Abstract
Morphine is among the most prevalent analgesics prescribed for chronic pain. However, prolonged morphine treatment results in the development of analgesic tolerance. An abundance of evidence has accumulated indicating that CNS glial cell activity facilitates pain transmission and opposes morphine analgesia. While the midbrain ventrolateral periaqueductal gray (vlPAG) is an important neural substrate mediating pain modulation and the development of morphine tolerance, no studies have directly assessed the role of PAG-glia. Here we test the hypothesis that morphine-induced increases in vlPAG glial cell activity contribute to the development of morphine tolerance. As morphine is primarily consumed for the alleviation of severe pain, the influence of persistent inflammatory pain was also assessed. Administration of morphine, in the absence of persistent inflammatory pain, resulted in the rapid development of morphine tolerance and was accompanied by a significant increase in vlPAG glial activation. In contrast, persistent inflammatory hyperalgesia, induced by intraplantar administration of Complete Freund’s Adjuvant (CFA), significantly attenuated the development of morphine tolerance. No significant differences were noted in vlPAG glial cell activation for CFA-treated animals versus controls. These results indicate that vlPAG glia are modulated by a persistent pain state, and implicate vlPAG glial cells as possible regulators of morphine tolerance.
Perspective
The development of morphine tolerance represents a significant impediment to its use in the management of chronic pain. We report that morphine tolerance is accompanied by increased glial cell activation within the vlPAG, and that the presence of a persistent pain state prevented vlPAG glial activation and attenuated morphine tolerance.
Keywords: opioids, tolerance, pain, inflammation, astrocytes, microglia
Introduction
Chronic pain, defined as pain lasting more than 3–6 months, will affect more than one in three Americans at some point in their life64, 66. Although morphine is one of the most commonly prescribed analgesics76, secondary side effects (e.g., tolerance) limit its efficacy for long-term chronic pain treatment8, 28, 48, 49. In the absence of pain, morphine tolerance, defined as the requirement for steadily larger doses of opioids to achieve the same analgesic effect, develops quite rapidly19, 48, 54. In contrast, clinical studies have consistently reported that the latency to develop morphine tolerance is increased in chronic pain sufferers, although dose escalation is eventually required for the maintenance of analgesic efficacy77. Dose escalation leads to increased risk of developing additional negative side effects, including anti-analgesia, addiction, withdrawal, and respiratory depression76, and is not always sufficient to overcome tolerance and reinstate analgesic efficacy77. As over 90% of chronic pain sufferers are treated with opioids76, including morphine, elucidation of the mechanisms by which morphine tolerance develops warrants investigation.
The midbrain ventrolateral periaqueductal gray (vlPAG) and its descending projections to the rostral ventromedial medulla (RVM) and spinal cord, comprises an important neural circuit for both endogenous and exogenous opioid-mediated analgesia4–7, 24. In rats, chronic subcutaneous injections of morphine result in tolerance to subsequent doses of morphine, an effect that is eliminated by intra-vlPAG injections of the opioid receptor antagonist naltrexone42. In addition, chronic intra-vlPAG injections of morphine induce tolerance, and this effect remains when the RVM, the primary downstream target of the PAG, is inhibited with the GABA agonist muscimol42.
An abundance of evidence has accumulated indicating that systemic morphine administration activates glial cells, including microglia and astrocytes31, 51, 52, 60, 70, 75, 84. Song and Zhou (2001) reported that chronic morphine administration results in the activation of astrocytes within the cingulate cortex, hippocampus and spinal cord, and that blockade of glial activation within the spinal cord attenuates the development of morphine tolerance. Since then, a myriad of studies have been published implicating glia activation in the development of morphine tolerance84, 85 and pain facilitation11, 61, 82. While it is clear that the activation of microglia and astrocytes contribute to the development of morphine tolerance, no studies have examined the role of activated glia within the PAG, a primary site of morphine action. Similarly, the influence of a persistent inflammatory pain state on PAG glial cell activation has not been assessed. The present study tested the hypothesis that morphine-induced increases in vlPAG glial cell activity contribute to the development of morphine tolerance, and that persistent inflammatory pain alters this activation, resulting in the attenuation of morphine tolerance.
Materials and Methods
Subjects
Weight-matched (250–350g) male Sprague Dawley rats (Charles River, Wilmington, MA) were pair-housed on a 12:12 hour light: dark cycle. Access to food and water was available ad libitum throughout the experiments except during behavioral testing. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Georgia State University, and performed in strict compliance with Ethical Issues of the International Association for the Study of Pain (IASP) and National Institute of Health (NIH). All efforts were made to reduce the number of animals used in these experiments and to minimize any possible suffering by the animal.
Persistent Inflammatory Hyperalgesia
In a subset of animals, persistent inflammatory hyperalgesia was induced by injection of complete Freund’s adjuvant (CFA; Mycobacterium tuberculosis; Sigma; 200 μl), suspended in an oil/saline (1:1) emulsion, into the plantar surface of the right hindpaw as previously described46, 48, 81. As intraplantar saline administration results in a short-term inflammatory response, control animals were restrained in a similar manner but did not receive an intraplantar injection.
Experiment 1: Influence of Persistent Inflammatory Pain on Morphine Tolerance
Twenty-four hours following intraplantar CFA injection or handling, animals were administered morphine (5 mg/kg, sc; NIDA) or saline (1 ml/kg, sc) once a day for three consecutive days (CFA+Morphine; CFA+Saline; Handled+Morphine; Handled+Saline). The 5 mg/kg dose was chosen based on our previous studies demonstrating this to be the 50% effective dose (ED50) for systemic morphine in male rats47, 53, 81. Baseline nociceptive thresholds were measured before morphine or saline injections, and 15 minutes following the first and last injection (Injection 1 and Injection 3, respectively). Tolerance was assessed on Day 5 (Day 1 being CFA administration), by injecting cumulative doses of morphine every 20 min, resulting in doses of 3.2, 5.6, 8.0, 10.0 and 18.0 mg/kg as previously described48. Nociception was assessed using the paw thermal stimulator29 15 min after each injection81. Briefly, for this test, the rat is placed in a clear Plexiglas box resting on an elevated glass plate maintained at 30°C. A radiant beam of light is positioned under the hindpaw and the time for the rat to remove the paw from the thermal stimulus is electronically recorded as the paw withdrawal latency (PWL). The intensity of the beam was set to produce basal withdrawal rates of 7–9 seconds. A maximal PWL of 20.48 seconds was used to prevent excess tissue damage due to repeated application of the noxious thermal stimulus. Animals were acclimated to the testing apparatus (30 minutes a day for 3 consecutive days) at the start of the experiment. All behavioral testing took place between 12:00pm and 5:00pm (lights on at 7:00am). All testing was conducted blind with respect to group assignment (i.e., morphine or saline treatment).
Behavioral data analysis and presentation
Behavioral data are expressed in raw seconds. Paw withdrawal latency data were analyzed using repeated measures ANOVA for significant main effect of pain (CFA or handled) and treatment (morphine or saline) across dose. Pre-planned t-tests were used to determine specific group and dose differences when a significant main effect was observed. All values are reported as Mean ± S.E.M.; p≤ 0.05 was considered statistically significant.
Experiment 2: Anatomical Assessment of Morphine Tolerance
Twenty-four hours following intraplantar CFA or handling, animals were administered morphine (5 mg/kg; sc) or saline (1 ml/kg; sc) once a day for three consecutive days as described above (CFA+Morphine, CFA+Saline, Handled+Morphine, Handled+Saline). One hour following the last injection of morphine or saline, animals were given a lethal dose of Nembutal (160 mg/kg; i.p.) and transcardially perfused with 250 ml of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator to remove blood from the brain. Immediately following blood removal, 300 ml of 4% paraformaldehyde in 0.1M phosphate buffer containing 2.5% acrolein (Polysciences Inc.; Warrington, PA) was perfused through the brain as a fixative. A final rinse with 250 ml of sodium chloride/sodium nitrite solution was perfused through the brain to remove any residual acrolein. Brains were removed and placed in a 30% sucrose solution and stored at 4°C until sectioning. To examine the acute effects of morphine on vlPAG glia activation, a separate group of animals received one sc injection of morphine (or saline) and were sacrificed 1 or 24 hours later. A separate group of animals (CFA+Morphine, CFA+Saline, Handled+Morphine, Handled+Saline) were decapitated immediately following treatment for western blot analysis. Brains were removed, flash frozen in 2-methyl-butane on dry ice, and stored at −80°C.
Immunohistochemistry
Hallmarks of glial cell activity include increased cytokine release that correlates with increased expression of the protein markers glial fibrillary acetic protein (GFAP; astrocytes), and CD-11b (OX-42; microglia)60. Further, increased glial cell activity is evidenced by a profound shift in morphology that can be easily visualized using immunohistochemistry for GFAP and OX-4216. Perfused brains were sectioned into 25μm coronal sections with a Leica 2000R freezing microtome and stored free-floating in cryoprotectant-antifreeze solution44 at −20°C. A 1:6 series through the rostrocaudal axis of each brain was processed for GFAP and OX-42 immunoreactivity using standard immunohistochemical techniques56. Briefly, sections were rinsed extensively in potassium phosphate-buffered saline (KPBS) immediately followed by a 20-minute incubation in 1% sodium borohydride. The tissue was then incubated in primary antibody solution (rabbit anti-GFAP 1:5,000 or rabbit anti-OX42 1:1000; Abcam) in KPBS containing 1.0% Triton-X for one hour at room temperature followed by 48 hours at 4°C. After rinsing with KPBS, the tissue was incubated for one hour in secondary antibody (biotinylated IgG goat anti-rabbit 1:600), rinsed with KPBS, and then incubated for one hour in an avidin-biotin peroxidase complex (1:10; ABC Elite Kit, Vector Labs). After rinsing in KPBS and sodium acetate (0.175 M; pH 6.5), GFAP or OX-42 immunoreactivity was visualized as a black reaction product using nickel sulfate intensified 3,3’-diaminobenzidine (DAB) solution (2 mg/10ml) containing 0.08% hydrogen peroxide in sodium acetate buffer. After 15 minutes, tissue was rinsed in sodium acetate buffer followed by KPBS. In a subset of sections, GFAP (Rabbit anti-GFAP 1:3,000; Abcam) or OX-42 (Mouse anti-CD11b 1:3000; Serotec) was visualized using a fluorescent secondary antibody (goat anti-rabbit Dylight488 1:50 for GFAP and rabbit anti-mouse Cy3 1:50 for CD11b; Jackson Immunoresearch Laboratories). Following secondary incubation, sections were rinsed in KPBS. DAB and fluorescent sections were mounted out of KPBS onto gelatin-subbed slides, air-dried and dehydrated in a series of graded alcohols. Tissue-mounted slides were then cleared in Xylenes and glass cover-slipped using Permount for DAB reactions or Krystalon for fluorescence.
Western blotting
Flash frozen brains were sectioned at 300µm on a cryostat (Leica, Buffalo Grove, IL) and mounted onto slides. One-millimeter bilateral micropunches were taken from 6 levels for the vlPAG (Bregma −8.52, −8.28, −7.92, −7.68, −7.20, and −6.96)58 and 6 levels of the superior colliculus (Bregma −7.68, −7.20, −6.96, −6.60, −6.24, and −5.80)58, and homogenized in a 10mM HEPES buffer (pH 7.2). Equal amounts of protein (2µg) along with a standard marker (Bio-Rad, Hercules, CA) were run at 100V for 2 hours through 10% Tris-HCl polyacrylamide gels (Bio-Rad, Hercules, CA), and electro-transferred at 4°C on ice at 250mA for 2 hours onto PVDF membranes (0.2µm pore size; Bio-Rad, Hercules, CA). Membranes were blocked with 5% milk in TBS-Tween 20 (1%) at 4°C overnight, and probed with rabbit anti-GFAP primary antibody (1:300,000; Abcam) in 2% milk/TBS-Tween 20 (1%) for 3 hours at room temperature followed by a 30 minute incubation in HRP-conjugated goat anti-rabbit secondary (1:5000; Abcam) in 2% milk/TBS-Tween 20 (1%). Rabbit anti-β–actin primary (1:10,000; Novus Biologicals) was included as a control for protein loading. Membranes were stripped and reprobed with mouse anti-rat CD11b (OX-42, 1:700; Serotec) followed by HRP-conjugated goat anti-mouse (OX-42; 1:5000; Abcam) and HRP-conjugated goat anti-rabbit (β-actin; 1:5000; Abcam) secondaries.
Anatomical data analysis and presentation
Levels of GFAP and OX-42 immunoreactivity in the vlPAG were compared across treatment groups using semi-quantitative densitometry as previously described43, 48. To determine if the observed changes in glia activation were limited to the vlPAG, sections through the superior colliculus (SC), a region containing a high density of µ opioid receptors (MOR) but not implicated in opioid modulation of pain, were also analyzed. 12-bit grayscale images that included the region of interest (ROI) were captured using a QImaging Retiga EXi CCD camera (Surrey, BC, Canada) and iVision Image analysis software (Biovision Technologies, Exton, PA). Grayscale values for each image were inverted so that higher values were representative of increased staining levels. Images of three slices through each ROI for each animal were analyzed and data sampled unilaterally. Data sampling occurred by using the drawing tools in iVision to outline the ROI and using the “measure” function to determine an average grayscale pixel value for the outlined area. ROI measures were corrected for nonspecific binding by subtraction of background measure taken from gray matter adjacent to the ROI. Data were analyzed across 3 representative levels through the rostral-caudal axis of the vlPAG (Bregma −7.08, −7.68, and −8.30)58 and superior colliculus (Bregma −7.68, −6.24, and −5.80) 58 as previously described48. Densitometry values are presented as the mean ± S.E.M. density of immunoreactivity. Data were analyzed using an ANOVA to determine significant main effects of treatment (morphine, saline) and pain (CFA, handled). Fisher’s post hoc tests were used to determine specific group differences when a significant main effect was observed; p < 0.05 was considered statistically significant. For Western blots, band intensities for tissue from the vlPAG and superior colliculus were visualized at 55kD (GFAP) and 160kD (CD11b), and quantified using ImageJ (NIH, USA) analysis software, as a relative intensity of GFAP or CD11b band divided by the intensity of the β–actin band. Data are expressed as the mean ratio ± S.E.M of protein of interest/β–actin. Data were analyzed for significant main effects of treatment (morphine, saline) and pain (CFA, handled) using an ANOVA, and Fisher’s HSD was used for post-hoc analysis; p ≤ 0.05 was considered statistically significant.
Results
Experiment 1: Persistent Peripheral Inflammation Attenuated Morphine Tolerance
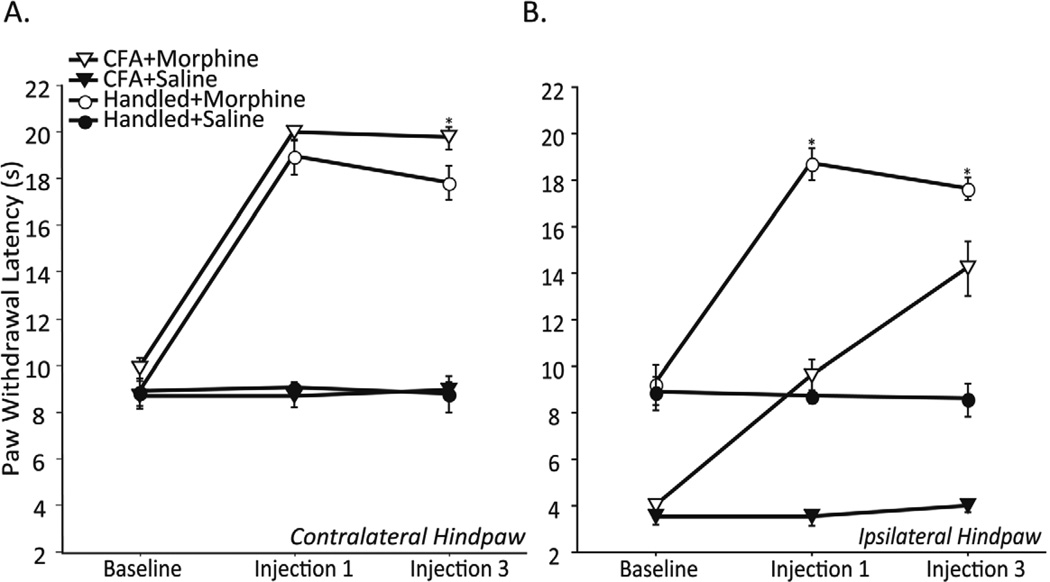
To assess the initial analgesic potency of morphine, and the degree and time course for development of morphine tolerance, paw withdrawal latencies (PWL) were determined for both the injured (ipsilateral; right) and uninjured (contralateral; left) hindpaws at baseline, and after Day 1 and Day 3 of morphine or saline. Contralateral (uninjured) PWL did not differ between CFA+Saline and Handled+Saline groups at any time point (Figure 1A). By contrast, intraplantar CFA significantly decreased ipsilateral PWL 24, 48, and 96 hours post injection as compared with handled controls (CFA+Saline versus Handled+Saline; Figure 1B) indicating the development of persistent hyperalgesia. Administration of morphine on Day 1 and 3 significantly increased both contralateral and ipsilateral PWLs as compared with saline controls (Figure 1A and B, respectively). For the contralateral paw, administration of morphine (Day 1) produced comparable levels of analgesia in the CFA+Morphine and Handled+Morphine groups (Figure 1A). However, the degree of analgesia produced by morphine on Day 3 was significantly attenuated in the Handled+Morphine vs. the CFA+Morphine animals, suggesting the development of morphine tolerance (Figure 1A). In the ipsilateral hindpaw, administration of morphine on Day 1 produced anti-hyperalgesia in CFA treated animals as indicated by a return to normal baseline PWL (CFA+Morphine; Handled+Saline, Figure 1B). In contrast to the decreased analgesia observed in Handled+Morphine animals on Day 3, morphine produced a significant increase in ipsilateral PWL of injured animals on Day 3 as compared to Day 1 (CFA+Morphine; Injection 1 and Injection 3, Figure 1B) indicating lack of tolerance development.
Figure 1.
Contralateral (A) and ipsilateral (B) PWL (in seconds) following intraplantar CFA or handling (Baseline), and after the first and third injection of morphine or saline in CFA+Morphine (n=6), CFA+Saline (n=7), Handled+Morphine (n=6), and Handled+Saline (n=5) treated male rats. The first and third injection of morphine caused an increase in contralateral and ipsilateral PWL as compared with saline controls (p< 0.05; A & B, respectively). Contralateral PWL did not differ between CFA+Saline and Handled+Saline groups at any time point (p> 0.05; A). CFA treatment caused a significant decrease in ipsilateral PWL at all time points as compared with handled controls (p< 0.05; CFA+Saline; Handled+Saline; B). While uninjured animals treated with morphine showed a decrease in analgesia to the third injection as compared with the first (p< 0.05; A), CFA treated animals showed an increase in antihyperalgesia to the third injection (p< 0.05; B). Asterisks indicate significant differences between CFA+Morphine and Handled+Morphine groups.
Assessment of morphine tolerance
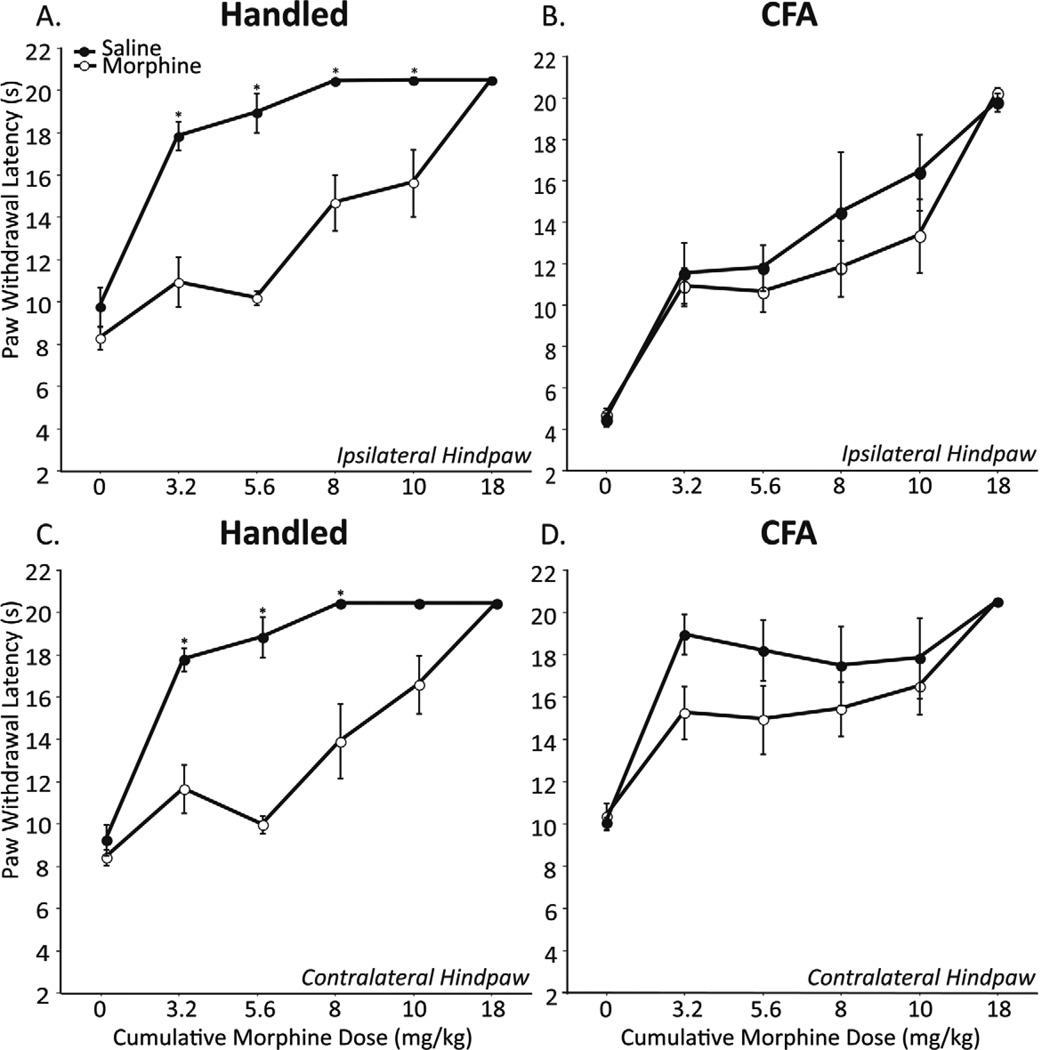
Morphine tolerance, assessed on Day 5 using a cumulative dosing paradigm, was only observed in non-CFA treated animals. As shown in Figure 2, the antinociceptive potency of morphine was significantly decreased in both the ipsilateral and contralateral hindpaw of uninjured animals that received 3 consecutive days of morphine (Handled+Morphine) as compared with uninjured animals that received saline (Handled+Saline; Figures 2A and C). Indeed, animals that received 3 days of saline reached 100% maximum possible analgesia (MPE) at the 8 mg/kg dose. In contrast, 100% MPE was not noted until the 18 mg/kg dose in animals that received 3 prior days of morphine. Neither the antinociceptive nor the antihyperalgesic potency of morphine was different in CFA+Morphine treated animals as compared with CFA+Saline treated animals (Figures 2B and D) indicating lack of tolerance development. Indeed, no differences in PWLs produced by morphine were noted for all doses tested. Together these data indicate that persistent inflammatory pain attenuates the development of tolerance to morphine. As glia activation in the spinal cord has been implicated in the development of morphine tolerance, the next series of experiments examined if glia were similarly activated within the vlPAG, and if persistent inflammatory pain altered their activation.
Figure 2.
PWL (in seconds) as a function of cumulative doses of morphine in handled (A & C), and CFA treated (B & D) male rats. Both ipsilateral (A & B) and contralateral (C & D) PWL data are presented. Animals received 3 consecutive days of morphine (5 mg/kg; sc, open circles) or saline (1 ml/kg; sc, filled circles). CFA+Morphine treated animals (n=7) did not differ from CFA+Saline treated animals (n= 4) in response to cumulative morphine on day 5 (ipsilateral; F1,9=1.128, p=0.3159 & contralateral; F1,9=1.470, p=0.2563). Handled+Morphine treated animals (n= 9) showed a significant decrease in PWL in response to cumulative morphine on day 5 as compared with Handled+Saline animals (n=5; ipsilateral; F1,12 =21.702, p=0.0006 & contralateral; F1,12 =20.373, p=0.0007).
Experiment 2: Morphine Tolerance is Associated with Increased Glial Cell Activation in the vlPAG
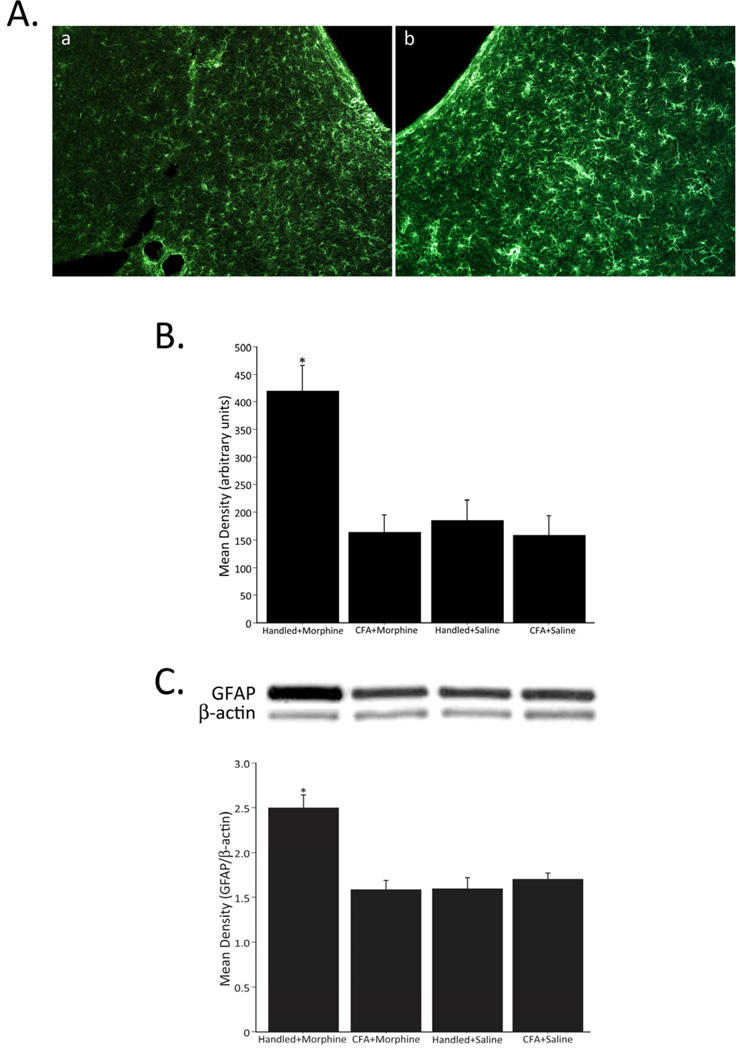
Increased activity of astrocytes, as evidenced by an increase in GFAP immunoreactivity, was only observed in non-CFA treated animals that received morphine (Handled +Morphine, Figure 3B). A representative example of vlPAG GFAP staining in animals administered CFA+Morphine versus Handled+Morphine is shown in Figure 3A. Western blots confirmed increased activity of astrocytes, as evidenced by an increase in relative band intensity of GFAP/ β-actin, was only observed in non-injured animals that received morphine (Handled+Morphine, Figure 3C).
Figure 3.
Representative fluorescent photomicrographs of GFAP immunoreactivity in the vlPAG of animals treated with CFA+Morphine (a) and Handled+Morphine (b) (A). Densitometry of GFAP immunoreactivity in the vlPAG (B). Administration of morphine, in the absence of CFA (Handled+Morphine; n=7), resulted in a significant increase in GFAP immunoreactivity within the vlPAG (F3,22=10.022, p=0.0002). No differences in GFAP levels were noted for the CFA+Morphine (n=11), CFA+Saline (n=4) or Handled+Saline control groups (n=4). Relative band intensity of GFAP/β-actin in the vlPAG (C). Administration of morphine, in the absence of CFA (Handled+Morphine; n=5), resulted in a significant increase in relative band intensity of GFAP/β-actin in the vlPAG (F3,19=10.256, p=0.0003). No differences in GFAP levels were noted for the CFA+Morphine (n=5), CFA+Saline (n=7) or Handled+Saline (n=6) control groups.
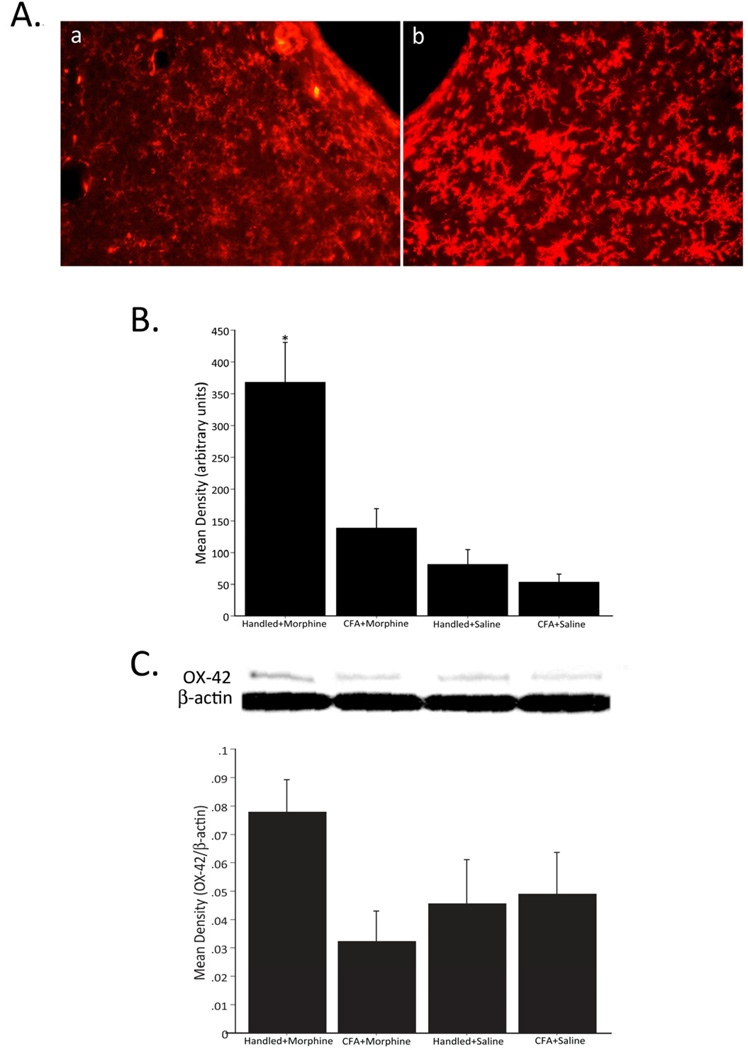
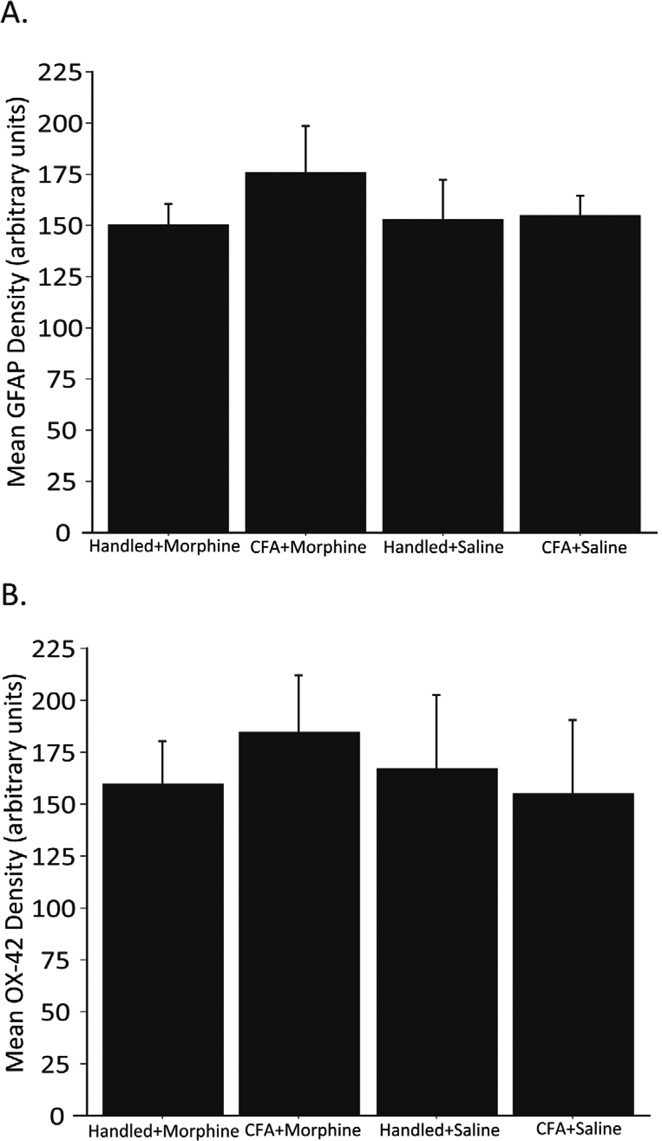
Similar to what was noted for astrocytes, microglia activity, as evidenced by OX-42 immunoreactivity, was significantly increased in animals that received morphine in the absence of pain (Handled+Morphine, Figure 4B). A representative example of vlPAG OX-42 staining in animals administered CFA+Morphine versus Handled+Morphine is shown in Figure 4A. A trend towards increased activity of microglia, as evidenced by an increase in relative band intensity of OX-42/β-actin, was only observed in animals that received morphine in the absence of pain (Handled+Morphine, Figure 4C); however, it did not reach statistical significance. Peripheral inflammation induced by intraplantar CFA did not illicit significant increases in vlPAG glial cell activity (Figures 3 and 4). Importantly, one injection of morphine (5 mg/kg) was not sufficient to alter vlPAG GFAP (Figure 5A) or OX-42 levels (Figure 5B) at 24 hours post-morphine. Similarly, no increase in GFAP or OX-42 levels were noted 1 hour post-morphine (data not shown). No significant group differences were noted in superior colliculus GFAP or OX-42 immunoreactivity (F3,23=2.089, p=0.1295 and F3,19=1.416, p=0.2690, respectively) or protein level (F3,28=0.232, p=0.8730 and F3,6=1.822, p=0.2435, respectively), indicating that changes in vlPAG glial cell activity are region specific (data not shown).
Figure 4.
Representative fluorescent photomicrographs of OX-42 immunoreactivity in the vlPAG of animals treated with CFA+Morphine (a) and Handled+Morphine (b) (A). Densitometry of OX-42 immunoreactivity in the vlPAG (B). Administration of morphine, in the absence of CFA (Handled+Morphine; n=8), significantly increased OX-42 immunoreactivity within the vlPAG (F3,19=9.270, p=0.0005). No differences in OX-42 levels were noted for the CFA+Morphine (n=7), CFA+Saline (n=4) or Handled+Saline (n=4) control groups. Relative band intensity of OX-42/β-actin in the vlPAG (C). Administration of morphine, in the absence of CFA (Handled+Morphine; n=5), resulted in an increase in relative band intensity of OX-42/β-actin in the vlPAG (F3,10=2.544, p=0.1151); however, it did not reach significance.
Figure 5.
Densitometry of GFAP (A) and OX-42 (B) immunoreactivity in the vlPAG in Handled+Morphine (n=3), CFA+Morphine (n=4), Handled+Saline (n=4), and CFA+Saline (n=5) animals 24 hours following one morphine or saline injection. Neither CFA nor morphine (5 mg/kg, sc) increased vlPAG GFAP (F(3,12)=0.494; p=0.693) or OX-42 (F(3,12)=0.162; p=0.9198) levels in the vlPAG as compared to handled and saline controls.
Discussion
The present experiments tested the hypothesis that vlPAG glial cell activity contributes to the development of morphine tolerance. Clinical studies indicate that chronic pain attenuates the development of morphine tolerance37, 77; however, animal studies have yielded variable results35, 65, 78. Therefore, the impact of persistent inflammatory hyperalgesia on morphine tolerance development and glial activation were also investigated. Here we report that (1) short-term daily administration of an ED50 dose of morphine was sufficient to induce morphine tolerance; (2) persistent inflammatory pain induced by intraplantar CFA significantly attenuated morphine tolerance; and (3) increased vlPAG microglia and astrocyte activity was only observed in those animals made tolerant to morphine. Together, these data suggest a potential role for vlPAG microglia and astrocytes in the development of morphine tolerance, and suggest that persistent inflammatory pain attenuates morphine tolerance by inhibiting morphine-induced vlPAG glial cell activation.
Increases in vlPAG microglia and astrocyte activity correlate with the development of morphine tolerance
Many mechanisms have been proposed to account for opioid tolerance, including decoupling, internalization and/or down-regulation of µ opioid receptors67, 68, upregulation of NMDA receptor function1–3, down-regulation of glutamate transporters9, 59, and production of nitric oxide (a known mediator of NMDAR function)84. These mechanisms were all thought to implicate some form of ‘neuronal adaptation’84. However, it is becoming increasingly clear that activated glia mediate many of these ‘neuronal adaptations’ that contribute to morphine tolerance84. Consistent with previous reports, here we find that tolerance to morphine developed rapidly in the absence of pain41, 42, 48. Indeed, one ED50 dose of morphine (5 mg/kg) injected subcutaneously for three days was sufficient to induce behaviorally defined tolerance. Paralleling the development of tolerance, GFAP and OX-42 protein levels increased significantly within the vlPAG, suggesting the activation of astrocytes and microglia, respectively.
A large body of evidence has accumulated implicating opioids as activators of spinal astrocytes and microglia31, 60, 63, 86. In both mice and rats, morphine increases spinal GFAP and OX-42 protein levels,20, 33 as well as glially derived proinflammatory cytokines32, 63. Inhibition of spinal glia or cytokine release increases the analgesic efficacy of morphine32, 63, and attenuates morphine tolerance31, 51, 52, 60, 70. Our novel findings in the vlPAG parallel the data from studies of spinal cord glia, and indicate that supraspinal glial cell activity may also contribute to the development of morphine tolerance.
Under basal conditions glia survey the environment for pathogens, debris, and regulate ion and neurotransmitter levels in the synapse to modulate neuronal excitability86. The activation of glia results in the release of excitatory substances that oppose morphine analgesia (e.g., proinflammatory cytokines)86. Glial release of cytokines increases with chronicity of morphine administration84, making these excitatory substances key players in the development of morphine tolerance. Glially-derived cytokine release, particularly tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β), results in increased density and conductance of neuronal AMPA57, 71 and NMDA79 receptors, decreased astrocytic glutamate transporter proteins (GLT-1, GLAST)86, and down-regulation of neuronal GABA receptors71. These cytokine-induced changes, among others39, 55, effectively increase neuronal excitability. Morphine binds to neuronal µ opioid receptors (MOR) in the vlPAG that are primarily located on GABAergic neurons14, 18, 38; MOR binding in the vlPAG disinhibits GABAergic PAG-RVM projection neurons25, resulting in the net excitation of the PAG-RVM-spinal cord descending pain modulatory circuit. Glial-induced increases in the excitability of vlPAG MOR-containing neurons may act to alter the inhibitory properties of morphine, thereby decreasing analgesic efficacy and contributing to the development of morphine tolerance.
Persistent inflammatory pain prevented morphine-induced increases in vlPAG glial cell activity and attenuated the development of morphine tolerance
The results of the present study demonstrate that the presence of persistent pain alters both the development of morphine tolerance and morphine-induced vlPAG glial cell activation. The finding that persistent peripheral inflammation attenuates morphine tolerance is consistent with the clinical literature demonstrating that opioid tolerance is attenuated in chronic pain sufferers17, 23, 26. Indeed, clinical studies have repeatedly shown that morphine tolerance develops most robustly in those individuals consuming morphine in the absence of pain19, 54. In the present study, male rats given CFA 24 hours before the 3-day morphine administration regimen showed significant increases in analgesia to all challenge doses of morphine, as compared with non-injured animals. Several factors may contribute to the pain-induced attenuation in morphine tolerance. First, morphine, given in conjunction with peripheral inflammation, failed to illicit the increases in vlPAG microglia and astrocyte activity observed in non-CFA treated animals given morphine. Indeed, peripheral inflammatory pain blocked both morphine tolerance and morphine-induced glial cell activation within the vlPAG. As glia are not activated, no cytokine release would be expected, and, therefore, no net change in neuronal excitability. Alternatively, cannabinoids, which are released within the PAG during peripheral pain80, have been shown to influence both glial activity and morphine analgesia. Cannabinoid receptor is robustly expressed within the vlPAG, with approximately 32% of cannabinoid receptor 1 (CB1) expressing neurons also expressing µ opioid receptor88. Functionally, intra-PAG administration of a CB1 agonist enhances morphine analgesia87, and systemic administration of cannabinoids, along with morphine, leads to the attenuation of morphine tolerance15, 69, 87. Endocannabinoids also possess potent anti-inflammatory properties22, which would likely block the activation of glia. Indeed, systemic administration of the cannabinoid receptor agonist WIN 55,212-2 prevents microglia and astrocyte activation, and decreases the release of the proinflammatory cytokines interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in the spinal cord10.
In the present study, no glia activation was noted following administration of CFA alone. These results are in contrast with previous reports that peripheral pain, including CFA13, 50, 62, 72, peripheral neuropathy16, 21, 27, 62, 73, 74, 83, formalin45, and spinal nerve ligation30 induce significant glia activation within the spinal cord. However, given the roles of the spinal cord and PAG in pain facilitation and pain modulation, respectively, it is not entirely surprising that there would be differential pain-induced regulation of glial activation in these two sites. Together, these studies suggest that inflammation elicits differential glial responses in a CNS region-dependent manner, and prevents morphine-induced increases in vlPAG glial cell activity.
How opioids activate glia
Opioid hyperalgesia is still observed in neuronal opioid receptor (mu, delta, and kappa) knockout mice36, suggesting that the anti-analgesic affects of morphine (e.g., anti-analgesia and tolerance) are mediated by non-neuronal opioid receptors. Indeed, it was recently discovered that morphine analgesia is modulated not only by classical neuronal opioid receptors but also by non-classical glial receptor activity34. Opioids have been shown to bind to Toll-like receptor 4 (TLR4)34, an innate immune receptor located on microglia and astrocytes, and an abundance of evidence has accumulated indicating that TLR4 activity opposes morphine analgesia34, 86. Functionally, animals that receive TLR4 antagonism, as well as TLR4 knockout mice, exhibit increased responsiveness to the analgesic properties of acute morphine administration86. Similarly, systemic administration of TLR4 antagonists attenuates morphine tolerance34. To date, the specific role of TLR4 in morphine tolerance development has not been elucidated. However, given our findings that the development of morphine tolerance correlates with increased vlPAG glial cell activation, and the evidence showing that TLR4 is expressed on rat PAG glia12, 40, future studies investigating the potential role of vlPAG TLR4 in the development of morphine tolerance are warranted.
Conclusions
There is extensive literature supporting a critical role for glial cell activation in the development of morphine tolerance. Our findings that increased vlPAG glial activity is concurrent with the development of morphine tolerance, and that pain inhibits both vlPAG glial reactivity and morphine tolerance development suggests that vlPAG glia play a significant role in the development of morphine tolerance. Taken together, our results may provide a direct neurobiological mechanism whereby chronic inflammatory pain attenuates the development of morphine tolerance, and implicate vlPAG glial cells as key regulators of this phenomenon.
ACKNOWLEDGEMENTS
National Institutes of Health grant DA16272 awarded to AZM supported this work. Morphine sulfate was kindly provided by the National Institute on Drug Abuse (NIDA) drug supply program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES. The authors have no financial conflict of interest.
Literature Cited
- 1.Adam F, Bonnet F, Le Bars D. Tolerance to morphine analgesia: Evidence for stimulus intensity as a key factor and complete reversal by a glycine site-specific nmda antagonist. Neuropharmacology. 2006;51:191–202. doi: 10.1016/j.neuropharm.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Adam F, Dufour E, Le Bars D. The glycine site-specific NMDA antagonist (+)-ha966 enhances the effect of morphine and reverses morphine tolerance via a spinal mechanism. Neuropharmacology. 2008;54:588–596. doi: 10.1016/j.neuropharm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Allen RM, Dykstra LA. Role of morphine maintenance dose in the development of tolerance and its attenuation by an NMDA receptor antagonist. Psychopharmacology (Berl) 2000;148:59–65. doi: 10.1007/s002130050025. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Fields HL. Endogenous pain control mechanisms: Review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 5.Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 6.Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- 7.Beitz AJ. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivisions. J Comp Neurol. 1985;237:445–459. doi: 10.1002/cne.902370403. [DOI] [PubMed] [Google Scholar]
- 8.Bernabei R, Gambassi G. The sage database: Introducing functional outcomes in geriatric pharmaco-epidemiology. J Am Geriatr Soc. 1998;46:251–252. doi: 10.1111/j.1532-5415.1998.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 9.Bogulavsky JJ, Gregus AM, Kim PT, Costa AC, Rajadhyaksha AM, Inturrisi CE. Deletion of the glutamate receptor 5 subunit of kainate receptors affects the development of morphine tolerance. J Pharmacol Exp Ther. 2009;328:579–587. doi: 10.1124/jpet.108.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgos E, Gomez-Nicola D, Pascual D, Martin MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist win 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol. 2012;682:62–72. doi: 10.1016/j.ejphar.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter S, Carlson T, Dellacasagrande J, Garcia A, Gibbons S, Hertzog P, Lyons A, Lin LL, Lynch M, Monie T, Murphy C, Seidl KJ, Wells C, Dunne A, O'Neill LA. Tril, a functional component of the TLR4 signaling complex, highly expressed in brain. J Immunol. 2009;183:3989–3995. doi: 10.4049/jimmunol.0901518. [DOI] [PubMed] [Google Scholar]
- 13.Chen FL, Dong YL, Zhang ZJ, Cao DL, Xu J, Hui J, Zhu L, Gao YJ. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res Bull. 2012;87:60–66. doi: 10.1016/j.brainresbull.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- 16.Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 17.Collett BJ. Opioid tolerance: The clinical perspective. Br J Anaesth. 1998;81:58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of muopioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Cowan DT, Allan LG, Libretto SE, Griffiths P. Opioid drugs: A comparative survey of therapeutic and"street" use. Pain Med. 2001;2:193–203. doi: 10.1046/j.1526-4637.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 21.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 22.Downer EJ. Cannabinoids and innate immunity: Taking a toll on neuroinflammation. ScientificWorldJournal. 2011;11:855–865. doi: 10.1100/tsw.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: Immpact recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Fields HL, Basbaum AI. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- 25.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 26.Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. Morphidex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: Three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain. 2005;115:284–295. doi: 10.1016/j.pain.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Gazda LS, Milligan ED, Hansen MK, Twining CM, Poulos NM, Chacur M, O'Connor KA, Armstrong C, Maier SF, Watkins LR, Myers RR. Sciatic inflammatory neuritis (sin): Behavioral allodynia is paralleled by peri-sciatic proinflammatory cytokine and superoxide production. J Peripher Nerv Syst. 2001;6:111–129. doi: 10.1046/j.1529-8027.2001.006001111.x. [DOI] [PubMed] [Google Scholar]
- 28.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 30.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 31.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwai S, Kiguchi N, Kobayashi Y, Fukazawa Y, Saika F, Ueno K, Yamamoto C, Kishioka S. Inhibition of morphine tolerance is mediated by painful stimuli via central mechanisms. Drug Discov Ther. 2012;6:31–37. [PubMed] [Google Scholar]
- 36.Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–444. doi: 10.1016/j.neuroscience.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to gabaergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol. 1998;392:528–547. [PubMed] [Google Scholar]
- 39.Kleibeuker W, Ledeboer A, Eijkelkamp N, Watkins LR, Maier SF, Zijlstra J, Heijnen CJ, Kavelaars A. A role for g protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci. 2007;25:1696–1704. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- 40.Laflamme N, Rivest S. Toll-like receptor 4: The missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. Faseb J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 41.Lane DA, Tortorici V, Morgan MM. Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience. 2004;125:63–69. doi: 10.1016/j.neuroscience.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis ME, Khachaturian H, Schafer MK, Watson SJ. Anatomical approaches to the study of neuropeptides and related mrna in the central nervous system. Res Publ Assoc Res Nerv Ment Dis. 1986;64:79–109. [PubMed] [Google Scholar]
- 45.Lin T, Li K, Zhang FY, Zhang ZK, Light AR, Fu KY. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192:40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: A potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: A potential mechanism for sex differences in antinociception. Neuroscience. 2007;147:456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi WL, Mao-Ying QL, Wang XW, Li X, Yang CJ, Jiang JW, Yu J, Wang J, Liu Q, Wang YQ, Wu GC. Involvement of spinal neurotrophin-3 in electroacupuncture analgesia and inhibition of spinal glial activation in rat model of monoarthritis. J Pain. 2011;12:974–984. doi: 10.1016/j.jpain.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- 52.Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun. 2009;23:75–84. doi: 10.1016/j.bbi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morioka N, Inoue A, Hanada T, Kumagai K, Takeda K, Ikoma K, Hide I, Tamura Y, Shiomi H, Dohi T, Nakata Y. Nitric oxide synergistically potentiates interleukin-1 beta-induced increase of cyclooxygenase-2 mRNA levels, resulting in the facilitation of substance p release from primary afferent neurons: Involvement of cgmp-independent mechanisms. Neuropharmacology. 2002;43:868–876. doi: 10.1016/s0028-3908(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 56.Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- 57.Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (tnf-alpha) induces rapid insertion of CA2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-a/k) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1977. [DOI] [PubMed] [Google Scholar]
- 59.Popik P, Kozela E, Pilc A. Selective agonist of group ii glutamate metabotropic receptors, ly354740, inhibits tolerance to analgesic effects of morphine in mice. Br J Pharmacol. 2000;130:1425–1431. doi: 10.1038/sj.bjp.0703438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: Mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 62.Raghavendra V, Tanga FY, DeLeo JA. Complete freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the cns. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 63.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawalinduced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- 64.Rauck RL. What is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9:468–479. doi: 10.1111/j.1533-2500.2009.00320.x. [DOI] [PubMed] [Google Scholar]
- 65.Romero A, Hernandez L, Garcia-Nogales P, Puig MM. Deletion of the inducible nitric oxide synthase gene reduces peripheral morphine tolerance in a mouse model of chronic inflammation. Fundam Clin Pharmacol. 2010;24:317–323. doi: 10.1111/j.1472-8206.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 66.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: Implications for clinical management. Anesth Analg. 2004;99:510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. table of contents. [DOI] [PubMed] [Google Scholar]
- 67.Sim-Selley LJ, Scoggins KL, Cassidy MP, Smith LA, Dewey WL, Smith FL, Selley DE. Region-dependent attenuation of mu opioid receptor-mediated G-protein activation in mouse CNS as a function of morphine tolerance. Br J Pharmacol. 2007;151:1324–1333. doi: 10.1038/sj.bjp.0707328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res. 2002;958:28–35. doi: 10.1016/s0006-8993(02)03394-2. [DOI] [PubMed] [Google Scholar]
- 69.Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 71.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 73.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- 74.Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, Deleo JA. Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience. 2006;140:1003–1010. doi: 10.1016/j.neuroscience.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 75.Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- 76.Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, Jasper JF, Singh V, Jordan AE, Johnson BW, Cicala RS, Dunbar EE, Helm S, 2nd, Varley KG, Suchdev PK, Swicegood JR, Calodney AK, Ogoke BA, Minore WS, Manchikanti L. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician. 2006;9:1–39. [PubMed] [Google Scholar]
- 77.Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11:S181–S200. [PubMed] [Google Scholar]
- 78.Uhelski ML, Boyette-Davis JA, Fuchs PN. Chronic inflammatory pain does not attenuate the development of tolerance to chronic morphine in adult male rats. Pharmacol Biochem Behav. 2011;98:325–330. doi: 10.1016/j.pbb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances nmda receptor-mediated intracellular calcium increase through activation of the SRC family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci U S A. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 83.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: New players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 84.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: Novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman cousins lecture. Glia as the "bad guys": Implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The"toll" of opioid-induced glial activation: Improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson AR, Maher L, Morgan MM. Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology. 2008;55:1219–1225. doi: 10.1016/j.neuropharm.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. 2012;213:191–200. doi: 10.1016/j.neuroscience.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]