http://guideline.gov/browse/guideline_index.aspx.
- 21.Woolf SH, DiGuiseppi CG, Atkins D, Kamerow DB. Developing evidence-based clinical practice guidelines: lessons learned by the US Preventive Services Task Force. Ann Rev Public Health. 1996;17:511–38. doi: 10.1146/annurev.pu.17.050196.002455. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman RM, Papenfuss MR, Buller DB, Moon TE. Attitudes and practices of primary care physicians for prostate cancer screening. Am J Prev Med. 1996;12(4):277–81. [PubMed] [Google Scholar]
- 23.Raich PC, Zoeter MA, Hagan M, et al. Perception of preventive health needs in a prostate-cancer screening population: a preliminary report. J Cancer Educ. 1997;12(4):224–8. doi: 10.1080/08858199709528493. [DOI] [PubMed] [Google Scholar]
- 24.Bunting PS, Goel V, Williams JI, Iscoe NA. Prostate-specific antigen testing in Ontario: reasons for testing patients without diagnosed prostate cancer. CMAJ. 1999;160(1):70–5. [PMC free article] [PubMed] [Google Scholar]
- 25.McKnight JT, Tietze PH, Adcock BB, Maxwell AJ, Smith WO, Nagy MC. Screening for prostate cancer: a comparison of urologists and primary care physicians. South Med J. 1996;89(9):885–8. [PubMed] [Google Scholar]
- 26.Voss JD, Schectman JM. Prostate cancer screening practices and beliefs. J Gen Intern Med. 2001;16(12):831–7. doi: 10.1111/j.1525-1497.2001.10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks RJ, Hamm RM, Bemben DA. Prostate cancer screening: what family physicians believe is best. Arch Fam Med. 1995;4(4):317–22. doi: 10.1001/archfami.4.4.317. [DOI] [PubMed] [Google Scholar]
- 28.Williams RB, Boles M, Johnson RE. Use of prostate-specific antigen for prostate cancer screening in primary care practice. Arch Fam Med. 1995;4(4):311–5. doi: 10.1001/archfami.4.4.311. [DOI] [PubMed] [Google Scholar]
- 29.Austin OJ, Valente S, Hasse LA, Kues JR. Determinants of prostate-specific antigen test use in prostate cancer screening by primary care physicians. Arch Fam Med. 1997;6(5):453–8. doi: 10.1001/archfami.6.5.453. [DOI] [PubMed] [Google Scholar]
- 30.Kripalani S, Weinberg AD, Cooper HP. Screening for breast and prostate cancer: a survey of Texas primary care physicians. Tex Med. 1996;96(12):59–67. [PubMed] [Google Scholar]
- 31.Lawson DA, Simoes EJ, Sharp D, et al. Prostate cancer screening—a physician survey in Missouri. J Community Health. 1998;23(5):347–58. doi: 10.1023/a:1018745821888. [DOI] [PubMed] [Google Scholar]
- 32.Edlefsen KL, Mandelson MT, McIntosh MW, Andersen MR, Wagner EH, Urban N. Prostate-specific antigen for prostate cancer screening: do physician characteristics affect its use? Am J Prev Med. 1999;17(1):87–90. doi: 10.1016/s0749-3797(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 33.Little B, Ho KJ, Gormley G, Young M. PSA testing in general practice. Prostate Cancer Prostatic Dis. 2003;6(2):154–8. doi: 10.1038/sj.pcan.4500646. [DOI] [PubMed] [Google Scholar]
- 34.Moran WP, Cohen SJ, Preisser JS, Wofford JL, Shelton VJ, McClatchey MW. Factors influencing use of the prostate-specific antigen screening test in primary care. Am J Manag Care. 2000;6(3):315–24. [PubMed] [Google Scholar]
- 35.Sirovich BE, Schwartz L, Woloshin S. Screening men for prostate and colorectal cancer in the United States. JAMA. 2003;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 36.Han PK, Coates RJ, Uhler RJ, Breen N. Decision making in prostate-specific antigen screening National Health Interview Survey, 2000. Am J Prev Med. 2006;30(5):394–404. doi: 10.1016/j.amepre.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Jordan TR, Price JH, King KA, Masyk T, Bedell AW. The validity of male patients’ self-reports regarding prostate cancer screening. Prev Med. 1999;28(3):297–303. doi: 10.1006/pmed.1998.0430. [DOI] [PubMed] [Google Scholar]
- 38.Chan ECY, Vernon SW, Ahn C, Greisinger A. Do men know that they have had a prostate-specific antigen test? Accuracy of self-reports of testing at 2 sites. Am J Public Health. 2005;94(8):1336–8. doi: 10.2105/ajph.94.8.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnad SS, Kurichi JE, Guerra CE. Facilitators in successful guideline implementation; Paper presented at: the 27th Annual Meeting of the Society for Medical Decision Making; San Francisco, CA. 2005; [Accessed 8 November 2007]. Available from: URL: http://smdm.confex.com/smdm/2005ca/techprogram/P2305.HTM. [Google Scholar]
- 40.Bero LA, Grilli R, Grimshaw JM, et al. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317(7156):465–8. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemelin J, Hogg W, Baskerville N. Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care. CMAJ. 2001;164(6):757–63. [PMC free article] [PubMed] [Google Scholar]
- 42.Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180(6 Suppl):S57–60. doi: 10.5694/j.1326-5377.2004.tb05948.x. [DOI] [PubMed] [Google Scholar]
- 43.Oxman AD, Thompson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. Can Med Assoc J. 1995;153(10):1423–31. [PMC free article] [PubMed] [Google Scholar]
- 44.Restrepo MI, Anzueto A. Guidelines for the diagnosis and treatment of adult lower respiratory tract infections: a true “European cooperative effort.”. Eur Respir J. 2005;26:979–81. doi: 10.1183/09031936.05.00102105. [DOI] [PubMed] [Google Scholar]
- 45.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124(2):544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 46.Merenstein D. Winners and losers. JAMA. 2004;291(1):15–6. doi: 10.1001/jama.291.1.15. [DOI] [PubMed] [Google Scholar]
- 47.Dresselhaus TR, Peabody JW, Luck J, Bertenthal D. An evaluation of vignettes for predicting variation in the quality of preventive care. J Gen Intern Med. 2004;19(10):1013–8. doi: 10.1007/s11606-004-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peabody J, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 49.Elstein AS, Holmes MM, Ravitch MM, Rovner DR, Holzman GB, Elstein AS. Medical decisions in perspective: applied research in cognitive psychology. Perspect Biol Med. 1983;26:486–501. doi: 10.1353/pbm.1983.0068. [DOI] [PubMed] [Google Scholar]
- 50.Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141(10):771–80. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]