Abstract
Dopamine (DA) neurons in sporadic Parkinson disease (PD) display dysregulated gene expression networks and signaling pathways that are implicated in PD pathogenesis. Micro (mi)RNAs are regulators of gene expression, which could be involved in neurodegenerative diseases. We determined the miRNA profiles in laser microdissected DA neurons from postmortem sporadic PD patients’ brains and age-matched controls. DA neurons had a distinctive miRNA signature and a set of miRNAs was dysregulated in PD. Bioinformatics analysis provided evidence for correlations of miRNAs with signaling pathways relevant to PD, including an association of miR-126 with insulin/IGF-1/PI3K signaling. In DA neuronal cell systems, enhanced expression of miR-126 impaired IGF-1 signaling and increased vulnerability to the neurotoxin 6-OHDA by downregulating factors in IGF-1/PI3K signaling, including its targets p85β, IRS-1, and SPRED1. Blocking of miR-126 function increased IGF-1 trophism and neuroprotection to 6-OHDA. Our data imply that elevated levels of miR-126 may play a functional role in DA neurons and in PD pathogenesis by downregulating IGF-1/PI3K/AKT signaling and that its inhibition could be a mechanism of neuroprotection.
Keywords: miRNAs, miR-126, dopamine neurons, Parkinson disease, insulin, IGF-1 signaling, PI3K, laser capture microdissection, postmortem, 6-OHDA neurotoxicity, cell systems
1. Introduction
Parkinson disease (PD) is a severe neurological disorder and one of its main characteristics is the progressive loss of substantia nigra (SN) dopamine (DA) neurons (Braak and Del Tredici, 2008). Its pathogenesis is characterized by genetic and environmental factors, which affect key signaling pathways in DA neuronal cell function (Schapira and Jenner, 2011, Wirdefeldt, et al., 2011). Recently we, and others, determined the gene expression profiles of laser microdissected (LMD) SN DA neurons from sporadic PD patients demonstrating a prominent dysregulation of expression networks that are related to major signaling pathways in PD pathogenesis (Cantuti-Castelvetri, et al., 2007, Elstner, et al., 2011, Simunovic, et al., 2009, Simunovic, et al., 2010). The mechanisms of these pathway dysregulations, however, are largely unknown.
Micro (mi)RNAs are short non-coding RNAs that regulate gene expression on the pre- or post transcriptional level (Bartel, 2009). Evidence suggests that these molecules are involved in the pathology of neurodegenerative diseases, including PD, but their functions are still poorly understood (Du and Pertsemlidis, 2011, Eacker, et al., 2009, Sonntag, 2010, Sonntag, et al., 2012). Here, we hypothesized that miRNAs might be factors in the dysregulated gene expression network in PD-affected DA neurons. In the current study, we show that SN DA neurons have a unique miRNA expression profile, and that miRNAs were dysregulated in PD. One miRNA, miR-126, that was upregulated in PD has previously been associated with Insulin-like growth factor/Phosphoinositide 3-kinase (IGF-1/PI3K) signaling in endothelial cells, cancer, hepato- and myocytes (reviewed in Sonntag, et al., 2012), and this pathway has also been implicated in the pathogenesis of aging and neurodegenerative diseases, including Alzheimer’s disease (AD) and PD (Bomfim, et al., 2012, Craft and Watson, 2004, de la Monte, 2012, Deak and Sonntag, 2012, Kim and Feldman, 2012, Talbot, et al., 2012, Torres-Aleman, 2010). Functional analysis of miR-126 in DA neuronal cell systems demonstrated that its overexpression conveys neurotoxicity by impairing IGF-1/PI3K/AKT signaling, while its inhibition increases the trophic effects of IGF-1 and confers neuroprotection. Altogether, our data point to a novel mechanism in DA neurons to modulate the insulin/IGF-1 signaling pathway and vulnerability or protection to toxic insult by miR-126, and suggest a functional role of miRNAs in the pathogenesis of PD.
2. Method
2.1. Subjects, material collection, and miRNA profiling
Subjects are listed in Supplementary Table 1 and additional information is publicized at the National Brain Databank (http://national_databank.mclean.harvard.edu/brainbank) or has been published elsewhere (Simunovic, et al., 2009, Simunovic, et al., 2010). Eight normal controls and eight PD samples (5 males and 3 females in each group) with an average age of 74.8 and 78.6, respectively, were used for miRNA profiling. An additional sample per group (C11 and PD12) (Supplementary Table 1) was used to establish the methodology and determine reproducibility of the miRNA arrays. Material collection, preparation, and data generation for the miRNA arrays were conducted according to (Benes, et al., 2007, Pietersen, et al., 2011, Simunovic, et al., 2009, Simunovic, et al., 2010). The Human MicroRNA TaqMan® qRT-PCR Arrays A v1 or 2.0 (Life Technologies, Foster City, CA) were used for miRNA analysis of total RNA derived from the laser-captured DA neurons. miRNAs were analyzed according to published protocols using RNU44 or global mean normalization, or ABI’s R package for quantitative real-time polymerase chain reaction (qRT-PCR) analysis (ABqPCR) in Bioconductor (D’Haene, et al., 2012, Deo, et al., 2011, Mestdagh, et al., 2009). Only miRNAs with cycle threshold (Ct) values < 35 were included in the analyses.
2.2. Computational data analysis
Potential miRNA targets were identified using TargetScan (http://www.targetscan.org) (Friedman, et al., 2009, Grimson, et al., 2007, Lewis, et al., 2005) and TargetCombo (http://diana.pcbi.upenn.edu/cgi-bin/TargetCombo.cgi) (Sethupathy, et al., 2006), and subjected to negative correlation enrichment analysis based on their individual miRNA and mRNA expression profiles using data from previously published microarray analysis (Simunovic, et al., 2009, Simunovic, et al., 2010). Pathway enrichment was conducted according to previously published methodologies (Simunovic, et al., 2009, Simunovic, et al., 2010, Yi, et al., 2009) using Biocarta, GOBP, GSEA, KEGG, and WIKI. Data mining was performed on published literature and using resources such as the MIRECORDS database (http://mirecords.umn.edu) (Xiao, et al., 2009), National Center for Adult Stem Cell Research - Parkinson’s Disease Review Database (https://ncascr.griffith.edu.au/pdreview/2009) and www.pdgene.org.
2.3. Construction of lentivirus vectors and cell transduction
The third generation lentivirus system was kindly provided by Drs. D. Trono and R. Zufferey, University of Geneva, Switzerland (Zufferey, et al., 1998, Zufferey, et al., 1997). For conditional and cell-specific expression of miR-126, two lentivirus vectors were constructed: The first contained a modified Tet-On reverse transactivator rtTA3 (kindly provided by Dr. A. Das, University of Amsterdam, The Netherlands (Das, et al., 2004)) driven by either phosphoglycerine kinase (PGK) or Synapsin promoter (kindly provided by Dr. Atsushi Miyanohara (UCSD)), and an Internal Ribosomal Entry Site – green fluorescent protein (IRES-GFP) cassette downstream of the rtTA3 cDNA (Lenti.PGK/Syn.rtTA3.IRES.GFP). The second virus contained the Tet response element promoter 2 (pTRE2; Clontech Laboratories, Mountain View, CA) and approximately 270 to 300 bp upstream and downstream sequences of either rat or human miR-126 pre-miRNA (Lenti.pTRE2.rno-/hsa-miR-126). The following primers were used to amplify the miRNA sequences from genomic DNA by PCR (given without flanking sequences for restriction sites): hsa-miR-126 5′: ACAGGTAAACAGCCCTGGCTGTG; hsa-miR-126 3′: CTTCATTGCACTGTCCACTCCTG; rno-miR-126 5′: GCACTATGCTGAGGGCTGATTC; rno-miR-126 3′: TTCTACACCTCCTCTCTCACC. All cloning experiments were based on standard molecular biological techniques. Virus production and cell transductions were performed as described (Sastry, et al., 2002, Seo, et al., 2004, Seo, et al., 2007). Cell transductions were performed with multiplicity of infections (MOI) of 10–20 in the presence of 5–7 μg/ml hexadimethrine bromide (Polybrene; Sigma Aldrich, St. Louis, MO). Cells were incubated with virus and polybrene for 5–6 hrs before changing to fresh media. Expression of virus vectors were determined by GFP fluorescence and qRT-PCR using the miR-126 TaqMan® MicroRNA Assay from Life Technologies Corporation (Cat. # 4427975). Transduced SH-SY5Y and PC12 cells were expanded and stocks were frozen. Cell batches were frequently monitored for GFP and miR-126 expression.
2.4. Cell culture and determination of cell proliferation and toxicity
SH-SY5Y human neuroblastoma and rat PC12 cells were obtained from ATCC (Manassas, VA) and differentiated according to (Presgraves, et al., 2004) or (Chung, et al., 2010), respectively. For assessing cell proliferation, virus-transduced cells were plated at 5,000–8,000 cells/well in 96-well plates and serum-starved for 24 hrs before treatment with different concentrations of IGF-1 (Peprotech, Rocky Hill, NJ) for 48 hrs. miR-126 expression was induced by 200 ng/ml doxycycline (Dox; Sigma, St Louis, MO) at cell plating. In the toxicity assays, undifferentiated or differentiated neuroblastoma cells were treated with 20 ng/ml IGF-1 for 24 hrs (SH-SY5Y), or in case of PC12 cells, throughout the entire (full) or only in the second phase of cell differentiation (half) (see Supplementary Fig. 5A), followed by different concentrations of 6-OHDA (Sigma) for 24 hrs. miR-126 expression was induced during cell differentiation and 6-OHDA treatment with 200 ng/ml Dox. For miRNA inhibition, cells were transfected with 100 nM scrambled controls or miR-126 targeting Locked Nucleic Acids (LNA™, Exiqon, Woburn, MA) using lipofectamine (Life Technologies).
Rat ventral mesencephalon (VM) primary neurons were obtained from embryonic day 14 (E14) rat embryos (Sprague-Dawley, Charles River, MA) as previously described (Chung, et al., 2005). Cells were transduced with lentiviruses 8 days after plating, in the absence or presence of 200 ng/ml Dox. Six days after transduction, the cells were treated with 0, 50, or 90 μM 6-OHDA and 20 ng/ml IGF-1 was added 2 days prior to 6-OHDA treatment in serum-free conditions.
Determination of cell proliferation was performed by adding 15 μl of MTT solution (Sigma) into each well followed by incubation for 3.5 hrs at 37 °C. MTT formazan crystals were dissolved in 150 μl of MTT solvent (4 mM HCl and 0.1 % Nonidet P-40 in isopropanol), and the absorbance was measured at 570 nm after 15 min incubation at room temperature. Cell viability in the toxicity assays was determined using the activity of lactate dehydrogenase (LDH) in collected cell culture medium, according to the manufacturer’s instructions (Roche, Indianapolis, IN). LDH activities of control or 6-OHDA treated cells were measured by absorbance at 450 nm.
2.5. Protein sample preparation and Western blot
Protein sample preparation and Western blots were performed as previously described (Seo, et al., 2004) using the following primary antibodies: PI3-kinase p85α (1:1,250; Millipore, Billerica, MA), PI3-kinase p85β (1:1,250; Santa Cruz Biotechnology, Santa Cruz, CA), IRS-1 (1:1,250; Cell Signaling, Danvers, MA), SPRED1 (1:1,250; Abcam, MA, UK), AKT, phospho-AKT, ERK, phospho-ERK (1:1,250; Cell Signaling), and β-actin (1:10,000; Covance, Princeton, NJ), and Alkaline phosphatase (AP)-conjugated anti-mouse or -rabbit secondary antibodies (1:2,500; Invitrogen), and Immun-Star™ AP Substrate (Bio-Rad, Hercules, CA) for protein detection. Quantification of the immunoreactive bands was performed using ImageJ (NIH, http://rsb.info.nih.gov/ij/). Experiments were performed at least in triplicate.
2.6. Immunocytochemistry and cell counting
Cells were fixed in 4% paraformaldehyde (Fisher Scientific) and rinsed with phosphate-buffered saline (PBS). Cells were then incubated with blocking buffer (10 % normal goat serum and 0.1 % Triton X-100) for 30 min at room temperature. Immunostaining was performed using primary antibodies against tyrosine hydroxylase (TH, 1:1,000; Pel-Freez, Rogers, AR) and green fluorescence protein (GFP, 1:500; Millipore), followed by incubation in Alexa Fluor 488 or Alexa Fluor 568 conjugated anti-mouse or -rabbit secondary antibodies (1:1,000; Invitrogen). After counterstaining with 1 μg/ml Hoechst (Sigma) for 2 min, cover glasses were mounted onto glass slides using Gel-Mount anti-fade media (Electron Microscopy Sciences, Hatfield, PA). Total numbers of TH+ or TH+/GFP− neurons were counted from images taken with an inverted Zeiss Axiovision microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) connected to a fluorescence light source and digital camera (Zeiss AxioCam HRc). Five to 72 images at 30x magnification that contain at least one TH+ neuron were taken for each coverslip and condition. Three investigators, blinded to the treatment groups, independently performed counting and duplicate analyses.
2.7. Statistical analysis
Microsoft Excel software (Microsoft Corp., Redmond, WA) was used for statistical analysis. Data were compared between different experimental groups or within a group using Student’s t-test. Differences of comparison were considered statistically significant when P values were less than 0.05 (P < 0.05).
3. Results
3.1. SN DA neurons have a distinctive miRNA expression profile that is dysregulated in PD
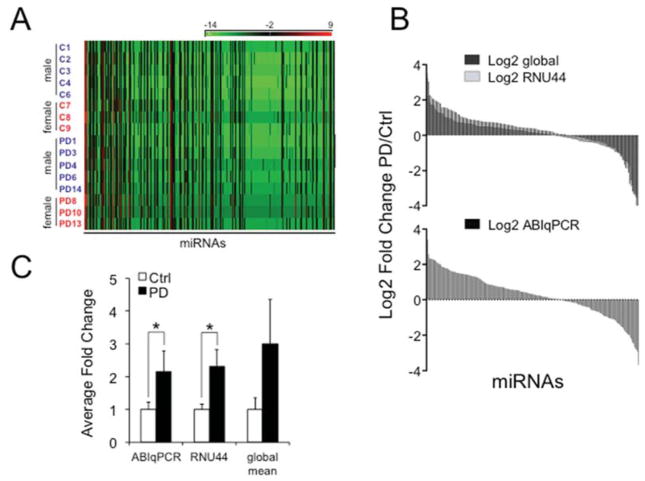
We first established the technology to detect miRNAs in LMD DA neurons from one normal control and one PD patient’s brain. Direct comparison of Ct values between two replicates in each group showed high reproducibility with > 95% (R = 0.954) overlap for the samples from normal controls and > 96% (R = 0.967) for those from PD brains (Supplementary Fig. 1A, B). We then determined the expression profiles from 8 normal controls and 8 PD brain samples (5 males and 3 females in each group) (Supplementary Table 1). Analysis of ΔCt values demonstrated that the DA neurons had a distinctive miRNA signature (Fig. 1A), a high correlation of expression profiles between samples within each group, and different expression levels for individual samples within and across each group (Supplementary Fig. 1C, D). To assess miRNA expression levels we used three independent normalization methods according to published literature (D’Haene, et al., 2012, Deo, et al., 2011, Mestdagh, et al., 2009), including the endogenous snoRNA RNU44, global mean normalization, and ABI’s R package for qRT-PCR analysis (ABqPCR). Normalized values with a cut-off of Ct < 35 were then used to determine fold changes (FC) of miRNA expression between the normal control and PD samples. These data revealed high overlap of average FC in all methods, a set of significantly dysregulated miRNAs, and a trend of more up- than downregulated miRNAs in PD (Fig. 1B).
Figure 1.
miRNA expression profiles in LMD DA neurons. (A) Heat maps of 379 miRNAs for 8 control and 8 PD samples after profiling with the TaqMan® Human MicroRNA A Array v2.0 plotted as −ΔCt values (−(Ct miRNA − Ct RNU44)) according to miRNA numbers show that aged human postmortem DA neurons have a distinctive miRNA expression profile. (B) Average fold change (FC) expression for each miRNA with Ct values < 35 after data analysis with three independent methods according to published protocols (D’Haene, et al., 2012, Deo, et al., 2011, Mestdagh, et al., 2009). miRNAs were normalized using the endogenous control RNU44, global mean, or ABIqPCR and average FC between controls and PD samples are plotted as Log2 scales. In all methods there was a trend of more up- than downregulated miRNAs in PD. (C) Average FC for miR-126 based on ABIqPCR (*P = 0.022), RNU44 (*P = 0.03), and global mean (not significant) normalization.
3.2. miR-126 is upregulated and can be associated with dysregulated IGF-1/PI3K signaling in PD DA neurons
To determine potential associations of miRNAs with the gene expression networks in the DA neurons, we performed negative correlation enrichment analysis linking miRNAs with mRNA targets that are dysregulated in the same cells (Simunovic, et al., 2009, Simunovic, et al., 2010), followed by pathway enrichment analysis. These studies demonstrated that a set of miRNAs had significant negative correlations with terms that are associated with dysregulated gene expression networks related to PD. This included the insulin/IGF-1 and PI3K signaling pathways (Supplementary Fig. 2A). miR-126 was one of the miRNAs that were upregulated in the PD samples (Fig. 1C) and negatively or positively correlated with some of its targets, including p85β (phosphoinositide-3-kinase regulatory subunit 2, PIK3R2) (Fish, et al., 2008, Guo, et al., 2008, Zhu, et al., 2011), insulin receptor substrate 1 (IRS-1) (Ryu, et al., 2011, Zhang, et al., 2008), regulator of v-crk sarcoma virus CT10 oncogene homolog (CRK) (Crawford, et al., 2008, Guo, et al., 2008), and Myb protein 1 (TOM1) (Oglesby, et al., 2010) (reviewed in Sonntag, et al., 2012) (Supplementary Fig. 2B).
3.3. Overexpression of miR-126 in VM neurons increases their vulnerability to 6-OHDA toxicity
To identify a causal relationship of miR-126 with Insulin/IGF-1/PI3K signaling in the DA neuronal context, we generated a Dox-inducible lentivirus system (Supplementary Fig. 3) and cell-specifically expressed GFP and miR-126 using the Synapsin promoter in rat E14 VM cells (Fig. 2A). After cell transduction, 73% of TH+ neurons expressed GFP (TH+/GFP+) and the Dox-induced expression levels of miR-126 in the virus transduced cultures were 3 fold (Fig. 2B, C). Cells were then treated with the neurotoxin 6-OHDA and IGF-1, and cell numbers were quantified. We found less numbers of TH+ DA neurons in response to 6-OHDA when miR-126 was overexpressed, and miR-126 expressing cells were less protected by IGF-1 than controls (Fig. 2D and Supplementary Fig. 4, upper panel). These effects were specific to miR-126 transduced cells, since no differences in cell counts were seen for untransduced TH+ neurons (TH+/GFP-) between virus control and miR-126 conditions (Supplementary Fig. 4, lower panel).
Figure 2.
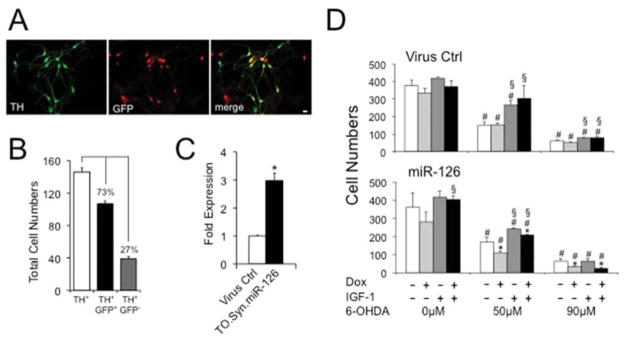
Overexpression of miR-126 compromises survival of VM primary DA neurons. (A) TH+ DA neurons (green) transduced with Syn.rtTA3.IRES.GFP (Virus Ctrl) or the dual TetOn.Syn.miR-126 virus system (miR-126) express GFP (red). Images are at 40 X magnification and scale bars represent 20 μm. (B) 73% of all TH+ cells were virus-transduced (TH+GFP+). (C) In TetOn.Syn.miR-126 transduced cultures, miR-126 expression is 3-fold upregulated in the presence of 200 ng/ml Dox compared to Syn.rtTA3.IRES.GFP transduced controls (*P < 0.05). (D) Transduced cells were cultured in the absence or presence of 50 or 90 μM 6-OHDA, 200 ng/ml Dox, or 20 ng/ml IGF-1, and TH+ cell numbers were counted. Shown are data from one out of two experiments (*P < 0.05 comparing Dox-treated (Dox+) to Dox-untreated (Dox−) condition; #P < 0.05 comparing 6-OHDA-treated to 6-OHDA-untreated (0 μM) condition; §P < 0.05 comparing IGF-1-treated (IGF-1+) to IGF-1-untreated (IGF-1-) condition).
3.4. Overexpression of miR-126 reduces and its inhibition promotes the trophic effects of IGF-1 in neuroblastoma cells
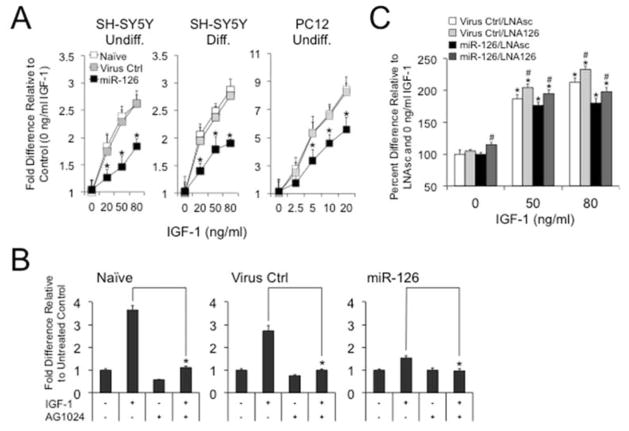
To gain further insight into the molecular functions of miR-126, we analyzed its effects on IGF-1 signaling in SH-SY5Y or PC12 cell lines that were generated with the inducible miR-126 virus system (Supplementary Fig. 3). When we treated the cells with IGF-1, we found that immature or retinoic acid (RA) and 12-O-tetradecanoylphorbol-13-acetate (TPA) differentiated SH-SY5Y cells exhibited a reduction in trophic support when miR-126 was induced with Dox, and similar results were obtained from PC12 cells (Fig. 3A). These effects could be abrogated when cells were treated with the IGF-1 receptor (IGF-1R) antagonist AG1024 (Fig. 3B) demonstrating that miR-126 specifically acts on the IGF-1 signaling pathway. Moreover, both miR-126 transduced and control SH-SY5Y cells that were transfected with miR-126 targeting LNAs exhibited an increase in IGF-1 activated cell proliferation (Fig. 3C).
Figure 3.
Overexpression of miR-126 impairs and its inhibition promotes IGF-1 signaling in neuroblastoma cells. (A) MTT assays on SH-SY5Y or PC12 cells that are transduced with the dual virus system show reduced cell proliferation in response to IGF-1 when miR-126 expression is induced by 200 ng/ml Dox. Results are plotted as fold differences relative to 0 ng/ml IGF-1 for Dox+ over Dox− conditions (*P < 0.05 comparing miR-126 to Naïve or Virus Ctrl.). (B) The effects of IGF-1 could be abrogated by AG1024. Dox-treated (200 ng/ml) undifferentiated SH-SY5Y cells were cultured in the absence or presence of 80 ng/ml IGF-1 or 1 μM AG-1024 and cell proliferation was measured by MTT. Data are plotted as fold differences relative to untreated controls (*P < 0.05 comparing IGF-1+ to IGF-1− condition). (C) Inhibition of miR-126 increases trophic support of IGF-1. Dox-treated Virus Ctrl and miR-126 transduced cells were transfected with scrambled or miR-126 targeting LNAs (LNAsc and LNA126, respectively) and cultured in the absence or presence of IGF-1. Data are plotted as percent cell death relative to 0 ng/ml IGF-1 in LNAsc transfected cells (*P < 0.05 comparing IGF-1+ to IGF-1 conditions, #P < 0.05 comparing LNA126 to LNAsc).
3.5. Overexpression of miR-126 conveys vulnerability and its inhibition neuroprotection to 6-OHDA toxicity
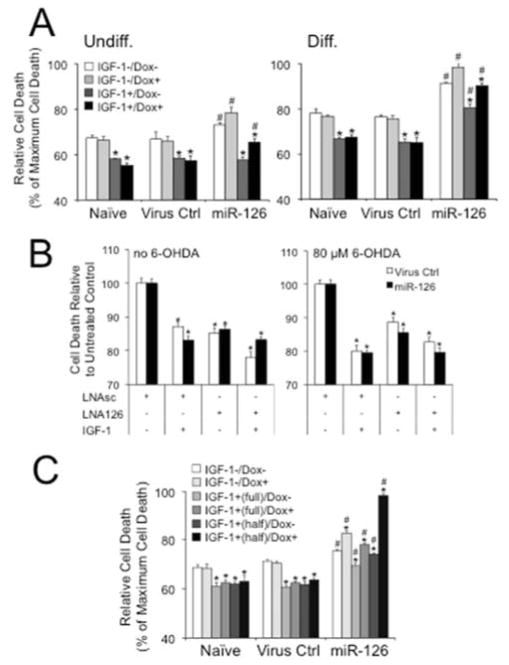
We next assessed the effects of miR-126 on neurotoxicity mediated by 6-OHDA. As demonstrated in the VM cultures, miR-126 overexpressing SH-SY5Y cells demonstrated higher vulnerability to the toxin and were less protected by IGF-1 than naïve and virus controls, and this effect was more pronounced in the differentiated neuroblastoma cells (Fig. 4A, Supplementary Fig. 5A). Cell death in these cultures was confirmed by analysis of caspase 3 expression (Supplementary Fig. 5B). Conversely, inhibition of miR-126 with miR-126 targeting LNAs increased cell survival in both untreated and 6-OHDA toxicity conditions (Fig. 4B). We also analyzed the effects of miR-126 on 6-OHDA toxicity in differentiated PC12 cells according to a recently published protocol by Chung et al., (Chung, et al., 2010). Cell differentiation was induced with nerve growth factor (NGF) in an early transient stimulation that induced an extracellular signal-regulated kinase (ERK)- and transcription-dependent latent process, and a second sustained NGF stimulation driving the fast neurite extension process by ERK- and PI3K-dependent activities (Supplementary Fig. 6). To test a function of miR-126 on the protective effects of IGF-1 in either phase, we added IGF-1 throughout (“full”) or only in the late phase (“half”) of differentiation. In both conditions, overexpression of miR-126 alone was neurotoxic, increased the toxic effects of 6-OHDA and impaired IGF-1 when compared to naïve and virus controls (Fig. 4C).
Figure 4.
Overexpression of miR-126 conveys vulnerability and its inhibition neuroprotection to 6-OHDA toxicity in neuroblastoma cells. (A) Treatment of undifferentiated (Undiff.) and differentiated (Diff.) SH-SY5Y cells with 80 μM 6-OHDA in the absence or presence of 20 ng/ml IGF-1 shows higher toxicity and less protective effects of the trophic factor in Dox+ (200 ng/ml) miR-126 transduced cells when compared to Naïve or Virus Ctrl. Results are from LDH assays and plotted as relative cell death to Triton-X (1%) induced maximum cell death (*P < 0.05 comparing IGF-1+ to IGF-1− condition; #P < 0.05 comparing miR-126 to Naïve or Virus Ctrl). (B) Inhibition of miR-126 increases cell survival of untreated and 6-OHDA treated SH-SY5Y cells. Dox-treated Virus Ctrl and miR-126 transduced cells were transfected with LNAsc or LNA126 and cultured in the absence or presence of 80 μM 6-OHDA and 20 ng/ml IGF-1. Data are plotted as percent cell death relative to untreated LNAsc controls (*P < 0.05 comparing IGF-1 and LNA126 to untreated LNAsc conditions). (C) Treatment of differentiated PC12 cells with 250 μM 6-OHDA in the absence or presence of 20 ng/ml IGF-1 shows higher toxicity and less protective effects of the trophic factor in Dox+ (200 ng/ml) miR-126 transduced cells when compared to Naïve or Virus Ctrl. Cells were differentiated according to a protocol by Chung et al., (Chung, et al., 2010), and a protective effect of IGF-1 was tested throughout (“full”) or only in the late phase (“half”) of differentiation (see Supplementary Fig. 5A) (*P < 0.05 comparing IGF-1+ to IGF-1− condition; #P < 0.05 comparing miR-126 to Naïve or Virus Ctrl).
3.6. Overexpression of miR-126 downregulates factors in the IGF-1/PI3K and ERK signaling cascades
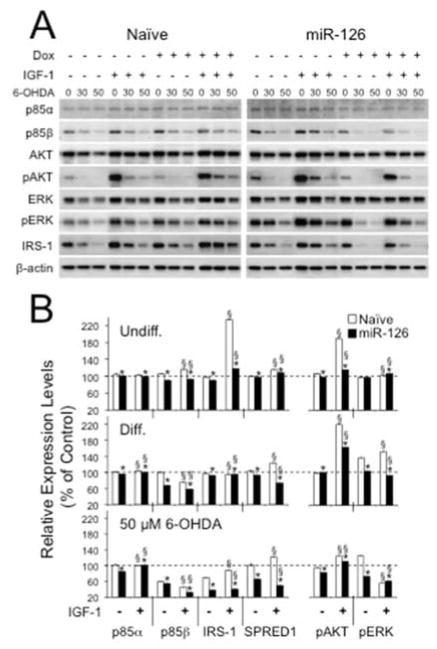
To gain insight into target regulation of miR-126, we then assessed the expression of factors in IGF-1/PI3K signaling, including AKT and its phosphorylation (pAKT), and the validated miR-126 targets p85β (Fish, et al., 2008, Guo, et al., 2008, Zhu, et al., 2011) and IRS-1 (Ryu, et al., 2011, Zhang, et al., 2008). In addition, we evaluated levels of Sprouty-related, EVH1 domain-containing protein 1 (SPRED1), ERK and pERK, because SPRED1 is another validated target of miR-126 and a known inhibitor of mitogen-activated protein kinase (MAPK)/ERK signaling (Fish, et al., 2008, S. Wang, et al., 2008). Compared to naïve and virus controls, miR-126 overexpressing cells exhibited lower expression levels of p85β, IRS-1, and SPRED1 and a reduction in phosphorylated AKT and ERK (Fig. 5). These impacts were markedly exacerbated after treatment with 6-OHDA.
Figure 5.
Overexpression of miR-126 downregulates expression levels of molecules in IGF-1/PI3K signaling. The expression levels of miR-126 targets and downstream molecules of IGF-1/PI3K signaling in lentivirus transduced SH-SY5Y cells were analyzed by semi-quantitative Western blots. (A) Representative example of a Western blot for differentiated SH-SY5Y cells in presence or absence of 200 ng/ml Dox, 20 ng/ml IGF-1, and 30 or 50 μM 6-OHDA. (B) Expression levels were calculated as Dox+ over Dox− and plotted as percent changes relative to untreated (IGF-1−/Dox−) condition. In the case of phosphorylated proteins, converted values of phosphorylated proteins were divided by converted values of total proteins. Relative protein expression levels in PGK.rtTA3.IRES.GFP (Virus Ctrl) transduced SH-SY5Y cells had similar expression patterns to Naïve. In both Undiff. and Diff. SH-SY5Y cells, expression levels of p85β, IRS-1, and SPRED1, and phosphorylation of AKT and ERK were reduced in Dox+ miR-126 transduced cells with an exacerbated downregulation in Diff. cells treated with 50 μM 6-OHDA (*P < 0.05 within a group (IGF-1− or IGF-1+); §P < 0.05 between IGF-1− and IGF-1+ conditions).
4. Discussion
Our study demonstrates that DA neurons in late stage PD and aged-matched controls have a distinctive signature (“fingerprint”) of miRNA expression and that a set of miRNAs was dysregulated and predominantly upregulated in PD. These results extend data from previous microarray and qRT-PCR analyses on the same sample populations, which showed a prominent downregulation of gene expression in PD (Elstner, et al., 2011, Simunovic, et al., 2009), suggesting that midbrain DA neurons exhibit unique mRNA/miRNA expression networks that are dysregulated in PD.
So far, several miRNAs have been associated with PD. In cell culture or in mice, miR-7 and miR-153 downregulated α-Synuclein, a protein associated with PD (Doxakis, 2010, Junn, et al., 2009), miR-433 inhibited translation of the DA neuron-associated mitogen FGF20 in vitro (G. Wang, et al., 2008), miR-133b was a regulator of midbrain DA cell development in mice by targeting the transcriptional activator Pitx3 (Kim, et al., 2007), and miR-34b/c might affect mitochondrial function through an indirect mechanism that could include Parkin and DJ1 (Minones-Moyano, et al., 2011). In the latter two studies, miR-133b and miR-34b/c were identified from profiling human postmortem tissue and both were downregulated in the midbrain of sporadic PD patients. In our data set, there was a trend of miR-433 downregulation in PD samples, however, this was statistically not significant (FC 0.6; P = 0.8). miR-133b, miR-153, and miR-34b/c were expressed below detection threshold (average Ct values ~38), and miR-7 was not present on the TaqMan® Human MicroRNA A Array v2.0. Taken together, our results don’t provide strong support for a function of these miRNAs in DA neurons or PD, and this is consistent with recent data for miR-133b demonstrating that miR-133b mutant mice have normal midbrain dopaminergic function (Heyer, et al., 2012). Regarding the observed differences in expression levels of miR-133b and miR-34b/c between our study and the studies by Kim et al. (Kim, et al., 2007) and Miñones-Moyano et al. (Minones-Moyano, et al., 2011), it should be noted that these authors used dissected midbrain tissue. Therefore, it could be possible that the documented expression of the miRNAs might have occurred in different cell populations. On a technical note, some miRNAs in our profiles were recently detected independently in midbrain DA neurons. For example, in a study by Nelson et al., pigmented neurons in the SN labeled strongly for miR-320, but weakly for miR-107 in in situ hybridization experiments (Nelson, et al., 2008). This is consistent with our qRT-PCR data, which showed strong expression of miR-320 (average Ct values ~27) and very little expression of miR-107 (average Ct values ~38).
Our data set provides a platform for identifying possible miRNA/mRNA associations that could be related to cellular processes involved in PD pathogenesis. This included correlative evidence for an association of miR-126 with IGF-1/PI3K signaling, a pathway that has been implicated in PD (discussed in Craft and Watson, 2004), and its dysregulation was also observed in laser-microdissected PD DA neurons (Cantuti-Castelvetri, et al., 2007, Elstner, et al., 2011, Simunovic, et al., 2009, Simunovic, et al., 2010). On a functional level we now demonstrate that upregulation of miR-126 in the DA neuronal phenotype impairs the insulin/IGF-1/PI3K signaling pathway and negatively affects cell survival to neurotoxic insult and protection by IGF-1. Together with the data from the human postmortem material, these results suggest that (dys)regulation of insulin signaling by miR-126 may be a contributing factor in PD pathogenesis. However, whether this is cause or consequence of disease or a reflection of age remains to be determined. An association of miR-126 with insulin/IGF-1/PI3K signaling has previously been described in myocytes (Wang, et al., 2009), adipocytes (Ling, et al., 2009), endothelial cells, cancer, and hepatocytes (reviewed in (Sonntag, et al., 2012)). Interestingly, in hepatocytes, Rotenone-induced mitochondrial dysfunction caused upregulation of several miRNAs, including miR-126, which in turn contributed to impaired insulin signaling by downregulating IRS-1 (Ryu, et al., 2011). Oxidative stress is a key mechanism in PD pathogenesis, and although not directly addressed in our studies, it could be that a combination of dysfunctional mitochondria with miRNA-modulated metabolic dysfunction contributes to the demise of DA neurons during aging and in PD. Of interest is our observation that blocking miR-126 function in Naïve or miR-126 overexpressing cells increased the trophic effects of IGF-1 and cell survival in untreated cells or 6-OHDA toxicity, indicating that inhibition of miR-126 may be a mechanism of neuroprotection.
Other than miR-126, several miRNAs have been linked to regulating insulin/IGF-1 signaling. For example, 11 miRNAs that potentially target IRS-1 were increased in the mitochondrial-stressed hepatocytes (Ryu, et al., 2011), and 4 of them were elevated in the PD DA neurons in our study. Other examples include miR-470, -669b, and -681, which were recently identified as potential suppressors of IGF-1R and AKT in the hippocampus of aged Ames dwarf and growth hormone receptor knock out mice (Liang, et al., 2011), and miR-7 and miR-98, which inhibit IGF-1/AKT signaling by targeting IRS-1 in glioblastoma or N2A cells, respectively (Hu, et al., 2013, Kefas, et al., 2008). In our miRNA profiles, miR-7, -470, -669b, and 681 were not included in the TaqMan® Human MicroRNA A Array v2.0., and miR-98 was expressed below detection threshold. However, another miRNA, miR-320, which has been shown to influence IGF-1 signaling through regulation of IGF-1/2, IGF-1R, p85α (PIK3R1), and the glucose transporter 4 (SLC2A4) in myocytes (Wang, et al., 2009) and adipocytes (Ling, et al., 2009), was upregulated in the PD DA neurons (FC 1.9, P = 0.09; FC 2.8, P = 0.2; FC 2.2, P = 0.8 in RNU44, global mean normalization, and ABIqPCR, respectively). In the negative correlation analysis, miR-320 was associated with dysregulated IGF-1, IGF-1R, and PI3KR2 indicating that this miRNA could be also involved in (dys)regulating insulin/IGF-1/PI3K signaling in DA neurons and PD.
Aside of targeting insulin/IGF-1/PI3K signaling, other targets of miR-126 have been described (summarized in (Sonntag, et al., 2012)), including SPRED1 (Fish, et al., 2008), which was downregulated in the miR-126 overexpressing DA neurons. SPRED1 is an inhibitor of MAPK/ERK signaling and this pathway is involved in neuronal cell function, aging, and degeneration (Kim and Choi, 2010), suggesting that miR-126 may have additional functions in the neuronal context.
In summary, the distinctive miRNA signature in SN DA neurons, their dysregulation in late stage PD, the identification of miRNAs that maybe associated with dysregulated signaling pathways related to PD, and a functional role of miR-126 in IGF-1/PI3K signaling in the DA neuronal context, indicate that miRNAs could be involved in the function of (aged) DA neurons and in PD pathogenesis. The modulation of IGF-1/PI3K/AKT signaling by miR-126 suggests a novel mechanism in regulating trophic support in DA neurons and their degeneration. This might not be restricted to DA neurons as IGF-1/PI3K/AKT signaling is an important pathway in multiple neuronal phenotypes and its impairment has been attributed to aging and other neurodegenerative diseases (Bomfim, et al., 2012, Craft and Watson, 2004, de la Monte, 2012, Deak and Sonntag, 2012, Kim and Feldman, 2012, Talbot, et al., 2012, Torres-Aleman, 2010). In fact, recent data from our studies on cortical and hippocampal primary cultures indicated that overexpression of miR-126 increased their vulnerability to neurotoxic Aβ1–42 peptides by downregulating the IGF-1/insulin signaling pathway (Kim et al., unpublished results). Therefore, (dys)regulating this pathway by miRNAs could have broader implications for normal and abnormal neuronal cell function.
Supplementary Material
Acknowledgments
We want to thank Drs. Bruce Cohen, Donna McPhie, Anna M. Krichevsky, Kwang-Soo Kim, Tsung-Ung Wilson Woo, Andrii Domanskyi, and Ilya Vinnikov for intellectual, stimulating discussions, reading the manuscript, and additional help in data analysis. Dr. D. Trono (EPFL) for the third generation lentivirus vector system, Dr. A. Das (University of Amsterdam) for the Tet-On reverse transactivator rtTA3, Dr. A. Miyanohara (UCSD) for the Synapsin promoter, and Drs. Cohen and Kim for additional financial support and resources. K.C.S. was involved in all aspects of the study, including conception, design, data acquisition, analysis, interpretation, presentation and write-up for publication; W.K., Y.L., N.M.K., F.S., and H.S. were directly involved with data acquisition and/or manuscript preparation; B.K. and Y.W. performed the miRNA profiling, M.Y. and R.S. the bioinformatics analyses, and R.J.R. additional computational miRNA quantification. This research was supported by a grant from the Massachusetts’ Alzheimer’s Disease Research Center and the Harvard NeuroDiscovery Center, and National Institute of Neurological Disorders and Stroke R21NS067335 (K.C.S), and the National Research Foundation of Korea (NRF) and a grant of the Korean Government, MSIP, No 2011-0030775 to H.S.
Abbreviations
- AD
Alzheimer’s disease
- AKT
Protein kinase B
- Ct
Cycle threshold
- CRK
regulator of v-crk sarcoma virus CT10 oncogene homolog
- ERK
Extracellular signal-regulated kinases
- DA
Dopamine
- Dox
Doxycyline
- GFP
Green fluorescent protein
- IGF-1
Insulin-like growth factor 1
- IGF-1R
Insulin-like growth factor 1 receptor
- IRES
Internal ribosomal entry side
- IRS-1
Insulin receptor substrate 1
- LDH
Lactate dehydrogenase
- LMD
Laser microdissection
- MAPK
Mitogen-activated protein kinase
- MOI
Multiplicity of infection
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- NGF
Nerve growth factor
- PD
Parkinson disease
- PGK
Phosphoglycerine kinase promoter
- PI3K
Phosphoinositide 3-kinase
- PIK3R1/2 (p85α/β)
Phosphoinositide-3-kinase receptor subunit 1/2
- pTRE2
Tetracycline-response element 2 promoter
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RA
Retinoic acid
- rtTA
Reverse transactivator
- SN
Substantia nigra
- SPRED1
Sprouty-related, EVH1 domain-containing protein 1
- TH
Tyrosine hydroxylase
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRE2
Tet-response element promoter 2
- VM
Ventral mesencephalon
Appendix A. Supplementary Material
Detailed Material and Methods and additional Supplementary Material is available in the online version.
Footnotes
Disclosure statement
The authors declare no potential conflict of interest.
The authors declare no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104(24):10164–9. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. The Journal of clinical investigation. 2012;122(4):1339–53. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70(20):1916–25. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis. 2007;26(3):606–14. doi: 10.1016/j.nbd.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14(13):1709–25. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kubota H, Ozaki Y, Uda S, Kuroda S. Timing-dependent actions of NGF required for cell differentiation. PLoS One. 2010;5(2):e9011. doi: 10.1371/journal.pone.0009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–78. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- Crawford M, Brawner E, Batte K, Yu L, Hunter MG, Otterson GA, Nuovo G, Marsh CB, Nana-Sinkam SP. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373(4):607–12. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- D’Haene B, Mestdagh P, Hellemans J, Vandesompele J. miRNA expression profiling: from reference genes to global mean normalization. Methods Mol Biol. 2012;822:261–72. doi: 10.1007/978-1-61779-427-8_18. [DOI] [PubMed] [Google Scholar]
- Das AT, Zhou X, Vink M, Klaver B, Verhoef K, Marzio G, Berkhout B. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. The Journal of biological chemistry. 2004;279(18):18776–82. doi: 10.1074/jbc.M313895200. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Current Alzheimer research. 2012;9(1):35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67(6):611–25. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo A, Carlsson J, Lindlof A. How to choose a normalization strategy for miRNA quantitative real-time (qPCR) arrays. Journal of bioinformatics and computational biology. 2011;9(6):795–812. doi: 10.1142/s0219720011005793. [DOI] [PubMed] [Google Scholar]
- Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. The Journal of biological chemistry. 2010;285(17):12726–34. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Pertsemlidis A. Cancer and neurodegenerative disorders: pathogenic convergence through microRNA regulation. J Mol Cell Biol. 2011;3(3):176–80. doi: 10.1093/jmcb/mjq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10(12):837–41. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner M, Morris CM, Heim K, Bender A, Mehta D, Jaros E, Klopstock T, Meitinger T, Turnbull DM, Prokisch H. Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol. 2011;122(1):75–86. doi: 10.1007/s00401-011-0828-9. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47(11):939–46. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer MP, Pani AK, Smeyne RJ, Kenny PJ, Feng G. Normal Midbrain Dopaminergic Neuron Development and Function in miR-133b Mutant Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(32):10887–94. doi: 10.1523/JNEUROSCI.1732-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YK, Wang X, Li L, Du YH, Ye HT, Li CY. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neuroscience bulletin. 2013 doi: 10.1007/s12264-013-1348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–7. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68(10):3566–72. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- Kim B, Feldman EL. Insulin resistance in the nervous system. Trends in endocrinology and metabolism: TEM. 2012;23(3):133–41. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et biophysica acta. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Liang R, Khanna A, Muthusamy S, Li N, Sarojini H, Kopchick JJ, Masternak MM, Bartke A, Wang E. Post-transcriptional regulation of IGF1R by key microRNAs in long-lived mutant mice. Aging cell. 2011;10(6):1080–8. doi: 10.1111/j.1474-9726.2011.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HY, Ou HS, Feng SD, Zhang XY, Tuo QH, Chen LX, Zhu BY, Gao ZP, Tang CK, Yin WD, Zhang L, Liao DF. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clinical and experimental pharmacology & physiology. 2009;36(9):e32–9. doi: 10.1111/j.1440-1681.2009.05207.x. [DOI] [PubMed] [Google Scholar]
- Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, Vandesompele J. A novel and universal method for microRNA RT-qPCR data normalization. Genome biology. 2009;10(6):R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minones-Moyano E, Porta S, Escaramis G, Rabionet R, Iraola S, Kagerbauer B, Espinosa-Parrilla Y, Ferrer I, Estivill X, Marti E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet. 2011;20(15):3067–78. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18(1):130–8. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, Greene CM. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010;184(4):1702–9. doi: 10.4049/jimmunol.0902669. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Lim MP, Macey L, Woo TU, Sonntag KC. Neuronal type-specific gene expression profiling and laser-capture microdissection. Methods Mol Biol. 2011;755:327–43. doi: 10.1007/978-1-61779-163-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves SP, Ahmed T, Borwege S, Joyce JN. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res. 2004;5(8):579–98. doi: 10.1007/BF03033178. [DOI] [PubMed] [Google Scholar]
- Ryu HS, Park SY, Ma D, Zhang J, Lee W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS One. 2011;6(3):e17343. doi: 10.1371/journal.pone.0017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene therapy. 2002;9(17):1155–62. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2011;26(6):1049–55. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- Seo H, Sonntag KC, Isacson O. Generalized brain and skin proteasome inhibition in Huntington’s disease. Ann Neurol. 2004;56(3):319–28. doi: 10.1002/ana.20207. [DOI] [PubMed] [Google Scholar]
- Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O. Proteasome Activator Enhances Survival of Huntington’s Disease Neuronal Model Cells. PLoS ONE. 2007;2:e238. doi: 10.1371/journal.pone.0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods. 2006;3(11):881–6. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- Simunovic F, Yi M, Wang Y, Macey L, Brown LT, Krichevsky AM, Andersen SL, Stephens RM, Benes FM, Sonntag KC. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain. 2009;132(Pt 7):1795–809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic F, Yi M, Wang Y, Stephens R, Sonntag KC. Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One. 2010;5(1):e8856. doi: 10.1371/journal.pone.0008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010;1338:48–57. doi: 10.1016/j.brainres.2010.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Woo TU, Krichevsky AM. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Experimental neurology. 2012;235(2):427–35. doi: 10.1016/j.expneurol.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70(5):384–96. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82(2):283–9. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clinical and experimental pharmacology & physiology. 2009;36(2):181–8. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37(Database issue):D105–10. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Mudunuri U, Che A, Stephens RM. Seeking unique and common biological themes in multiple gene lists or datasets: pathway pattern extraction pipeline for pathway-level comparative analysis. BMC Bioinformatics. 2009;10:200. doi: 10.1186/1471-2105-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Du YY, Lin YF, Chen YT, Yang L, Wang HJ, Ma D. The cell growth suppressor, mir-126, targets IRS-1. Biochem Biophys Res Commun. 2008;377(1):136–40. doi: 10.1016/j.bbrc.2008.09.089. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, Bai Y, Shen Y, Yuan W, Jing Q, Qin Y. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351(1–2):157–64. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–80. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nature Biotechnology. 1997;15:871–5. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.