Abstract
Objectives
The main goals of this study were: (i) to examine genotypic association of the COMT val158met polymorphism with anxiety-related traits via a meta-analysis; (ii) to examine sex and ethnicity as moderators of the association, and (iii) to evaluate whether the association differed by particular anxiety traits.
Methods
Association studies of the COMT val18met polymorphism and anxiety traits were identified from the PubMed or PsycInfo databases, conference abstracts and listserv postings. Exclusion criteria were: (a) pediatric samples, (b) exclusively clinical samples, and (c) samples selected for a non-anxiety phenotype. Standardized mean differences in anxiety between genotypes were aggregated to produce mean effect sizes across all available samples, and for subgroups stratified by sex and ethnicity (Caucasians vs. Asians). Construct-specific analysis was conducted to evaluate the association of COMT with neuroticism, harm avoidance, and behavioral inhibition.
Results
Twenty seven eligible studies (N=15,979) with available data were identified. Overall findings indicate sex-specific and ethnic-specific effects: Val homozygotes had higher neuroticism than Met homozygotes in studies of Caucasian males ( , 95%CI: 0.02 – 0.25, p = 0.03), and higher harm avoidance in studies of Asian males ( , 95%CI: 0.14 – 0.72, p = 0.004). No significant associations were found in women and effect sizes were diminished when studies were aggregated across ethnicity or anxiety traits.
Conclusions: This meta-analysis provides evidence for sex and ethnicity differences in the association of the COMT val158met polymorphism with anxiety traits. Our findings contribute to current knowledge on the relation between prefrontal dopaminergic transmission and anxiety.
Keywords: Catechol-O-methyltransferase, COMT, anxiety, neuroticism, harm avoidance, meta-analysis, genetic association studies
INTRODUCTION
Catechol-O-methyltransferase (COMT) is an enzyme that plays a key role in inactivating catecholamine neurotransmitters (dopamine, epinephrine, norepinephrine), their metabolites, catechol estrogens and catechol drugs via methylation. In humans, the gene coding for COMT is located on chromosome 22, between bands q11.1 and q11.2 (Grossman et al., 1992). COMT mRNA transcripts encode two isoforms: soluble cytoplasmic (S-) and membrane-bound (MB-) COMT. The latter represents 70% of the total COMT polypeptides in the human brain (Tenhunen et al., 1994) and has a 10-fold higher affinity for dopamine and norepinephrine than S-COMT; therefore, MB-COMT is more effective in metabolizing catecholamines (Lotta et al., 1995; Roth, 1992) and highly relevant for the study of psychiatric and cognitive phenotypes.
Regulation of COMT enzyme activity depends in part on a COMT gene polymorphism involving a change in amino acid from valine (GTG, or Val) to methionine (ATG, or Met) at codon 108 of S-COMT or codon 158 at MB-COMT (Lachman et al., 1996). Val homozygotes have higher COMT enzyme activity than Met homozygotes (Chen et al., 2004), with heterozygotes demonstrating intermediate activity (Weinshilboum et al., 1999). The role of COMT on dopamine catabolism in the prefrontal cortex (PFC) provides the basis for its association with cognitive and emotional processes. Across COMT val158met genotypes, Met/Met is linked to the lowest level of COMT enzyme activity and yields the highest level of extracellular dopamine in the PFC. It is thought of as being positioned at the peak of the putative inverted-U shaped curve of PFC function and dopamine transmission. Higher COMT activity of Val-carriers result in lower levels of PFC dopamine and prefrontal function at baseline, thus Val-carriers are placed on the up slope of the inverted U, with Val/Val at the leftmost position (Goldman-Rakic et al., 2000; Tunbridge et al., 2006).
Bilder et al.’s (2004) extension of the tonic-phasic dopamine hypothesis (Grace, 1991) provides a framework for understanding the effects of COMT val158met on vulnerability to neuropsychiatric disorders. COMT activity has differential effects on dopamine transmission in the PFC and the nucleus accumbens. In the PFC, high COMT activity by the Val allele results in greater dopamine metabolism, weaker postsynaptic D1 receptor-mediated excitation of pyramidal neurons, and lower PFC-accumbens glutamate transmission compared to the low-activity Met allele. In the accumbens, Val-allele is hypothesized to yield less glutamate-stimulated tonic dopamine release into the extrasynaptic space. High COMT activity by Val allele results in lower extracellular (tonic) dopamine in this region, which stimulates higher D2 transmission and is associated with stronger phasic response to external stimuli. The Val-linked, stronger D2 stimulation in subcortical systems facilitate set-switching, updating neural networks with novel information, and inhibiting previously rewarded responses (i.e., allowing extinction of conditioned responses). This has led some to speculate that the Val allele confers protection from distressing stimuli via rapid disengagement of the cortical circuits from the stimuli. The low-activity COMT Met allele, on the other hand, is linked to sustained D1 activation, which helps maintain stable neural networks by preventing attentional distraction. However, the Met allele is also considered to index risk for psychiatric disorders due to a hypothesized inflexibility in switching set away from negative stimuli (Stein et al., 2006; also see Goldman, 2012; Mier et al., 2010).
Rodent studies using knock-out and transgenic models have linked COMT deficiency with higher anxiety responses, including heightened startle activity, greater pain perception, exaggerated stress-induced hyperthermia, less exploratory behavior, and blunted stress response (e.g., Papaleo et al., 2008). Reports of sex differences are inconsistent, with some studies finding effects of lower COMT on higher anxiety just in females (e.g., Gogos et al., 1998) or just in males (e.g., Papaleo et al., 2008).
Human studies of the COMT val158met polymorphism have provided mixed evidence for an association with anxiety disorders. A meta-analysis of eight case-control studies found that men but not women having the Met allele were at greater risk for obsessive-compulsive disorder (Pooley et al., 2007). In a meta-analysis of COMT and panic disorder (PD) based on six Caucasian and Asian case-control samples, Zintzaras and Sakelaridis (2007) reported no evidence for an association. The association was also non-significant when samples were stratified by sex or by ethnicity. However, using the same set of studies, Domschke et al. (2007) further stratified the samples by sex and ethnicity and found that the Val allele was associated with higher risk of PD among Caucasian women, but lower risk in Asian women. There was no evidence of an association between COMT val158met polymorphism and PD in men. A recent study examining the effects of COMT genotype on symptom trajectories in a sample of Caucasian PD patients receiving cognitive-behavioral therapy found that Val-carriers (Val/Val or Val/Met) endorsed more anxiety and depressive symptoms than Met homozygotes at baseline, but also experienced greater symptom improvement (Lonsdorf et al., 2010). These results are consistent with earlier findings linking the Met/Met genotype to difficulties extinguishing conditioned fear (Lonsdorf et al., 2009).
Anxiety-Related Personality Traits in Genetic Association Studies
Association studies have also investigated the genetic basis for anxiety-related personality traits (abbreviated as “anxiety traits” in this report). Anxiety traits are theoretically informative because of their overlapping genetic and environmental vulnerability with a range of psychiatric diagnoses (Ducci et al., 2007; Fanous et al., 2007; Hettema et al., 2006). Anxiety traits also meet the criteria for endophenotypes for anxiety disorders outlined by Gottesman and Gould (2003): First, Individuals with high scores on neuroticism and harm avoidance have higher risk of anxiety disorders (Khan et al., 2005; Starcevic et al., 1996). Second, these traits are heritable. Studies in populations of Western European ancestry find genetic factors underlie 26% to 51% of individual differences in neuroticism (Jardine et al., 1984; Lake et al., 2000; Pedersen et al., 1988; Viken et al., 1994; Wray et al., 2007), and 32% to 49% of harm avoidance (Heath et al., 1994; Heiman et al., 2003; Stallings et al., 1996). Third, elevations in these traits precede illness and are manifested regardless of whether an illness is “active.” Fourth, genetic correlations between neuroticism and several anxiety disorders are high (0.67 – 0.82; Hettema et al., 2006), supporting a shared genetic basis. Practical and methodological benefits of focusing on anxiety traits rather than diagnoses include: availability of psychometrically-validated self-reported instruments for anxiety traits, ease of collecting data from large samples, greater statistical power derived from normally distributed traits compared to categorical (diagnostic) entities, and less sampling bias that are sometimes associated with clinical samples.
Neuroticism and harm avoidance have been frequently studied as anxiety-related personality traits in candidate gene studies. In the personality literature, neuroticism has been conceptualized under two theoretic models. In Eysenck’s personality system, neuroticism reflects a labile autonomic nervous system that triggers frequent, negative emotional distress and expression (Eysenck and Eysenck, 1985). Eysenck neuroticism is indicated by feeling anxious, depressed, moody, irritable, guilty, shy and being interpersonally sensitive. In the NEO system (e.g., NEO-PI; Costa and McCrae, 1985) based on the Five-Factor Model of personality, neuroticism is a somewhat broader construct, with items tapping anxiety, hostility, depression, self-consciousness, impulsiveness and stress vulnerability.
In Cloninger’s psychobiological personality model, harm avoidant individuals are characterized as fearful, cautious, insecure, pessimistic, easily tired and sensitive to punishment (Cloninger, 1987). Harm avoidance is theoretically proximal to Gray’s behavioral inhibition system (BIS; 1976), which is triggered by novel experience, stimuli associated with aggression, punishment, reward omission or termination, and social interactions. Activation of the BIS results in inhibition of ongoing behaviors and gives rise to anxiety. Anxious individuals are introverted, sensitive to BIS triggers, and respond to them with high degrees of arousal and distress.
Studies of COMT val158met and Anxiety Traits
Findings from association studies of the COMT val158met polymorphism with anxiety traits are inconsistent. The Val allele has been associated with higher levels of phobic anxiety (McGrath et al., 2004), harm avoidance (Kim et al., 2006), negative emotionality (Chen et al., 2011), and neuroticism (Hettema et al., 2008). Other reports indicate the Met allele is associated with anxiety traits, including neuroticism (Eley et al. 2003; Hoth et al., 2006) and harm avoidance (Enoch et al. 2003, Hashimoto et al., 2007; Ishii et al., 2007).
Some of the variation in findings may be due to gender composition of the samples, given sex differences in COMT activity (Boudíková et al.,1990) and downregulation of COMT gene transcription by estrogen (Xie et al., 1999). Some association studies have been conducted on combined-sex or single-sex samples for which results might not be directly comparable. Among studies reporting results for both sexes, Reuter et al. (2006) reported that Val homozygotes had higher behavioral inhibition than Met-carriers in men, but lower behavioral inhibition in women.
Ethnic heterogeneity within and between studies is another challenge in interpreting the existing literature. There are marked population differences in the val158met allele frequency, such that the two alleles are nearly equal in frequency among European populations but Val is more common than Met in Asian populations (Palmatier et al., 1999). Differences in language and measures used in studies conducted in ethnic populations also make direct comparisons difficult.
A lack of association between COMT val158met and anxiety traits reported in many samples may be due to insufficient power. For example, an 80% power to detect an effect size of 0.3 for the Met/Met vs. Val-carrier contrast requires N=340 Caucasians or N=884 Asians (accounting for ethnicity-specific genotype frequencies) (Faul et al., 2007). Studies conducted on some Caucasian (Calati et al., 2011; Golimbet et al, 2007; Reuter and Hennig, 2005) and Asian (Tsai et al., 2004; Tochigi et al., 2006; Urata et al., 2007) samples were likely underpowered.
The inconsistency in this literature motivated us to conduct a meta-analysis to synthesize the results of studies of the genotypic association of the COMT val158met polymorphism with anxiety-related traits. In particular, we test whether evidence for the association differs across sex, ethnicity, and different anxiety-related traits.
MATERIALS AND METHODS
Search Strategy
We identified eligible studies by searching the National Library of Medicine’s PubMed and PsycInfo databases. Articles from the first date available through December, 2011, were screened. Keywords used in the search included a combination of the terms “COMT” or “Catechol-O-methyltransferase” with “anxiety”, “personality”, “neuroticism”, “behavioral inhibition”, “harm avoidance”, “OCD”, “obsessive compulsive”, “panic”, “phobia”, “social anxiety”, “agoraphobia”, “posttraumatic stress”, “PTSD”, “generalized anxiety” or “worry.” Identified abstracts were read to select studies examining the association between the COMT val158met polymorphism and anxiety traits. Relevant articles were retrieved and read to determine their appropriateness for inclusion in the meta-analysis; their bibliographies were also searched for potentially eligible studies. We also performed a search for relevant unpublished sources among presentation abstracts1 from the World Congress on Psychiatric Genetics between 2002 and 2011 and posting a request on the electronic listserv of the Behavioral Genetics Association.
Selection of Studies for Inclusion in Meta-Analysis
Association studies of the COMT val158met polymorphism with anxiety traits were included in the meta-analysis. We were interested in the full range of anxiety as expressed among adults in the general population. We included population-based studies that did not screen participants for psychiatric diagnoses (Bækken et al., 2008; Benjamin et al., 2000a; Eley et al., 2003; Hatzimanolis et al., 2010; Harris et al., 2005; Henderson et al., 2000; Hettema et al., 2008; Middeldorp et al., 2010; Olsson et al., 2005, 2007; Wray et al., 2008) to ensure that the analyzed samples are representative of the general population and to avoid range restriction of anxiety traits.
We excluded studies if the samples were: (A) Pediatric (age <18); (B) exclusively based on clinically anxious samples; (C) samples originally selected for having a non-anxiety phenotype (e.g., alcoholics). We excluded samples with anxiety disorders (Criterion B) because specific diagnoses represent etiologically heterogeneous, disorder-specific characteristics in addition to the anxiety traits of interest here, and they may confound the association with COMT Val158Met. Similarly, the decision to exclude samples selected for having other (non-anxiety) phenotypes (Criterion C) addresses the possibility that any detected association between COMT and anxiety would be attributable to their common association with the non-anxiety phenotype (c.f., Enoch et al., 2003). Control samples used in case-control studies (Calati et al., 2011; Desmeules et al., 2011) were included if the sample did not meet any of the exclusion criteria. We did not exclude studies conducted within one sex or ethnicity group.
Data Extraction and Data Requests
We extracted the following data from each study: (1) authors, (2) year of publication, (3) sex, (4) ethnicity, (5) country of origin, (6) sample size by genotype (and by sex and ethnicity within genotype, when possible), (7) mean age by sex, (8) relatedness of participants, (9) unselected vs. extreme-scoring design, (10) anxiety measure(s), (11) means and standard deviations of each anxiety measure by genotype and, when provided, by sex and ethnicity within genotype. Alternatively, for a subset of studies, genotype-specific odds ratios (ORs) or cell frequencies (by sex and ethnicity, when available) were extracted. For each study, we further coded (12) whether anxiety data were available as mean scores or ORs, and (13) whether genotype frequencies deviated from Hardy-Weinberg equilibrium (HWE) for each ethnicity and sex-by-ethnicity subgroup.
Our outcome of interest is the standardized mean differences effect size (ESSM), which reflects the mean difference in anxiety scores between two genotype groups in standard deviation units. Authors were contacted directly to request further data if publications reported: (1) anxiety scores and/or age data collapsed across sexes and/or ethnicity, or (2) results in OR format. Specifically, we requested summary statistics (mean, standard deviation, N) for each anxiety measure by genotype, sex and ethnicity, and mean age by sex and ethnicity. Authors were contacted by email at least twice for each data request.
Data Analysis
Data analysis steps were guided by the work of Lipsey and Wilson (2001) and conducted using the Comprehensive Meta-Analysis statistical software Version 2.2.064 (Borenstein et al., 2011). Hedges (1981) showed that ESSM tends to overestimate the true population parameter, especially in small samples. To correct for this bias, ESSM was multiplied by , with n1 and n2 defined as the sample sizes for the two genotype groups, to yield Hedge’s g (1981), the study-specific effect size entered into the meta-analysis. Hedge’s g is a commonly used mean difference effect size in meta-analyses (e.g., see Borenstein et al., 2009). Three genotypic comparisons were made: Met/Met (MM) vs. Val/Val (VV), Met/Met vs. Val-carriers (Val/*), and Met-carriers (Met/*) vs. Val/Val. A positive g indicates higher anxiety scores for the Met group than the Val group.
OR effect sizes (ESOR) instead of ESSM were calculated for a subset of studies (see Table 1) because the study sampled participants based on extreme scores on an anxiety measure (Eley et al., 2003, Hettema et al., 2008, Wray et al., 2008), or the anxiety measure had a skewed distribution (Bækken et al., 2008; Olsson et al., 2005), or we were unable to acquire data in the desired format from the authors (Urata et al., 2007). ORs were calculated as the odds of having high anxiety in one genotype versus another genotype. For each OR based on an extreme-scoring sample (e.g., top vs. bottom decile), we calculated an OR based on a continuous distribution (e.g., top 10% vs. bottom 90%) calculated from expected genotypic N of unsampled individuals in the middle of the distribution to mitigate the issue of inflated effect size due to extreme groups sampling. Genotypic N for unsampled individuals in the middle of the distribution were estimated based on published population frequencies of the COMT val158met polymorphism for that sample’s ethnicity (Palmetier et al., 1999) while accounting for genotypic frequencies in the extreme groups. Details of this procedure are illustrated with an example in Supplementary Document 1. It is acknowledged that this method of estimating an OR for an extreme-scoring sample is sensitive to the population genotypic frequencies used in the calculation, thus we conducted sensitivity analysis (described later) to test whether any significant associations based on extreme-scoring samples remained after excluding these samples. To allow for direct comparison between effect sizes in mean differences and OR formats, we natural log-transformed each ESOR into ESLOR, and applied the Cox (1970) method by dividing ESLOR by the constant 1.65 to yield ESCox. The sampling variance of ESLOR is estimated as , where Og1,H represents the number of individuals with genotype 1 (e.g., Met/Met) and high anxiety, Og2,L represents the number of individuals with genotype 2 (e.g., Val/Vet) and low anxiety, and so on. An example of this transformation is provided in Supplementary Document 1. ESCox has been found to give a practically unbiased estimation of the population standardized mean difference and closely estimate ESSM when the trait underlying the measure is normally distributed (J. Sánchez-Meca, personal communication, April 2, 2009; Sánchez-Meca et al., 2003).
Table 1.
Descriptive information on studies included in the meta-analysis.
| Study | Year | Sex | Mean Age | Location | Measure (construct) | All N | Male N | Female N |
|---|---|---|---|---|---|---|---|---|
| Benjamin | 2000aR | MF▲ | 26.95 | Israelu | TPQ (HA) | 454 | -- | -- |
|
| ||||||||
| Henderson | 2000 | MF | 42.05 | Australiac | BIS (BI) | 870 | 407 | 463 |
| DSSI (Anx) | 870 | 407 | 463 | |||||
| EPQ-R-S (N) | 868 | 405 | 463 | |||||
| Goldberg (Anx) | 870 | 407 | 463 | |||||
| PANAS (NA) | 965 | 502 | 463 | |||||
|
| ||||||||
| Eley | 2003E,X | F | 36.59 | Germanyc | NEO-FFI (N) | 71 | -- | 71 |
|
| ||||||||
| Kim (A) | 2004H | MF | 25.77 | USc | TCI (HA) | 349 | 164 | 185 |
|
| ||||||||
| Kim (B) | 2004 | F | 24.73 | USa | TCI (HA) | 64 | -- | 64 |
|
| ||||||||
| Tsai | 2004 | F | 20.00 | Taiwana | TPQ (HA) | 120 | -- | 120 |
|
| ||||||||
| Harris | 2005 | MF | 79.06 | Scotlandc | HADS (Anx) | 533 | 223 | 310 |
|
| ||||||||
| Olsson | 2005X | MF | 24.09 | Australiac | CIS-R (Anx) | 803 | 338 | 465 |
| 2007 | NEO (N) | 801 | 338 | 463 | ||||
|
| ||||||||
| Reuter | 2005 | MF | 24.39 | Germanyc | NEO-FFI (N) | 363 | 101 | 262 |
| TCI (HA) | 363 | 101 | 262 | |||||
|
| ||||||||
| Hoth | 2006 | MF | 39.68 | (mixed)c | DASS (Anx) | 402 | 221 | 181 |
| NEO-FFI (N) | 374 | 204 | 170 | |||||
|
| ||||||||
| Ivanova | 2006 | MF | 36.82 | Bulgaria c | TCI (HA) | 102 | 40 | 62 |
|
| ||||||||
| Kim | 2006 | MF | 23.00 | Koreaa | TCI (HA) | 286 | 138 | 148 |
|
| ||||||||
| Reuter | 2006 | MF | 25.57 | Germanyc | BIS (BI) | 295 | 143 | 152 |
|
| ||||||||
| Tochigi | 2006 | MF▲ | 37.40 | Japana | NEO-PI-R (N) | 248 | -- | -- |
|
| ||||||||
| Golimbet | 2007 | MF | 31.55 | Russiac | EPI (N) | 270 | 98 | 172 |
| MMPI (Pt) | 269 | 99 | 170 | |||||
| TCI (HA) | 211 | 68 | 143 | |||||
|
| ||||||||
| Hashimoto | 2007H | MF | 36.32 | Japana | TCI (HA) | 139 | 47 | 92 |
|
| ||||||||
| Ishii | 2007 | MF | 29.30 | Japana | TCI (HA) | 478 | 246 | 232 |
|
| ||||||||
| Urata | 2007X | F | 20.00 | Japana | NEO-FFI (N) | 235 | -- | 235 |
|
| ||||||||
| Bækken | 2008X | MF | 50.61 | Norwayc | HADS (Anx) | 5,651 | 2,629 | 3022 |
|
| ||||||||
| Hettema (A) | 2008E,X | MF | 37.41 | USc | EPQ-R-S (N) | 359 | 186 | 173 |
|
| ||||||||
| Hettema (B) | 2008E,X | MF | 36.47 | USc | EPQ-R-S (N) | 712 | 471 | 241 |
|
| ||||||||
| Montag | 2008C | F | 22.11 | Germanyc | BIS (BI) | 96 | -- | 96 |
|
| ||||||||
| Wray | 2008E,X | MF | 40.14 | Australiac | EPQ-R-S (N) | 954 | 403 | 551 |
|
| ||||||||
| Arias | 2010 | MF | 21.87 | Spainc | STAI-T (Anx) | 456 | 200 | 256 |
|
| ||||||||
| Hatzimanolis | 2010 | F | 39.60 | Greecec | EPQ (N) | 381 | -- | 381 |
|
| ||||||||
| Middeldorp | 2010 | MF | 56.46 | Netherlandsc | YASR (Anx) | 274 | 124 | 150 |
|
| ||||||||
| Calati | 2011 | MF | 45.22 | Germanyc | TCI (HA) | 289 | 123 | 166 |
|
| ||||||||
| Chen | 2011 | MF | 20.47 | Chinaa | BAI (Anx) | 556 | 250 | 306 |
| BIS (BI) | 556 | 250 | 306 | |||||
| TCI (HA) | 556 | 250 | 306 | |||||
|
| ||||||||
| Desmeules | 2011 | F | 50.00 | Switzerlandc | STAI-T (Anx) | 74 | -- | 74 |
|
| ||||||||
| TOTAL (27 studies / 29 independent samples) | 15,979 | 6,648 | 8,630 | |||||
Abbreviations (measures): BAI = Beck Anxiety Inventory (Beck and Steer, 1990); BIS = Behavioral Inhibition System Scale (Carver and White, 1994); CIS-R = Clinical Interview Schedule – Revised (Lewis et al., 1988); DASS = Depression Anxiety Stress Scales (Anxiety subscale; Lovibond and Lovibond, 1995); DSSI = Delusions-Symptoms-States Inventory (Anxiety scale; Bedford et al., 1976); EPI = Eysenck Personality Inventory (Eysenck and Eysenck, 1964); EPQ = Eysenck Personality Questionnaire (Eysenck and Eysenck, 1975); EPQ-R-S = EPQ-Revised-short form (Eysenck and Eysenck, 1991); Goldberg = Goldberg anxiety scale (Goldberg et al., 1988); HADS = Hospital Anxiety and Depression Scale (anxiety subscale; Zigmond and Snaith, 1983); MMPI-Pt = Minnesota Multiphasic Personality Inventory (Psychasthenia subscale) (Hathaway and McKinley, 1940); NEO = Neuroticism, Extraversion, and Openness Personality Inventory (Costa & McCrae, 1985); NEO-FFI = NEO Five Factor Inventory (Costa & McCrae, 1992); NEO-PI-R = NEO Revised (Costa et al., 1991); PANAS = Positive and Negative Affect Schedule (short form; Mackinnon et al., 1999); STAI-T = State Trait Anxiety Inventory (trait form; Spielberger, 1983); TCI = Temperament and Character Inventory (Cloninger et al., 1994); TPQ = Tridimensional Personality Questionnaire (Cloninger et al., 1991); YASR = Young Adult Self-Report (anxious depression scale; Achenbach, 1990).
Abbreviations (constructs): HA = harm avoidance; N = neuroticism; BI = behavioral avoidance; Anx = anxiety; Dep = depression.
Abbreviations (others):a = Asian, c = Caucasian, u = unknown ethnicity. H = HWE flag indicates deviation of genotype frequency from HWE, X = effect sizes provided in odds ratio format and transformed with the Cox method; E = extreme-scoring samples; R = related participants. M = male, F = female, MF = both sexes included; ▲ = studies that did not provide separate statistics for males and females.
- When a sample included multiple measures, N for measure with biggest sample size was used to calculate the total N reported here. Sex-specific N only includes studies that provided sex-specific data. For clarity, each independent sample is placed within the same shaded/unshaded row.
- Olsson et al. (2005) and Olsson et al. (2007) were treated as one study because of the nearly identical samples; they were based on the same study sample (Victorian Adolescent Health Cohort Study. Effect sizes on CIS-R, but not NEO-FFI, were provided in odds ratio format and transformed with the Cox method. On the CIS-R, individuals who reported heightened generalized anxiety symptoms for =>3 waves were coded as “high anxiety” to approximate a median split of the sample.In the 2005 sample, “cases” vs. controls were coded as individuals with 3+ vs. 0–3 waves of generalized anxiety.
- Kim (A) and Kim (B) represent the Caucasian and Asian samples in Kim et al. (2004), respectively. In this study, Hardy-Weinberg equilibrium (HWE) was violated in the Caucasian sample. We excluded the entire sample (Caucasian and Asian) from HWE sensitivity analyses. This study included Asian males, but this subgroup was excluded from our meta-analysis due to N=0 with the rs4680 met/met genotype.
- Hettema (A) and Hettema (B) represent the two-stage independent samples in Hettema et al. (2008).
- In Hashimoto et al. (2007), deviation from HWE was observed only in the combined sex sample, but to be conservative, we removed the study from all HWE sensitivity analyses.
We used the inverse variance method to aggregate effect sizes across studies, as it adjusts for the error variance and size of each sample. The mean effect size across studies ( ), was calculated as the sum of inverse variance weighted effect sizes divided by the sum of inverse variance weights. Data were analyzed under a random effects framework, which accounts for between-study variation in effect sizes in determining and its error variance. We also report study heterogeneity using Cochran’s Q (1954) quantified with the I2 index for all significant findings. Between-study heterogeneity is considered low with I2 < 25%, moderate with I2 = 25–50% and large with I2 > 50% (Higgins et al., 2003).
Moderator and Construct-Based Analyses
We investigated sex (three subgroups: combined, male, female) and ethnicity (three subgroups: any ethnicity, Caucasians, Asians) as moderators of the association between COMT val158met genotype and anxiety-related traits. Subgroup analysis stratified by sex and ethnicity was conducted only if data from at least three studies were available. Samples for which genotype frequencies deviated from the Hardy-Weinberg equilibrium (HWE) were excluded from analysis.
Initially, we examined the association of COMT val158met with any anxiety traits across studies. When multiple anxiety measures were administered to a sample, the average effect size across measures for the sample was entered into the meta-analysis (Schinka et al., 2004).
Next, we investigated whether the COMT val158met genotype was associated with specific anxiety traits: (1) Eysenck’s neuroticism; (2) NEO neuroticism;(3) harm avoidance; and (4) behavioral inhibition. (See Table 1 for a list of measures assessing each trait included in the meta-analysis.) For some analyses, we combined the Eysenck and NEO measures of neuroticism as these are moderately to strongly correlated (r = 0.52 – 0.71; McCrae and Costa, 1983) and have high genetic correlations (0.82 to 0.9; Wray et al., 2007).
Sensitivity Analysis and Assessment of Publication Bias
When a statistically significant (at α =0.05 level) association was detected, we conducted sensitivity analyses to test whether the association could be attributed to the following design features: (1) use of extreme-scoring samples; (2) effect sizes in OR format; or (3) within-sample relatedness (see Table 1). Of note, for the family-based sample in Middeldorp et al. (2010), meta-analyses included data only from parents, but not offspring.
We assessed publication bias with two methods. First, for each genotypic contrast, we conducted meta-regression of individual effect sizes (collapsed across sex, ethnicity and measures within study) against the year of publication. Second, we created funnel plots by plotting the standard error of effect size against the effect size for each study, and used Egger’s test (Egger et al., 1997) to evaluate funnel plot symmetry.
RESULTS
Of 74 studies identified by our literature search, 43 were excluded for the following reasons: Three studies were based exclusively on clinically anxious individuals (i.e., exclusion criterion B; Hohoff et al., 2008; Lochner et al., 2005, 2008; Lonsdorf et al., 2010). Four studies were based on clinically anxious individuals and their family members, i.e., individuals with a high genetic risk and therefore “selected” for anxiety disorders (Alsobrook et al., 2002; Hamilton et al., 2002; Schindler et al., 2000; Walitza et al., 2008;). Ten studies were selected for a non-anxiety phenotype (i.e., exclusion criterion C; Barr et al., 1999; Birklein et al., 2008; Enoch et al., 2003; Kolassa et al., 2010; Gothelf et al., 2007a, 2007b; Light et al., 2007; Max et al., 2006; Michaelovsky et al., 2008; Zinkstok et al., 2008). Thirteen studies did not include a measure of trait anxiety (Cavallini et al., 2000; Domschke et al., 2004; Freitag et al., 2006; Karayiorgiou et al., 1997; O’Hara et al., 1998; Pooley et al., 2007; Poyurovsky et al., 2005; Rothe et al., 2006; Rotondo et al., 2002; Rujescu et al., 2003; Samochowiecz et al., 2004; Strug et al., 2010; Woo et al., 2002). In five studies, the target measure reflected an underlying anxiety disorder, such as a checklist of panic disorder symptoms (Erdal et al., 2003; McGrath et al., 2004; Maron et al., 2008; Meira-Lima et al., 2004; Niehaus et al., 2001). In three studies, the target phenotype was not an anxiety trait (Lang et al., 2007; Lonsdorf et al., 2009; Smolka et al., 2005). The sample from one study was already represented by another study included in our meta-analysis (Khan et al., 2005). One study appeared to examine a different locus on the COMT gene than Val158Met (Woo et al., 2004); attempts to contact the authors for clarification were unsuccessful. Additionally, one study was excluded because the anxiety trait measure was administered only to cases (of irritable bowel syndrome) but not to controls (Karling et al., 2011). Another study was excluded because the experimental procedure (pain induction via infusion of saline into the masseter muscle) possibly affected anxiety ratings (Zubieta et al., 2003). Finally, there was insufficient information from one study (Kulikova et al., 2008) and one poster abstract (Kenis et al., 2010) to determine their eligibility and we were unable to reach the corresponding authors for clarification.
Of 31 studies deemed eligible for the meta-analysis, attempts to request data from authors of seven studies were unsuccessful. We incorporated partial data from three of the seven based on what was in the publication (Benjamin et al., 2000a; Tochigi et al., 2006; Urata et al., 2007), but had insufficient information for four others so these were not included (Åberg et al., 2011; Drabant et al., 2006; Lee et al., 2011; Stein et al., 2005). The remaining 27 studies contributed k=29 independent samples, yielding a total sample size of N=15,979 for the meta-analysis. . Sex-specific data were unavailable for two samples (Benjamin et al., 2000a; Tochigi et al., 2006). Data were available from k=20 independent samples (N=6,648) of men and k=27 independent samples (N=8,630) of women. Of the 29 independent samples, k=20 were Caucasian (N=13,399), k=8 were Asian (N=2,126), and k =1 (N=454) did not report sample ethnicity (Benjamin et al., 2000a). Table 1 provides details for each sample including the country of origin and available measures. Table 2 provides the distribution of genotype frequencies by sex and ethnicity, combined across the available samples.
Table 2.
Genotype frequencies by sex and ethnicity.
| Met/Met | Val/Met | Val/Val | Met/Met | Val/Met | Val/Val | Total | |
|---|---|---|---|---|---|---|---|
| ALL INDEPENDENT SAMPLES (k=29)* |
Any Ethnicity | -- | |||||
| 4,064 (25.43%) | 7,713 (46.26%) | 4,202 (26.30%) | -- | -- | -- | 15,979 | |
| ALL INDEPENDENT SAMPLES (k=28) |
Caucasians (k=20) | Asians (k=8)^ | (k=28) | ||||
| 3,796 (28.33%) | 6,650 (49.63%) | 2,953 (22.04%) | 181 (8.51%) | 833 (39.18%) | 1,112 (52.30%) | Caucasians: 13,399 Asians: 2,126 Total: 15,525 |
|
| MALE (k=20) | Caucasians (k=16) | Asians (k=4) | (k=20) | ||||
| 1,749 (29.31%) | 2,969 (49.76%) | 1,249 (20.93%) | 58 (8.52%) | 250 (36.71%) | 373 (54.77%) | Caucasians: 5,967 Asians: 681 Total: 6,648 |
|
| FEMALE (k=27) | Caucasians (k=20) | Asians (k=7) | |||||
| 2,047 (27.54%) | 3,683 (49.55%) | 1,703 (22.91%) | 95 (7.94%) | 478 (39.93%) | 624 (52.13%) | Caucasians: 7,433 Asians: 1,197 Total: 8,630 |
|
Notes:
See list in Table 1.
Includes one study that did not specify genotype frequencies by sex.
1. When a sample included multiple measures, N for measure with biggest sample size (specific to each sex category) was used to calculate the Ns reported here. Sex-specific Ns are only based on studies that provided sex-specific data.
2. Percentages given as row total for each ethnicity.
The first set of meta-analysis considered the overall association of COMT val158met genotype with anxiety traits combined across all studies. In the overall analysis of 20 combined-sex samples of any ethnicity (Table 3a, top left), the association between anxiety-related traits and genotype was non-significant. In subgroup analyses, we found that among Asian male samples (k=3) higher anxiety was associated with Val-carriers across all three genotypic contrasts, with mean effect sizes between -0.18 and -0.44 (Table 3a). There was minimal evidence for between-study heterogeneity for any of the genotypic contrasts (MM-VV: Q = 0.74; MM-V*: Q = 0.93; M* - VV: Q = 0.06; all df = 2, p ≥ 0.63, I2 = 0%). Sensitivity analysis was not applicable to these samples. Results for other sex*ethnicity subgroups were non-significant. (Study-specific effect sizes for the three genotypic contrasts by sex for each sample are available as Supplementary Table 1. Forest plots of the meta-analyses conducted by each sex subgroup and across ethnicity for the MM-VV contrast are available as Supplementary Figures 1a-c.)
Table 3a–f.
Summary of effect sizes (95% UCI, 95% LCI) by sex, ethnicity and genotypic contrasts; by constructs.
| (a) Any anxiety-related traits: | |||
|---|---|---|---|
| Any Ethnicity | Caucasian | Asian | |
| Both Sexes | k=20 | k=15 | k=4 |
| MM - VV | −0.06 (−0.14, 0.02), p=0.14 | −0.05 (−0.14, 0.04), p=0.30 | −0.17 (−0.46, 0.13), p=0.26 |
| MM - V* | −0.02 (−0.07, 0.02), p=0.34 | −0.02 (−0.07, 0.03), p=0.49 | −0.10 (−0.34, 0.14), p=0.41 |
| M* - VV | −0.05 (−0.12, 0.02), p=0.16 | −0.03 (−0.12, 0.06), p=0.52 | −0.13 (−0.28, 0.03), p=0.10 |
|
| |||
| Male | k=18 | k=15 | k=3 |
| MM - VV | −0.09 (−0.20, 0.01), p=0.07 | −0.06 (−0.16, 0.04), p=0.21 | −0.44 (−0.73, −0.15), P=0.003 |
| MM - V* | −0.05 (−0.12, 0.02), p=0.16 | −0.03 (−0.10, 0.04), p=0.42 | −0.36 (−0.65, −0.08), P=0.01 |
| M* - VV | −0.07 (−0.15, 0.01), p=0.07 | −0.04 (−0.13, 0.05), p=0.38 | −0.18 (−0.34, −0.03), P=0.02 |
|
| |||
| Female | k=24 | k=19 | k=5 |
| MM - VV | 0.02 (−0.11, 0.14), p=0.78 | −0.001 (−0.13, 0.12), p=0.99 | 0.08 (−0.42, 0.58), p=0.75 |
| MM - V* | 0.05 (−0.04, 0.13), p=0.28 | 0.04 (−0.05, 0.12), p=0.41 | 0.11 (−0.31, 0.52), p=0.61 |
| M* - VV | −0.03 (−0.13, 0.06), p=0.50 | −0.04 (−0.14, 0.07), p=0.51 | −0.03 (−0.26, 0.21), p=0.83 |
| (b) Neuroticism: | |||
|---|---|---|---|
| Any Ethnicity | Caucasian | Asian | |
| Both Sexes | k=9 | k=8 | k=1 |
| MM - VV | −0.08 (−0.22, 0.05), p=0.23 | −0.08 (−0.23, 0.06), p=0.27 | -- |
| MM - * | −0.04 (−0.11, 0.03), p=0.28 | −0.04 (−0.12, 0.04), p=0.30 | -- |
| M* - V | −0.06 (−0.20, 0.08), p=0.37 | −0.05 (−0.21, 0.10), p=0.50 | -- |
|
| |||
| Male | (all Caucasian) | k=8 | k=0 |
| MM - V | -- | −0.13 (−0.25, −0.02), P=0.03 | -- |
| MM - V* | -- | −0.07 (−0.17, 0.03), p=0.18 | -- |
| M* - V | -- | −0.10 (−0.21, 0.004), p=0.06 | -- |
|
| |||
| Female | k=11 | k=10 | k=1 |
| MM - VV | −0.03 (−0.21, 0.16), p=0.79 | −0.04 (−0.23, 0.16), p=0.72 | -- |
| MM - V* | 0.02 (−0.07, 0.11), p=0.67 | 0.02 (−0.08, 0.11), p=0.71 | -- |
| M* - V | −0.04 (−0.22, 0.14), p=0.66 | −0.05 (−0.25, 0.14), p=0.59 | -- |
| (c) Neuroticism as measured by Eysenck scales: | |
|---|---|
| Caucasian | |
| Both sexes | k=5 |
| MM-VV | −0.16 (−0.33, 0.01), p=0.07 |
| MM-V* | −0.04 (−0.11, 0.04), p=0.33 |
| M* - VV | −0.13 (−0.33, 0.07), p=0.21 |
|
| |
| Male | k=5 |
| MM-VV | −0.17 (−0.31.−0.04), p=0.01 |
| MM-V* | −0.05 (−0.16, 0.06), p=0.39 |
| M* - VV | −0.17 (−0.29, −0.05), p=0.004 |
|
| |
| Female | k=6 |
| MM-VV | −0.11 (−0.39, 0.18), p=0.46 |
| MM-V* | −0.004 (−0.12, 0.11), p=0.95 |
| M* - VV | −0.11 (−0.40, 0.19), p=0.47 |
| (d) Neuroticism as measured by the NEO scales: | |||
|---|---|---|---|
| Any Ethnicity | Caucasian | Asian | |
| Both sexes | k=4 | k=3 | k=1 |
| MM - VV | 0.02 (−0.19, 0.24), p=0.83 | 0.05 (−0.22, 0.32), p=0.72 | -- |
| MM - V* | −0.02 (−0.19, 0.15), p=0.84 | −0.02 (−0.23, 0.19), p=0.87 | -- |
| M* - VV | 0.02 (−0.14, 0.18), p=0.80 | 0.07 (−0.11, 0.24), p=0.44 | -- |
|
| |||
| Male | (all Caucasian) | k=3 | k=0 |
| MM - VV | -- | −0.01 (−0.33, 0.31), p=0.96 | -- |
| MM - V* | -- | −0.09 (−0.33, 0.15), p=0.45 | -- |
| M* - VV | -- | 0.06 (−0.16, 0.28), p=0.60 | -- |
|
| |||
| Female | k=5 | k=4 | k=1 |
| MM - VV | 0.06 (−0.11, 0.23), p=0.47 | 0.06 (−0.13, 0.25), p=0.53 | -- |
| MM - V* | 0.07 (−0.10, 0.23), p=0.42 | 0.07 (−0.12, 0.27), p=0.46 | -- |
| M* - VV | 0.04 (−0.09, 0.18), p=0.53 | 0.03 (−0.12, 0.18), p=0.67 | -- |
| (e) Harm avoidance: | |||
|---|---|---|---|
| Any Ethnicity | Caucasian | Asian | |
| Both sexes | k=8 | k=4 | k=3 |
| MM - VV | −0.06 (−0.22, 0.10), p=0.47 | 0.08 (−0.10, 0.25), p=0.40 | −0.25 (−0.63, 0.14), p=0.21 |
| MM - V* | −0.05 (−0.17, 0.07), p=0.40 | 0.01 (−0.14, 0.15), p=0.95 | −0.17 (−0.48, 0.14), p=0.27 |
| M* - VV | −0.04 (−0.15, 0.07), p=0.52 | 0.10 (−0.04, 0.24), p=0.16 | −0.14 (−0.32, 0.03), p=0.10 |
|
| |||
| Male | k=7 | k=4 | k=3 |
| MM - VV | −0.17 (−0.43, 0.08), p=0.19 | 0.10 (−0.20, 0.40), p=0.52 | −0.43 (−0.72, −0.14), P=0.004 |
| MM - V* | −0.20 (−0.38, −0.02), P=0.03 | −0.10 (−0.34, 0.14), p=0.43 | −0.34 (−0.62, −0.06), P=0.02 |
| M* - VV | −0.06 (−0.26, 0.14), p=0.54 | 0.19 (−0.06, 0.45), p=0.13 | −0.23 (−0.39, −0.07), P=0.004 |
|
| |||
| Female | k=8 | k=4 | k=4 |
| MM - VV | 0.08 (−0.20, 0.35), p=0.58 | 0.15 (−0.07, 0.37), p=0.19 | −0.01 (−0.64, 0.63), p=0.99 |
| MM - V* | 0.08 (−0.15, 0.30), p=0.51 | 0.11 (−0.08, 0.30), p=0.26 | 0.035 (−0.50, 0.57), p=0.90 |
| M* - VV | 0.01 (−0.15, 0.16), p=0.91 | 0.09 (−0.09, 0.26), p=0.32 | −0.06 (−0.35, 0.23), p=0.68 |
| (f) Behavioral inhibition: | |||
|---|---|---|---|
| Any Ethnicity | Caucasian | Asian | |
| Both sexes | k=3 | k=2 | k=1 |
| MM - VV | 0.02 (−0.13, 0.16), p=0.83 | -- | -- |
| MM - V* | 0.03 (−0.10, 0.15), p=0.69 | -- | -- |
| M* - VV | 0.01 (−0.10, 0.11), p=0.91 | -- | -- |
|
| |||
| Male | k=3 | k=2 | k=1 |
| MM - VV | −0.08 (−0.64, 0.48), p=0.78 | -- | -- |
| MM - V* | −0.06 (−0.51, 0.38), p=0.78 | -- | -- |
| M* - VV | −0.02 (−0.31, 0.27), p=0.90 | -- | -- |
|
| |||
| Female | k=4 | k=3 | k=1 |
| MM - VV | 0.21 (−0.07, 0.49), p=0.15 | 0.15 (−0.20, 0.51), p=0.39 | -- |
| MM - V* | 0.19 (−0.07, 0.45), p=0.16 | 0.15 (−0.18, 0.47), p=0.37 | -- |
| M* - VV | 0.09 (−0.04, 0.23), p=0.18 | 0.06 (−0.12, 0.23), p=0.52 | -- |
Notes for Tables 3a–f:
1. COMT val158met genotype: MM = Met/Met, VV = Val/Val, M* = Met-carriers, V* = Val-carriers.
2. Bold font indicates significant effect size at p ≤ .05.
3. Samples with genotype frequency in violation of Hardy-Weinberg equilibrium have been excluded.
4. Analyses were not conducted when the number of available samples (k) was lower than three.
5. For (c), all samples administered the Eysenck scales were Caucasian.
Analyses of Specific Anxiety Traits
Neuroticism
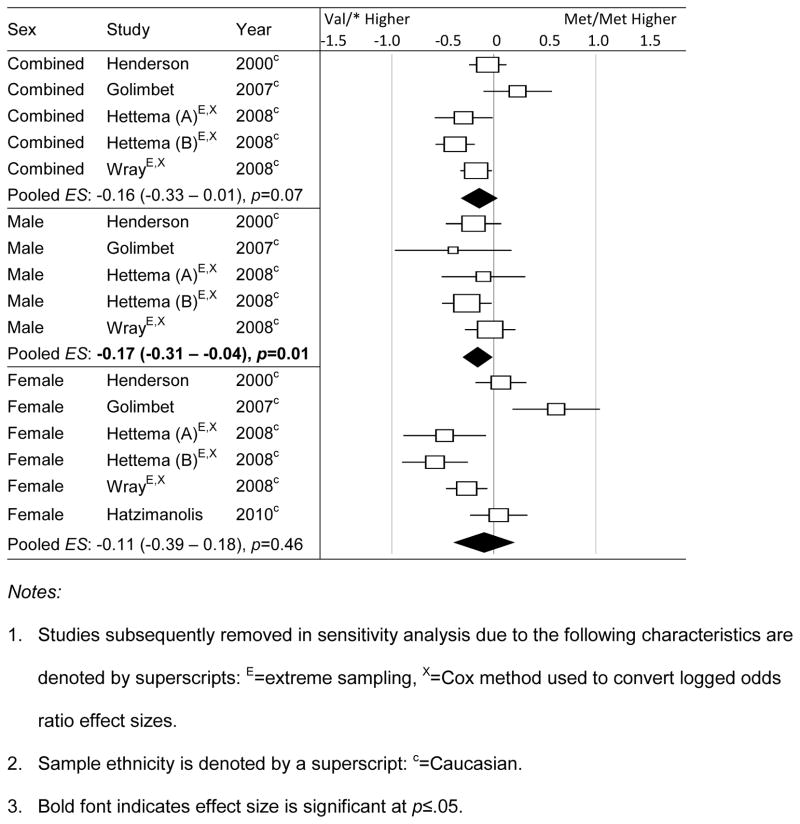
Results for neuroticism as measured by variants of the NEO-PI and EPI questionnaires are shown in Table 3b. Across all possible sex-by-ethnicity subgroups, Val-carriers had higher neuroticism than met-carriers among Caucasian male samples, but the difference was statistically significant only for the MM-VV comparison ( , Q = 7.23, df = 7, p = 0.41, I2 = 3.13%). Sensitivity analysis entailed excluding three studies using an extreme groups design, after which only the MM-V* contrast was significant, , 95%CI = −0.27, −0.01, p = 0.04), with little evidence for between-study heterogeneity (Q = 3.98, df = 4, p = 0.41, I2 = 0%). Findings among Caucasian females were non-significant and we were unable to examine the association in Asians due to limited data (k = 0 to 1 across sex subgroups).
To follow up on the significant association, we separately explored genotype in relation to neuroticism as measured by versions of the EPI versus the NEO scales. Figure 1 is a forest plot of the Met/Met vs. Val/* results by sex. It can be seen that all samples with Eysenck neuroticism data were Caucasian. As shown in Table 3c, significant associations were identified in male samples: men with Val/Val genotype had higher neuroticism than men with Met/Met (MM-VV: ) and Met-carrying genotypes (M*-VV: ). Between-study heterogeneity was large (MM-VV: Q = 26.55, df = 5, p < 0.001, I2 = 81.17%; M*-VV: Q = 42.77, df = 5, p < 0.001, I2 = 88.3%). As shown in Figure 1, sensitivity analysis would have required repeating the meta-analysis after eliminating three samples with extreme-sampling design and ESOR, but this was not feasible due to the limited number of remaining studies (k=2). No other significant association was observed for Caucasian female or combined-sex samples.
Figure 1.
Forest plot of meta-analysis results by sex for Eysenck Neuroticism measures; Met/Met vs. Val/* contrast.
Table 3d summarizes meta-analysis results for neuroticism as measured by the NEO scales. We were unable to test the association in Asians due to limited samples. In the remaining sex*ethnicity subgroups, there was no significant association of genotype with NEO-neuroticism.
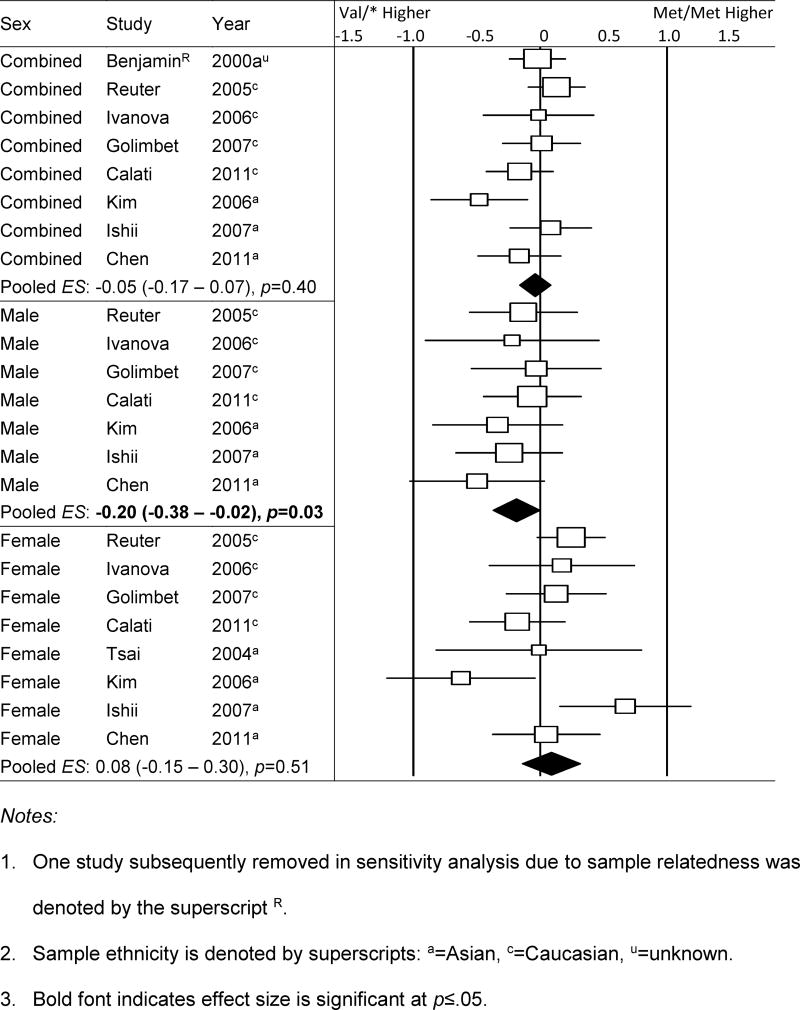
Harm Avoidance
Table 3e summarizes the meta-analysis results and Figure 2 is a forest plot for the Met/Met vs. Val/* contrast by sex for harm avoidance. Significant associations were initially detected among male samples of any ethnicity, such that Val-carrying men had higher levels of harm avoidance than their Met/Met counterpart (MM-V*: ). There was little between-study heterogeneity (Q = 2.42, df = 6, p = 0.88, I2 = 0%) and no sensitivity analysis was warranted. Further stratifying the male samples by ethnicity showed that the significant association was unique to Asians and evident across all three genotypic contrasts: MM-VV: ; MM-V*: M*=VV, . Again, there was little between-study heterogeneity (MM-VV: Q = 0.63; MM-V*: Q = 0.51; M*-VV: Q = 1.01; all df = 2, p ≥ 0.60, I2 = 0%). No sensitivity analysis was warranted. The association was not significant in any female and combined-sex subgroups.
Figure 2.
Forest plot of meta-analysis results by sex for harm avoidance measures; Met/Met vs. Val/* contrast.
Behavioral Inhibition
Data on COMT val158met polymorphism and behavioral inhibition were available on k=3 combined sex samples, k=3 male samples, and k=4 female samples (see Table 3f). Among samples of any ethnicity, there was no evidence of an association in any of the three sex groupings. Due to limited data, stratified analysis by ethnicity was possible only for Caucasian females (k=3), for whom the association was not significant.
Publication Bias
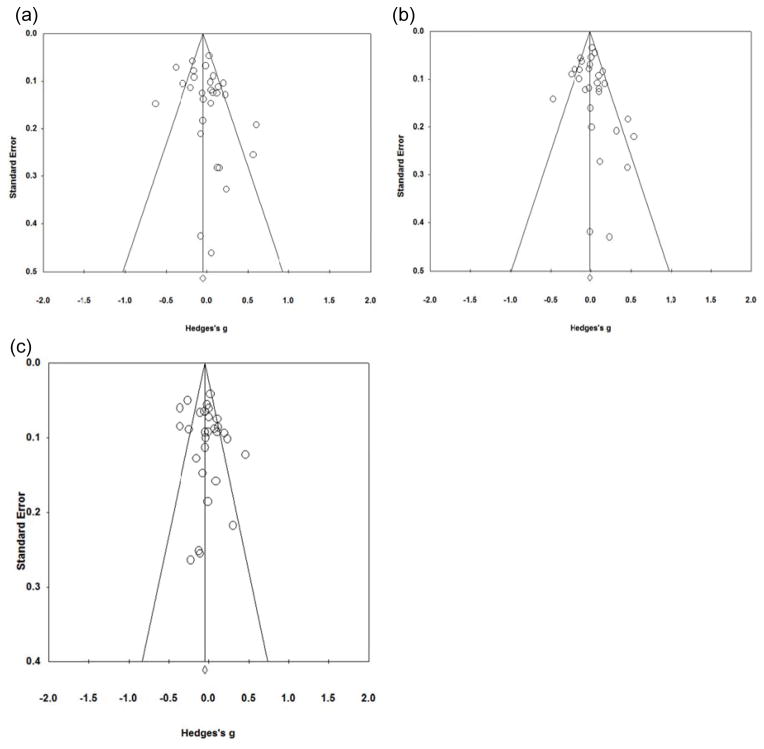
To examine these data for publication bias, we first conducted a meta-regression of average effect size across all anxiety measures for each independent sample against year of publication. There was no evidence of an association between effect size and publication year for each genotypic contrast: MM-VV: bpub_yr = −0.006 (0.01), p = 0.39; MM-V*: bpub_yr = −0.002 (.01), p = 0.78; M*-VV: bpub_yr = −0.003 (0.01), p = 0.56.
Next, we inspected funnel plots for evidence that effect sizes were biased in a particular direction by studies with small sample sizes or large error variances. Funnel plots are shown in Figures 3a–c. Results from Egger’s test were consistent with the null hypothesis of symmetrical funnel plots because the intercepts did not significantly deviate from (0, 0): MM-VV: intercept = 0.99 (0.72), 2-tailed p = 0.18; MM-V*: intercept = 0.59 (0.55), 2-tailed p = 0.30; M*-VV: intercept = 1.08 (0.94), 2-tailed p = 0.26. In sum, we did not find any evidence for publication bias across all samples entered into the meta-analysis.
Figure 3.
Funnel plots of meta-analysis results across k=29 independent samples: (a) Met/Met vs. Val/Val, (b) Met/Met vs. Val/*, (c) Met/* vs. Val/Val.
DISCUSSION
We conducted a meta-analysis of 27 studies (N=15,979) to examine the association of the COMT val158met polymorphism with anxiety traits. Overall, our findings indicate sex-specific effects and some variation associated with anxiety phenotype. Compared to men who were Met homozygotes, Val-carrying men had higher levels of anxiety as measured by neuroticism in Caucasians and harm avoidance in Asians. The ethnic specificity of these effects could not be evaluated fully as there were no studies of neuroticism in Asian males. However, there was evidence suggesting the absence of a COMT-harm avoidance association in Caucasian men was not due to low statistical power, but the association was specific to Asian males. There was no evidence for an association between COMT val158met polymorphism and any anxiety traits in women, regardless of ethnicity.
Association of COMT Val Allele with Anxiety Traits
The association between the COMT Val allele and higher anxiety may be related to COMT effects on dopaminergic tone in the prefrontal cortex (PFC) and their relation to cognition. As mentioned earlier, the stronger phasic response to external stimuli and higher D2 transmission linked to the Val allele has led to speculations that Val protects against distressing stimuli via rapid disengagement of the cortical circuits from the stimuli (e.g., Bilder et al., 2004). Supporting this hypothesis, a recent meta-analysis concluded that Met homozygotes were less efficient than Val homozygotes when engaging in tasks requiring emotional processing, but more efficient on tasks based primarily on cognitive processing (Mier et al., 2010). Specifically, Mier et al. (2010) reported a large effect (d = −1.0) indicating stronger PFC activation by Met homozygotes than Val homozygotes on emotion processing paradigms. Examining the details of the emotional processing tasks used in the four studies included in the Mier et al. (2010) meta-analysis (Domschke et al., 2008; Drabant et al., 2006; Smolka et al., 2005; Yacubian et al., 2007) and more recent works suggests a more nuanced interpretation of these findings. Carriers of the Met allele tended to have greater limbic response in emotional processing tasks that are less effortful, as evidenced by the stronger PFC activation during passive viewing of affectively valenced vs. neutral pictures (e.g., Smolka et al. 2005) and during a facial matching task (Drabant et al. 2006). Domschke et al. (2008) also used a passive-viewing paradigm; however, in contrast to findings by Smolka et al. (2005) and Drabant et al. (2006), they found that Val carriers have greater amygdala and PFC activation in response to emotional faces than Met homozygotes. It may be that a passive facial viewing task was more effortful for the clinically anxious sample in Domschke et al.’s (2008) study than for the healthy controls in the other two studies. Yacubian et al.’s (2007) examined PFC activation in response to reward anticipation during a gambling task. Their task is less comparable to other emotional processing paradigms included in Mier et al.’s (2010) meta-analysis.
One speculation is that during emotional tasks with a high cognitive load, Val carriers may be at a disadvantage. For example, using an affect labeling task that is more effortful in nature than the aforementioned passive viewing paradigms, Kempton et al. (2009) reported less amygdala deactivation in Val/Val men compared to Met/Met men, suggesting less efficient prefrontal inhibition by Val homozygotes. On an attentional task with negatively valenced distractors, Val allele dosage was positively associated with PFC activation to negative distractors relative to neutral distractors (Bishop et al., 2006), suggesting inefficient resource allocation by Val carriers while engaging in a cognitive task and being simultaneously distracted by unpleasant stimuli. One interpretation of these findings is that whereas Met carriers may initially exhibit an exaggerated limbic response to affective stimuli, Val carriers are less efficient at subsequently modulating or inhibiting amygdala response when required to manipulate the emotional information.
Although higher COMT activity and lower PFC dopaminergic tone of Val carriers have been considered as mechanisms that protect against anxiety based on a cognitive advantage, our finding of an association between higher anxiety and the COMT Val allele contradicts this notion. We suggest that our results may be partly attributable to a cognitive profile marked by greater distractibility and unstable cognitive sets among Val carriers. This is consistent with earlier work linking lower cortical dopamine and D1 stimulation (associated with Val) to low cortical signal-to-noise ratio and poor differentiation of target from background in cognitive tasks (Winterer and Weinberger, 2004). Additionally, Val allele dosage has been associated with poor sensorimotor gating (Roussos et al., 2008) and poor accuracy and efficiency in attentional tasks (Blasi et al., 2005). Both neuroticism and harm avoidance are characterized by irritability, hypervigilance, and poor concentration. The higher distractibility associated with the Val allele may facilitate a hypervigilant attentional style and threat-scanning behaviors. Difficulties staying “on task” or engaging in complex cognitive tasks when faced with emotional stimuli may exacerbate distress and increase tendencies toward neurotic and harm avoidant behaviors. If the effect of COMT val158met on anxiety is mediated through cognitive mechanisms, the modest effect sizes observed here are not surprising.
Additional factors may explain the small effect sizes of the association between the COMT val158met polymorphism and anxiety. Other COMT functional loci may account for additional variation in anxiety that is not captured in this study. Cis-regulatory elements (Zhu et al., 2004) and interactions of COMT val158met with two non-synonymous COMT SNPs (Diatchenko et al., 2005) contribute to COMT gene expression beyond the effect of val158met. . For example, association studies of anxiety traits have identified COMT haplotypes containing the val158met polymorphism that were more strongly related to neuroticism than val158met alone (Hettema et al., 2008; Stein et al., 2005). Genetic epistasis and gene-environment interactions are other pathways by which COMT may influence anxiety (e.g., Benjamin et al., 2000b; Kolassa et al., 2010). Research investigating genetic networks may help identify how multiple variants are co-expressed and influence behavioral phenotypes (e.g., Zhang and Horvath, 2005).
The small effect sizes in the current meta-analysis highlight the challenges that exist when conducting association studies of single functional variants with complex phenotypes. The self-report paper-and-pencil measures used to assess anxiety in the studies included in this meta-analysis are indirect indices of a psychological trait. Effect sizes for genetic association are typically larger as one moves from complex behaviors and traits to endophenotypes such as neuroimaging and molecular phenotypes (Zhou et al, 2008).
Sex Differences in the COMT-Anxiety Association
There are several possible explanations for our finding of sex differences in the association of COMT genotype with neuroticism and harm avoidance, including insufficient statistical power, methodological differences and true differences. For each significant association reported for men, the sample size for the corresponding subgroup analysis in women was always larger. All male-based analyses had comparable female samples assessed with the same methodology. Given the reported interaction of COMT val158met genotype and endogenous estrogen fluctuation on cognitive functioning in women (Jacobs and D’Esposito, 2011), a lack of adjustment for female participants’ menstrual phase or use of hormonal birth control in most studies in our meta-analysis might contribute to greater measurement error in female-specific findings. However, the effect sizes estimated for female and male samples within the same studies were generally of similar precision, suggesting the observed sex differences are not due to power or methodological variation.
Several studies have identified sex differences in COMT activity in dopamine-related pathways, including higher COMT enzyme activity in blood and liver tissues among males than females (Cohn and Axelrod, 1971; Boudíková et al., 1990) and lower proportion of dopamine neurons making up mesocortical projections in male than female rats (Kritzer and Creutz, 2008). This implies Val/Val males relative to Met-carrying males or to females are more likely to have low PFC dopaminergic transmission and the associated cognitive and emotional processing vulnerability. Relatedly, greater affective sensitivity to dopamine has been found in men compared to women (Riccardi et al., 2011). The finding of higher anxiety in Val-carrying men but not women may therefore be attributable to their lower levels of PFC dopamine and greater affective sensitivity to dopamine.
Our null finding of a lack of association between COMT val158met genotype and any anxiety traits in women differs from the report by Enoch et al. (2003) of elevated harm avoidance scores in Met/Met women compared to Val-carrying women2. Enoch et al. (2003) used pedigrees that had been selected for alcoholism and an alcohol-related electroencephalogram trait. Differences in sampling may therefore explain the different findings.
Our findings are in contrast to Papaleo et al.’s (2008) finding of blunted stress response, lower pain sensitivity, less exploration and more avoidant behaviors when exposed to novel objects in male mice with lower COMT activity. Our findings and those of Papaleo et al.’s (2008) are in contrast to Gogos et al.’s (1998) report of COMT-deficient female mice exhibiting greater anxiety-like behaviors than wild-type females. In Gogo et al.’s (1998) study, the effect of COMT on anxiety-like behaviors was nonsignificant in male mice. Complete COMT deficiency, as in the knock-out mice used in these animal studies, is not directly analogous to low COMT activity among human Met homozygotes; the same can be said for the comparison between transgenic mice overexpressing the COMT Val allele in Papaleo et al.’s study and human Val homozygotes. Mouse models of anxiety were developed primary in males and sex differences on the same phenotypes can be attributable to numerous factors (see review by Palanza, 2001). For these reasons, we believe that these landmark studies are best used to implicate COMT variation as contributing to risk for neuropsychiatric disorders, but caution is warranted in extrapolating specific findings to humans.
Ethnicity- and Measure-Specific Findings
We found evidence that ethnicity moderates the association between COMT and harm avoidance in men, with higher levels of harm avoidance in Val-carriers than non-Val carriers among males of East Asian descent. The absence of a parallel finding in Caucasian males does not appear to be due to lower statistical power. Calculations using G-power (Faul et al., 2007) and taking ethnic differences in genotype frequency into consideration found the Caucasian male sample size of N=174 had 80% power to detect an effect as large as that found for the MM-VV comparison in the Asian sample (−0.43 based on N=397 Asians). For the MM-V* and M*-VV comparisons, the Caucasian sample size of N=332 had 79% and 43% power, respectively, to detect the corresponding effect sizes found in the Asian sample of N=634.
Another goal of our study was to explore whether several anxiety-related traits differed in their strength of the association with the COMT val158met polymorphism. For neuroticism, the association with COMT val158met was significant for studies using the Eysenck scale but not the NEO scales. Between neuroticism and harm avoidance, the strength of association with COMT val158met was somewhat stronger for the latter. As discussed below, differences in the strength of association is likely attributable to true differences, study methodology and ethnic differences in COMT action on anxiety. However, it was impossible to directly compare the strength of association across constructs because the use of different measures was confounded with ethnicity.
For neuroticism, effect sizes for the MM-VV contrast was −0.13 in the analysis considering both Eysenck and NEO scales, and slightly higher (−0.17) when only considering Eysenck neuroticism. The somewhat higher effect sizes in the Eysenck-only analysis may be due to the inclusion of extreme-scoring samples, as evidenced by the significant between-study heterogeneity in effect sizes. For the MM-V* and M*-VV contrasts, effect sizes of the COMT-neuroticism (Eysenck+NEO, Eysenck only) association ranged from −0.05 to −0.17. The nonsignificant association between COMT val158met polymorphism and NEO neuroticism is likely due to a combination of a weaker true effect and smaller sample sizes in the NEO-only analysis.
For harm avoidance in Asian males, the effect size was −0.43 for the MM-VV contrast, −0.34 and −0.23 in the MM-V* and M*-VV contrasts, respectively. Ethnic differences in COMT action on anxiety and smaller measurement error in the harm avoidance measures may explain the slightly larger effect size for harm avoidance than neuroticism. In particular, despite language differences, all three studies contributing to the significant harm avoidance finding used the same version of the scale (TCI; Cloninger et al., 1993). For Eysenck neuroticism, different versions of the scale (EPQ-R-S vs. EPI) used across studies may contribute to greater effect size heterogeneity across studies, thus reducing the pooled effect size. A twin study investigating the genetic overlap across measures found that 53% and 65% of the covariance between neuroticism and harm avoidance was attributable to genetic factors in men and women, respectively (Heath et al., 1994), so it is not surprising that we found an association of COMT with both traits.
Limitations
There are several limitations to this study. First, despite our attempts to locate all relevant studies, we had insufficient information for several. It is likely that there are others we missed. Our analyses of publication bias indicated that publication year, sample size and error variance were not associated with effect sizes, thus alleviating concerns regarding biased samples entered into the meta-analysis.
Second, despite the relatively large number of studies and pooled sample size contributing to the overall meta-analysis, some of the sex-by-ethnicity subgroup analyses for specific constructs were underpowered. This restricted our ability to evaluate whether the COMT-neuroticism association among Caucasian males also applies to Asian males. Similarly, we were unable to directly compare the magnitude of the association across constructs as the use of different measures was confounded with differences in ethnicity.
Third, we observed significant heterogeneity among studies included in the analysis of Eysenck neuroticism in Caucasian men. Such heterogeneity is likely due to inclusion of three studies that used an extreme-groups design. We were unable to formally estimate the effect size after excluding these samples as only two samples remained. We used two strategies to ameliorate the impact of these studies: calculation of ESOR after imputing the genotypes of unsampled individuals in the middle of these distributions and use of a random-effects analytic framework. Furthermore, in the Eysenck+NEO neuroticism analyses, we obtained similar results before and after excluding the extreme samples.
Summary
This is the first meta-analysis of genetic association of the COMT val158met polymorphism with anxiety-related traits. The total sample of more than 15,000 individuals provided an opportunity to assess whether the association differed by sex and ethnicity. There was a sex-specific association across anxiety traits, such that Val-carriers had higher anxiety than Met homozygotes only among males. The COMT-harm avoidance association was observed among Asian but not Caucasian men. These findings highlight the importance of considering sex and ethnicity in genetic association studies.
Supplementary Material
Acknowledgments
Sources of Support: LOL was supported by NIH AG-031691, AG-039343 and AG-032037.
The authors are grateful to the investigators who have generously contributed data to this study. We would like to thank Dr. Julio Sánchez-Meca and Dr. David B. Wilson for statistical advice, Dr. David Goldman and Dr. Biing-Jiun Shen for helpful comments on earlier versions of this paper, and Ms. Mayuko Onuki for translating email correspondence with Japanese investigators.
Footnotes
Data based on three conference posters were incorporated into the meta-analysis reported here (Arias et al., 2010; Hatzimanolis et al., 2010; Ivanova et al., 2006). Data from two are included in peer-reviewed publications that appeared after the cut-off date of our literature search (Alemany et al., 2013; Hatzimanolis et al., 2013).
Of note, after consultation with the study’s lead author, we chose not to include the Enoch et al. (2003) study in our meta-analysis primarily because it met our exclusion criterion C (sample selection based on a non-anxiety phenotype). Both samples included individuals selected for alcoholism or a putative alcoholism-related phenotype (low voltage alpha EEG) Consequently, any association observed between COMT and measures of trait anxiety could be due to a common association with alcoholism.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Åberg E, Fandiño-Losada A, Sjöholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord. 2011;29:158–166. doi: 10.1016/j.jad.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. The Young Adult Self Report. Burlington, VT: University of Vermont, Department of Psychiatry; 1990. [Google Scholar]
- Alemany S, Arias B, Fatjo-Filas M, Villa H, Moya J, Ibanez MI, et al. Psychosis-inducing effects of cannabis are related to both childhood abuse and COMT genotypes. Acta Psychiatr Scand. 2013 Feb 28; doi: 10.1111/acps.12108. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Alsobrook JP, 2nd, Zohar AH, Leboyer M, Chabane N, Ebstein RP, Pauls DL. Association between the COMT locus and obsessive-compulsive disorder in females but not males. Am J Med Genet. 2002;114:116–120. doi: 10.1002/ajmg.10040. [DOI] [PubMed] [Google Scholar]
- Arias B, Fatjo-Filas M, Estrada G, Mitjans M, Moya J, Ibapez I, et al. The analysis of genetic variability at COMT, CNR1, CNR2, FAAH and CRHNA7 genes on cannabis use, schizotypy and psychotic-like experiences: a study in a Spanish general population. Poster presented at the XVIIIth World Congress on Psychiatric Genetics; Athens, Greece. 2010. [Google Scholar]
- Bækken PM, Skorpen F, Stordal E, Zwart JA, Hagen K. Depression and anxiety in relation to catechol-O-methyltransferase Val158Met genotype in the general population: The Nord Trøndelag Health Study (HUNT) BMC Psychiatry. 2008;8:48. doi: 10.1186/1471-244X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CL, Wigg KG, Sandor P. Catechol-O-methyltransferase and Gilles de la Tourette syndrome. Mol Psychiatry. 1999;4:492–495. doi: 10.1038/sj.mp.4000549. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Bedford A, Foulds GA, Sheffield BF. A new personal disturbance scale (DSSI/sAD) Br J Soc Clin Psychol. 1976;15:387–394. doi: 10.1111/j.2044-8260.1976.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH, et al. Association between tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: dopamine receptor D4 (DRD4), serotonin transporter promoter regions (5-HTTLPR) and catechol-O-methyltransferase. Mol Psychiatry. 2000a;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Lichtenberg P, Bachner-Melman R, Gritsenko I, Kotler M, et al. An interaction between the catechol-O-methyltransferase and serotonin transporter promoter region polymorphisms contributes to Tridimensional Personality Questionnaire persistence scores in normal subjects. Neuropsychobiology. 2000b;41:48–53. doi: 10.1159/000026632. [DOI] [PubMed] [Google Scholar]
- Birklein F, Depmeier C, Rolke R, Hansen C, Rautenstrauss B, Prawitt D, et al. A family-based investigation of cold pain tolerance. Pain. 2008;138:111–118. doi: 10.1016/j.pain.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Cohen JD, Fossella J, Casey BJ, Farah MJ. COMT genotype influences prefrontal response to emotional distraction. Cogn Affect Behav Neurosci. 2006;6:62–70. doi: 10.3758/cabn.6.1.62. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2.2.064. Englewood, NJ: Biostat; 2011. [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Introductive to Meta-Analysis. West Sussex, UK: John Wiley and Sons; 2009. [Google Scholar]
- Boudíková B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Calati R, Porcelli S, Giegling I, Hartmann AM, Möller HJ, De Ronchi D, et al. Catechol-o-methyltransferase gene modulation on suicidal behavior and personality traits: review, meta-analysis and association study. J Psychiatr Res. 2011;45:309–321. doi: 10.1016/j.jpsychires.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- Cavallini MC, Di Bella D, Catalano M, Bellodi L. An association study between 5-HTTLPR polymorphism, COMT polymorphism, and Tourette's syndrome. Psychiatry Res. 2000;97:93–100. doi: 10.1016/s0165-1781(00)00220-1. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen C, Moyzis R, Dong Q, He Q, Zhu B. Sex modulates the associations between the COMT Gene and personality traits. Neuropsychopharmacology. 2011;36:1593–1598. doi: 10.1038/npp.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Syrakic DM, Wetzel RD. The temperament and character inventory (TCI): A guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1994. [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. Normative Data. Psychol Rep. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Cohn CK, Axelrod J. The effect of estradiol on catechol-O-methyltransferase activity in rat liver. Life Sci I. 1971;10:1351–1354. doi: 10.1016/0024-3205(71)90335-3. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO personality inventory manual. Odessa, FL: Psychological Assessment Resources; 1985. [Google Scholar]
- Costa PT, McCrae RR. The revised NEO personality inventory (NEO PI-R). Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, McCrae RR, Dye DD. Facet scales for agreeableness and conscientiousness: A revision of the NEO Personality Inventory. Pers Indiv Differ. 1991;12:887–898. [Google Scholar]
- Cox DR. Analysis of binary data. New York: Chapman & Hall/CRC; 1970. [Google Scholar]
- Desmeules J, Piguet V, Besson M, Chabert J, Rapiti E, Rebsamen M, et al. Psychological distress in fibromyalgia patients: A role for Catechol-O-Methyl-transferase Val158Met polymorphism. Health Psychol. 2011;31:242–249. doi: 10.1037/a0025223. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Domschke K, Deckert J, O’Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbäumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, et al. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol. 2004;7:183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Domschke K, Ohrmann P, Braun M, Suslow T, Bauer J, Hohoff C, et al. Influence of the catechol-O-methyltransferase val158met genotype on amygdala and prefrontal cortex emotional processing in panic disorder. Psychiatry Res. 2008;163:13–20. doi: 10.1016/j.pscychresns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Funt S, Virkkunen M, Albaugh B, Goldman D. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol. 2007;41:3–12. doi: 10.1016/j.alcohol.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, et al. Association analysis of MAOA and COMT with neuroticism assessed by peers. Am J Med Genet Neuropsychiatr Genet. 2003;120B:90–96. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman DA. Genetic origins of anxiety in women: a role for a functional catechol-o-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Erdal ME, Tot S, Yazici K, Yazici A, Herken H, Erdem P, et al. Lack of association of catechol-O-methyltransferase gene polymorphism in obsessive-compulsive disorder. Depress Anxiety. 2003;18:41–45. doi: 10.1002/da.10114. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SGB. The Manual of the Eysenck Personality Inventory. London: University of London Press; 1964. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the EPQ (Eysenck Personality Questionnaire) San Diego, CA: Educational and Industrial Testing Service; 1975. [Google Scholar]
- Eysenck HJ, Eysenck MW. Personality and Individual Differences: A Natural Science Approach. New York: Plenum; 1985. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual for the EPQ-R. San Diego, CA: EdITS; 1991. [Google Scholar]
- Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychol Med. 2007;37:1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Domschke K, Rothe C, Lee YJ, Hohoff C, Gutknecht L, et al. Interaction of serotonergic and noradrenergic gene variants in panic disorder. Psychiatr Genet. 2006;16:59–65. doi: 10.1097/01.ypg.0000199443.69668.b1. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-Omethyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. Br Med J. 1988;297:897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Warriors and worriers. In: Goldman D, editor. Our Genes, Our Choices: How Genotype and Gene Interactions Affect Behavior. San Diego, CA: Academic Press; 2012. pp. 153–162. [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Golimbet VE, Alfimova MV, Gritsenko IK, Ebstein RP. Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci Behav Physiol. 2007;37:601–606. doi: 10.1007/s11055-007-0058-8. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M, et al. Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. Int J Neuropsychopharmacol. 2007;10:301–308. doi: 10.1017/S1461145706006699. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;4:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioral inhibition system: A possible substrate for anxiety. In: Feldman MP, Broadhurst A, editors. Theoretical and Experimental Bases of the Behavior Therapies. London: John Wiley & Sons; 1976. pp. 3–41. [Google Scholar]
- Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1 → 22q11.2. Genomics. 1992;12:822–825. doi: 10.1016/0888-7543(92)90316-k. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, Heiman GA, Deng Z, Haghighi F, Klein DF, et al. Evidence for a susceptibility locus for panic disorder near the catechol-O-methyltransferase gene on chromosome 22. Biol Psychiatry. 2002;51:591–601. doi: 10.1016/s0006-3223(01)01322-1. [DOI] [PubMed] [Google Scholar]
- Harris SE, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The functional COMT polymorphism, Val158Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci Lett. 2005;385:1–6. doi: 10.1016/j.neulet.2005.04.104. [DOI] [PubMed] [Google Scholar]
- Hatzimanolis A, Vitoratou S, Mandelli L, Vaiopoulos C, Nearchou FA, Stefanis CN, et al. Potential role of membrane-bound COMT gene polymorphisms in female depression vulnerability. J Affect Disord. 2013;148:316–322. doi: 10.1016/j.jad.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Noguchi H, Hori H, Ohi K, Yasuda Y, Takeda M, et al. A possible association between the Val158Met polymorphism of the catechol-O-methyltransferase gene and the personality trait of harm avoidance of harm avoidance in Japanese healthy subjects. Neurosci Lett. 2007;428:17–20. doi: 10.1016/j.neulet.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Hathaway SR, McKinley JC. A multiphasic personality schedule (Minnesota): I. Construction of the schedule. J Psychol. 1940;10:249–254. [Google Scholar]
- Hatzimanolis A, Mandelli L, Vaiopoulos C, Nearchou N, Stefanis CN, Serretti A, et al. Regulatory COMT gene variants associate with depression in females. Poster presented at the XVIIIth World Congress on Psychiatric Genetics; Athens, Greece. 2010. [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: A comparison of the personality systems of Cloninger and Eysenck. J Pers Soc Psychol. 1994;66:762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6:107–128. [Google Scholar]
- Heiman N, Stallings MC, Hofer SM, Hewitt JK. Investigating age differences in the genetic and environmental structure of the Tridimensional Personality Questionnaire in later adulthood. Behav Genet. 2003;22:171–180. doi: 10.1023/a:1022558002760. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Korten AE, Jorm AF, Jacomb PA, Christensen H, et al. COMT and DRD3 polymorphisms, environmental exposures, and personality traits related to common mental disorders. Am J Med Genet. 2000;96:102–107. doi: 10.1002/(sici)1096-8628(20000207)96:1<102::aid-ajmg20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, Bukszar J, van den Oord EJCG, Neale MC, Kendler KS. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry. 2008;64:302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks J, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohoff C, Domschke K, Schwarte K, Spellmeyer G, Vögele C, Hetzel G, et al. Sympathetic activity relates to adenosine A2A receptor gene variation in blood-injury phobia. J Neural Trasm. 2008;116:659–662. doi: 10.1007/s00702-008-0089-5. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Paul RH, Williams LM, Dobson-Stone C, Todd E, Schofield PR, et al. Associations between the COMT Val/Met polymorphism, early life stress, and personality among healthy adults. Neuropsychiatr Dis Treat. 2006;2:219–225. doi: 10.2147/nedt.2006.2.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii G, Suzuki A, Oshino S, Shiraishi H, Matsumoto Y, Otani K, et al. Association study of catechol-O-methyltransferase Val158met polymorphiasm with personality traits in Japanese healthy volunteers. Eur Psychiatry. 2007;22:462–5. doi: 10.1016/j.eurpsy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ivanova M, Chorbov V, Kostov C, Milanova V, Kremensky I, Kaneva R. Association analysis of polymorphisms in serotonine and dopamine related genes and personality traits in Bulgarian subjects. Poster presented at the XIVth World Congress on Psychiatric Genetics; Cagliari, Italy. 2006. [Google Scholar]
- Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genet Epidemiol. 1984;1:89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, et al. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 1997;94:4572–5. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karling P, Danielsson Å, Wikgren M, Söderström I, Del-Favero J, Adolfsson R, et al. The relationship between the val158met catechol-O-methyltransferase (COMT) polymorphism and irritable bowel syndrome. PLoS One. 2011;6:e18035. doi: 10.1371/journal.pone.0018035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Joqia J, Christodoulou T, Powell J, Collier D, et al. The effects of gender and COMT Val158Met polymorphism on fearful facialaffect recognition: a fMRI study. Int J Neuropsychopharmacol. 2009;12:371–381. doi: 10.1017/S1461145708009395. [DOI] [PubMed] [Google Scholar]
- Kenis G, Köhler S, Prickaerts J, van Winkle R, Smeets B, Bekers O, et al. Interaction between depression and genotype of COMT and BDNF on cognitive decline in a community dwelling cohort. Poster presented at the XVIIIth World Congress of Psychiatric Genetics; Athens, Greece. 2010. [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Kim SY, Lee HS, Kim CH. An association study of catechol-O-methyltransferase and oxidase A polymorphisms and personality traits in Koreans. Neurosci Lett. 2006;401:154–158. doi: 10.1016/j.neulet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJF. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val158Met polymorphism. Biol Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kritzer MH, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova MA, Trushkin EV, Timofeeva MA, Shlepzova VA, Schegolkova JV, Maluchenko NV, et al. Genetic markers of predisposition to increased anxiety. Bull Exp Biol Med. 2008;146:774–778. doi: 10.1007/s10517-009-0373-x. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lake RIE, Eaves LH, Maes HHM, Heath AC, Martin NG. Further evidence against the environmental transmission of individual differences in neuroticism from a collaborative study of 45,850 twins and relatives on two continents. Behav Genet. 2000;30:223–233. doi: 10.1023/a:1001918408984. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Sander T, Gallinat J. Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology. 2007;32:1950–1955. doi: 10.1038/sj.npp.1301335. [DOI] [PubMed] [Google Scholar]
- Lee P, Holmes A, Fagerness J, Roffman J, Purcell S, Buckner R, et al. Genome-wide analyses of neurocognitive/brainimaging phenotypes reveal enriched association of neuronal and psychiatric candidate genes. Poster presented at the XIXth World Congress of Psychiatric Genetics; Washington, DC. 2011. [Google Scholar]
- Lewis G, Pelosi AJ, Glover E, Wilkinson G, Stansfeld SA, Williams P, et al. The development of a computerized assessment for minor psychiatric disorder. Psychol Med. 1988;18:737–745. doi: 10.1017/s0033291700008448. [DOI] [PubMed] [Google Scholar]
- Light KJ, Joyce PR, Luty SE, Mulder RT, Carter JD, Frampton CM, et al. An association study of DRD2 and COMT polymorphisms with novelty seeking and harm avoidance scores, in two independent samples of depressed patients. Behav Brain Funct. 2007;3:3. doi: 10.1186/1744-9081-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Lochner C, Hemmings SM, Kinnear CJ, Nel D, Hemmings SM, Seedat S, et al. Cluster analysis of obsessive-compulsive symptomatology: identifying obsessive-compulsive disorder subtypes. Isr J Psychiatry Relat Sci. 2008;45:164–176. [PubMed] [Google Scholar]
- Lochner C, Kinnear CJ, Hemmings SM, Seller C, Niehaus DJ, Knowles JA, et al. Hoarding in obsessive-compulsive disorder: clinical and genetic correlates. J Clin Psychiatry. 2005;66:1155–1160. doi: 10.4088/jcp.v66n0911. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Öhman A. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Rück C, Bergströnm J, Andersson G, Öhman A, Lindefors N. The COMTval158met polymorphisms is associated with symptom relief during exposure-based cognitive-behavioral treatment in panic disorder. BMC Psychiatry. 2010;10:99–107. doi: 10.1186/1471-244X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol-o-methyltransferase: A revised mechanism of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney: Psychology Foundation; 1995. [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Pers Indiv Differ. 1999;27:405–416. [Google Scholar]
- Maron E, Tõru I, Tasa G, Must A, Toover E, Lang A, et al. Association testing of panic disorder candidate genes using CCK-4 challenge in healthy volunteers. Neurosci Lett. 2008;446:88–92. doi: 10.1016/j.neulet.2008.09.052. [DOI] [PubMed] [Google Scholar]
- Max MB, Wu T, Atlas SJ, Edwards RR, Haythornthwaite JA, Bollettino AF, et al. A clinical genetic method to identify mechanisms by which pain causes depression and anxiety. Mol Pain. 2006;2:14. doi: 10.1186/1744-8069-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Joint factors in self reports and ratings: Neuroticism, extraversion and openness to experience. Pers Indiv Differ. 1983;4:245–255. [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. Am J Psychiatry. 2004;161:1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- Meira-Lima I, Shavitt RG, Miguita K, Ikenaga E, Miguel EC, Vallada H. Association analysis of the catechol-o-methyltransferase (COMT), serotonin transporter (5-HTT) and serotonin 2A receptor (5HT2A) gene polymorphisms with obsessive-compulsive disorder. Genes Brain Behav. 2004;3:75–79. doi: 10.1046/j.1601-1848.2003.0042.x. [DOI] [PubMed] [Google Scholar]
- Michaelovsky E, Gothelf D, Korostishevsky M, Frisch A, Burg M, Carmel M, et al. Association between a common haplotype in the COMT gene region and psychiatric disorders in individuals with 22q11.2DS. Int J Neuropsychopharmacol. 2008;11:351–363. doi: 10.1017/S1461145707008085. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Slof-Op't Landt MCT, Medland SE, van Beijsterveldt CEM, Bartels M, Willemsen G, et al. Anxiety and depression in children and adults: influence of serotonergic and neurotrophic genes? Genes Brain Behav. 2010;9:808–816. doi: 10.1111/j.1601-183X.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, et al. COMT genetic variation affects fear processing: Psychophysiological evidence. Behav Neurosci. 2008;122:901–9. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Niehaus DJ, Kinnear CJ, Corfield VA, du Toit PL, van Kradenburg J, Moolman-Smook JC, et al. Association between a catechol-o-methyltransferase polymorphism and obsessive-compulsive disorder in the Afrikaner population. J Affect Disord. 2001;65:61–65. doi: 10.1016/s0165-0327(00)00246-9. [DOI] [PubMed] [Google Scholar]
- Ohara K, Nagai M, Suzuki Y, Ochiai M, Ohara K. No association between anxiety disorders and catechol-O-methyltransferase polymorphism. Psychiatry Res. 1998;80:145–148. doi: 10.1016/s0165-1781(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Anney RJL, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet. 2005;15:109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Byrnes GB, Anney RJL, Collins V, Hemphill SA, Williamson R, et al. COMT Val158Met and 5HTTLPR functional loci interact to predict persistence of anxiety across adolescence: Results from the Victorian Adolescent Health Cohort Study. Genes Brain Behav. 2007;6:647–652. doi: 10.1111/j.1601-183X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Palmetier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, McClearn GE, Friberg L. Neuroticism, extraversion, and related traits in adult twins reared apart and reared together. J Pers Soc Psychol. 1988;55:950–957. doi: 10.1037//0022-3514.55.6.950. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Fineberg N, Harrison PJ. The met158 allele of catechol-O-methyltransferase is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry. 2007;12:556–561. doi: 10.1038/sj.mp.4001951. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Michaelovsky E, Frisch A, Knoll G, Amir I, Finkel B, et al. COMT Val158Met polymorphism in schizophrenia with obsessive-compulsive disorder: a case-control study. Neurosci Lett. 2005;389:21–24. doi: 10.1016/j.neulet.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. NeuroReport. 2005;16:1135–1138. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schitz A, Corr P, Hennig J. Molecular genetics support Gray’s personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int J Neuropsychopharmacol. 2006;9:155–166. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MB, Schmidt D, et al. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA. Membrane-bound catechol-o-methyltransferase: A reevaluation of its role in the o-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol. 1992;120:1–29. doi: 10.1007/BFb0036121. [DOI] [PubMed] [Google Scholar]
- Rothe C, Koszycki D, Bradwejn J, King N, Deluca V, Tharmalingam S, et al. Association of the Val158Met catechol O-methyltransferase genetic polymorphism with panic disorder. Neuropsychopharmacology. 2006;31:2237–2242. doi: 10.1038/sj.npp.1301048. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Mazzanti C, Dell'Osso L, Rucci P, Sullivan P, Bouanani S, et al. Catechol o-methyltransferase, serotonin transporter, and tryptophan hydroxylase gene polymorphisms in bipolar disorder patients with and without comorbid panic disorder. Am J Psychiatry. 2002;159:23–29. doi: 10.1176/appi.ajp.159.1.23. [DOI] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol Med. 2008;38:1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Gietl A, Hartmann AM, Möller HJ. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Stepie G, Grzywacz A, et al. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Res. 2004;128:21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J, Chacón-Moscoso S, Marín-Martínez F. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–467. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- Schindler KM, Richter MA, Kennedy JL, Pato MT, Pato CN. Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. Am J Med Genet. 2000;96:721–4. doi: 10.1002/1096-8628(20001204)96:6<721::aid-ajmg4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schinka A, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase Val158Met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stallings MC, Hewitt JK, Cloninger C, Heath AC, Eaves LJ. Genetic and environmental structure of the Tridimensional Personality Questionnaire: Three or four temperament dimensions? J Pers Soc Psychol. 1996;70:127–140. doi: 10.1037//0022-3514.70.1.127. [DOI] [PubMed] [Google Scholar]
- Starcevic V, Uhlenhuth EH, Fallon S, Pathak D. Personality dimensions in panic disorder and generalized anxiety disorder. J Affect Disord. 1996;37:75–9. doi: 10.1016/0165-0327(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warrior versus worrier: The role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Strug LJ, Suresh R, Fyer AJ, Talati A, Adams PB, Li W, et al. Panic disorder is associated with the serotonin transporter gene (SLC6A4) but not the promoter region (5-HTTLPR) Mol Psychiatry. 2010;15:166–176. doi: 10.1038/mp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol-O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–59. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Otowa T, Hibino H, Kato C, Otani T, Umekage T, et al. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci Res. 2006;54:180–185. doi: 10.1016/j.neures.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Yu YW, Chen TJ. Association study of catechol-O-methyltransferase gene and dopamine D4 receptor gene polymorphisms and personality traits in healthy young Chinese females. Biol Psychiatry. 2004;50:153–156. doi: 10.1159/000079107. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Urata T, Takahashi N, Hakamata Y, Iijima Y, Kuwahara N, Ozaki N, et al. Gene-gene interaction analysis of personality traits in a Japanese population using an electrochemical DNA array chip analysis. Neurosci Lett. 2007;414:209–212. doi: 10.1016/j.neulet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Kaprio J, Koskenvuo M. A developmental genetic analysis of adult personality: Extraversion and Neuroticism from 18 to 59 years of age. J Pers Soc Psychol. 1994;66:722–730. doi: 10.1037//0022-3514.66.4.722. [DOI] [PubMed] [Google Scholar]
- Walitza S, Scherag A, Renner TJ, Hinney A, Remschmidt H, Herpertz-Dahlmann B, et al. Transmission disequilibrium studies in early onset of obsessive-compulsive disorder for polymorphisms in genes of the dopaminergic system. J Neural Transm. 2008;115:1071–1078. doi: 10.1007/s00702-008-0051-6. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-Methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine, and cortical signal-to-noise ratio in schizophrenia. Trends in Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH. The association between panic disorder and the L/L genotype of catechol-O-methyltransferase. J Psychiatr Res. 2004;38:365–70. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Yu BH. Catechol O-methyltransferase genetic polymorphism in panic disorder. Am J Psychiatry. 2002;159:1785–1787. doi: 10.1176/appi.ajp.159.10.1785. [DOI] [PubMed] [Google Scholar]
- Wray NR, Birley AJ, Sullivan PF, Visscher PM, Martin NG. Genetic and phenotypic stability of measures of neuroticism over 22 years. Twin Res Hum Genet. 2007;10:695–702. doi: 10.1375/twin.10.5.695. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Dumenil T, Handoko HY, Lind PA, Montgomery GW, et al. Association study of candidate variants of COMT with neuroticism, anxiety and depression. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1314–1318. doi: 10.1002/ajmg.b.30744. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho S-L, Ramsden D. Characterization and implications of estrogenic downregulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, et al. Gene–gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article 17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Lipsky RH, Xu K, Ali S, Hyde T, Kleinman J, et al. Differential expression of human COMT alleles in brain and lymphoblasts detected by RT-coupled 5’ nuclease assay. Psychopharmacology. 2004;177:178–84. doi: 10.1007/s00213-004-1938-z. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, van Nimwegen L, van Amelsvoort T, de Haan L, Yusuf MA, Baas F, et al. Catechol-O-methyltransferase gene and obsessive-compulsive symptoms in patients with recent-onset schizophrenia: preliminary results. Psychiatry Res. 2008;157:1–8. doi: 10.1016/j.psychres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Zintzaras E, Sakelaridis N. Is 472G/A catechol-O-methyltransferase gene polymorphism related to panic disorder? Psychiatr Genet. 2007;17:267–273. doi: 10.1097/YPG.0b013e3280d6472e. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.