Abstract
WRKY transcription factors form one of the largest transcription factor families and function as important components in the complex signaling processes that occur during plant stress responses. However, relative to the research progress in model plants, far less information is available on the function of WRKY proteins in cotton. In the present study, we identified the GhWRKY40 gene in cotton (Gossypium hirsutum) and determined that the GhWRKY40 protein is targeted to the nucleus and is a stress-inducible transcription factor. The GhWRKY40 transcript level was increased upon wounding and infection with the bacterial pathogen Ralstonia solanacearum. The overexpression of GhWRKY40 down-regulated most of the defense-related genes, enhanced the wounding tolerance and increased the susceptibility to R. solanacearum. Consistent with a role in multiple stress responses, we found that the GhWRKY40 transcript level was increased by the stress hormones salicylic acid (SA), methyl jasmonate (MeJA) and ethylene (ET). Moreover, GhWRKY40 interacted with the MAPK kinase GhMPK20, as shown using yeast two-hybrid and bimolecular fluorescence complementation systems. Collectively, these results suggest that GhWRKY40 is regulated by SA, MeJA and ET signaling and coordinates responses to wounding and R. solanacearum attack. These findings highlight the importance of WRKYs in regulating wounding- and pathogen-induced responses.
Introduction
Stress is perceived and transduced through a series of signaling molecules that ultimately affect the regulation of stress-inducible genes to initiate the synthesis of different types of proteins, including transcription factors, enzymes, molecular chaperones, ion channels, and transporters, or to alter their activities [1]. Among these proteins, transcription factors (TFs) are crucial in eliciting stress responses by modulating the expression of specific target genes in a temporal and spatial manner; they are also necessary for normal development and proper responses to physiological or environmental stimuli [2]–[5]. WRKY proteins are a class of zinc finger-containing TFs that are encoded by large gene families in all higher plants and are reported to play a pivotal role in many physiological processes. WRKY TFs share a highly conserved sequence of approximately 60 amino acids called the WRKY domain, which contains the conserved amino acid sequence motif WRKYGQK at the N-terminus and a novel zinc finger-like motif at the C-terminus. Based on their domain structures, WRKY TFs are classified into three major groups (I, II, and III) [6]. Additionally, WRKY TFs act as transcriptional regulators by binding to the W-box, which is present in the promoter regions of various stress-related genes, thus regulating the expression of many genes, resulting in stress tolerance [7].
WRKY TFs have mostly been studied with respect to their participation in the regulation of defense against biotic stresses or against tolerance of abiotic stresses [8]. Some WRKY proteins are reported to be involved in the coordination of multiple biological processes. For example, AtWRKY33 regulates disease resistance, NaCl tolerance and thermotolerance [9]–[11], while CaWRKY40 modulates tolerance to heat stress and resistance to Ralstonia solanacearum infection [12]. This suggests that some WRKY proteins serve as nodes in a crosstalk between different physiological processes. However, the functions of the majority of WRKY family members and their possible roles in signaling crosstalk are limited.
Previous studies have shown that several wound-responsive WRKY genes are also regulated by pathogen infection [13]–[16]. These reports have provided useful research methods and broadened our knowledge on the function of plant WRKYs. However, so far, few reports have addressed the mechanistic details of the crosstalk between wounding and pathogen infection. The prominent role of WRKYs in stress signaling indicates a promising target for applied studies in crop species. Moreover, dissecting the crosstalk between different pathways is critical to understanding the plant response to environmental cues. Wounding presents a threat to plant survival because it not only physically destroys plant tissues but also provides a pathway for pathogen invasion. Therefore, it is necessary to map the interaction between the wounding response and pathogen infection and to identify novel genes involved in these processes. In particular, the molecular mechanisms involved in these processes should be examined in different genetic backgrounds.
WRKY proteins have been linked to the MAP kinase (MAPK) cascade in Arabidopisis; AtWRKY22 and AtWRKY29 are thought to function downstream of MPK3/MPK6 [17]. The MAPK cascade is the basic module for transmitting signals from upstream ligand receptors to downstream substrates in response to various biotic and abiotic stress signals, hormones, and growth and developmental processes [18]. In tobacco, WRKY8 is a physiological substrate of SIPK, NTF4, and WIPK [19]. In rice, OsWRKY30 interacts with and is phosphorylated by OsMPK3 [20]. Thus, only a limited number of upstream WRKY components have been identified, particularly in crops, and whether MAPKs interact with WRKYs in cotton should be investigated.
Cotton is one of the most economically important crops worldwide and is an excellent source of fiber and oil. However, its growth and yield are severely inhibited under various biotic and abiotic stress conditions. The applied study of cotton WRKYs will provide new insight that may aid in creating cotton plants that are better able to adapt to environmental challenges. However, the majority of WRKY TFs in cotton have not been characterized. In the present study, a group II a WRKY gene from cotton (Gossypium hirsutum), GhWKRY40, was isolated and characterized. GhWRKY40 expression was induced by various abiotic and biotic stresses. We obtained information on the ability of GhWRKY40 overexpression to alter the responses to wounding and infection with the bacterial pathogen R. solanacearum in Nicotiana benthamiana. Moreover, we demonstrated that GhWRKY40 interacted with GhMPK20 but not GhMK6a, two MAPKs that were previously identified by our group, using a yeast two-hybrid system and bimolecular fluorescence complementation (BiFC). This study suggests that the transcriptional responses of GhWRKY40-overexpressing plants to wounding may be related to defense signaling pathways.
Materials and Methods
Cloning of the full-length GhWRKY40 cDNA
Total RNA was extracted from the leaves of seven-day old cotton seedlings using a modified cetyltrimethylammonium bromide (CTAB) protocol [21]. Reverse transcription-PCR (RT-PCR) and RACE-PCR were used to amplify the full-length GhWRKY40 cDNA. A pair of degenerated primers (MP1/MP2) was designed to isolate WRKY family members from the cotton cotyledons. According to the obtained fragment, specific primers (5P1/5P2, 3P1/3P2, and QC1/QC2) were used for 5′ rapid amplification of cDNA ends (RACE), 3′ RACE and the identification of the full-length cDNA sequence. The general PCR procedures and primers are shown in Table S1 and Table S2, respectively. The PCR product was purified, cloned into the pEasy-T1 vector, and transformed into competent Escherichia coli cells for sequencing. The amino acid sequence alignment of GhWRKY40 and its homologues was conducted using BLAST (http://www.ncbi.nlm.gov/blast) and DNAman software 5.2.2. The phylogenetic tree was performed in MEGA version 4.1 (http://megasoftware.net) using the neighbour-joining method.
Amplification of the GhWRKY40 genomic sequence and promoter
For the amplication of GhWRKY40 genomic sequence, one pair of primers (QG1 and QG2), which was designed and synthesized based on the full-length GhWRKY40 cDNA, was used. Genomic DNA was isolated from seedling leaves using the CTAB method. Inverse-PCR (I-PCR) was performed to obtain the promoter sequence. Three restriction endonucleases (NdeI, SspI and VspI) were used to digest the cotton seedling genomic DNA, and T4 DNA ligase was used to self-ligate the DNA fragments into circles, which were used as templates to amplify the promoter region. Three promoter fragments were amplified using six pairs of primers (Nde1/2 and Nde3/4, Ssp1/2 and Ssp3/4, Vsp1/2 and Vsp3/4). The deduced portion of the promoter was subsequently verified using the special primers WP1 and WP2. The sequences of the primers are provided in Table S2. The programme PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html) and PLACE (http://www.dna.affrc.go.jp/PLACE/) was used to analyze the GhWRKY40 promoter sequence.
Subcellular localization of GhWRKY40
The coding sequence of GhWRKY40 without the termination codon was amplified by PCR using the primers Wgf1 (5′-GGATCCATGGATACTTCTTCATGGGTGG-3′, BamHI site underlined) and Wgf2 (5′-CTCGAGCTTATAGTTGACAAAATCATAGAAAC-3′, XhoI site underlined). Then, the coding sequence was ligated into the binary vector pBI121-GFP, which contains the Cauliflower mosaic virus (CaMV) 35S promoter, to yield the expression vector p35S::GhWRKY40-GFP. The resulting expression plasmid, pBI121-GhWRKY40-GFP, or the pBI121-GFP control plasmid was transformed into onion (Allium cepa) epidermis cells via biolistic bombardment transformation using the Biolistic PDS-1000/He system (Bio-Rad, USA) with gold particles (1.0 µl) and a helium pressure of 1,100 psi. And then plated on MS agar medium in the dark condition at 28°C for 24 h, the nuclei were stained with 100 µg/ml of 4′,6-diamidino-2-phenylindole (DAPI) in phosphate-buffered saline for 4 min, and the fluorescence signal of the GhWRKY40-GFP fusion protein was imaged using a fluorescence microscope using excitation wavelength of 488 nm and 350 nm, respectively. The vector p35S::GFP was used as a control.
Transactivation assay
The transactivation activity of the GhWRKY40 protein was investigated in the Y2HGold yeast strain, which contains the HIS3, ADE2 and MEL1 reporter genes and the GAL4 promoter. The coding region of GhWRKY40 was amplified using the primers Wbd1 and Wbd2, which possess EcoRI and BamHI sites (underlined), 5′-GAATTCATGGATACTTCTTCATGGGTGG-3′ and 5′- GGATCCTTCAACTGGACTTTGCTGAAAC-3′ to build the pGBKT7-GhWRKY40 vector (Clontech, TaKaRa) containing the GAL4 DNA-binding domain. The plasmid pGBKT7-GhWRKY40 and pGBKT7 (negative control) was transformed into Y2HGold yeast cells. The transformed yeast cells were streaked on SD/-Trp and SD/-Trp-Ade-His medium plates to observe yeast growth at 30°C for 3–4 days. An assay of α-galactosidase activity was performed using X-α-gal.
Cotton growth conditions and GhWRKY40 expression assay
Cotton (Gossypium hirsutum L. cv. lumian 22) seeds were grown under greenhouse conditions at 25±1°C with a 16 h light/8 h dark cycle (relative humidity of 60–75%), and seven-day-old seedlings were used for the following treatments. For the pathogen treatment, cotton seedlings were inoculated with R. solanacearum suspensions using the root dip method. For the H2O2 treatment, cotton seedlings were sprayed with 10 mM H2O2. The wounding, MeJA, SA and ET treatments were performed as described previously [22]. The treated cotyledons were collected, frozen directly in liquid nitrogen and stored at −80°C for RNA extraction and further analysis. Each treatment was repeated at least three times.
Total RNA was extracted from the treatment samples using a modified cetyltrimethylammonium bromide (CTAB) protocol [21] and then treated with RNase-free DNaseI to remove any potential genomic DNA contamination. First-strand cDNA was synthesized using the EasyScript First-Strand cDNA Synthesis SuperMix. The GhWRKY40 (KC414679) gene primer pairs WRT1/WRT2 and Ghubiquitin (EU304080) primer pairs Ub1/Ub2 were used for quantitative real-time PCR (qPCR) with the SYBR PrimeScript RT-PCR Kit in a CFX96TM Real-time System. The PCR programme was as follows: predenaturation at 95°C for 30 s; 40 cycles of 95°C for 5 s, 55°C for 15 s and 72°C for 15 s; and a melt cycle from 65°C to 95°C. The data was analyzed using the CFX Manager software, version 1.1, and significant differences were determined using the Statistical Analysis System (SAS) software (version 9.1). All reactions were performed with three technical replicates.
Wounding analysis of transgenic plants
The GhWRKY40 coding region was amplified with primers WOE1 (5′-GGATCCATGGATACTTCTTCATGGGTGG-3′, BamHI site underlined) and WOE2 (5′-GAGCTCTTCAACTGGACTTTGCTGAAAC-3′, SacI site underlined). Then this fragment was subcloned into pBI121 under the control of the CaMV35S promoter. The recombinant plasmid was introduced into the Agrobacterium tumefaciens (strain LBA4404) for Nicotiana benthamiana (N. benthamiana) transformation using the leaf disc method as described previously [23]. The transgenic seedlings were screened on Murashige and Skoog (MS) agar medium containing 100 mg/L of kanamycin and further confirmed by PCR. Eight independent transgenic T1 native tobacco lines were obtained. Three independent GhWRKY40-OE lines (OE1, OE2 and OE3) and wild-type plants were used for the following experiments. The transgenic T2 lines were used in the experiments.
Transgenic N. benthamiana seeds were surface sterilized and planted on MS medium for germination under greenhouse conditions. Four-leaf stage seedlings were transplanted into soil and maintained under greenhouse conditions. For the wounding treatment, the third leaf from the top of 8-week old seedlings was cut with scissors. After wounding, the leaves were harvested at the indicated timepoints for histochemical staining and the preparation of RNA. The experiment was repeated at least three times.
After wounding treatment, the OE and WT leaves were incubated with 3,3′-Diaminobenzidine (DAB, 1 mg/mL, pH 3.8) or Nitro Blue tetrazolium (NBT, 0.1 mg/mL) for 36 h at 25°C in the dark. Then, the leaves were boiled in ethanol (95%) for 5 min. After cooling, the leaves were soaked and preserved in fresh ethanol at room temperature and photographed. To examine oxidative tolerance, leaf discs (1.3 cm in diameter) were detached from healthy, fully expanded leaves of OE and WT plants, floated in methyl viologen (MV) (0, 200, 400 or 600 µM) solutions for 64 h, and immersed in 95% ethanol for 40 h to extract chlorophyll for spectrophotometric measurement.
Disease resistance analysis of transgenic plants
For the disease resistance analysis, R. solanacearum strains were cultured at 200 rpm and 37°C in Luria-Bertani (LB) broth. The bacterial cell density was diluted to OD600 = 0.6–0.8. A total of 20 µL of the resulting R. solanacearum suspension was injected into the third leaf from the top of each plant of 8-week old seedlings using a syringe with a needle. The leaves were harvested at the indicated timepoints for the preparation of RNA. The experiment was repeated at least three times.
Expression analysis of defense-related genes in transgenic and WT lines
Total RNA of all samples was extracted with TRIzol reagent. qPCR was performed using cDNA as the template, which was synthesized from total RNA extracted from the transgenic or WT N. benthamiana lines after wounding and disease treatments. N. benthamiana β-actin (Nbβ-actin) genes were used as the standard controls. The GenBank accession numbers of the defense-related genes examined in the qPCR analysis are as follows: JQ256516.1 (β-actin), ACY30445.1 (JAZ1), BAG68657.1 (JAZ3), X84040.1 (LOX1), AF392978 (ACS6), U15933.1 (APX), AB093097 (SOD), D10524 (GST), X12485.1 (PR1a) M60460.1 (PR2), EH365959.1 (PR4), and Y07563 (HIN1). The primers of the defense-related genes examined in the qPCR are listed in Table S3.
Yeast two-hybrid and BiFC assay
For the yeast two-hybrid assay, the GhWRKY40 cDNA fragment was cloned into the pGADT7 vector in-frame with the GAL4 activation domain. The GhMPK6a and GhMPK20 cDNA fragments were cloned into the pGBKT7 vector in-frame and proximal to the binding domain. These vectors were co-transformed into the Y2HGold yeast strain using the Matchmaker Gold Yeast Two-Hybrid System. Positive clones were plated onto selective SD medium (DDO: SD/-Leu/-Trp, QDO: SD/-Ade/-His/-Leu/-Trp and QDO/X/A: QDO with X-α-gal and aureobasidin A). For the BiFC assay, the GhWRKY40 and GhMPK20 cDNA fragments were cloned into pUC-SPYCE-35S and pUC-SPYNE-35S, respectively. These two BiFC constructs were co-transformed into onion epidermal cells using the particle bombardment method, and the fluorescent signal of the resultant proteins was detected using a confocal microscope.
Statistical analysis
Data were shown as the mean ± standard deviation (SD) with n = 3. The results were analyzed using multiple comparisons by analysis of variance (ANOVA), and means were separated by the Duncan's Multiple Range test. ANOVA was performed using Statistical Analysis System (SAS) version 9.1 software.
Results
Characterization of GhWRKY40
The full-length cDNA of GhWRKY40 was determined to be 1463 bp. It contained an open reading frame (ORF) of 945 bp, encoding 314 amino acids, an untranslated region of 203 bp at the 5′ end and 315 bp of the noncoding region at the 3′ end. The relative molecular mass and theoretical pI of the predicted protein were 34.4 kDa and 8.45, respectively. According to the nomenclature for plant WRKYs as well as the alignment this WRKY sequence with related sequences, it was found to share 48.0% and 47.48% identity with AtWRKY40 (NP_178199.1) and BnWRKY40 (ACQ76806.1), respectively. Therefore, we termed the cDNA clone as GhWRKY40 (GenBank accession number: KC414679).
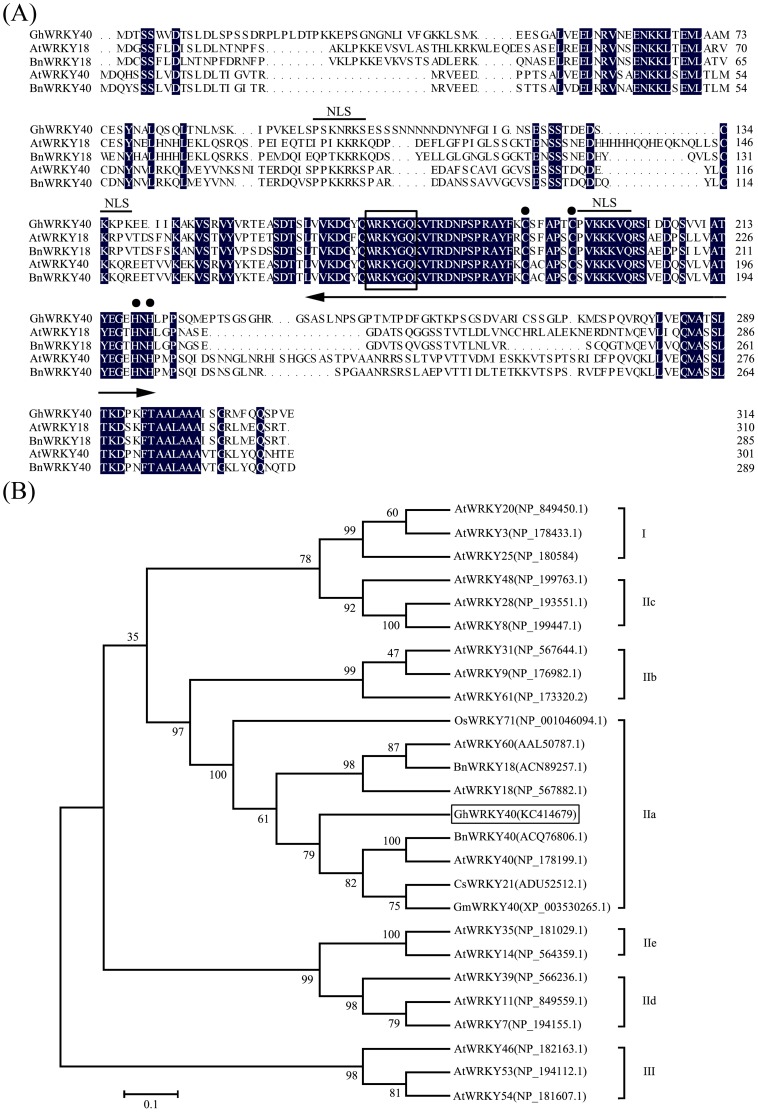
According to our multiple alignment and phylogenetic analyses of the WRKY proteins, GhWRKY40 was placed into group IIa of the WRKY superfamily (Fig. 1). The GhWRKY40 protein possesses fully canonical motif structures, including a typical DNA binding domain, the WRKY domain and a putative zinc finger structure (C-X4–5-C-X22–23-H-X1-H). The phylogenetic relationships among various WRKY IIa subgroup members from different organisms were further analyzed by comparing the protein sequences of their conserved WRKY domains.
Figure 1. Characterization and sequence analysis of GhWRKY40.
(A) Alignment of the amino acid sequences of GhWRKY40 and the representative related proteins AtWRKY18 (NP_567882), BnWRKY18 (ACN89257), AtWRKY40 (NP_178199) and BnWRKY40 (ACQ76806). Amino acids with 100% identity are shaded in black. The approximately 60-amino acid WRKY domain and the C and H residues in the zinc-finger motif (C-X4–5-C-X22–23-H-X1-H) are marked by a two-headed arrow and dot, respectively. The highly conserved amino acid sequence WRKYGQK in the WRKY domain is boxed. The putative nuclear localization signals are marked by lines. (B) Phylogenetic relationship between GhWRKY40 and other plant WRKY proteins. A neighbor-joining phylogenetic tree was created using MEGA 4.1 software. GhWRKY40 is boxed. Each gene name is followed by its protein ID. The abbreviations of the gene names are indicated as follows: Gh, Gossypium hirsutum; At, Arabidopsis thaliana; Os, Oryza sativa; Bn, Brassica napus; Cs, Cucumis sativus and Gm, Glycine max.
To analyze the structure of GhWRKY40, we isolated a 1936 bp genomic fragment (GenBank accession number: KC414680) from cotton genomic DNA. Sequence comparison revealed that GhWRKY40 has four introns. This intron number differs from that of AtWRKY40 but is similar to that of AtWRKY18, AtWRKY60 and is characteristic of group IIa of the WRKY superfamily.
GhWRKY40 is localized to the nucleus
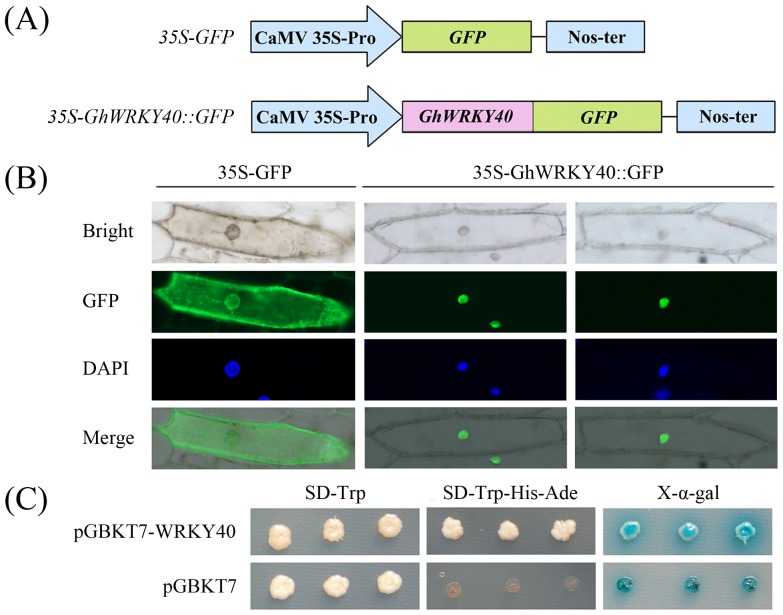
The PSORT program predicted that GhWRKY40 is localized to the nucleus. Sequence analysis using WoLF PSORT (http://wolfpsort.org/) indicated that the predicted GhWRKY40 protein contains three putative nuclear localization signals (98PSKNRKS104, 135KKPK138, 195PVKKKVQ201; Fig. 1A). To confirm its nuclear localization, we generated a construct for the expression of GhWRKY40 fused to green fluorescent protein (GFP) under the control of the constitutive CaMV35S promoter (Fig. 2A). Typical results indicated the exclusive localization of GhWRKY40-GFP in the nucleus, whereas GFP alone occurred throughout in the cell (Fig. 2B). This result suggests that GhWRKY40 has a nuclear localization.
Figure 2. Subcellular localization of the GhWRKY40 protein and transcriptional activation of the GhWRKY40 gene.
(A) Schematic diagram of the 35S-GhWRKY40::GFP fusion protein construct and the 35S-GFP construct. (B) Transient expression of the 35S-GFP and 35S-GhWRKY40::GFP constructs in onion epidermal cells. Green fluorescence corresponding to the expressed proteins was observed with a fluorescence microscope 24 h after particle bombardment. The nuclei of the onion cells were visualized by DAPI staining. (C) Transactivation of the GhWRKY40 gene in yeast. The vector pGBKT7 was used as a control. The transformed yeast culture was streaked onto SD/-Trp or SD/-Trp-His-Ade medium, and the α-galactosidase activity was determined. Three independent experiments were performed.
GhWRKY40 functions as a potential transcriptional activator
To determine whether the GhWRKY40 protein has transcriptional activity in eukaryotic cells, a plasmid containing the GAL4 DNA binding domain and the whole ORF of GhWRKY40 was constructed (pGBKT7-GhWRKY40). The plasmid pGBKT7-GhWRKY40 or pGBKT7 (negative control) was transformed into Y2HGold yeast cells. All transformants containing pGBKT7-GhWRKY40 and pGBKT7 grew well on selective medium without tryptophan (SD/-Trp). As shown in Fig. 2C, yeast transformed with pGBKT7-GhWRKY40 grew on selective medium without tryptophan, histidine and adenine (SD/-Trp-His-Ade). In addition, α-galactosidase activity was detected in these cultures, indicating that the expression of the reporter genes (HIS3, ADE2 and MEL1) was activated, whereas neither nor HIS3 were activated in yeast transformants containing the negative control plasmid (pGBKT7). These results indicated that GhWRKY40 is a transcriptional activator.
Expression profile of GhWRKY40 under stress conditions
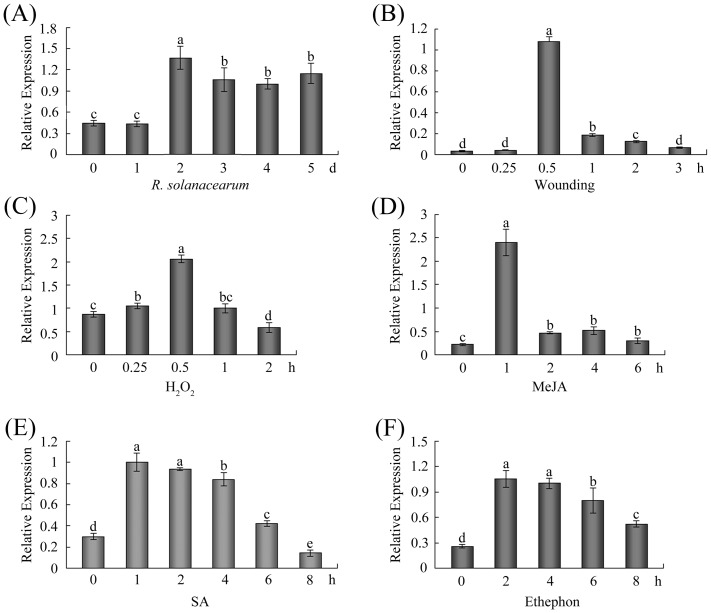
To test if GhWRKY40 is involved in the plant response to abiotic and biotic stresses, the transcript levels of GhWRKY40 were measured by qPCR after the treatment of cotton seedlings with R. solanacearum, wounding or H2O2. The GhWRKY40 transcript level was up-regulated in response to infection with the bacterial pathogen R. solanacearum (Fig. 3A). The GhWRKY40 transcript level was also increased at 0.5–2 h after wounding, with peak expression 0.5 h (Fig. 3B). During H2O2 treatment, the GhWRKY40 transcript level was noticeably elevated at 0.5 h (Fig. 3C). These results suggest that the expression of the GhWRKY40 gene is induced by environmental stimuli and may play an important role in the stress response.
Figure 3. Expression of the GhWRKY40 gene in response to stress.
Seven-day-old cotton seedlings in hydroponic culture were treated with R. solanacearum (A), wounding (B), 100 µM H2O2 (C), 100 µM MeJA (D), 2 mM SA (E) or 5 mM ET released from ethephon (F). Total RNA was isolated at the indicated times after the treatment and subjected to qPCR analysis. The ubiquitin gene was employed as an internal control. This experiment was repeated at least twice. The values indicated by the different letters are significantly different at P<0.01, as determined using Duncan's multiple range tests.
Phytohormones, such as SA, JA and ET, serve as significant signalling molecules in the regulation of plant defense responses against biotic and abiotic stresses and play vital roles in mediating the expression of downstream defense genes [24]. To determine the possible involvement of GhWRKY40 in the signaling cascades, the GhWRKY40 transcript levels were determined by qPCR in seven-day old cotton seedlings that were exogenously treated with MeJA, SA or ET. In response to 100 µM MeJA, the transcript level of GhWRKY40 was enhanced from 1–6 h, with maximum expression at approximately 1 h (Fig. 3D). GhWRKY40 mRNA was also induced and reached a peak at 1–2 h with 2 mM SA, (Fig. 3E). Application of 5 mM ET increased the GhWRKY40 transcript level at 2–8 h, reaching maximal levels from 2–4 h (Fig. 3F). The strong induction of GhWRKY40 expression by these signaling molecules suggests that this gene is involved in signaling pathways in stress resistance.
GhWRKY40 promoter analysis
To determine whether the GhWRKY40 gene is induced by stress, we isolated a 782 bp fragment from the upstream region of the GhWRKY40 gene by I-PCR. Analysis of this region using the PlantCARE and PLACE databases revealed many putative cis-elements, suggesting that GhWRKY40 plays a role in the plant response to environmental stress. The elements in this region include pathogen/elicitor-related elements, such as ARE, RAV1AAT and WBOXATRNPR1 and WBOX71OS, abiotic stress responsive elements, such as MBS, CCAAT-box, WBOXNTERF3, OSE2ROOTNODULE and CURECORECR, and tissue-specific and development-related elements, such as the circadian, Skn-1 motif, POLLEN1LELAT52 and WBOXHVISO1. All of the identified cis-elements are listed in Table 1.
Table 1. Putative cis-elements in the GhWRKY40 promoter.
| cis-element | Position | Sequence (5′-3′) |
| Abiotic stress response elements | ||
| MBS | −116(+) | TAACTG |
| CURECORECR | −490(+) | GTAC |
| CCAAT-box | −615(−) | CAACGG |
| WBOXNTERF3 | −304 (−),−372(+) | TGACY |
| Pathogen/elicitor response elements | ||
| ARE | −411(−) | TGGTTT |
| RAV1AAT | −307(+) | CAACA |
| WBOXATNPR1 | −371(+),−305(−) | TTGAC |
| WRKY71OS | −55(+),−141(+),−372(+),−305(−) | TGAC |
| Tissue-specific and development-related elements | ||
| circadian | −683(−) | CAANNNNATC |
| Skn-1_motif | −53(−),−139(−) | GTCAT |
| OSE2ROOTNODULE | −216(+),−323(−),−530(−),−652(−),−342(−),−352(−) | CTCTT |
| POLLEN1LELAT52 | −1004(+),−828(+),−604(+),−569(+),−271(+),−253(+),−28(+),−9(+),−861(−) | AGAAA |
| WBOXHVISO1 | −372(+),−304(−) | TGACT |
| Light regulation elements | ||
| AE-box | −654(−) | AGAAACAA |
| GA-motif | −347(+) | AAGGAAGA |
| I-box | −578(−) | GATAAGAATA |
| Sp1 | −327(+) | CC(G/A)CCC |
Overexpression of GhWRKY40 in transgenic plants affects the expression of defense-related genes in response to wounding
The GhWRKY40 transcript patterns suggest a role for this gene in defense against biotic and various abiotic stresses. To assess the significance of this gene in the response to these stresses, we generated transgenic native tobacco T2 lines that overexpress GhWRKY40 driven by the CaMV35S promoter. Except for the later germination of the transgenic plants relative to the WT plants (Fig. S1), we observed no differences between the plants. Wounding is a common plant injury and presents a potential threat to plant survival because it not only damages tissues but also provides means for pathogen invasion. Three independent GhWRKY40-OE lines and wild-type plants were used to understand the wounding response.
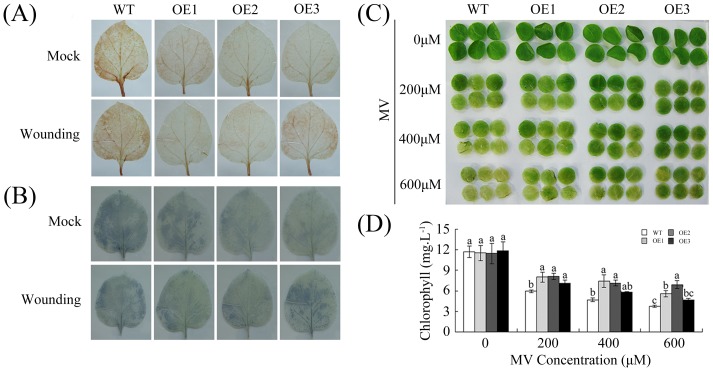
We first examined the wounding response by the DAB staining of H2O2 accumulation and the NBT staining of O2 − accumulation in wounded leaves. Compared with WT plants, the GhWRKY40-OE lines exhibited clearly decreased DAB staining intensities in their wounded leaves, reflecting low levels of H2O2 accumulation (Fig. 4A). These lines also exhibited decreased NBT intensities staining in the treated leaves compared with the WT plants (Fig. 4B). Meanwhile, the GhWRKY40-OE lines exhibited slightly lower H2O2 and O2 − accumulation compared with the WT plants in the absence of wounding treatment. Additionally, leaf discs from the overexpression lines were used to illustrate whether GhWRKY40 plays a role in oxidative resistance. Leaf discs were soaked in solutions containing various concentrations (0, 200, 400 or 600 µM) of methyl viologen (MV). The results presented in Fig. 4C and 4D show that the transgenic plants exhibited intense oxidative resistance. Leaf discs from both the OE and WT plants showed signs of chlorosis, but neither of these plants showed abnormalities in water. However, MV treatment led to more severe damage in the leaf discs from the WT plants. This result was further confirmed by measuring the leaf disc chlorophyll content before and after MV treatment. Taken together, GhWRKY40 overexpression appears to enhance the defense response to wounding, resulting in decreased H2O2 and O2 − levels.
Figure 4. Analysis of ROS accumulation after wounding in WT and OE plants.
(A–B) Wound-induced H2O2 and O2 − accumulation, as detected via DAB staining and NBT staining, respectively. (C) The phenotype of leaf disks from WT and OE plants that were incubated in different concentrations of MV (0, 200, 400 or 600 mM). (D) Relative chlorophyll content in the leaf disks from (C). Disks floated in water were used as a control. The presented data are the means ± standard error of three independent experiments. The different letters above the columns indicate significant differences (P<0.01) according to Duncan's multiple range test, which was performed using SAS version 9.1 software.
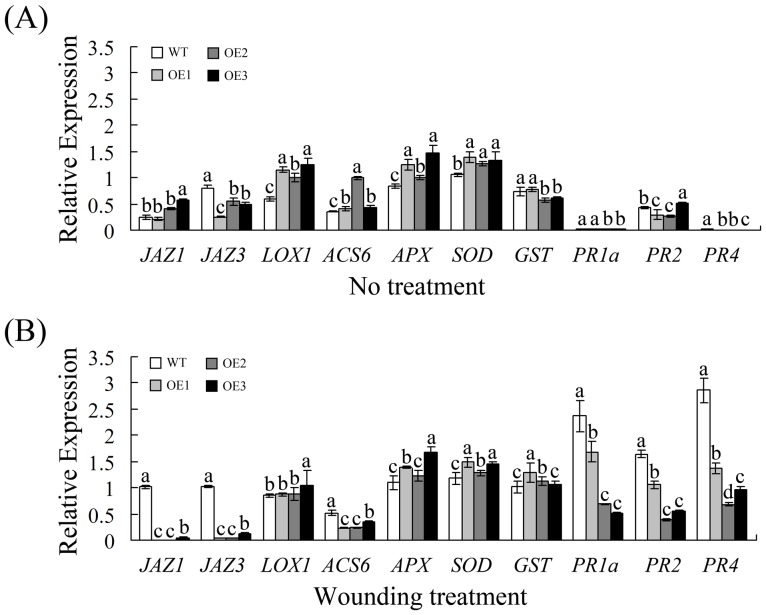
To further confirm the role of GhWRKY40 in the wounding response and to elucidate its possible mechanism of action, the transcriptional responses of known defense genes to GhWRKY40 overexpression were investigated by qPCR (Fig. 5). We examined the transcript levels of the JA-responsive genes JAZ1, JAZ3, and LOX1, the ET production-associated gene ACS6, the reactive oxygen species (ROS) detoxification-associated genes APX, GST, and SOD, and the SA-responsive genes PR1a, PR2, and PR4. Previous studies have shown that each of the tested genes is up-regulated in response to wounding [16], [25]. We found that the transcript levels of the two JAZ genes were clearly decreased in the OE plants after wounding compared with the WT plants. In contrast, we did not find a difference in the LOX1 transcript level between the WT and OE plants in response to wounding. The transcript expression of the ET production-associated genes, which have been identified as early wound-response genes [26]–[28], was inhibited in the OE plants in response to wounding. Interestingly, GhWRKY40 does not appear to significantly enhance the expression of APX, GST, or SOD. In the WT plants, the transcript levels of PR1a, PR2, and PR4 were up-regulated in response to wounding. However, this induction was attenuated by the overexpression of GhWRKY40. Based on the above analysis, it was proposed that wounding may elicit the activation of pathways that interact with the defense response and possibly other signaling pathways. Most importantly, GhWRKY40 may play a crucial role in the wounding response.
Figure 5. qPCR analysis of stress-related gene expression in WT and OE plants under normal conditions (A) and after wounding (B).
The data are presented as the mean ± standard error of three independent experiments. The values indicated by the different letters are significantly different at P<0.01, as determined using Duncan's multiple range tests.
Overexpression of GhWRKY40 increases the susceptibility to R. Solanacearum in transgenic plants
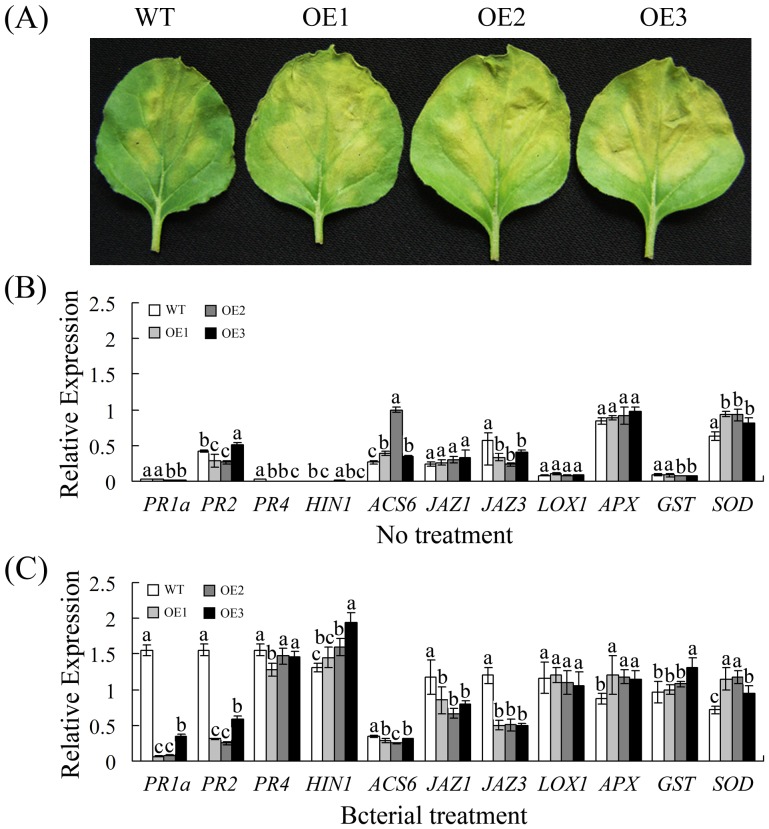
The up-regulation of the GhWRKY40 transcript in response to R. solanacearum suggests a role for this gene in the defense response. To analyze the role of GhWRKY40 in plant basal defense, the bacterial pathogen R. solanacearum was used to infect the OE and WT plants. Five days after infection, all three of the tested transgenic lines exhibited enhanced wilting symptoms and chlorosis (Fig. 6A), indicating that GhWRKY40 overexpression enhances the susceptibility of native tobacco plants to R. solanacearum.
Figure 6. GhWRKY40 overexpression enhances susceptibility to R. Solanacearum in transgenic plants.
(A) Phenotype of WT and OE lines after 5 days of incubation with R. solanacearum. (B–C) Relative transcript levels of defense-related genes in non-infected and infected WT and OE plants were analyzed by qPCR. The data are presented as the mean ± standard error of three independent experiments. The values indicated by the different letters are significantly different at P<0.01, as determined using Duncan's multiple range tests.
Consistent with the enhanced susceptibility of the GhWRKY40-OE plants, they also exhibited reduced transcript levels of the SA production-associated genes PR1a and PR2 relative to the WT plants after R. solanacearum infection. Similarly, the transcript level of ET-responsive gene ACS6 was decreased. After R. solanacearum infection, the JA-responsive genes JAZ1 and JAZ3 were lower in the OE plants than in the WT plants. The ROS detoxification-associated genes APX, GST and SOD exhibited increased transcript levels in the OE plants after R. solanacearum infection, and their transcripts accumulated to higher levels in the OE plants relative to the WT plants. Expression of the HR-associated gene HIN1 was obviously induced, as its transcript accumulated to higher levels in the OE plants than in the WT plants. However, the transcript levels of PR4 and LOX1 did not show any significant difference in the OE plants relative to the WT plants (Fig. 6 B–C).
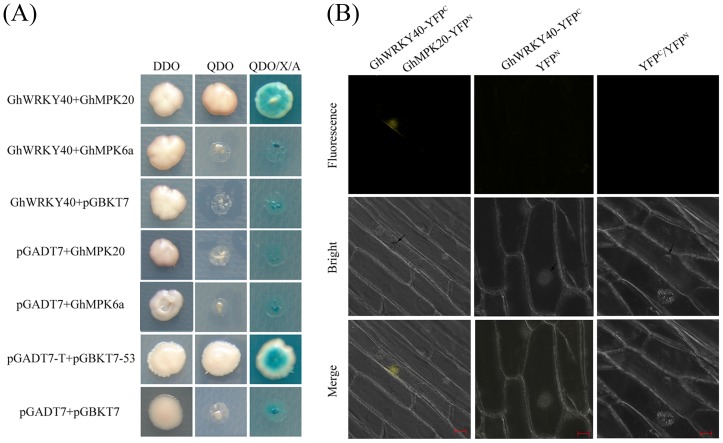
GhWRKY40 interacts with GhMPK20 but not with GhMPK6a
Yeast two-hybrid and BiFC systems were used to determine the interactions between GhWRKY40 and GhMPK6a (HM055511)/GhMPK20 (HQ828072). In the yeast two-hybrid system, positive clones expressing GhMPK20 and GhWRKY40 were able to grow on QDO and QDO/X/A plates, indicating that GhWRKY40 interacted with GhMPK20 due to the activation of the reporter genes AbA and MEL1. However, yeast cells cotransformed with GhMPK6a and GhWRKY40 were unable to grow on QDO and QDO/X/A plates (Fig. 7A). These interactions were further confirmed with the BiFC system, in which two plasmids were constructed for the expression of GhWRKY40-yellow fluorescent protein (YFP)C and GhMPK20-YFPN, reseparately, and then cotransformed into onion epidermal cells by particle bombardment. The YFP fluorescence signal in the onion epidermal cells transfected with GhWRKY40-YFPC and GhMPK20-YFPN was exclusively nuclear (Fig. 7B). Notably, GhMPK20 was detected in both the cytoplasm and nucleus (unpublished data). These data demonstrate that GhWKY40 interacts with GhMPK20 in the nucleus.
Figure 7. Interaction between GhWRKY40 and GhMPK20/GhMPK6a in yeast and onion epidermal cells.
(A) Transformants grown on DDO, QDO or QDO/X/A. (B) In vitro BiFC analysis of the GhWRKY40 -interacting protein GhMPK20 in co-transformed into onion epidermal cells. The yellow fluorescence indicates the interaction between GhWRKY40 and GhMPK20. The fluorescent signals were observed using a confocal microscope. Scale bar = 20 µm.
Discussion
To gain an increased understanding of WRKY transcription factors in cotton, we cloned a WRKY gene from G. hirsutum. Our sequence and phylogenetic tree analyses indicated that the GhWRKY40 gene belongs to subgroup IIa (Fig. 1B). Our subcellular localization experiment with GhWRKY40-GFP indicated that GhWRKY40 is located in the nucleus (Fig. 2B), which is consistent with previous studies on WRKY transcription factors from other species [29]. Moreover, consistent with the putative role of WRKY proteins as transcription factors, three nuclear targeting sequences were identified (Fig. 1A). Transcriptional activation analysis in yeast showed that the full-length GhWRKY40 protein is transcriptionally active (Fig. 2C). These results suggest that GhWRKY40 is a member of the WRKY family in cotton and may serve as a transcriptional activator.
Because plants are sessile, they are constantly affected by their environmental conditions in the form of different abiotic and biotic stresses. Wounding is a common injury in plants that occurs as a result of abiotic stress factors, such as wind, rain and hail, and biotic factors, especially insect feeding. A previous microarray study that focused on the transcriptional profiling of genes after wounding indicated that wounding induces the expression of WRKY family proteins in Arabidopsis [16]. In this study, we found that the GhWRKY40 transcript level is induced by wounding treatment (Fig. 3B) and that GhWRKY40 overexpression affects the expression of defense-related genes in response to wounding (Fig. 5B). The transcriptional induction of JAZ1 and JAZ3 in wounded GhWRKY40-OE plants is lower than in wild-type plants, suggesting that GhWRKY40 negatively controls the expression of certain JAZ genes. Jasmonate ZIM-domain (JAZ) genes, key repressors of JA signaling, are primary response genes in the JA signaling pathway. Most members of the JAZ gene family are highly expressed in response to mechanical wounding [30]. The promoters of JAZ genes contain several W boxes, which can be bound by WRKY genes. In addition, the overexpression of GhWRKY40 was also found to significantly inhibit the expression of PR genes. PRs, defense-related genes often associated with SA-mediated defense responses. Moreover, the expression of APX, SOD and GST is slightly enhanced in response to wounding. PRs and GST are induced by wounding and have been identified as late response genes [16]. In addition, the ROS levels were lower in OE lines. Thus, the wounding tolerance of OE plants might be correlated with oxidative tolerance. Both wounding and pathogenic attack induce the expression of WRKYs, and the responses to wounding and pathogenic infection in plants share a number of signal transduction pathway components [31]. PRs and JAZs have been reported to be such components. The expression of PRs and JAZs were found to be significantly inhibited by the overexpression of GhWRKY40 after R. solanacearum infection (Fig. 6C), and lead to the susceptibility of transgenic plants to R. solanacearum. Similarly, AtWRKY40 affects JA signaling by directly controlling the expression of a subset of JAZs upon plant-pathogen interaction, transcriptional reprogramming regulated by WRKY40 facilitates powdery mildew infection of Arabidopsis [32]. Taken together, GhWRKY40 may be a key component in response to wounding and R. solanacearum attack, and JAZs and PRs may play roles in GhWRKY40-mediated crosstalk between wounding and pathogen defense responses.
As discussed earlier, the signaling molecules SA, JA and ET play important roles in the regulation of the complex defense mechanisms [33]. Previous studies have revealed that responses against biotrophic pathogens are generally regulated by SA, while responses to necrotrophs are mediated by JA and ET [34]–[35]. SA, JA and ET have been shown to activate different sets of plant PR genes and to act either synergistically or antagonistically during defense signaling [36]–[38]. In numerous plants, the transcription of WRKY genes is strongly and rapidly upregulated in response to pathogen infection or defense-related plant hormones, such as SA and JA. In cotton, GhWRKY40 was upregulated by the R. solanacearum, MeJA and SA (Fig. 3). Moreover, the overexpression of GhWRKY40 decreased the resistance of transgenic plants to R. solanacearum infection, as well as PR and JAZ gene transcripts (Fig. 6). SA accumulates in infected leaves after infection with biotrophic pathogens and mediates the induced expression of defense genes, resulting in an enhanced state of defense known as systemic acquired resistance (SAR) [39]. PRs are often used as molecular markers for SAR. In Arabidopsis, the enhanced susceptibility of transgenic plants overexpressing WRKY8 to Pseudomonas syringae was associated with reduced expression of PR1 [40]. Moreover, Arabidopsis WRKY8 is a wounding-induced WRKY gene. In addition to their involvement in disease resistance signaling, SA, JA and ET have been reported to be involved in the wounding response [16], . We showed that GhWRKY40 is transcriptionally inducible by wounding (Fig. 3) and that the overexpression of GhWRKY40 represses the expression of the SA-dependent genes PR1a, PR2 and PR4 and the JA-responsive genes JAZ1 and JAZ3 upon wounding (Fig. 5). Therefore, we speculate that SA/JA induce GhWRKY40 expression which leads to the reduction in expression of downstream defense genes.
WRKY TFs exhibit autoregulation and crossregulation activities and also interact with different proteins, such as MAP kinases, to carry out diverse plant functions [8], [42]. The last decade of research has shown that MPKs regulate WRKY TFs in response to multiple stresses. In previous studies, we functionally identified two MAPK genes from cotton, GhMPK6a [43] and GhMPK20 (unpublished). The results obtained in the present study indicate that GhWRKY40 interacts with GhMPK20 but not with GhMPK6a (Fig. 7). In Arabidopsis, the group II WRKY proteins of WRKY6 and WRKY22 were found to interact with MPK10 and MPK3/MPK6, respectively [44], [42]. However, the identification of the upstream components that regulate WRKY TFs is difficult due to cellular interactions, redundancy, and functional pleiotropy. Our results provide valuable information that aids our understanding of the relationship between MAPKs and the WRKY family proteins in cotton and enhances our understanding of the molecular mechanism of signal transduction in cotton plants under stress.
In conclusion, our results suggest that GhWRKY40 responds to a variety of stresses and that the overexpression of GhWRKY40 in N. benthamiana affects defense-related gene express, enhances the resistance to wounding and the susceptibility to bacterial pathogen. Furthermore, we show that GhWRKY40 interacts with GhMPK20 both in vivo and in vitro. The elucidation of the regulatory mechanism of GhWRKY40 overexpression may reveal a converging node in the regulatory pathways involved the plant responses to wounding and pathogenic infection. Understanding the biological function of GhWRKY40 in cotton enriches our knowledge concerning WRKY function in crops. As we learn more about WRKY regulation, potential applications in genetic improvement should become possible in crops.
Supporting Information
Comparison of the seed germination and post germination of WT and OE plants. (A) Seeds germination phenotype of WT and OE lines on MS medium. (B) The germination rate (greening cotyledon ratio) of the seeds under normal condition. Germination was scored daily. (C) The mass of thousand grains of WT and OE plants. (D) The fresh weight (weight of twenty seedlings) of the seedlings was recorded 10 d after sowing. The data shown indicate the means ± standard errors of three independent experiments. Different letters above the columns indicate significant differences (P<0.01) according to Duncan's multiple range test using SAS version 9.1 software.
(TIF)
The full-length cDNA sequence and primers on the sequence of GhWRKY40. The primers mentioned in the text were underlined. The initiation codon and termination codon was bold.
(TIF)
Polymerase chain reaction amplification conditions.
(DOC)
Primers used for gene cloning.
(DOC)
The primers used for qPCR.
(DOC)
Funding Statement
This work was financially supported by the Genetically Modified Organisms Breeding Major Projects of China (2009ZX08009-092B) and the National Science Foundation of China (grant no. 31171837; 30970225). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgentic tobacco. Proc Nat Acad Sci U S A 101: 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh K, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5: 430–436. [DOI] [PubMed] [Google Scholar]
- 3. Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Plant Biol 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803. [DOI] [PubMed] [Google Scholar]
- 5. Ren X, Chen Z, Liu Y, Zhang H, Zhang M, et al. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis . Plant J 3: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5: 199–206. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal PK, Agarwal P, Reddy MK, Sopory SK (2006) Roles of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25: 1263–1274. [DOI] [PubMed] [Google Scholar]
- 8. Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends in Plant Science 15: 247–258. [DOI] [PubMed] [Google Scholar]
- 9. Birkenbihl RP, Diezel C, Somssich IE (2012) Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol 159 (1)266–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Y, Deyholos MK (2009) Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69 (1–2)91–105. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Fu Q, Chen L, Huang W, Yu D (2011) Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 233 (6)1237–1252. [DOI] [PubMed] [Google Scholar]
- 12. Dang FF, Wang YN, Yu L, Eulgem T, Lai Y, et al. (2012) CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ 36 (4)757–774. [DOI] [PubMed] [Google Scholar]
- 13. Chen W, Provart N, Glazebrook J, Katagiri F, Chang HS, et al. (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, et al. (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26: 403–409. [DOI] [PubMed] [Google Scholar]
- 15. Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, et al. (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci U S A 97: 11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, et al. (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis . Plant Physiol 129 (2)661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, et al. (2002) MAP kinase signalling cascade in Arobidopisis innate immunity. Nature 415: 977–983. [DOI] [PubMed] [Google Scholar]
- 18. Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis . J Biol Chem 281: 38697–38704. [DOI] [PubMed] [Google Scholar]
- 19. Ishihama N, Yamada R, Yoshioka M, Katou S, Yoshioka H (2011) Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell 23: 1153–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen H, Liu C, Zhang Y, Meng X, Zhou X, et al. (2012) OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol Biol 80 (3)241–253. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Xiao H, Chen G, Zhao X, Huang C, et al. (2011) Isolation of high-quality RNA from Reaumuria soongorica, a desert plant rich in secondary metabolites. Mol Biotechnol 48: 165–172. [DOI] [PubMed] [Google Scholar]
- 22. Yu F, Huaxia Y, Lu W, Wu C, Cao X, et al. (2012) GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biol 12: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horsch RB, Rogers SG, Fraley RT (1985) Cold Spring Harbor Symposia on Quantitative Biology. Transgenic plants 50: 433–437. [DOI] [PubMed] [Google Scholar]
- 24. Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, et al. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9 (4)436–442. [DOI] [PubMed] [Google Scholar]
- 25. León J, Rojo E, Sanchez-Serrano JJ (2001) Wounding signaling in plants. J Exp Bot 52 (354)1–9. [DOI] [PubMed] [Google Scholar]
- 26. O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, et al. (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917. [DOI] [PubMed] [Google Scholar]
- 27. Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268 (5211)667–675. [DOI] [PubMed] [Google Scholar]
- 28. Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411. [DOI] [PubMed] [Google Scholar]
- 29. Zhang CQ, Xu Y, Lu Y, Yu HX, Gu MH, et al. (2011) The WRKY transcription factor OsWRKY78 regulates stem elongation and seed development in rice. Planta 234 (3)541–554. [DOI] [PubMed] [Google Scholar]
- 30. Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, et al. (2008) Regulation and function of Arabidopsis JASMONATE ZIM-Domain genes in response to wounding and herbivory. Plant Physiol 146 (3)952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA binding proteins in Arabidopsis . Plant J 24: 837–847. [DOI] [PubMed] [Google Scholar]
- 32. Pandey SP, Roccaro M, Schön M, Logemann E, Somssich IE (2010) Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis . Plant J 64 (6)912–923. [DOI] [PubMed] [Google Scholar]
- 33. Spoel SH, Johnson JS, Dong X (2007) Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci U S A 104: 18842–18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- 35. Farmer EE, Alméras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6: 372–378. [DOI] [PubMed] [Google Scholar]
- 36. Leon-Reyes A, Du Y, Koorneef A, Proietti S, Körbes AP, et al. (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant-Microbe Interact 23: 187–197. [DOI] [PubMed] [Google Scholar]
- 37. Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227. [DOI] [PubMed] [Google Scholar]
- 40. Chen L, Zhang L, Yu D (2010) Wounding-induced WRKY8 is involved in basal defense in Arabidopsis . Mol Plant Microbe Interact 23 (5)558–565. [DOI] [PubMed] [Google Scholar]
- 41. Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin. Plant Biol 1: 404–411. [DOI] [PubMed] [Google Scholar]
- 42. Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, et al. (2009) MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev 1:23 (1)80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Zhang L, Wang X, Zhang W, Hao L, et al. (2013) Cotton GhMPK6a negatively regulates osmotic tolerance and bacterial infection in transgenic Nicotiana benthamiana, and plays a pivotal role in development. FEBS J 280 (20)5128–5144. [DOI] [PubMed] [Google Scholar]
- 44. Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the seed germination and post germination of WT and OE plants. (A) Seeds germination phenotype of WT and OE lines on MS medium. (B) The germination rate (greening cotyledon ratio) of the seeds under normal condition. Germination was scored daily. (C) The mass of thousand grains of WT and OE plants. (D) The fresh weight (weight of twenty seedlings) of the seedlings was recorded 10 d after sowing. The data shown indicate the means ± standard errors of three independent experiments. Different letters above the columns indicate significant differences (P<0.01) according to Duncan's multiple range test using SAS version 9.1 software.
(TIF)
The full-length cDNA sequence and primers on the sequence of GhWRKY40. The primers mentioned in the text were underlined. The initiation codon and termination codon was bold.
(TIF)
Polymerase chain reaction amplification conditions.
(DOC)
Primers used for gene cloning.
(DOC)
The primers used for qPCR.
(DOC)