Abstract
The neural crest is a highly migratory cell population, unique to vertebrates, that forms much of the craniofacial skeleton and peripheral nervous system. In exploring the cell biological basis underlying this behavior, we have identified an unconventional myosin, myosin-X (Myo10) that is required for neural crest migration. Myo10 is highly expressed in both premigratory and migrating cranial neural crest (CNC) cells in Xenopus embryos. Disrupting Myo10 expression using antisense morpholino oligonucleotides leads to impaired neural crest migration, but only a slight delay in induction. In vivo grafting experiments reveal that Myo10-depleted CNC cells migrate a shorter distance and fail to segregate into distinct migratory streams. Finally, in vitro cultures and cell dissociation-reaggregation assays suggest that Myo10 may be critical for production of cell protrusions and cell-cell adhesion. These results demonstrate an essential role for Myo10 in normal cranial neural crest migration and suggest a link to cell-cell interactions and formation of processes.
Keywords: Myosin-X, cranial neural crest, migration, adhesion
Introduction
The neural crest is a transient population of migratory cells present in all vertebrate embryos. Originating at the border of neural plate and non-neural ectoderm, this population becomes specified during gastrulation and neurulation via a complex cascade of signaling pathways and transcription factors (Mayor and Aybar, 2001; Sauka-Spengler and Bronner-Fraser, 2006, 2008). Immediately following neurulation, presumptive neural crest cells are initially contained within the central nervous system. They first become morphologically identifiable when they delaminate from the dorsal neural tube and begin migrating along defined pathways throughout the periphery. Upon reaching their destinations, they differentiate into a wide variety of cell types, including neurons and glia of the peripheral nervous system, pigment cells, and much of the craniofacial bone and cartilage. The molecular and cell biological factors underlying neural crest migration and differentiation are subjects of much interest.
In screening for novel players involved in neural crest induction and migration, we have identified an unconventional myosin, Myosin-X (Myo10), in premigratory and migrating cranial neural crest (CNC) cells. Like all myosins, Myo10 contains a motor domain that interacts with actin and hydrolyzes ATP. In addition, it possesses three IQ motifs that can bind to calmodulin or calmodulin-like light chain, and a coiled-coil domain for dimerization. The tail of Myo10 has a PEST region for proteolytic cleavage, three pleckstrin homology (PH) domains implicated in signaling through phosphatidylinositol phospholipids, and at the C-terminus, a myosin tail homology 4 (MyTH4) domain and a FERM domain important for binding microtubules and β-integrin (Sellers, 2000; Sousa and Cheney, 2005). This structure suggests that Myo10 may mediate membrane-cytoskeleton interactions. Studies in cultured mammalian cells show that Myo10 is expressed at the edges of lamellipodia, membrane ruffles, and the tips of filopodia. Consistent with its location, Myo10 is required for the formation and extension of filopodia. It not only induces the formation of filopodia by convergence of actin bundles through dimerization, but also promotes filopodial extension and stabilization by transporting integrin and actin binding proteins Mena/VASP to their tips (Berg et al., 2000; Berg and Cheney, 2002; Tokuo and Ikebe, 2004; Zhang et al., 2004; Bohil et al., 2006; Tokuo et al., 2007). In addition, Myo10 has been implicated in other processes such as neurite outgrowth (Zhu et al., 2007), endothelial cell migration (Pi et al., 2007), and the assembly of meiotic and mitotic spindles (Weber et al., 2004; Woolner et al., 2008).
Here, we explore the role Myo10 in embryonic development and find that it is highly expressed in premigratory and migrating CNC cells in Xenopus. Furthermore, loss-of-function experiments establish a critical role for Myo10 in the migration of CNC cells in vivo and in vitro, likely due to alterations in cell adhesive properties.
Materials and Methods
Embryo manipulations, morpholino oligonucleotides and RNA preparation
Xenopus embryos, both pigmented and albino, were microinjected with capped RNAs or MOs as described (Chang et al., 1997), using a standard control MO (Gene Tools Inc.) and two Myo10 MOs (Myo10-MO1: 5'-CCCCTCTGTGAAATATTCCTCCATG-3' and Myo10-MO2: 5'-TCTCCCTCTGCTCAGCCAGTATATA-3') that hybridize to −1 to 24 position and −26 to −2 position relative to the translational start site of XMyo10 (Genbank accession no. AY616034). RNAs of nuclear beta-galactosidase (nβGal), nuclear EGFP, and Myo10 open reading frame (kindly provided by Dr. William M. Bement), Wnt3a, noggin, membrane-tethered EGFP (kindly provided by Dr. Chenbei Chang) were synthesized with Ambion mMessage mMachine kit. To selectively target the neural crest, MOs and mRNAs were injected into dorsal animal region of 4–8 cell stage embryos. MOs were injected at 10 ng per embryo and 50–100 pg of Myo10 RNA was coinjected in rescue experiment. For tracing purpose, nβGal or EGFP was injected at 0.2 ng per embryo.
Animal Cap Assay
In screening for genes involved in neural crest development, Wnt3a conditioned media was obtained as previously described (Shibamoto et al., 1998) and a stock was prepared in DMEM containing 0.5% BSA. Recombinant mouse noggin (R&D Systems) was used at a concentration of 0.5 μg/ml. Animal caps were collected at late stage 9 and cultured in Wnt3a-conditioned media and noggin protein or in control media with noggin alone. To test myo10's requirement for neural crest induction, mRNAs encoding Wnt3a and noggin, with or without myo10-MO were injected into animal region of two-cell stage embryos. Animal caps were dissected at late blastula stages and cultured in 0.5xMMR till collected at late gastrula to early neurula stages for in situ hybridization.
Red-Gal staining, in situ hybridization, immunohistochemistry, TUNEL assay, and cartilage staining
For lineage tracing, embryos coinjected with nβGal were fixed for half an hour in MEMFA, rinsed twice with PBS and stained with the Red Gal substrate (Research Organics) until they turned visibly red in color. The embryos were re-fixed for 2 hours in MEMFA and in situ hybridization or whole mount immunohistochemistry was performed as previously described (Chang et al., 1997). Anti-phosphorylated histone H3 antibody was used at 5 μg/ml. The secondary antibodies were used at 1:1000 to 1:2000 dilutions. TUNEL assay was performed as described by Hensey and Gautier (1998). Phosphohistone and TUNEL stained embryos were either cleared in benzyl benzoate/benzyl alcohol solution and imaged directly, or embedded in paraffin and sectioned via microtome before imaging. Cartilage staining was performed according to Dr. Richard Harland's lab protocol (http://tropicalis.berkeley.edu/home/gene_expression/cartilage-stain/alcian.html).
Reverse transcription and polymerize chain reaction (RT-PCR)
Total RNA was extracted from cranial neural crest explants with RNAqueous-Micro kit (Ambion), reverse transcribed and PCR for myo10 expression. The following primer pairs were used: Myo10-U: 5'-TGGGAACTTTGGATGTGGGTCTCA-3'; Myo10-D: 5'-TGAGCAGTTTGGTGTAGAGCCTGT-3'; EF1α-U: 5'-CAGATTGGTGCTGGATATGC-3'; EF1α-D: 5'-ACTGCCTTGATGACTCCAAG-3'.
Cranial neural crest explant cultures and grafting experiment
Cranial neural crest (CNC) explants were dissected from stage 13–16 embryos as previously described (Borchers et al., 2000; Alfandari et al., 2003; DeSimone et al., 2005). Explants were plated onto fibronectin (FN, 20μ/ml in PBS)-coated dishes in MBSH (High Salt Modified Barth's Saline) media and their behavior was photographically documented every 2–3 hours for up to 8 hours. Spreading and segregation of the explants were compared by measuring the relative surface area and counting the number of segments. For observation of cell migration and membrane protrusions, CNC explants labeled with membrane-EGFP were plated on FN-coated coverslips and cell behaviors were recorded by time-lapse movies (3 hours with 3 minute frame intervals) using a Zeiss LSM5 PASCAL confocal microscope. For grafting experiment, cranial neural crest explants were dissected similarly from EGFP-labeled donor embryos and inserted isotopically and isochronically into unlabelled host embryos from which cranial neural crest tissue was removed. The grafted embryos were allowed to heal in MBSH media for 3 hours and then transferred to 0.1xMMR before imaged at late tailbud stages.
Cell adhesion assays
To analyze cell-cell adhesion, CNC explants were dissected from stage 14–16 embryos and dissociated for one hour in calcium- and magnesium-free buffer (CMFB). Loose cells were transferred to 0.5xMMR in agarose-coated dishes and allowed to aggregate by orbital horizontal shaking overnight. The number of large cell clusters that indicate cell reaggregation was counted and compared. To observe cell behavior on fibronectin substrates, CNC explants were dissected and dissociated as described above. Loose cells were then plated onto FN-coated dish in high salt buffer MBSH. Cells were given one hour to settle on FN before been imaged with Zeiss Axiovert35. Cells that became polarized on FN were counted and compared.
Results
Isolation of Xenopus Myo10 as a gene up-regulated during neural crest induction
To identify novel genes essential for neural crest induction and/or early migration, we took advantage of animal cap assays, in which neural crest genes are robustly expressed in naive ectodermal tissue treated with a combination of Wnt signals and BMP inhibitors. Treating animal caps with BMP antagonists, chordin or noggin, induces neural genes such as NCAM whereas treating animal caps with both BMP antagonist and Wnt induces neural crest genes such as Snail2 (Slug) (LaBonne and Bronner-Fraser, 1998). Animal caps were dissected at late stage 9 and cultured in standard media containing noggin protein alone or both noggin and Wnt3a proteins until control embryos reached stage 18. Total RNA and cDNA were prepared from animal caps receiving each treatment and subjected to cDNA microarray analysis as previously described (Baldessari et al., 2005; Peiffer et al., 2005). In this manner, we identified genes up-regulated under conditions of neural crest induction compared with neural induction. One of these, Myo10 was up-regulated 2.3 fold. This set the stage for examining the molecular mechanisms of Myo10 function during neural crest development.
Myo10 is expressed in neural crest cells during early Xenopus development
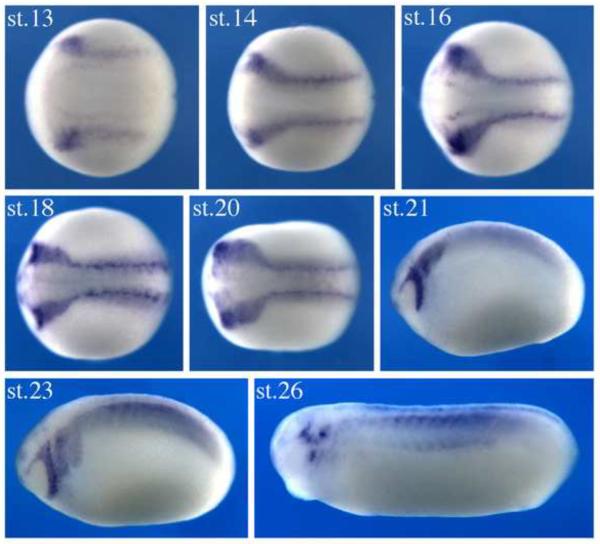
Since Myo10 expression was enhanced as a consequence of in vitro neural crest induction, we next examined its temporal and spatial expression pattern to better understand the time during which it may be functionally important. To this end, we performed in situ hybridization in embryos ranging from gastrula to tailbud stages (Figure1). The onset of Myo10 expression occurred at late gastrula stages (st.12.5–13), where transcripts were expressed at the border of the forming neural plate, the site of initial neural crest induction. During neurula stages (st.14–st.18), as the neural folds fuse to form the neural tube, the intensity of Myo10 increased in the cranial neural crest (CNC) region. Myo10 demarcated both premigratory neural crest cells within the dorsal neural tube as well as streams of migrating neural crest cells. By late neurula stages, Myo10 expressing neural crest cells were migrating towards the forming branchial arches. During tailbud stages, its expression decreased in the cranial neural tube but remained notable in cranial ganglia. In addition to the neural crest, Myo10 was also detected in the developing somites and in portions of the brain.
Figure 1. Myo10 is expressed in neural crest tissues during Xenopus development.
Myo10 expression is first observed in Xenopus embryos at late gastrula stages, at the borders of the forming neural plate, where the neural crest will arise. Expression subsequently increases in the cranial neural crest and continues as they migrate towards the branchial arches. By tailbud stages, the expression decreases, mainly remaining in the cranial ganglia. All embryos are oriented with anterior to the left.
Myo10 MO disrupts the expression of neural crest genes
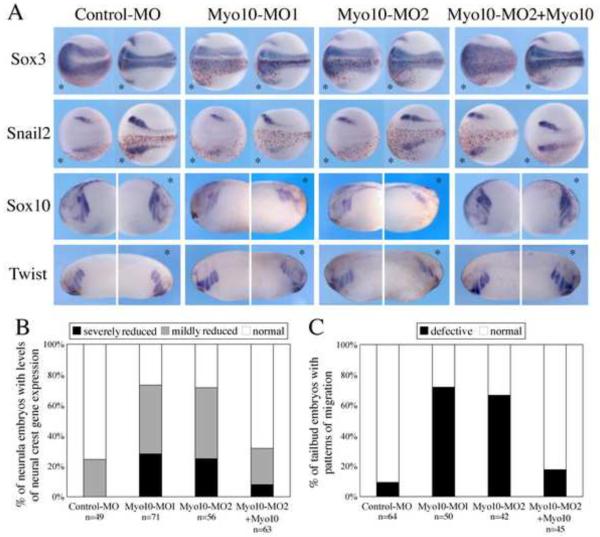
The expression pattern of Myo10 in both premigratory and migratory CNC cells is consistent with a possible role in their formation and/or migration. To test its functional importance, we performed loss-of-function experiments using anti-sense morpholino oligonucleotides (MOs) against Myo10. Myo10-MOs, together with nuclear beta-galactosidase (nβGal) RNA as a lineage tracer, were injected into one side of the embryo during early cleavage stages (2–8 cell stages), such that the contralateral uninjected side served as an internal control. Sister embryos were injected with control-MO in an analogous manner. In control embryos at neurula and tailbud stages, in situ hybridization with probes against neural crest markers Snail2, Sox10 and Twist revealed symmetric patterns on both injected and uninjected sides. In contrast, neural crest marker genes appeared to be down-regulated in Myo10-MO injected embryos (Figure 2A). At neurula stages, Snail2, Sox10, and Twist expression levels were markedly reduced on the Myo10-MO injected side in ~3/4 of injected embryos (68%, 76%, and 76% of embryos for Snail2, Sox10 and Twist, respectively; quantified in Figure 2B).
Figure 2. Myo10-MO disrupted expression of neural crest genes.
(A) Embryos were injected with control or Myo10-MO (10ng) in one cell at the two-cell stage, thus labeling one side (labeled with red-gal staining, bottom half in dorsal view and right panel in lateral view, marked by *). The subsequent expression of neural or neural crest genes was examined at later stages. While the expression of neural marker Sox3 was unaffected, the levels of neural crest genes Snail2, Sox10 and Twist were altered in the half of the embryo that received Myo10-MO. Co-expression of a low dose (50–100pg) Myo10 mRNA efficiently rescued the defect. (B) Percentages of embryos with different levels of neural crest gene expression (combining Snail2, Sox10 and Twist) at neurula stages. While over 75% of control-MO injected embryos showed symmetric neural crest gene expression, Myo10-MOs reduced the neural crest gene expression severely in over 25% of embryos and altered the expression mildly in 45% of the embryos. Nearly 70% of the embryos were rescued to normal levels by coexpressing Myo10 RNA. (C) Percentage of embryos with defected neural crest migration indicated by the expression of Sox10 and Twist at tailbud stages. Myo10-MOs lead to shorter and disorganized CNC migration in about 70% of the embryos and co-injection of Myo10 reduced that to 18%.
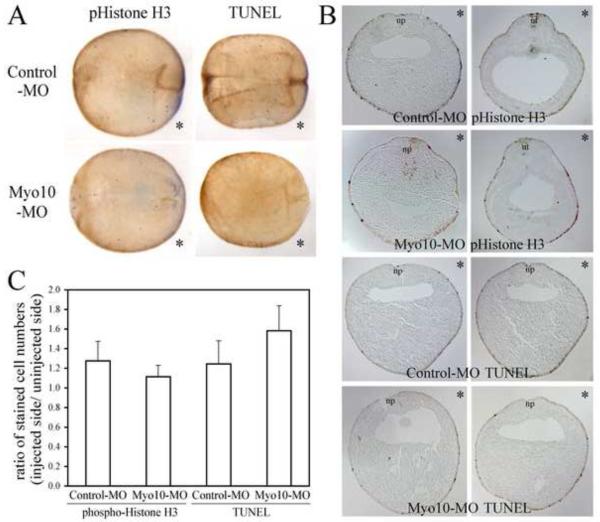
The reduction in transcript levels could reflect either a decrease in cell number due to alterations in cell proliferation/death or diminution of transcript levels. To discriminate between these possibilities, we sectioned embryos to count the number of cells on control versus experimental sides (Figure 3). We noted that the cell number was not significantly altered but the levels of transcript appeared diminished. In addition, cell proliferation was assayed with phosphohistone H3 and cell death with TUNEL staining. Neither was significantly affected (Figure 4). This diminution in transcript levels likely reflects a developmental delay, since neural crest markers were expressed at normal levels by tailbud stages.
Figure 3. High-resolution images of Snail2 expression.
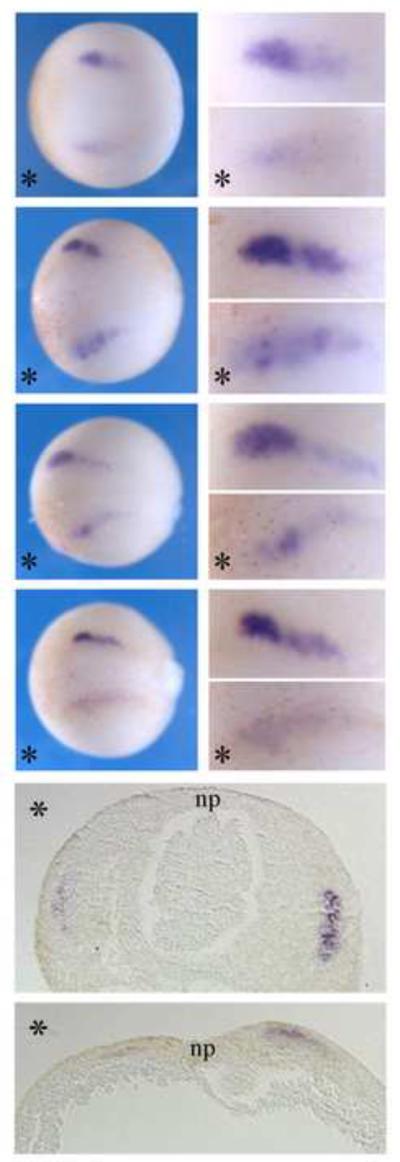
Myo10-MO injected Embryos stained with Snail2 (as in Figure 2) were imaged either at higher magnification or after transverse section. Upper panels were dorsal views of embryos in whole mount, with anterior to the left; bottom panels were 10 m sections through the embryos with dorsal to the top. * marks the injected side. np, neural plate. Although the numbers of cells expressing transcript were approximately the same on left and right sides, the level of expression was markedly lower on the Myo10-MOinjected side of the embryo.
Figure 4. Cell proliferation and cell death is not affected by Myo10-MO.
Embryos were stained with antibody to phospho-histone H3 to mark proliferating cells or processed for TUNEL staining to detect cells undergo apoptosis. (A) Whole embryos were cleared to visualize the internal staining. Embryos are shown in dorsal view, with anterior to the left. Injections were done in the bottom half (marked by *). (B) Transverse sections were oriented with dorsal side up, injected side to the right (marked by *). Closing neural plate and closed neural tube were marked as np or nt. (C) Numbers of proliferating or dying cells on the injected side versus uninjected side were compared. Sections of 10 embryos were counted for each group and no significant changes were observed (p=0.50, 0.36 for cell proliferation and cell death).
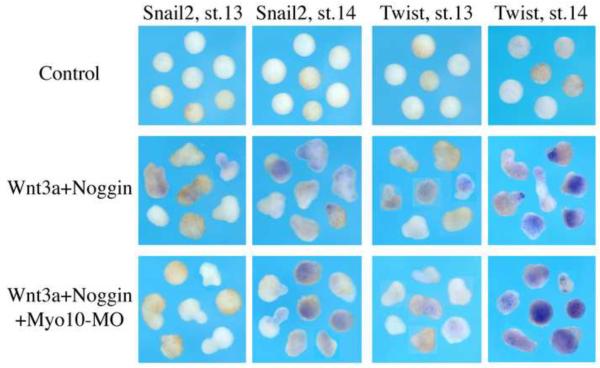
To directly test the possible effects of Myo10-MO on neural crest induction, we turned to an animal cap assay. Animal caps were dissected at late stage 9 from embryos injected with Wnt3a and Noggin mRNA, with or without Myo10-MO. In situ hybridization analysis reveals that Snail2 and Twist are expressed in a slightly delayed fashion in the presence of Myo10-MO, but identical to positive controls by stage 14 (equivalent stage of sibling embryos; Figure 5). These results suggest that Myo10 knock-down does not significantly affect induction of neural crest markers.
Figure 5. Myo10 is not required for neural crest induction in AC assay.
Animal caps were dissected from untreated embryos as well as those exposed to Wnt3a plus noggin, with or without Myo10-MO. Caps were stained against neural crest markers Snail2 and Twist at st.13 and st.14. While control explants remain round and did not express any neural crest genes, explants receiving Wnt3a and noggin started to express Snail2 and Twist weakly at st.13 and strongly at st.14 and the shapes of the caps become irregular. Myo10-MO did not inhibit either the expression of neural crest genes in the explants or the shape changes of the explants.
In contrast, severe migration defects were noted. As CNC cells migrated laterally to populate the branchial arches on the control side, Sox10- and Twist-positive neural crest cells on the Myo10-MO treated side appeared highly disorganized (>70% of embryos exhibited defective neural crest migration; 71% and 73% for Sox10 and Twist, respectively; Figure 2A&C). Moreover, the distance traversed by CNC cell migration was reduced and their paths of migration often appeared irregular. At both early and late stages, pan-neural markers Sox3 and NCAM were expressed normally (Figure 2A and data not shown), suggesting that the effect is specific to neural crest tissue. A second Myo10-MO yielded similar results, with reduced expression of neural crest genes in 71% of neurula stage embryos and disrupted CNC migratory patterns in 67% of tailbud stage embryos. This defect was partially rescued by simultaneous injection of Myo10-MO2 with low levels of Myo10 mRNA (only 32% and 18% of embryos with abnormal neural crest marker expression or migratory patterns at neurula and tailbud stages, respectively), confirming that the MO effect is specific to the down-regulation of Myo10. In contrast to loss-of-function, over-expression of Myo10 had only minor effects on levels of Snail2 expression (data not shown).
Cranial neural crest cells migration and stream formation are abnormal after Myo10 knockdown
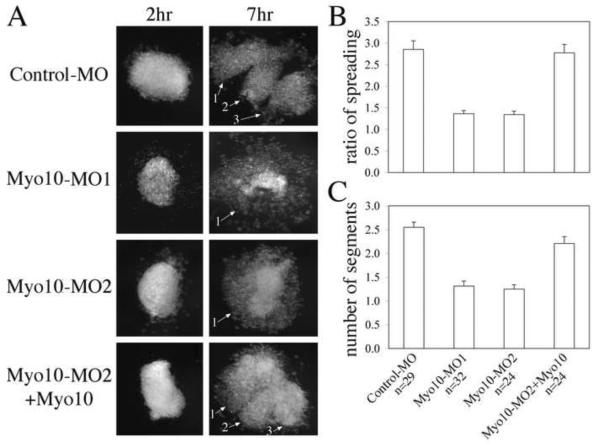
To examine the effects of Myo10 knock-down on neural crest cells in the absence of surrounding tissue, CNC explants were dissected from early neurula stage embryos (st. 13–14) and plated on fibronectin (FN) -coated dishes. After 6–8 hours, CNC cells of control-MO injected explants spread extensively on the substrate and segregated into several apparent lobes, reflecting the separate streams that, in vivo, migrate toward the branchial arches. In contrast, the majority of Myo10-MO injected CNC explants failed to spread well. Instead, the cells tended to dissociate from each other and remained round (Figure 6A). To quantify this effect, the area of coherent cell spreading was measured for each explant at the start and end point of the experiment and relative changes in area were calculated as a measure of the degree of spreading. In addition, the number of distinct cell segments was counted for each explant. The results, summarized in Figure 6B&C, reveal marked differences between control-MO injected explants, which spread 2.85X and formed ~2.6 streams, versus Myo10-MO injected explants, which only spread 1.36X and formed ~1.3 streams. When 50–100pg of Myo10 RNA was co-injected with its MO, both cell spreading and segregation were partially rescued (2.77X; 2.2 segments, respectively). Student t-test confirmed that Myo10-MOs significantly affected both the degree of explant spreading and the segregation into streams. This effect was abrogated by co-injection of a rescue mRNA (p-values below 10−7 when comparing Myo10-MO with control or rescue, above 0.05 when comparing control with rescue). This suggests that Myo10 is required for proper organization of neural crest migratory streams and that its knockdown alters the adhesion of CNC cells.
Figure 6. Myo10 is required for cranial neural crest (CNC) explants to spread and segregate on FN matrix.
(A) CNC explants were dissected from early neurula embryos receiving control or Myo10-MO and plated on FN. By 7 hours, control-MO expressing explants spread extensively and segregated into three cell streams. However, Myo10-MO expressing explants failed to spread on FN, but dissociated into loose and rounded cells. The addition of low dose Myo10 rescued the effect partially. Distinct cell streams were marked by numbers. (B) The surface areas of each explant at the beginning and the end of the experiment were compared and the ratios of explant spreading summarized. Myo10-MOs reduced the ratio of spreading from 2.85 folds to 1.36 folds, while coexpressing Myo10 mRNA restored it to 2.77 folds. (C) The number of segments formed for each explants was counted and compared. While control-MO expressing explants were able to segregate into an average of 2.6 pieces, Myo10-MO reduced this to 1.3. With the addition of low dose Myo10, explants made 2.2 segments on average. For both the spreading and segregation, the disruption by Myo10-MOs and the rescue by Myo10 were significant.
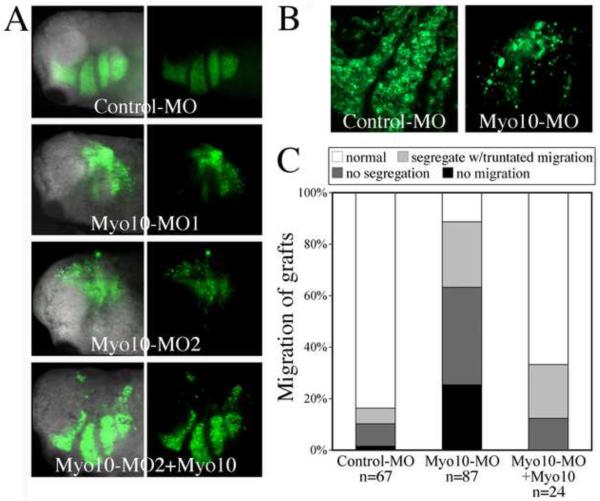
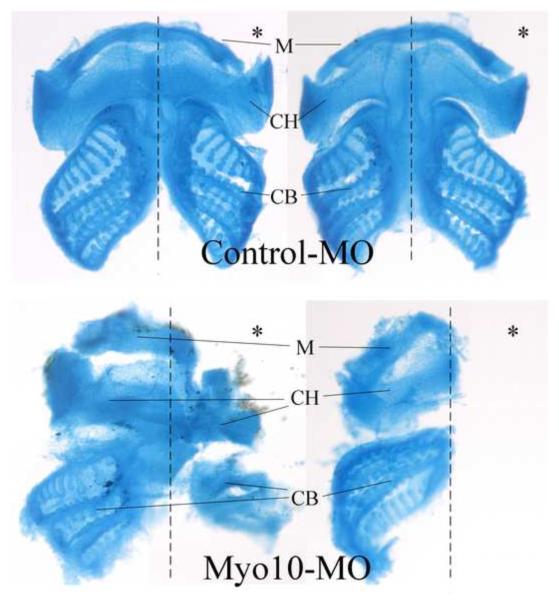
We next examined whether these defects were autonomous to the neural crest by grafting MO-treated CNC back into a normal environment. To this end, a piece of CNC was dissected from EGFP-labeled embryos at stages 14–16 and transplanted isotopically and homotopically into an unlabeled host embryo. At tailbud stages, grafted control-MO injected CNC cells migrated into the branchial arches in an organized manner (Figure 7A). In contrast, the migration of Myo10-MO injected cells was grossly abnormal. They not only failed to reach the ventral edge of the branchial arches, but also often migrated irregularly such that the streams of migrating cells were indistinct and appeared fused. Confocal images of the grafted cells suggest that there is a decrease in cell-cell adhesion between migrating cranial neural crest cells after loss-of-function of Myo10 (Figure 7B). This defect was rescued by co-injection of a low dose of Myo10 RNA, resulting in better separation of streams that extended further ventrally. The dorsolateral distance migrated by the grafted cells was measured in each embryo. While control-MO injected grafts migrated across over 75% of the dorsoventral body axis, Myo10-MO injected grafts only migrated 56% of the distance. With the addition of 50–100pg Myo10 RNA, the migration of the grafted cells recovered, such that they extended 78% of the lateral dimension. The grafts were also summarized into four categories according to their migration patterns (Figure 7C), revealing that not only the distance of migration was reduced, the segregation between cell streams was also disrupted. These results demonstrate that Myo10 is required in CNC cells themselves for proper migration to the branchial arches. Furthermore, in embryos allowed to develop to tadpole stages by which time craniofacial skeletal structures had formed, we found that neural crest derived Meckel's cartilage (from the mandibular stream), ceratohyal cartilage (from the hyoid stream), and ceratobranchial cartilage (from the branchial arches) were either malformed or missing (Figure 8). Thus, the early migration defects have long-term developmental consequences.
Figure 7. Myo10 is required for CNC migration in vivo.
(A) GFP labeled CNC explants were grafted into unlabeled host embryos and imaged at tailbud stages. Fluorescent images and merge images are shown side by side. While control-MO receiving grafts migrated laterally into mandibular arch, hyoid arch, and branchial arches, Myo10-MOs expressing grafts failed to migrate efficiently and the migration paths were often indistinguishable. Co-expression with Myo10 restored this to normal levels. (B) Confocal images of the grafts show that control cells interacted with each other and followed their routes of migration to segregate cleanly into three streams. In contrast, Myo10-MO expressing cells did not appear to interact with neighboring cells. (C) Grafts were placed into four categories based on their migration behavior. While over 80% of control grafts migrate and segregate normally, over 24% of the Myo10-MO expressing grafts failed to migrate at all. Another 60% migrated much shorter distances, half of which did not segregate. Co-expressing Myo10 rescued the migration phenotype, with nearly 70% of the grafts migrating normally. All embryos are shown in lateral view, with dorsal up, anterior left.
Figure 8. Myo10-MO inhibited the formation of cranial cartilage.
Cartilages in late tadpoles were stained with alcian blue. Ventral view of the cranial cartilage with anterior to the top, and injected side marked by *. Cartilage on the Myo10-MO injected side was often malformed or even completely missing. Neural crest derived cartilages are marked as: M, meckel's cartilage; CH, ceratohyal cartilage; CB, ceratobranchial cartilage.
Myo10 is required for cell adhesion and formation of protrusions between cranial neural crest cells in vitro
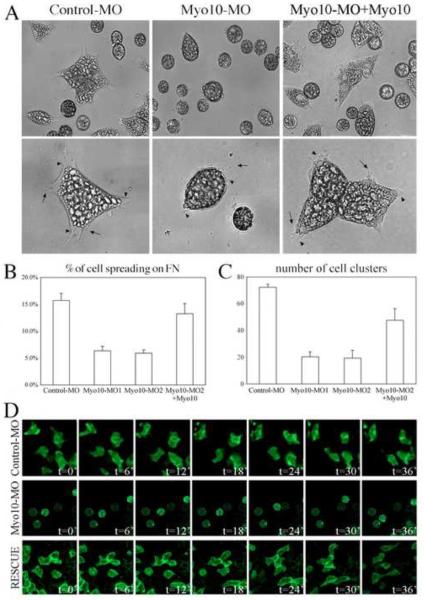
Cells actively modulate their adhesive properties in order to migrate properly. To directly test if Myo10 is required for CNC cell migration, we examined the behavior of individual neural crest cells plated onto fibronectin substrates in the presence and absence of Myo10-MO. Control morpholino-treated neural crest cells adhered to the substrate, spread, actively elicited processes, and were highly motile (Figure 9 and supplementary movie 1). In contrast, Myo10-MO-treated cells adhered but generally failed to spread. They remained rounded and underwent random small movements without directionality or polarization (Figure 9 and supplementary movie 2). In contrast, addition of Myo10-MO plus Myo10 mRNA rescued the cell spreading, process formation, and motility (Figure 9 and supplementary movie 3). These results suggest that Myo10 is important for eliciting cell processes that lead to cell polarization and directional movement.
Figure 9. Myo10-MO disrupted cell adhesion and polarization of CNC cells.
(A) CNC cells were dissociated and plated on FN-matrix. While control-MO expressing cells spread on FN and extend membrane protrusions actively, most Myo10-MO expressing cells remain round. A low dose of Myo10 mRNA together with Myo10-MO efficiently rescued the phenotype, resulting in normal cell morphology. Bottom panels are higher magnification views. Arrows point to the filopodial protrusions while arrowheads indicate lamellipodia. For those few Myo10-MO expressing cells that appeared polarized, only very short protrusions were observed. (B) Spreading cells were counted and their ratios among the entire population were calculated. Myo10-MO dropped the percentage of spreading cells from over 15% to around 6% while Myo10-MO plus Myo10 mRNA resulted in 13% cell spreading, significantly different from Myo10-MO but not from controls. (C) Cell-cell adhesion was examined by dissociation-reaggregation experiments. The same number of explants were used for each experiment, and the number of cell clusters with diameters over 100μm were counted at the end of aggregation assay. While an average of 72 large cell clusters were formed by control-MO cells, only ~20 large cell clusters were formed by Myo10-MOs treated cells. Coexpression of Myo10 increased the number to 48 in average, significantly different from that of MO alone. (D) Behaviors of labeled cells in CNC explant were recorded by time-lapse movies. Frames with 6' intervals were shown. While control cells migrated and extended membrane protrusions dynamically, Myo10-MO expressing cells remained round and failed to form protrusions. The addition of Myo10 mRNA efficiently rescued the ability to form protrusions and to migrate.
Finally, we performed a cell dissociation-reaggregation assay to examine effects on cell-cell adhesion. CNC explants were dissected from early neurula embryos and dissociated in calcium- and magnesium-free buffer. Loose cells then were transferred into 0.5xMMR and gently agitated to allow cell contact. The degree of cell reaggregation between experimental and control cultures was compared by counting the numbers of cell clusters with diameters over 100 μm, using 50 control or 50 experimental explants per experiment (3 repeats). On average, control-MO treated cells formed 72 large cell clusters while Myo10-MO treated cells formed only 19–20 cell clusters of similar size under identical conditions. Co-injection of MO with Myo10 mRNA gave partial rescue (average of 48 cell aggregates formed; Figure 9C). Student t-test showed that the Myo10-MOs caused a significant reduction in the ability of cell aggregation compared to controls and rescued conditions. These data suggest an important role for Myo10 in cell-cell adhesion between CNC cells. Taken together, the effects of cell process formation and intercellular adhesion may partially account for the effects of Myo10 knockdown on CNC cell migration.
Discussion
Our results show that Myo10 is selectively expressed in premigratory and migrating neural crest cells in the early Xenopus embryo. We further show that it plays a critical role in their migration, partly by influencing cell adhesive interactions. We hypothesize that these changes in adhesive behavior may underlie the role of Myo10 activity in influencing neural crest migration.
Myo10 in neural crest specification
At early neurula stages, Myo10-MO reduces the expression level of neural crest genes. Since the expression of these markers is recovered for the most part by tailbud stages, it seems likely that this reflects a delay rather than an inhibition of induction or specification. In contrast, loss-of-Myo10 function profoundly affects the subsequent migratory behavior of cranial neural crest cells.
During neural crest specification, Myo10 may be linked to the activity of signals involved in specifying the neural plate border and/or neural crest. For example, BMP6 has been shown to activate Myo10 in endothelial cells, and Myo10, in turn, provides positive feedback to BMP signals (Pi et al., 2007). Since BMP signaling is required for neural crest specification, an intriguing possibility is that Myo10 modulates BMP activity, causing a partial inhibition of specification. However, neither inhibition nor excess BMP appears to affect the expression of Myo10 in the early Xenopus embryo (Supplementary Figure 1), suggesting that these events may be uncoupled in early development. Alternatively, they may be compensated by other signaling pathways, such as Wnts, thus accounting for a delay rather than complete inhibition of the induction process upon knock-down of Myo10. Another intriguing possibility is that Myo10 may affect the amplification of neural crest progenitor pool. In Xenopus oocyte and epithelial cells, Myo10 has been shown to control the assembly of meiotic and mitotic spindles (Weber et al., 2004; Woolner et al., 2008). However, we failed to observed apparent effects of altering Myo10 activity on neural crest cell proliferation.
Myo10 in neural crest migration
Our results both in vitro and in vivo suggest that Myo10 is required for normal migration of neural crest cells and their cell-cell interactions. After Myo10 knock-down, CNC cells only migrate half the distance towards the branchial arches and exhibit an impaired ability to adhere to one another.
Several signaling pathways have been implicated in the control of neural crest migration. Repulsive signals are important for confining neural crest cells to appropriate pathways. In particular, receptor/ligand interactions of Eph/ephrin, Neuropilin/Semaphorin, and Slit/Robo play roles in regulating the migration of different neural crest populations (Smith et al., 1997; Helbling et al., 1998; Santiago and Erickson, 2002; Davy and Soriano, 2005; Jia et al., 2005; Gammill et al., 2006, 2007; Mellott and Burke, 2008). Interestingly, these ligand/receptor pairs are expressed in complementary patterns on neural crest cells and the neighboring mesoderm, where they function to prevent cells from entering prescribed regions adjacent to their migratory paths. Ephs have been shown to modify the activity of phosphatidylinositol-3 kinase (PI3K) and Rho GTPase Cdc42 in various systems (Fukushima et al., 2006; Bisson et al., 2007; Groeger and Nobes, 2007). Since Myo10 acts downstream of Cdc42 in filopodial formation and may mediate PI3K signal by its PH domains, an intriguing possibility is that Myo10 may interact with Ephs to control neural crest migration.
Attractive signals have also been suggested to positively regulate cell migration, though little is known about chemoattractive signals for the neural crest. A netrin receptor deleted in colorectal cancer (DCC) has been implicated in guiding neural crest cells as they migrate to the developing bowel and pancreas. During neurite outgrowth, Myo10 can regulate the cellular distribution of DCC (Jiang et al., 2003; Zhu et al., 2007). Non-canonical Wnt signaling plays an important role in directional migration of Xenopus neural crest cells. Inhibition of Wnt11 signaling dramatically blocks neural crest migration (Calisto et al., 2005; Matthews et al., 2008a, 2008b). In addition, non-canonical Wnt has been shown to underlie contact inhibition of locomotion in neural crest cells, thus regulating their migration (Carmona-Fontaine et al., 2008). This may occur by modulating cytoskeleton and cell protrusions mediated by Rho GTPases Rho and Rac. Whether or not there is cross-talk between Myo10 and Wnt signals remains to be determined.
Myo10 regulates cell adhesion
Cells require adhesive interactions with either each other and/or an extracellular substrate in order to actively migrate. During cell migration, cells first flatten and spread on the matrix to maximize their adhesions. The points of contact are stabilized at the leading edge of the cell while the adhesion between the trailing edge and the matrix is released so that the cell body can be pulled forward.
Our in vitro culture data suggests that Myo10 is required for adhesion of neural crest cells and to the extracellular matrix. After knockdown of Myo10, neural crest cells attached but fail to spread on fibronectin substrates, instead remaining rounded. In mammalian cells, Myo10 has been shown to translocate β1-integrin to the tips of filopodia (Zhang et al., 2004). A similar mechanism may operate in neural crest cells to regulate cell-matrix interaction.
Cell-cell adhesion is also important for cell movement. Groups of cells tend to move in a directional fashion (Winklbauer et al., 1992). In neural crest cells, their movement along prescribed pathways is mediated by contact inhibition of locomotion via non-canonical Wnt signaling pathways (Carmona-Fontaine et al., 2008). Our in vitro results suggest that Myo10 is involved in maintaining contacts between neural crest cells. Furthermore, knockdown of Myo10 in vivo results in intermingling of neural crest cells between different branchial arch streams and abnormal migration patterns. Although the downstream regulators of these adhesive events are not yet known, cadherins are likely candidates for players in cell-cell adhesion since neural crest cells express specific members of cadherins during different stages of development. In the chick, neural crest cells switch from cadherin 6B, which is down-regulated during delamination, to cadherin 7 during migration (Nakagawa and Takeichi, 1995). In Xenopus, cadherin 11 is turned on when the neural crest begins to migrate (Hadeball et al., 1998). Our results suggest that Myo10 may be involved directly or indirectly in controlling cell adhesive properties that sort cells into distinct migratory pathways, perhaps by modulating cadherins.
Myo10 and cell protrusions
The best-studied activity of Myo10 is in filopodial induction and extension. Filopodia are important membrane protrusive structures important for cell adhesion, movement and guidance. They sense the cell's surroundings and act as sites for signal transduction (Mattila and Lappalainen, 2008). In migrating neural crest cells, both filopodia and lamellipodia are actively extended and withdrawn. Studies in chick demonstrate that the front tier of cells in the migrating neural crest population are typically comprised of cells with filopodia distributed around their entire circumference, while the middle of the migratory streams contains cells with bipolar filopodia (Teddy and Kulesa, 2004). In Xenopus, it has been proposed that filopodia play important roles in short-range and long-range communication for path finding. For example, inhibition of Wnt11 signaling by Dsh-DEP+ causes massive formation of filopodia in neural crest cells, which consequently, fail to migrate (Calisto et al., 2005). Our results are consistent with the intriguing possibility that Myo10 may function as molecular motor in filopodial formation and extension during CNC cell migration, as previously shown in other system (Bohil et al., 2006).
Supplementary Material
RT-PCR experiment was performed with control CNC explants and CNC explants treated with BMP7 (kindly provided by Dr. David Anderson) or BMP inhibitor Dorsomorphin (Tocri bioscience) to compare the expression of Myo10. EF1α was used as a loading control. No obvious changes in Myo10 expression were observed. (Relative expression level was calculated by ImageJ from three parallel experiments. Comparing to control, Myo10 expression in 500ng/ml BMP7, 100ng/ml BMP7 and 5μM Dorsomorphin is 0.97, 1.10 and 0.93, suggesting that myo10 is not likely a downstream target of BMP.)
Migratory behavior of control CNC cells. Time-lapse movie recording cell behaviors in CNC explants at 3' intervals for 4.5 hours.
Migratory behavior of Myo10-MO expressing CNC cells. Time-lapse movie recording cell behaviors in Myo10-MO expressing CNC explants at 3' intervals for 4.5 hours.
Migratory behavior of CNC cells after Myo10-MO plus mRNA, which restored the migratory phenotype to one similar to that observed in control treated cells. 50pg of Myo10 was co-injected with 10ng Myo10-MO2. Time-lapse movie recording cell behaviors in CNC explants at 3' intervals for 3 hours.
Acknowledgments
We thank Dr. William M. Bement for providing us full-length Xenopus Myo10 constructs, Dr. Dominique Alfandari for providing protocols for grafting experiment, Dr. Chenbei Chang for providing Wnt3a and Noggin constructs, and Dr. Ken Cho for providing microarray analysis tool. This work was supported by NS36585 to MBF.
References
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin α5β1 supports the migration of Xenopus cranial neural crest of fibronectin. Dev. Biol. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- Baldessari D, Shin Y, Krebs O, König R, Koide T, Vinayagam A, Fenger U, Mochii M, Terasaka C, Kitayama A, Peiffer D, Ueno N, Eils R, Cho KW, Niehrs C. Global gene expression profiling and cluster analysis in Xenopus laevis. Mech. Dev. 2005;122:441–475. doi: 10.1016/j.mod.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J. Cell Sci. 2000;113:3439–3451. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- Bisson N, Poitras L, Mikryukov A, Tremblay M, Moss T. EphA4 signaling regulates blastomere adhesion in the Xenopus embryo by recruiting Pak1 to suppress Cdc42 function. Mol. Biol. Cell. 2007;18:1030–1043. doi: 10.1091/mbc.E06-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc. Natl. Acad. Sci. U S A. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A, Epperlein H, Wedlich D. An assay system to study migratory behavior of cranial neural crest cells in Xenopus. Dev. Genes Evol. 2000;210:217–222. doi: 10.1007/s004270050307. [DOI] [PubMed] [Google Scholar]
- Calisto JD, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signaling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlow A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: Look both ways. Dev. Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- DeSimone DW, Davidson L, Marsden M, Alfandari D. The Xenopus embryo as a model system for studies of cell migration. Methods Mol. Biol. 2005;294:235–245. doi: 10.1385/1-59259-860-9:235. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Ueno Y, Inoue J, Kanno N, Shimosegawa T. Filopodia formation via a specific Eph family member and PI3K in immortalized cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G812–G819. doi: 10.1152/ajpgi.00250.2005. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/Semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev. Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- Groeger G, Nobes CD. Co-operative Cdc42 and Rho signaling mediates ephrinB-triggered endothelial cell retraction. Biochem. J. 2007;404:23–29. doi: 10.1042/BJ20070146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeball B, Borchers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech. Dev. 1998;72:101–113. doi: 10.1016/s0925-4773(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Helbling PM, Tran CT, Brändli AW. Requirement for EphA receptor signaling in the segregation of Xenopus third and fourth arch neural crest cells. Mech. Dev. 1998;78:63–79. doi: 10.1016/s0925-4773(98)00148-8. [DOI] [PubMed] [Google Scholar]
- Hense C, Gautier J. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol. 1998;203:36–48. doi: 10.1006/dbio.1998.9028. [DOI] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev. Biol. 2005;282:411–421. doi: 10.1016/j.ydbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev. Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Matthews HK, Broders-Bondon F, Thiery JR, Mayor R. Wnt11r is required for cranial neural crest migration. Dev. Dyn. 2008a;237:3404–3409. doi: 10.1002/dvdy.21758. [DOI] [PubMed] [Google Scholar]
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008b;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mayor R, Aybar MJ. Induction and development of neural crest in Xenopus laevis. Cell Tissue Res. 2001;305:203–209. doi: 10.1007/s004410100369. [DOI] [PubMed] [Google Scholar]
- Mellott DO, Burke RD. Divergent roles for Eph and Ephrin in avian cranial neural crest. BMC Dev. Biol. 2008;8:56–71. doi: 10.1186/1471-213X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Peiffer DA, Von Bubnoff A, Shin Y, Kitayama A, Mochii M, Ueno N, Cho KW. A Xenopus DNA microarray approach to identify novel direct BMP target genes involved in early embryonic development. Dev. Dyn. 2005;232:445–456. doi: 10.1002/dvdy.20230. [DOI] [PubMed] [Google Scholar]
- Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, DiVito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J. Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr. Opin. Genet. Dev. 2006;16:360–366. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochimica et Biophysica Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Cheney RE. Myosin-X: a molecular motor at the cell's fingertips. Trends Cell Biol. 2005;15:533–539. doi: 10.1016/j.tcb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem. Biophys. Res. Commun. 2004;319:214–220. doi: 10.1016/j.bbrc.2004.04.167. [DOI] [PubMed] [Google Scholar]
- Tokuo H, Mabuchi K, Ikebe M. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J. Cell Biol. 2007;179:229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Selchow A, Nagel M, Angres B. Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Dev. Dyn. 1992;195:290–302. doi: 10.1002/aja.1001950407. [DOI] [PubMed] [Google Scholar]
- Woolner S, O'Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang C, Dai P, Xie Y, Song N, Liu Y, Du Q, Mei L, Ding Y, Xiong W. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat. Cell Biol. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR experiment was performed with control CNC explants and CNC explants treated with BMP7 (kindly provided by Dr. David Anderson) or BMP inhibitor Dorsomorphin (Tocri bioscience) to compare the expression of Myo10. EF1α was used as a loading control. No obvious changes in Myo10 expression were observed. (Relative expression level was calculated by ImageJ from three parallel experiments. Comparing to control, Myo10 expression in 500ng/ml BMP7, 100ng/ml BMP7 and 5μM Dorsomorphin is 0.97, 1.10 and 0.93, suggesting that myo10 is not likely a downstream target of BMP.)
Migratory behavior of control CNC cells. Time-lapse movie recording cell behaviors in CNC explants at 3' intervals for 4.5 hours.
Migratory behavior of Myo10-MO expressing CNC cells. Time-lapse movie recording cell behaviors in Myo10-MO expressing CNC explants at 3' intervals for 4.5 hours.
Migratory behavior of CNC cells after Myo10-MO plus mRNA, which restored the migratory phenotype to one similar to that observed in control treated cells. 50pg of Myo10 was co-injected with 10ng Myo10-MO2. Time-lapse movie recording cell behaviors in CNC explants at 3' intervals for 3 hours.