Abstract
Introduction
A common feature of many types of cells is their responsiveness to chemotactic gradients of factors for which they express the corresponding receptors. The most studied chemoattractants so far are peptide-based growth factors and a family of cytokines endowed with strong chemotactic properties, called chemokines. However, additional evidence has accumulated that, in addition to these peptide-based chemoattractants, an important role in cell migration is played by bioactive lipids.
Areas covered
Solid evidence has accumulated that two bioactive phosphorylated sphingolipids that are derivatives of sphingolipid metabolism, namely sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P), are potent chemoattractants for a variety of cells. In this review, we will discuss the effect of these two phosphorylated sphingolipids on the trafficking of normal and malignant cells, and, in particular, we will focus on their role in trafficking of normal hematopoietic stem/progenitor cells. Unlike other mediators, S1P under steady state conditions maintain a steep gradient between interstitial fluid and peripheral blood and lymph across the endothelial barrier, which is important in the egress of cells from bone marrow. Both S1P and C1P may be upregulated in damaged tissues, which may result in reversal of this gradient.
Expert opinion
S1P and C1P are important regulators of the trafficking of normal and malignant cells, and modification of their biological effects will have important applications in optimizing stem cell mobilization and homing, tissue organ/regeneration, and preventing cancer metastasis.
Keywords: Sphingosine-1-phosphate, ceramide-1-phosphate, stem cell mobilization, cancer metastasis, stem cell homing
1. Introduction
Sphingoplipids are important components of cell membranes and are derived from the metabolism of ceramide and the aliphatic amino alcohol sphingosine [1, 2]. The very important sphingolipids are sphigosine-1-phosphate (S1P) and ceramide-1 phosphate (C1P), which play an important role in intracellular signaling and in cell-to-cell communication [1, 3-6]. While S1P can be secreted from normal activated cells as an extracellular mediator, C1P is released mainly from damaged or “leaky” cells [7]. The concentration of both phosphorylated sphingolipids in peripheral blood (PB) and lymph is high, which supports their active production and secretion [8-11]. In addition, evidence has accumulated that both S1P and C1P are upregulated in peripheral tissues in response to injury (e.g., irradiation, toxic effects of chemotherapy, or ischemia) [12, 13].
S1P is the product of two sphingosine kinases (Sphk1 and Sphk2). While mice with knockout of one of these kinases are viable, which suggests that they have somewhat redundant functions, double knockout of Sphk1 and Sphk2 is embryonic-lethal due to defects in development of vasculature and neural tissue [14]. S1P is released from cells by a transporter-facilitated process and interacts with at least five Gαi protein-coupled seven-transmembrane-spanning receptors, S1P1–5, which vary in their repertoire of associated G proteins [1, 15]. While, S1P1-3 receptors are widely expressed in the cardiovascular and lymphohematopoietic systems, S1P4 is highly expressed in lymphoid tissue and S1P5 in oligodendrocytes. Deletion of the S1P1 receptor causes embryonic lethality due to excessive hemorrhaging [16], and this hemorrhagic phenotype occurs in response to selective knockout of this receptor in the endothelial cells of adult mice, suggesting an important role for this receptor in endothelial integrity. S1P1 also plays an important role in neurogenesis and brain development. As reported, while single deletion of other S1P receptors (S1P2 and S1P3) does not lead to embryonic lethality, simultaneous deletion of both of these receptors leads also to hemorrhagic complications in embryos, with a profound bleeding phenotype and early embryonic lethality, which, as expected, is also seen in triple knockout animals (S1P1-3 KO mice) [17-19]. Interestingly, signaling through the S1P2 receptor somewhat counterbalances the effects of activated S1P1 [20], suggesting the existence of a S1P1/S1P2 rheostat. Thus, the presence of multiple receptors and their various tissue distributions allows S1P to exert several pleiotropic effects. S1P is irreversibly degraded by S1P lyase (SPL) and dephosphorylated by lipid phosphate phosphatases (LPP1–3) and S1P-specific phosphatases (SPP1 and SPP2) [10, 21-23].
The structurally related bioactive lipid C1P is a product of ceramide (N-acyl sphingosine) phosphorylation by ceramide kinase (CERK). Mice with CERK knockout are alive and have an almost normal level of C1P [24], which suggests that there are other not-yet-identified, CERK-independent pathways for C1P synthesis. For example, studies of the synthesis of C1P in murine retina suggested the involvement of CERK-like (CerkL) kinase [25]. Thus, the relationship between genotype and phenotype in CERK-deficient mice may be analogous to that of Sphk-deficient animals, in which single-mutation mice do not show a significant defect because there are two isoforms (Sphk1 and Sphk2) with overlapping activity [14]. Like S1P, C1P is degraded by LPP1–3 [26, 27]. Unlike ceramide (which is often pro-apoptotic), C1P has been reported to promote cell growth, survival, and migration through an unknown receptor-initiated signaling pathway that is pertussis toxin-sensitive and therefore likely to involve as-yet-unidentified, GαI protein-coupled, seven-transmembrane-spanning receptors [28]. Thus, both bioactive lipids most likely engage structurally similar yet distinct families of GαI protein-coupled receptors.
Sphingolipids have well-characterized roles in intracellular membrane function, but it is now widely appreciated that extracellular S1P and possibly C1P, as specific receptor-directed bioactive lipids, are involved in inhibiting apoptosis and enhancing cell survival and proliferation, regulating stress responses, and enhancing the trafficking of normal and malignant cells [7, 13, 28-31]. In support of this latter notion, the concentration of both bioactive lipids in peripheral blood (PB) is relatively high, ~0.5–1 μM, and is much lower in interstitial fluids [8-11]. The concentration in lymph is also high; however, in a few cases it is lower than in PB. Thus, S1P forms a steep gradient across the endothelial barrier between tissues and both PB plasma and lymph [32].
S1P is transported in PB by erythrocytes, platelets, albumin, and high-density lipoproteins (HDL). Since the plasma half-life of S1P that is transported by albumin is ~15 min, S1P must be produced continuously to maintain its high concentration in blood and lymph, and as recently reported, erythrocytes are a major source of S1P in blood plasma [9, 33]. The level of S1P in PB increases additionally during intravascular hemolysis, as seen, for example, in paroxysmal nocturnal hemoglobinuria or sickle cell anemia [34, 35]. By contrast, the S1P level is lower in anemic patients [33, 36]. It has been proposed that the sphingosine present in PB plasma is actively absorbed by erythrocytes, phosphorylated to S1P, and stored in the plasma membrane of these cells [33]. Another source of S1P in PB is blood platelets [37]. However, since mice that have very low platelet counts (e.g., NF-E2-deficient mice) maintain a normal S1P level, it has been proposed that they are not the major source of plasma S1P [9, 38]. On the other hand, platelets may contribute to a local increase in S1P during platelet activation, as seen, for example, in thrombotic episodes. An important source of S1P is the vascular endothelium, and as reported, S1P can also be secreted into blood by endothelial cells in response to shearing forces [38]. By contrast, C1P is metabolized relatively slowly and thus is more stable in biological fluids and has a longer half-life than S1P [39, 40]. Further studies are required to see whether the level of bioactive lipids in PB shows a circadian rhythm. This is an important question, because of the involvement of bioactive lipids in the coagulation and inflammatory responses, and it is known that both the coagulation cascade and complement cascade show a circadian rhythm of activation [41-43].
S1P could be extracted from erythrocyte membranes by serum albumin (SA) and high-density lipoproteins (HDL), bound to SA and HDL, and may then stimulate its receptors on the surface of target cells [33]. On the other hand, S1P is a component of cell membrane-derived microvesicles or exosomes that are shed from activated cells and subsequently act as signal transmitters that circulate in the PB [44]. In this context, the S1P present in the membranes of microvesicles/exosomes is probably responsible for several biological effects exerted by these newly appreciated cell–cell contact mediators, such as inhibition of apoptosis or stimulation of angiogenesis [45].
Several small-molecule compounds have been developed that modulate the responsiveness of cells to an S1P gradient. The most important is a family of S1P1 agonists that at high doses binds to S1P1 receptors, leading to their internalization and thus desensitizing the responsiveness of cells to an S1P gradient (e.g., FTY720, also known as fingolimod, SEW2871, or CYM5542) or S1P1 receptor antagonists (W146 or NIBR-0213) [46-49]. Of note, FTY720 is already approved for clinical use to inhibit the migration of immune cells in patients suffering from multiple sclerosis [50]. There are other available compounds, such as 2-acetyl-4(5)-tetrahydroxybutyl imidazole (THI) or 4-deoxypyridoxine (DOP), which, by inhibiting SPL activity, increase the S1P level in tissues and thus inhibit egress of cells from BM into PB and lymph [10, 29, 51]. In addition, there are several other molecules that modulate S1P activity and its biological availability, which will be discussed latter on in this review.
We can expect that very soon more small-molecule compounds regulating the biological activity of S1P will also be admitted to the clinic. When C1P receptors are identified, we may also expect the development of potent modulators of C1P activity.
In our review, we will address the most important effects of S1P and C1P in stem trafficking of normal and malignant cells; however, we recommend for further reading other excellent reviews that have addressed specific aspects of this intriguing topic [1, 4, 52-54].
2. S1P as a potent chemoattractant for immune cells
The role of S1P in trafficking of lymphocytes was first suggested by data showing that FTY720 increases the homing of circulating lymphocytes to lymph nodes and is somehow involved in modulating the responsiveness of these cells to chemokines[55]. This and subsequent studies revealed that administration of FTY720 induces profound lymphopenia in experimental animals by inhibiting the egress of lymphocytes from the thymus, peripheral lymph nodes, and Peyer's patches into PB [55, 56]. This phenomenon has been subsequently explained by the realization that FTY720, in its phosphorylated form as FTY720-P, acts as a strong agonist for four S1P receptors (S1P1, S1P3, S1P4, and S1P5), which then become internalized and undergo ubiquitination [57]. Thus, FTY720 at high doses desensitizes the responsiveness of cells to an S1P gradient and inhibits their migration in response to this bioactive phospholipid. A decrease in S1P-mediated cell migration was also observed after administration in vivo in experimental animals of a specific S1P1 antagonist, SEW2971, which confirmed the major involvement of the S1P–S1P1 receptor axis in this phenomenon [47].
The involvement of the S1P1 receptor and the role of its desensitization due to the internalization process has recently been confirmed in knockin mice in which the C-terminal, serine-rich S1P1 motif, which plays an important role in internalization of the S1P1 receptor, was mutated [58]. These mutant mice displaying resistance to S1P1 internalization exhibited significantly delayed lymphopenia after administration of FTY720. Overall, as subsequently reported, S1P signaling modulates trafficking not only of naïve and central memory T lymphocytes but also B cells, dendritic cells, and NK cells [59-61].
In contrast to S1P, there are no parallel studies on the role of C1P in the trafficking of lymphocytes. Progress again is hampered by the fact that the C1P receptors have not yet been identified. We envision that C1P could like S1P also play an important role in the trafficking of immune cells. This however requires further studies.
3. S1P and C1P as chemoattractants for hematopoietic cells
Shortly after S1P was identified as a chemotactic factor for lymphocytes [62], it was suggested that S1P may be involved in the migration of hematopoietic stem/progenitor cells (HSPCs). In these initial experiments, instead of S1P, FTY720 was employed as a potential ligand for S1P receptors [63]. It was shown that pretreatment of FTY720 increases the chemotactic responsiveness of human CD34+ lineage-committed progenitor cells for mixed lineages, granulocyte-monocytes, and erythroid cells to a stromal-derived factor 1 (SDF-1) gradient[63]. This effect was also observed for more primitive cobblestone-area-forming cells (CAFCs) [63] but not for the most primitive population of CD34+CD38– HSPCs[63]. Accordingly, in direct Transwell migration experiments, S1P efficiently chemoattracted human PB CD34+ cells, and in addition, FTY720 exposure resulted in prolonged SDF-1-induced calcium flux and actin polymerization in these cells [63]. In further support of this effect, human PB-derived CD34+ cells engrafted better in immunodeficient NOD/SCID mice after systemic pretreatment by FTY720 [63]. Thus, it has been suggested that S1P somewhat modulates the responsiveness of HSPCs to a BM-directed SDF-1 homing gradient by increasing the sensitization of CXCR4 signaling; however, a more detailed molecular explanation of this phenomenon has not been provided. More importantly, in the followup of this study, the same authors demonstrated in a Transwell migration system that S1P directly chemoattracts human CD34+ progenitor cells [64].
In another study, based on data showing the involvement of S1P in the trafficking of lymphocytes and other immune cells, it was postulated that S1P is involved in the circulation of CFU-GM and lymphoid progenitors in PB and lymph under steady-state conditions [65]. According to this concept, steady-state circulation of CFU-GM and lymphoid progenitors is orchestrated by the S1P–S1P receptor axes. As postulated, HSPCs enter extramedullary tissues in response to S1P where they expand, giving rise to myeloid and dendritic cells and may, on the other hand, egress from extramedullary tissues into lymph in response to an S1P gradient and return again to BM [52]. Based on this concept, by patrolling peripheral tissues, HSPCs would be responsible for the local production of tissue-resident innate immune cells. A similar mechanism postulated by the authors could also be involved in the circulation of HSPCs during inflammation [66]. This concept, however, must address how circulating HSPCs can enter peripheral tissues against an S1P gradient (S1P concentration in the interstitial fluid is much lower than in PB) and take into consideration the negative effect of desensitization of S1P receptors on the surface of HSPCs while they circulate in PB and are exposed to high S1P levels. The mechanism for such S1P receptor desensitization, due to their internalization in the presence of high levels of S1P in PB or lymph, is very well demonstrated for circulating lymphocytes[58]. S1P also chemoattracts macrophages and as recently demonstrated apoptotic cells enhance S1P1 mediated migration of these cells [67].
Our personal interest in S1P was initiated during the analysis of potential chemoattractants in PB that direct egress of hematopoietic stem/progenitor cells (HSPCs) from BM during pharmacological mobilization. Pharmacological mobilization is one of the means of obtaining HSPCs for hematopoietic transplantation, and it is well known that some compounds, such as cytokine granulocyte stimulating factor (G-CSF), stimulate egress of HSPCs from BM into PB [68]. This G-CSF-mediated mobilization mechanism is exploited widely in the clinic to obtain HSPCs for transplantation [69].
For many years it was believed that the major chemoattractant in PB that directs egress of HSPCs is α-chemokine SDF-1, which interacts with the CXCR4 receptor on the surface of HSPCs and, as demonstrated, at high supraphysiologic doses is a potent chemoattractant for these cells [70, 71]. Chemotaxis of HSPCs toward an SDF-1 gradient can be inhibited by a small-molecule blocking agent for this receptor, the bicyclam AMD3100. However, while the SDF-1–CXCR4 axis plays a crucial role in retention of HSPCs in BM niches, several studies suggested that the plasma level of SDF-1 does not change during mobilization, and there is not a direct correlation between its level and good or poor HSPC mobilization status [29, 72]. This has also been confirmed in our own studies [7].
Therefore, we sought to determine which major chemottractant present in PB is responsible for the egress of HSPCs. We observed that in normal and mobilized mice, the PB plasma strongly chemoattracts HSPCs in an SDF-1-independent manner, which is known because i) the plasma SDF-1 level, as mentioned above, does not correlate with mobilization efficiency, ii) the chemotactic responsiveness of HSPCs to a plasma gradient are not affected by the presence of AMD3100, and iii) a factor present in mobilized PB plasma was resistant to denaturation by heat [29]. Overall, this observed resistance to denaturation by heat as well as loss of plasma chemotactic activity after charcoal stripping suggested the involvement of bioactive lipids, and we focused on sphingosine-1-phosphate (S1P), which was previously identified as a chemoattractant for HSPCs[29]. The pivotal role of S1P in mobilization of HSPCs has been recently confirmed in excellent papers published by other investigators [53, 66, 73].
Our data indicate that S1P i) creates in plasma a continuously present gradient for BM-residing HSPCs, ii) is, at physiologically relevant concentrations, a chemoattractant several magnitudes stronger than SDF-1, and iii) increases its plasma level during mobilization due to complement cascade (CC) activation and the interaction of the C5b-C9 membrane attack complex (MAC) with erythrocytes, which are a major reservoir for S1P. We concluded that the CC, via either non-lytic or lytic activity of MAC, induces additional release of S1P from erythrocyte membranes for optimal egress/mobilization of HSPCs [29, 74]. It is also likely that during CC activation anaphylatoxin C5a (known as a potent stimulator of endothelial cells [75]) is released, which induces release of S1P from endothelial cells into BM sinusoids, and this possibility is currently being investigated in our laboratory.
Nevertheless, since erythrocytes are a major source of plasma S1P [33], in our most recent studies we asked whether the massive hemolysis of erythrocytes induced by phenylhydrazine (PHZ), leading to an additional increase in plasma S1P level, triggers mobilization of HSPCs. We observed, however, that despite the fact that massive hemolysis doubled the PB plasma level of S1P (from 1 to 2 μM), this did not significantly enhance the egress of HSPCs from BM into PB. However, egress was dramatically enhanced when we blocked SDF-1–CXCR4-mediated retention of HSPCs in BM by combining administration of PHZ with AMD3100. This finding confirms our previous observation that under steady-state conditions, the S1P level in PB has already established a steep chemotactic gradient for BM-residing HSPCs [76], showing that hemolysis alone, even if it doubles the S1P level in PB, requires attenuation of CXCR4–SDF-1-mediated retention in BM niches.
This finding also demonstrates that retention of HSPCs in BM niches is an active process that counteracts the continuous chemotactic gradient of S1P present in PB. Identification of S1P as a major chemottractant for the HSPCs present in PB raises the question whether modulation of the S1P–S1P receptor axes could be exploited to optimize the mobilization of HSPCs [7, 52, 77]. In fact, mice that have the S1P1 receptor knocked out on their HSPCs, and thus do not respond efficiently to an S1P gradient, are poor mobilizers [73] in a similar way as mice in which the S1P level in BM has been upregulated after administration of DOP [29].
The fact that S1P receptors on the surface of HSPCs are highly sensitive to exposure to S1P, which results in their rapid internalization, may explain why HSPCs freshly isolated from murine BM respond robustly to an S1P gradient, in contrast to HSPCs recovered from PB, which are permanently exposed to a high level of S1P [77]. We observed a similar effect for human umbilical cord blood (UCB) and PB HSPCs [77], which clearly shows that HSPCs, depending on the source of their isolation (BM, PB or UCB), differ in responsiveness to an S1P gradient.
An interesting question related to the abovementioned concerns is whether S1P and C1P also play some role in the reverse process to mobilization, that is, homing of HSPCs into BM. Employing the most sensitive and accurate measurement for bioactive lipids, liquid chromatography electrospray ionization tandem mass spectrometry (HPLC ESI MS/MS), it has been shown that S1P and C1P levels increase in the BM microenvironment after conditioning for hematopoietic transplantation by lethal irradiation [7]. Therefore, both of these phosphorylated sphingolipids could play a role in directing migration of HSPCs from PB to BM niches. However, this mechanism seems to be more complicated, because as mentioned above, HSPCs are exposed in PB to high levels of circulating S1P and C1P and thus desensitized in their responses to potential homing gradients of both bioactive lipids [77], even if their level increases in the BM microenvironment after conditioning for transplantation. However, at the moment we do not have direct methods for measuring chemoattractant gradients (e.g., S1P or C1P) directly across the endothelial barrier that separates blood in the BM sinusoids from the BM microenvironment. Thus, it is difficult to judge whether this gradient works in favor of homing of cells to BM or not. Finally, upregulation of S1P in BM conditioned for transplantation may play another important role in the crosstalk between stromal and endothelial cells with HSPCs during the process of their homing and engraftment.
Finally, while much work has been done to address the role of S1P in the trafficking of HSPCs, the role of C1P, because of a lack of proper tools (e.g., potential receptors have not been identified), still awaits further study. Since the C1P concentration is also very high in PB, both bioactive lipids together may have an additive effect in this process. However, the potent chemotactic effect of C1P has already been well demonstrated in an elegant study employing macrophage migration assays [28] as well as by us in employing such assays with human and murine HSPCs [7]. Moreover, CERK-deficient mice were reported to have a reduced number of neutrophils in PB and spleen in an animal model of Streptococcus pneumonia with induced fulminant pneumonia [24]. Interestingly, as recently demonstrated C1P induces release of macrophage chemoattractant protein-1 (MCP-1) from J774A.1 macrophages, human THP-1 monocytes and 3T3-L1 preadipocytes and autocrine secreted MCP-1 has been identified as a major mediator of C1P-stimulated cell migration for these cells [78].
Further work is needed to elucidate better S1P- and C1P- mediated molecular mechanisms that regulate trafficking of cells. To address this important issue, both these phosphorylated sphingolipids stimulated in our hands in normal HSPCs, MSCs and HUVECs phosphorylation of MAPKp42/44 and AKT that are crucial in cell migration and adhesion as well as induced phosphorylation of MAPKp38, STAT1, STAT3 and STAT5 proteins [7, 13].
4. S1P and C1P regulate the migration of multipotent stromal cells (MSCs) and endothelial progenitor cells (EPCs)
It has already been demonstrated in several animal and clinical models that various stem cells are mobilized into PB in response to tissue/organ injury [79-82]. However, the potential involvement of these circulating stem cells in organ tissue regeneration is still not fully understood. While circulating endothelial progenitor cells (EPCs) could directly supply new vessels in damaged tissues, and so-called very small embryonic-like stem cells (VSELs) could directly contribute to replacement of damaged cells, other stem cell types, such as multipotent stromal cells (MSCs) or HSPCs, could play an important indirect role as a source of several soluble paracrine factors (e.g., growth factors, cytokines, or chemokines) and microvesicles/exosomes that play an important role in inhibiting apoptosis in damaged cells and promoting vascularization of damaged tissues [83, 84].
We became interested in the role of phosphorylated sphingolipids in the trafficking of nonhematopoietic cells, because S1P has been reported not only as a potent chemoattractant for HSPCs [29, 65] but also for neural stem cells [85], EPCs [16, 86], and MSCs [87]. In our studies, we have compared the responsiveness of endothelial cells (HUVEC), MSCs, and BM-residing VSELs, which express several markers of pluripotency, to gradients of various bioactive lipids, including S1P, C1P, lysophosphatidic acid (LPA), and lysophosphatidylcholine (LPC) and, in parallel, compared the responsiveness of these cells to known chemoattrractants, such as SDF-1 and fibroblast growth factor-2 (FGF-2) [13].
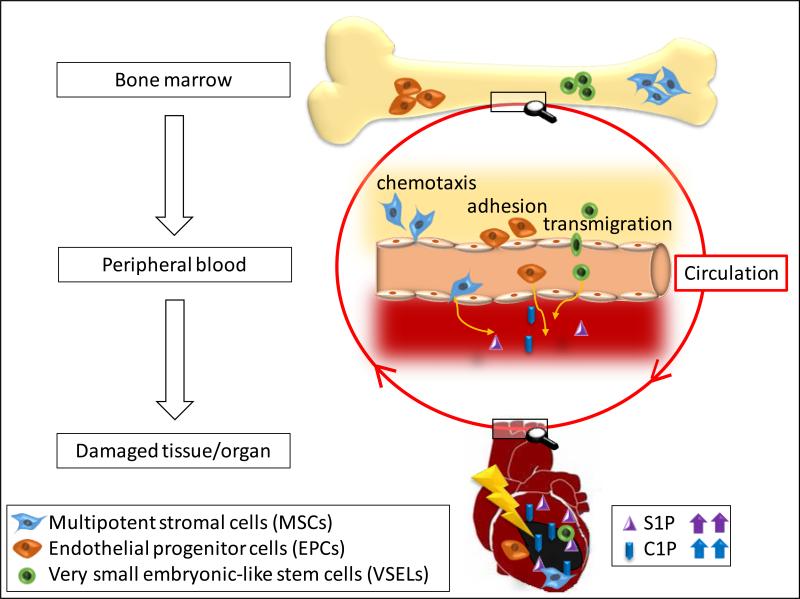
We observed that both S1P and C1P, at the physiological doses that are encountered in PB, are the most potent chemoattractants for all these cells [7]. More importantly, we demonstrated for the first time that C1P also strongly chemoattracts these cells to a similar degree as S1P [7, 13]. Thus, S1P and C1P play an underappreciated role in mobilization/egress of nonhematopoietic stem cells from BM into PB [13]. Furthermore, since S1P and C1P, as we have shown, are released from damaged or “leaky” cells (e.g., in heart tissue during acute hypoxia) [13], they may create a chemotactic gradient in damaged organs for stem cells mobilized and circulating in PB that could be involved in tissue/organ repair (Figure 1).
Figure 1. The role of S1P and C1P in chemoattracting circulating stem cells to damaged tissues.
Cells in damaged organs release S1P and C1P, which creates a chemotactic gradient for stem cells circulating in PB, such as multipotent stromal cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like stem cells (VSELs). For reasons of simplicity, other potential chemoattractants, such as SDF-1, are not shown in this scheme. Organ tissue damage may, for example, be the result of ischemia (e.g., stroke or heart infarct) or the result of hypoxia in a growing tumor. Therefore, C1P could, on the one hand, chemoattract circulating MSCs and EPCs for physiological process of regeneration and, on the other hand, may play an unwanted role in recruitment of these cells to an expanding tumor.
An important step in the regeneration of damaged organ tissues is promotion of neovascularization or angiogenesis. We demonstrated that C1P, like S1P, is a very strong proangiopoietic factor, being a potent chemoattractant for endothelial cells [13]. Furthermore, in in vitro functional assays we confirmed that C1P directly promotes tube formation, and, more importantly, our data in vivo in a murine model demonstrate that C1P also promotes vascularization of Matrigel implants [13]. This observation of the novel role of C1P in angiogenesis corroborates a previous report where it was shown that skin microendothelial cells isolated from CERK-deficient mice show defects of angiogenesis in in vitro assays [88]. Thus, C1P, like S1P, plays an important role as a novel pro-angiopoietic factor.
On the other hand, since, as mentioned above, evidence has accumulated that cell membrane-derived microvesicles/exosomes play an important role in tissue/organ regeneration by promoting survival of damaged target cells due to stimulation of angiogenesis [45], and since these circular membranous structures are reported to be highly enriched in S1P [44], further studies are needed to measure the content of C1P in these spherical membrane fragments to assess whether C1P delivered by microvesicles is involved in tissue and organ repair as well.
Interestingly, our experiments performed in serum-free media indicate that C1P, in contrast to macrophages and some myoblastic cells, does not affect proliferation of MSCs and HUVECs. Thus, the responsiveness of cells to C1P seems to be cell-specific and not always related to the stimulation of proliferation.
Based on these findings, we conclude (Figure 1) that cells in damaged organs release S1P and C1P, which together with other factors not shown in this scheme (e.g., SDF-1, S1P, VEGF, and HGF) creates a chemotactic gradient for MSCs, EPCs, and VSELs circulating in PB. Based on this mechanism, we propose that modification of S1P and C1P signaling in damaged tissues will lead to development of more optimal therapeutic strategies in regenerative medicine. Since we already know a lot about S1P and its receptors, it is important to invest effort in cloning the C1P receptor(s), which will be the first step in developing small-molecule C1P receptor agonists and antagonists that could be employed in the clinical setting, as is already the case for S1P receptors.
5. Novel potential approaches to enhancing the responsiveness of HSPCs to an S1P gradient – implications for enhancing the trafficking of normal stem cells
As mentioned above, we observed that S1P strongly chemoattracts HSPCs that reside in BM and loses this activity against HSPCs already mobilized into PB or into UCB. This could be explained by desensitization/downregulation of S1P receptors by S1P circulating in PB and UCB plasma [77]. We observed that incubation of HSPCs in S1P- and erythrocyte/platelet-free medium, which allows for re-expression of S1P receptors on the surface of HSPCs, may have a crucial role in regaining their responsiveness to an S1P gradient and thus will affect their homing/engraftment.
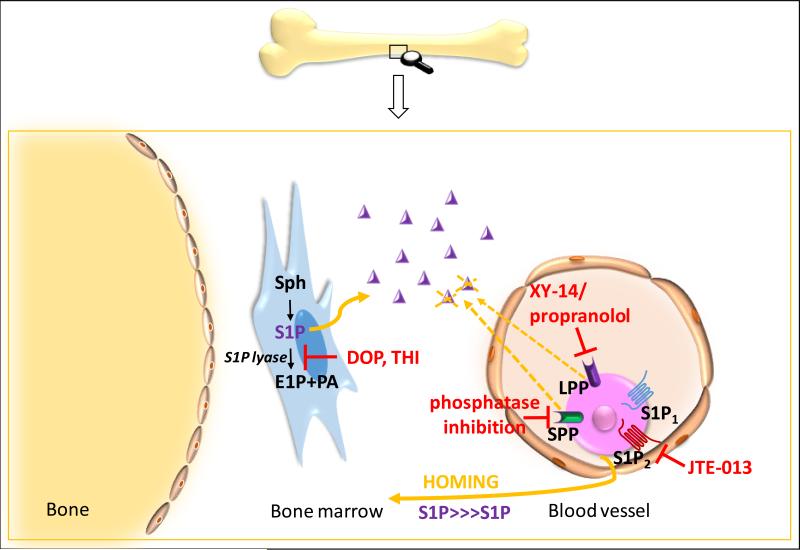
In Figure 2, we demonstrate additional possible strategies for improving the response of HSPCs to S1P and C1P gradients. Firstly, since S1P2 counteracts the biological effects of S1P1 and S1P3 receptors and mediates some inhibitory effects on the migration of HSPCs [15], we envision that by blocking S1P2 with a small-molecule antagonist, e.g., JTE-013, we may additionally enhance, as previously demonstrated for neural progenitors [85], S1P1- and S1P3-mediated chemotaxis of human and murine stem/progenitor cells in response to an S1P gradient. Secondly, it is known that HSPCs express on the cell surface lipid phosphate phosphatases (LPP1–3), which possess ecto-enzymatic activity against S1P and C1P [21-23]. Thus, LPP1–3 expressed on HSPCs could negatively affect the migration of these cells in response to gradients of C1P and S1P (dephosporylated sphingosine and ceramide do not chemoattract HSPCs). This concept is functionally somewhat analogous to the effect of HSPC-expressed dipeptidyl peptidase (CD26) on SDF-1-mediated chemotaxis [89]. Thus, it is worthwhile to test the hypothesis that inhibition of LPP1-3 on HSPCs would enhance both their mobilization as well as their homing responses to an S1P gradient. Finally, since the BM level of S1P may be additionally increased by inhibiting SP1 lyase (SPL) by DOP or THI [10, 29, 51], this effect should enhance the S1P tissue level and thus additionally improve the responsiveness of HSPCs to the S1P homing gradient in BM. Since inhibitors of S1P lyase are FDA-approved [51], these investigations may establish a new and relatively simple homing improvement strategy for HSPCs in which patients could be preconditioned before transplantation with an SPL inhibitor.
Figure 2. Potential strategies to improve the responsiveness of HSPCs to S1P by targeting the S1P–S1P receptor axis.
S1P-mediated homing of HSPCs to BM could be improved by upregulating the S1P level in the BM microenvironment by DOP or THI, which inhibit SPL activity by blockade of the S1P2 receptor on transplanted donor cells (e.g., employing JTE-013) or by inhibiting the LPP and SPP receptors on donor HSPCs (e.g., with XY-14). Similar strategies could be helpful in directing other types of stem cells involved in the regeneration of damaged tissues (e.g., MSCs, EPCs, and VSELs).
All these strategies proposed for HSPCs may also apply for improving the trafficking of other stem cells (e.g., EPCs, MSCs, or VSELs) that may be involved in tissue/organ regeneration. In support of this possibility, elevation of the S1P level by inhibiting SPL activity has been demonstrated to improve regeneration of pancreas [90] and skeletal muscles [91].
6. S1P and C1P as chemoattractants for tumor cells
Both bioactive lipids, and in particular S1P, have been reported to play a role in the progression of tumors by affecting several steps of malignant growth, such as increasing resistance to chemotherapy, inhibiting apoptosis, stimulating proliferation of tumor cells, and promoting angiogenesis, lymphoangiogenesis, and accumulation of stromal cells in tumor tissue [92-96]. This latter finding is supported by the evidence discussed above that S1P and C1P enhance migration of both endothelial cells and MSCs [13], which are responsible for unwanted vascularization and stromalization of tumor tissue.
It has also been reported that S1P has direct chemotactic activity against several types of malignancies, including thyroid follicular carcinoma [94], breast cancer [30], pancreatic carcinoma [92], and hepatocarcinoma [31]. In the latter two cases, evidence has been presented for a direct involvement of the S1P–S1P1 receptor axis in tumor invasion. However, in addition to the S1P–S1P1 receptor axis, the S1P–S1P3 axis also plays an important role in the motility of several types of tumor cells. Thus, both of these axes are potential targets for inhibition [97, 98].
In our own studies, we recently reported that S1P and C1P strongly enhance in vitro motility and adhesion of human rhabdomyosarcoma (RMS) cells [12]. This effect was observed at physiological concentrations of both bioactive lipids, which are present in blood plasma and lymph, and was much stronger than the effect observed in response to known RMS prometastatic factors, such as SDF-1 [99] or hepatocyte growth factor/scatter factor (HGF/SF) [99].
More importantly, we observed that the levels of S1P and C1P increase in several organs after γ-irradiation and chemotherapy, which indicates induction of an unwanted pro-metastatic environment related to treatment as a side effect of therapy [12]. This novel effect needs more attention, because it may play an important role in chemoattracting tumor cells that survive initial anti-cancer treatment to distant locations in which a pro-metastatic microenvironment involving S1P and C1P has been induced as a result of “collateral damage” induced by therapy [12].
This observation also indicates that S1P and C1P gradients became legitimate targets for anti-metastatic therapies. To cope with an unwanted S1P gradient during tumor therapy, S1P could, for example, be depleted from tissues and biological fluids by employing the S1P-specific monoclonal antibody LT1002 and the humanized form of this antibody known as LT1009 [100]. Another interesting strategy that we recently employed in our experiments is based on employing so- called spiegelmers, which are biostable and immunologically passive mirror-image (L-stereoisomer) oligonucleotides that can bind to pharmacologically relevant targets with high affinity and specificity. An anti-S1P spiegelmer, NOX-S93, which prevents S1P binding to its receptors, has been successfully employed by us to inhibit the metastasis of RMS cells in response to S1P in vivo [12]. Therefore, an anti-metastatic treatment with a specific S1P-binding scavenger such as spiegelmer NOX-S93 could become a part of standard radio/chemotherapy to ameliorate the responsiveness of tumor cells to an S1P gradient.
Based on our data obtained in the RMS metastatic model that bioactive lipids play a previously underappreciated role in dissemination of tumor cells due to the unwanted side effects of radio/chemotherapy, which creates a pro-metastatic microenvironment in collateral tissues, similar strategies depleting S1P gradients should also be tested for other tumors. In parallel with S1P neutralizing/blocking compounds (e.g., antibodies or spiegelmers), other molecules that may also affect S1P–S1P1 and S1P–S1P3 receptor axes should be tested as potential anti-metastatic drugs, e.g., FTY720, which at high doses desensitizes the responsiveness of cells to an S1P gradient, or some other small-molecule inhibitors of S1P1, such as W146, NIBR-0213, or CYM5442 and other S1P1/S1P3-directed reagents, such as VPC44116, VPC23019, and VPC25239 [46-49, 101]. In addition to preventing the migration of cancer cells, these inhibitors would also have a positive effect on the inhibition of tumor vascularization and stromalization.
Since in addition to S1P, C1P also shows strong chemotactic activity against tumor cells, the metastatic effect of bioactive lipids will be further ameliorated when the C1P receptor is cloned and appropriate inhibitors are developed. Finally, while bioactive lipids have been studied in some detail in solid-tumor malignancies, more work is needed to learn how they affect the expansion of leukemia and lymphoma cells.
Nevertheless, before S1P-S1P receptors modulators would be employed in the clinic as antimetastatic drugs, it will be important to assess their effect on migration and potential anti-tumor activity of immune cells involved in controlling tumor growth.
7. Conclusions
S1P and C1P have emerged as important regulators of stem cell trafficking. In PB they create a steep chemotactic gradient for normal and malignant cells, thus playing a crucial role in egress of hematopoietic and non-hematopoietic stem cells from BM into PB as well as directing the metastasis of cancer cells. Both bioactive phosphorylated sphingolipids are also upregulated in damaged tissue in response to several types of organ injuries. Besides their effects on stem cell trafficking, depending on cell type, they affect several other biological processes, such as inhibiting apoptosis, enhancing cell adhesion, and possibly inducing cell proliferation. In a growing tumor, bioactive phosphorylated sphingolipids increase the resistance of cells to chemotherapy and, by chemoattracting EPCs and MSCs, are involved in tumor vascularization and stromalization. Several potent small-molecule compounds modulating the S1P–S1P receptor axis have already been developed, and we can expect that as soon as receptors for C1P are cloned, similar molecules modulating the biological effects of C1P will be developed.
Expert Opinion
After many years in which stem cell trafficking has been studied, mainly by testing the effects of peptide-based chemoattractants, in recent years we have observed a shift in scientific interest to a greater focus on the role of the bioactive phospholipids as modulators of stem cell trafficking [13, 29, 52, 53, 77]. S1P is so far the best-studied bioactive phosphorylated sphingolipids [1, 7, 52, 53], though C1P has also emerged as a potent regulator of cell trafficking[7, 12, 13]. Research on C1P is hampered by the fact that receptor/s for C1P have not yet been identified and cloned, and mice deficient in CERK have turned out to have an almost normal level of C1P, suggesting that C1P is synthesized by additional unidentified pathways [24, 25].
An intriguing observation is that the concentration of both these bioactive phosphorylated sphingolipids increases in damaged tissues [7, 12, 13, 86], which suggests that S1P as well as C1P may play an important role in chemoattracting EPCs, MSCs, and other types of even-more-primitive stem cells (e.g., VSELs) for regeneration of damaged organs. This implies that by modulating the responsiveness of circulating stem cells to S1P and C1P gradients, we would be potentially able to enhance the mobilization as well as homing to damaged tissues of cells that have a beneficial effect on regeneration. In support of this notion, enhanced S1P- and C1P-mediated stem cell trafficking has already been described in an acute myocardial infarction model [86].
S1P and C1P have been demonstrated to play an important role in the egress of HSPCs from BM into PB. Thus, one can envision that additional pharmacological manipulations that increase the responsiveness of these cells to an S1P gradient may, on the one hand, enhance the egress of HSPCs from BM into PB, and, on the other hand, may also be helpful in enhancing the engraftment of HSPCs after transplantation. In support of this latter notion, it has been demonstrated that myeloablative conditioning for transplantation enhances the BM level of both bioactive lipids [7]. The level of S1P could also be additionally increased in the BM microenvironment after systemic administration of SPL inhibitors, such as DOP or THI [10, 29, 51].
There are also some clinically opposite situations in which it is important to inhibit the responsiveness of cells to an S1P gradient. Inhibition of S1P–S1P receptor signaling is an important strategy to inhibit migration of lymphocytic cells to organs affected by chronic inflammation [102]. On the other hand, taking into consideration that at physiological concentrations S1P and C1P play an important role as chemoattractants for tumor cells, inhibition of S1P-mediated migration may inhibit tumor metastasis [12]. Important from a clinical point of view are observations that both S1P and C1P are upregulated in distant tissues as unwanted side effects after exposure to radio-chemotherapy [12]. Therefore, to prevent spread and metastasis of cancer cells that have survived initial anticancer treatment to these S1P- and C1P-enriched locations, one might anticipate that anti-metastatic treatment by attenuation of S1P and C1P chemotactic effects will become a standard part of successful radio-chemotherapy.
Taking into consideration all these aspects of S1P and C1P activity, it is important to develop more potent and specific compounds that can modulate the biological effects of these bioactive phosphorylated sphingolipids. In particular, it will be important to develop strategies to modulate the biological effects of C1P. More work is also needed to understand the potential crosstalk between S1P and C1P and other chemoattractant axes (e.g., the SDF-1–CXCR4 axis). Interestingly, the recent data indicate also that C1P may trigger in non-receptor mediated mechanism pro-inflammatory generation of another group of bioactive lipids that are eicosanoids. This phenomenon is mediated by intracellular transfer of C1P between cell membranes in lipid transfer protein (CPTP) - dependent manner. CPTP resides in the cell cytosol but is also associated with the trans-Golgi membrane network as well as nucleus and plasma membranes [103]. Depletion of CPTP induced by RNA interference results in C1P decrease in plasma membranes and its increase in the Golgi complex. This leads to cPLA2α-mediated release of arachidonic acid and triggers as consequence generation of pro-inflammatory eicosanoids.
Finally, in addition to S1P and C1P, there are also other bioactive lipids, such as LPA and LPC, that also play a role in stem cell trafficking, and more studies are needed to understand their involvement in this process.
Article Highlights Box.
- Sphingosine-1-phosphate (S1P) and ceramide-1-phosphate have emerged as potent chemoattractants for a variety of cells.
- S1P and C1P are upregulated in damaged tissues in response to organ and tissue injuries, for example, as unwanted side effects of radio-chemotherapy or hypoxia related to acute myocardial infarction or stroke.
- Both S1P and C1P regulate the trafficking of hematopoietic stem/progenitor cells, endothelial progenitors, multipotent stromal cells, B and T lymphocytes, and NK cells and are involved in the metastasis of cancer cells.
- There are small-molecule agonists and antagonists available that modulate the responsiveness of cells to S1P, and the first of these has been employed in the clinic to modulate the biological effects of this phospholipid.
- S1P and C1P seem to have several overlapping functions and create additive chemotactic gradients. In contrast to S1P, receptors for C1P have still not been identified, which somewhat hampers the development of more efficient strategies to influence the biological effects of these bioactive phosphorylated sphingolipids.
- S1P and C1P are not the only bioactive phospholipids, and the role of other such lipids, for example, lysophosphatidic acid (LPA), in cell trafficking requires further study.
Acknowledgments
This work was supported by NIH grant 2R01 DK074720, NIH grant 1R01HL112788, the Stella and Henry Hoenig Endowment, and grant Maestro 2011/02/A/NZ4/00035 to MZR.
References
- 1*.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin Immunopathol. 2012 Jan;34(1):73–91. doi: 10.1007/s00281-011-0287-3. [State of art review on S1P.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010 Jul;6(7):489–97. doi: 10.1038/nchembio.392. [State of art review on biological effects of S1P.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005 Oct;15(118(Pt 20)):4605–12. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 4*.Gomez-Munoz A, Gangoiti P, Arana L, Ouro A, Rivera IG, Ordonez M, et al. New insights on the role of ceramide 1-phosphate in inflammation. Biochim Biophys Acta. 2013 Jun;1831(6):1060–6. doi: 10.1016/j.bbalip.2013.02.001. [Interesting review on a role of C1P in regulating biology of macrophages.] [DOI] [PubMed] [Google Scholar]
- 5.Lamour NF, Chalfant CE. Ceramide-1-phosphate: the “missing” link in eicosanoid biosynthesis and inflammation. Mol Interv. 2005 Dec;5(6):358–67. doi: 10.1124/mi.5.6.8. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–62. doi: 10.1146/annurev-biochem-062411-130916. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012 Jan;26(1):106–16. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010 Oct;51(10):3074–87. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007 Apr 13;316(5822):295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 10.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005 Sep 9;309(5741):1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 11.Hla T. Immunology. Dietary factors and immunological consequences. Science. 2005 Sep 9;309(5741):1682–3. doi: 10.1126/science.1118340. [DOI] [PubMed] [Google Scholar]
- 12.Schneider G, Bryndza E, Abdel-Latif A, Ratajczak J, Maj M, Tarnowski M, et al. Bioactive Lipids S1P and C1P Are Prometastatic Factors in Human Rhabdomyosarcoma, and Their Tissue Levels Increase in Response to Radio/Chemotherapy. Mol Cancer Res. 2013 Jul;11(7):793–807. doi: 10.1158/1541-7786.MCR-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, et al. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells--implications for tissue regeneration. Stem Cells. 2013 Mar;31(3):500–10. doi: 10.1002/stem.1291. [First report showing a role of C1P in trafficking of non-hematopoietic stem cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005 Dec;25(24):11113–21. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008 Oct;8(10):753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000 Oct;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001 Sep 7;276(36):33697–704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 18.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002 Jul 12;277(28):25152–9. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 19.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004 Jul 9;279(28):29367–73. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 20.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010 Dec 20;207(13):2793–8. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciorra VA, Morris AJ. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta. 2002 May 23;1582(1-3):45–51. doi: 10.1016/s1388-1981(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 22.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, et al. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005 Oct 1;391(Pt 1):25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008 Feb;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 24.Graf C, Zemann B, Rovina P, Urtz N, Schanzer A, Reuschel R, et al. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J Immunol. 2008 Mar 1;180(5):3457–66. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- 25.Graf C, Niwa S, Muller M, Kinzel B, Bornancin F. Wild-type levels of ceramide and ceramide-1-phosphate in the retina of ceramide kinase-like-deficient mice. Biochem Biophys Res Commun. 2008 Aug 15;373(1):159–63. doi: 10.1016/j.bbrc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Brindley DN, Waggoner DW. Mammalian lipid phosphate phosphohydrolases. J Biol Chem. 1998 Sep 18;273(38):24281–4. doi: 10.1074/jbc.273.38.24281. [DOI] [PubMed] [Google Scholar]
- 27.Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. J Lipid Res. 2009 Apr;50(Suppl):S225–30. doi: 10.1194/jlr.R800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granado MH, Gangoiti P, Ouro A, Arana L, Gonzalez M, Trueba M, et al. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell Signal. 2009 Mar;21(3):405–12. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 29**.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010 May;24(5):976–85. doi: 10.1038/leu.2010.53. [First report that C1P regulates trafficking of hematopoietic stem cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Galphaq coupling. J Cell Sci. 2011 Jul 1;124(Pt 13):2220–30. doi: 10.1242/jcs.076794. [DOI] [PubMed] [Google Scholar]
- 31.Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang S, et al. Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 2012 Feb;32(2):331–8. doi: 10.1111/j.1478-3231.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- 32.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008 Sep;1781(9):477–82. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode C, Sensken SC, Peest U, Beutel G, Thol F, Levkau B, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem. 2010 Apr 15;109(6):1232–43. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 34*.Ratajczak J, Kucia M, Mierzejewska K, Liu R, Kim CH, Natarajan N, et al. A novel view of paroxysmal nocturnal hemoglobinuria pathogenesis: more motile PNH hematopoietic stem/progenitor cells displace normal HSPCs from their niches in bone marrow due to defective adhesion, enhanced migration and mobilization in response to erythrocyte-released sphingosine-1 phosphate gradient. Leukemia. 2012 Jul;26(7):1722–5. doi: 10.1038/leu.2012.46. [Novel view on involvement of S1P in pathogenesis of PNH.] [DOI] [PubMed] [Google Scholar]
- 35.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One. 2008;3(5):e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selim S, Sunkara M, Salous AK, Leung SW, Berdyshev EV, Bailey A, et al. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (Lond) 2011 Dec;121(12):565–72. doi: 10.1042/CS20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997 May;121(5):969–73. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 38.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Mar 28. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102(6):669–76. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Gomez-Munoz A, Duffy PA, Martin A, O'Brien L, Byun HS, Bittman R, et al. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides. Mol Pharmacol. 1995 May;47(5):833–9. [Biological effects of C1P for first time described.] [PubMed] [Google Scholar]
- 40.Gangoiti P, Bernacchioni C, Donati C, Cencetti F, Ouro A, Gomez-Munoz A, et al. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie. 2012 Mar;94(3):597–607. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertolucci C, Pinotti M, Colognesi I, Foa A, Bernardi F, Portaluppi F. Circadian rhythms in mouse blood coagulation. J Biol Rhythms. 2005 Jun;20(3):219–24. doi: 10.1177/0748730405275654. [DOI] [PubMed] [Google Scholar]
- 42.Hoopes PC, McCall CE. The effect or cobra venom factor (CVJ) activation of the complement cascade on leukocyte circadian variation in the rat. Experientia. 1977 Feb 15;33(2):224–6. doi: 10.1007/BF02124080. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y, Pallansch M, Carandente F, Reissmann G, Halberg E, Halberg F, et al. Circadian and circannual aspects of the complement cascade - new and old results, differing in specificity. Chronobiologia. 1980 Apr-Jun;7(2):189–204. [PubMed] [Google Scholar]
- 44.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006 Sep;20(9):1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 45.Ratajczak MZ. The emerging role of microvesicles in cellular therapies for organ/tissue regeneration. Nephrol Dial Transplant. 2011 May;26(5):1453–6. doi: 10.1093/ndt/gfr165. [DOI] [PubMed] [Google Scholar]
- 46.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010 Nov;9(11):883–97. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 47.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005 Jun;12(6):703–15. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Obinata H, Hla T. Fine-tuning S1P therapeutics. Chem Biol. 2012 Sep 21;19(9):1080–2. doi: 10.1016/j.chembiol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD, et al. S1P(1) receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol. 2012 Feb;81(2):166–74. doi: 10.1124/mol.111.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010 Feb 4;362(5):402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 51.Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, et al. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1753–61. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Hsu A, Lee JF, Cramer DE, Lee MJ. To stay or to leave: Stem cells and progenitor cells navigating the S1P gradient. World J Biol Chem. 2011 Jan 26;2(1):1–13. doi: 10.4331/wjbc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bendall LJ, Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr Opin Hematol. 2013 Jul;20(4):281–8. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- 54.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010 Jul;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 55.Yanagawa Y, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer's patches by FTY720-induced lymphocyte homing. Immunology. 1998 Dec;95(4):591–4. doi: 10.1046/j.1365-2567.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005 Dec;108(3):308–19. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004 Jul;4(7):1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 58.Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010 Jul 5;207(7):1475–83. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007 Dec;8(12):1337–44. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 60.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005 Sep 1;175(5):2960–7. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 61.Vora KA, Nichols E, Porter G, Cui Y, Keohane CA, Hajdu R, et al. Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J Leukoc Biol. 2005 Aug;78(2):471–80. doi: 10.1189/jlb.0904487. [DOI] [PubMed] [Google Scholar]
- 62.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998 May 15;160(10):5037–44. [PubMed] [Google Scholar]
- 63*.Kimura T, Boehmler AM, Seitz G, Kuci S, Wiesner T, Brinkmann V, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004 Jun 15;103(12):4478–86. doi: 10.1182/blood-2003-03-0875. [First report that S1P regulates trafficking of hematopoietic stem cells.] [DOI] [PubMed] [Google Scholar]
- 64.Seitz G, Boehmler AM, Kanz L, Mohle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005 Jun;1044:84–9. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 65*.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012 Jan 19;119(3):707–16. doi: 10.1182/blood-2011-04-348904. [Interesting paper on involvement of S1P in mobilization of HSPCs.] [DOI] [PubMed] [Google Scholar]
- 66*.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007 Nov 30;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [Interesting paper on involvement of S1P in circulation of CFU-GM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weichand B, Weis N, Weigert A, Grossmann N, Levkau B, Brune B. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur J Immunol. 2013 Aug 12; doi: 10.1002/eji.201343441. doi: 10.1002/eji.201343441. [DOI] [PubMed] [Google Scholar]
- 68.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002 Jul;3(7):687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 69.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011 Feb;25(2):211–7. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 70.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997 Jan 6;185(1):111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratajczak MZ, Serwin K, Schneider G. Innate immunity derived factors as external modulators of the CXCL12-CXCR4 axis and their role in stem cell homing and mobilization. Theranostics. 2013;3(1):3–10. doi: 10.7150/thno.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozuka T, Ishimaru F, Fujii K, Masuda K, Kaneda K, Imai T, et al. Plasma stromal cell-derived factor-1 during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Bone Marrow Transplant. 2003 Apr;31(8):651–4. doi: 10.1038/sj.bmt.1703901. [DOI] [PubMed] [Google Scholar]
- 73*.Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012 Mar 15;119(11):2478–88. doi: 10.1182/blood-2011-06-358614. [Interesting paper on involvement of S1P as SDF-1 modulator in mobilization of HSPCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratajczak MZ, Borkowska S, Ratajczak J. An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate. Prostaglandins Other Lipid Mediat. 2013 Jul-Aug;104-105:122–9. doi: 10.1016/j.prostaglandins.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002 Aug 15;169(4):2102–10. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- 76.Mierzejewska K, Kucia M, Ratajczak J, Ratajczak MZ. Novel Evidence That Hematopoietic Stem/Progenitor Cells (HSPCs) Are Mobilized During Hemolysis in an Erythrocyte Lysis-Derived, Sphingosine-1-Phosphate (S1P)-Dependent manner—the Crucial Involvement of Complement Cascade (CC) Activation and Attenuation of CXCR4 Retention Signaling. Blood (ASH Annual Meeting Abstracts) 2012.
- 77.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012 Jan;26(1):63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arana L, Ordonez M, Ouro A, Rivera IG, Gangoiti P, Trueba M, et al. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: involvement in ceramide 1-phosphate-stimulated cell migration. Am J Physiol Endocrinol Metab. 2013 Jun 1;304(11):E1213–26. doi: 10.1152/ajpendo.00480.2012. [DOI] [PubMed] [Google Scholar]
- 79.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minguell JJ, Erices A. Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med (Maywood) 2006 Jan;231(1):39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 81.Ratajczak MZ, Liu R, Ratajczak J, Kucia M, Shin DM. The role of pluripotent embryonic-like stem cells residing in adult tissues in regeneration and longevity. Differentiation. 2011 Mar;81(3):153–61. doi: 10.1016/j.diff.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Wojakowski W, Kucia M, Liu R, Zuba-Surma E, Jadczyk T, Bachowski R, et al. Circulating very small embryonic-like stem cells in cardiovascular disease. J Cardiovasc Transl Res. 2011 Apr;4(2):138–44. doi: 10.1007/s12265-010-9254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010 Jun;14(6B):1605–18. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, et al. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008 Dec;39(12):3411–7. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 86*.Karapetyan AV, Klyachkin YM, Selim S, Sunkara M, Ziada KM, Cohen DA, et al. Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow-derived stem cells in patients with acute myocardial infarction. Stem Cells Dev. 2013 Jun 1;22(11):1645–56. doi: 10.1089/scd.2012.0488. [Interesting paper on a role of bioactive lipids in stem cell trafficking in acute myocardial infarction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quint P, Ruan M, Pederson L, Kassem M, Westendorf JJ, Khosla S, et al. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem. 2013 Feb 22;288(8):5398–406. doi: 10.1074/jbc.M112.413583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niwa S, Graf C, Bornancin F. Ceramide kinase deficiency impairs microendothelial cell angiogenesis in vitro. Microvasc Res. 2009 May;77(3):389–93. doi: 10.1016/j.mvr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006 Dec 15;108(13):4268–74. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moon H, Chon J, Joo J, Kim D, In J, Lee H, et al. FTY720 preserved islet beta-cell mass by inhibiting apoptosis and increasing survival of beta-cells in db/db mice. Diabetes Metab Res Rev. 2013 Jan;29(1):19–24. doi: 10.1002/dmrr.2341. [DOI] [PubMed] [Google Scholar]
- 91.Ieronimakis N, Pantoja M, Hays AL, Dosey TL, Qi J, Fischer KA, et al. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet Muscle. 2013;3(1):20. doi: 10.1186/2044-5040-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leong WI, Saba JD. S1P metabolism in cancer and other pathological conditions. Biochimie. 2010 Jun;92(6):716–23. doi: 10.1016/j.biochi.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Brocklyn J, Letterle C, Snyder P, Prior T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002 Jul 26;181(2):195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 94.Bergelin N, Blom T, Heikkila J, Lof C, Alam C, Balthasar S, et al. Sphingosine kinase as an oncogene: autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase C-alpha and ERK1/2. Endocrinology. 2009 May;150(5):2055–63. doi: 10.1210/en.2008-0625. [DOI] [PubMed] [Google Scholar]
- 95.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006 Mar;9(3):225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 96.Yoon CM, Hong BS, Moon HG, Lim S, Suh PG, Kim YK, et al. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008 Aug 15;112(4):1129–38. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li MH, Sanchez T, Yamase H, Hla T, Oo ML, Pappalardo A, et al. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett. 2009 Apr 18;276(2):171–9. doi: 10.1016/j.canlet.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, et al. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res. 2006 Jan;130(1):80–7. doi: 10.1016/j.jss.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, et al. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003 Nov 15;63(22):7926–35. [PubMed] [Google Scholar]
- 100.O'Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009 Nov;50(11):2245–57. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005 Mar 18;280(11):9833–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 102.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007 Jul;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 103*.Simanshu DK, Kamlekar RK, Wijesinghe DS, Zou X, Zhai X, Mishra SK, et al. Non vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013 Aug 22;(7463):463–467. doi: 10.1038/nature12332. [An interesting work that demonstrates a non-receptor mediated effect of C1P.] [DOI] [PMC free article] [PubMed] [Google Scholar]