Abstract
Study Design
Retrospective cohort study.
Objective
To identify factors that are independently associated with increased surgical drain output in patients who have undergone ACDF.
Summary of Background Data
Surgical drains are typically placed following ACDF to reduce the risk of complications associated with neck hematoma. The orthopaedic literature has repeatedly challenged the use of surgical drains following many procedures, and there are currently no guidelines for determining which patients are most likely to benefit from drain placement after ACDF.
Methods
Consecutive patients who underwent elective ACDF with surgical drain placement at a single academic institution between January 2011 and February 2013 were identified using billing records. Patient information was abstracted from the medical record. Patients were categorized based on normal or increased total drain output, with increased drain output defined as total drain output ≥ 50th percentile (30mL). A multivariate logistic regression was used to determine which factors were independently associated with increased drain output.
Results
A total of 151 ACDF patients met inclusion criteria. Total drain output ranged from 0 mL to 265 mL. The average drain output for this cohort was 42.3 ± 45.5 mL (mean ± standard deviation). Among all patients in the study, 80 patients had increased drain output (drain output ≥ 50th percentile or 30 mL).
Multivariate analysis identified three independent predictors of increased drain output: age ≥ 50 years (Odds Ratio [OR] = 3.9), number of levels (2 levels, OR = 2.7; 3–4 levels, OR = 17.0), and history of smoking (OR = 2.8). One patient developed a postoperative neck hematoma while a drain was in place.
Conclusion
Patients with the above-identified factors associated with increased drain output may benefit most from surgical drain placement after ACDF. Nonetheless, neck hematoma is still possible even with drain use.
Keywords: drain, closed-suction, anterior, cervical, discectomy, fusion, ACDF, outcomes, complications, wound, hematoma, airway obstruction, age, multilevel, smoking
Introduction
Anterior cervical discectomy and fusion (ACDF) is a well-accepted surgical procedure for the treatment of cervical spine pathology.1,2 In order to minimize the risk of hematoma following the procedure, the use of a closed-suction drain has become commonplace despite a lack of evidence establishing its efficacy.
The orthopaedic literature has repeatedly challenged the use of postoperative surgical drains.3 For hip and knee arthroplasty, several studies have shown no difference in the incidence of infection, blood loss, changes in hemoglobin and hematocrit, functional assessment, or other complications when the drainage group was compared with the no-drain group.4–8 In orthopaedic trauma studies, drains have also been found to be unnecessary.9,10
For lumbar spinal procedures, multiple studies have found the risk of wound infection and hematomas in single-level lumbar decompression surgery not to be influenced by use of a drain.11–14 Conversely, some studies have found an association between drains and increased postoperative blood transfusions and wound infection after spinal fusion.15–17 There is also evidence that drains may be associated with postoperative fevers after lumbar spine surgery.13,18
In light of the unique and potentially life-threatening complications associated with hematoma in the neck (such as airway obstruction), many spine surgeons are hesitant to forgo using a drain following ACDF.19–21 However, multiple studies of other operations involving the neck, including thyroidectomy, parathyroidectomy, and esophagectomy have found no advantage to drain use, and drains may actually be associated with increased incidence of wound infection and increased hospital length of stay in these procedures.22–25
Despite evidence challenging drain use for many other procedures, to our knowledge no prior studies have directly evaluated surgical drains after ACDF. The working hypothesis of this study is that drains with minimal output may not be necessary from the outset. If variables independently associated with greater drain output could be identified, it would suggest that a subset of patients with these variables might have a greater need for surgical drains after ACDF than others.
Materials and Methods
Data Source
Records of all consecutive patients who underwent ACDF by one of five attending surgeons (three orthopaedic surgeons and two neurosurgeons) at one institution from January 2011 to February 2013 were obtained from the institution’s billing department. In order to minimize selection bias, only surgeons who place a postoperative drain after every ACDF were used for this study.
ACDF procedures were identified using Current Procedural Terminology (CPT) codes 22551 (anterior discectomy and fusion), 22554 (anterior fusion), and 63075 (anterior discectomy). CPT code 22551 largely replaced codes 22554 and 63075 in January 2011, and while all subjects underwent ACDF after January 2011, several additional ACDF patients were captured using CPT codes 22554 and 63075.
Cases involving trauma, excision of malignancy, previous infection, patients under 18 years old, total disc replacement, concomitant posterior cervical arthrodesis, thoracic or lumbar spine surgery, or other unrelated procedures were excluded from analysis.
Data Collection
Once ACDF procedures were identified from billing records, patient information was abstracted from the electronic medical record for each case. Demographic and history data collected included: age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, preoperative hematocrit, history of smoking, and history of major medical comorbidities (non-spinal malignancy, diabetes mellitus, pulmonary disease, hypertension, heart disease, or bleeding disorder/currently taking aspirin).
Preoperative hematocrit was taken from patients’ preoperative lab values, however it was not drawn on every patient (generally due to low suspicion of an abnormal value). Patients with hematocrit at or above 36.0 or not drawn were defined as having normal preoperative hematocrit, and hematocrit below 36.0 was defined as low. Non-spinal malignancy was defined as either a current or previous history of treatment with radiation, chemotherapy, or surgery for a malignant tumor that did not involve the spine. Diabetes and hypertension were determined by a history of treatment for these conditions or by findings during the preoperative assessment. Pulmonary disease was defined as asthma requiring hospitalization, chronic obstructive pulmonary disease, chronic bronchitis, or a history of pulmonary embolism. Heart disease was defined as a history of arrhythmia, valvular abnormalities, coronary artery disease, myocardial infarction, or congestive heart failure. Bleeding disorder was defined as history of bleeding or clotting disorder such as factor deficiency, platelet disorder, antiphospholipid syndrome, or current aspirin use.
Procedural variables collected included: number of levels fused, use of iliac crest bone graft (ICBG), whether a corpectomy was performed, and operative time. Operative time was defined by time from the opening incision to the end of wound closure. The variable “extended operative time” was defined as greater than or equal to the 50th percentile operative time. In addition to the above variables, hospital records were reviewed to identify any issues potentially related to the surgical drains and/or hematomas.
Drain Output
Drain output was defined as the total output from the time of surgery until time of drain removal. This was measured in milliliters (mL). As drain output was checked at varying time intervals and drains were generally in place for limited time, it was deemed not accurate to divide the time of drain output into hourly, shift, or daily measures.
It is notable that when drains were removed, any residual volume in the drain reservoir may not have been recorded. However, drains were removed when there was minimal output, so significant drainage should not have been missed.
The primary outcome measure was increased drain output, a binary variable that we defined as positive when the drain output was greater than or equal to the median drain output for this cohort.
Statistical Analysis
Statistical analyses were conducted using STATA® version 11.2 (StataCorp, LP, College Station, Texas, USA). All tests were two-tailed and the statistical difference was established at a two-sided α level of 0.05 (p < 0.05).
Demographic, comorbidity, and procedural variables were tested for association with increased drain output using bivariate and multivariate logistic regression. The final multivariate model was constructed using a backwards stepwise process that initially included all potential variables and sequentially excluded variables with the highest p-value until only those with p < 0.20 remained. Variables with 0.05 < p < 0.20 were left in the model to control for potential confounding, but were not considered to be significantly associated with the outcome.
Results
A total of 151 ACDF patients met inclusion criteria. Demographic and surgical data are provided in Table 1. Age was 51.5 ± 11.5 years (mean ± standard deviation [SD]), BMI was 29.5 ± 6.2 kg/m2, and 55% of patients were female. The remaining variables were consistent with expected demographics, comorbidities, and surgical variation.
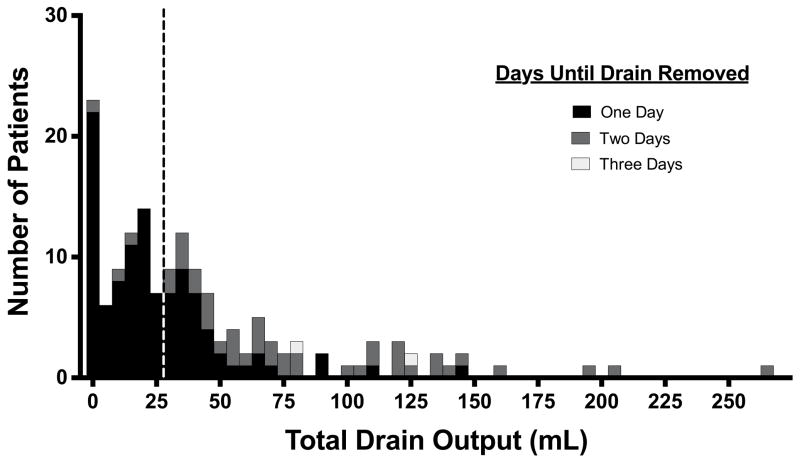
Total drain output for this population ranged from 0 to 265 mL. The average drain output for this cohort was 42.3 ± 45.5 mL. Figure 1 illustrates the distribution of drain output, with the number of days until drain removal indicated.
Figure 1.
Drain output after ACDF. An increased drain output was defined as one greater than or equal to the 50th percentile drain output, which was 30 mL. Patients with total drain output to the right of the vertical dashed line had increased drain output.
Overall, 80 patients had increased drain output (drain output ≥ 50th percentile or 30 mL). The percentage of patients with increased drain output is stratified by demographic, comorbidity, and surgical characteristics in Table 2.
Bivariate analysis examining the effects of each variable on odds of increased drain output is shown in the middle columns of Table 2. This analysis showed significant association between increased drain output and age, number of levels, corpectomy, history of smoking, and operative time.
Multivariate analysis was then used to simultaneously measure the effects of each variable on drain output while controlling for confounding variables (right columns of Table 2). This analysis showed that significant predictors of increased drain output (drain output ≥ 30 mL) were age ≥ 50 years (Odds Ratio [OR] = 3.9, 95% Confidence Interval [CI] = 1.8 – 8.5, p < 0.001), number of levels (2 levels OR = 2.7, 95% CI = 1.3 – 5.8; 3–4 levels OR = 17.0, 95% CI = 2.0 – 146.7; p = 0.004), and history of smoking (OR = 2.8, 95% CI = 1.3 – 6.1, p = 0.008). History of non-spinal malignancy was included in the final multivariate model to control for potential confounding (p < 0.20), but its association with drain output was not considered to be statistically significant (p > 0.05).
Corpectomy and extended operative time were associated with increased drain output on bivariate analysis, but this association dropped out of significance on multivariate analysis. This signifies covariance between these variables and those factors found to be significant in the multivariate model. Sex, BMI, ASA class, preoperative hematocrit < 36.0, history of non-spinal malignancy, diabetes, history of pulmonary disease, history of hypertension, history of heart disease, history of bleeding disorder or current aspirin use, and use of iliac crest bone graft were not significantly associated with drain output on either bivariate or multivariate analysis.
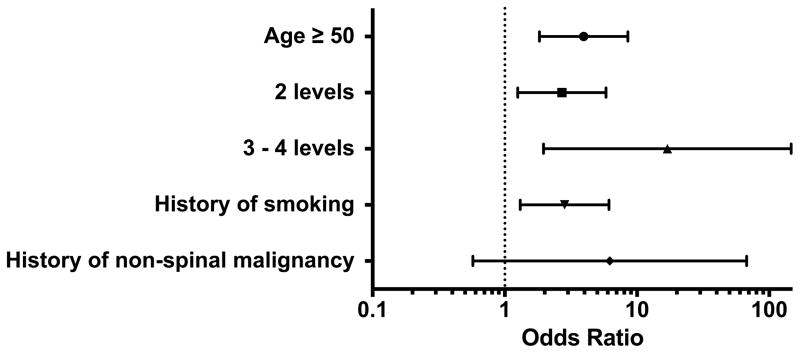
Figure 2 is a forest plot that shows odds ratios with 95% confidence intervals for each variable in the final multivariate model. Significant variables are those whose confidence interval does not cross the dotted line at OR = 1. As seen in Figure 2, as number of levels increases, so does the odds of increased drain output. Table 3 summarizes the effect of each variable on drain output (summarizes data from Table 2).
Figure 2.
Multivariate logisitic regression model for factors affecting drain output after ACDF. Odds ratios (OR) with confidence intervals are illustrated for each variable in the model. Variables with confidence intervals overlapping the dotted line at OR = 1 were not statistically significant predictors of increased drain output.
Review of postoperative data revealed that one patient had a significant neck hematoma on the second postoperative day after a two-level ACDF. This lead to airway obstruction, intubation and urgent neck exploration at the bedside, return to the operating room, and extended length of stay. The patient was 52 years old with a history of smoking. The patient still had a drain in place at the time of the above complication, and it had put out a total of 80 mL over the two days before the airway compromise occurred.
Discussion
ACDF is a common surgical procedure for which many surgeons routinely place a surgical drain with the goal of reducing complications due to post-operative hematoma.1,2,26,27 The efficacy of drains has been challenged for many surgical procedures; however, no study has assessed the necessity of drain use after ACDF.3–18,22–25 The current study was designed to identify factors that affect drain output after ACDF in order to assist surgeons with the decision of whether or not to place a drain postoperatively.
We found that age ≥ 50 years, number of levels fused, and smoking history were all independently associated with increased drain output (Tables 2 and 3). Sex, BMI, ASA class, preoperative hematocrit < 36.0, history of non-spinal malignancy, diabetes, history of pulmonary disease, history of hypertension, history of heart disease, history of bleeding disorder or current aspirin use, corpectomy, use of iliac crest bone graft, and operative time ≥ 150 minutes were not significantly associated with increased drain output after ACDF.
While there are no other studies for direct comparison, we found three studies that used multivariate analysis to identify factors associated with postoperative lumbar epidural hematoma formation after lumbar spine surgery. The most recent study by Amiri et al. found that alcohol consumption, a multilevel procedure, and previous spinal surgery are risk factors for developing spinal epidural hematoma.28 Sokolowski et al. found that advanced age, multilevel procedures, and international normalized ratio are independently associated with postoperative lumbar epidural hematoma volume.29 Kou et al. found that epidural hematoma after lumbar procedures was associated with multilevel procedures and presence of preoperative coagulopathy.30 While these results are not generalizable to hematoma formation following ACDF, they provide some basis for comparison.
In our study, patients with age ≥ 50 years were more likely to have increased drain output following ACDF. This resembles the results of the Sokolowski et al. study that found that advanced age is independently associated with postoperative lumbar epidural hematoma volume.29 This effect may be due to delayed wound healing associated with increased age.31
As the number of levels involved in the procedure increased, there was a corresponding rise in the odds of increased drain output. All three previously mentioned studies that identified factors associated with lumbar epidural hematoma formation found an association between multilevel procedures and hematoma formation.28–30 As multilevel procedures are generally associated with increased dissection and exposed bony surfaces, it would seem reasonable that this could translate into increased postoperative drainage.
Corpectomy and increased operative time were significantly associated with increased drain output after ACDF on bivariate analysis, yet most likely due to covariance with other predictor variables, both variables failed to show statistical association with increased drain output in the multivariate model (p > 0.05). Procedures that included corpectomy were counted as multilevel procedures for this analysis, so the contribution of number of levels could have trumped the contributions of corpectomy in the final multivariate model. Similarly, multilevel procedures are associated with increased operative time, so the contribution of operative time in the final multivariate model could have also been overcome by the effect of number of levels on drain output.
History of smoking was also independently associated with greater odds of increased drain output in our analysis. Smoking history has long been associated with poor wound healing, which could contribute to increased drain output.32 Additionally, history of smoking has previously been found to increase risk of postoperative bleeding in several other surgeries of the neck.33,34 Preoperative smoking cessation has been shown to decrease postoperative complications after orthopaedic surgery, particularly wound-related complications.35 This is yet another reason smoking cessation should be encouraged prior to ACDF.
One patient in this study did have a significant neck hematoma on the second postoperative day after a two-level ACDF, leading to airway obstruction, intubation and urgent neck exploration at the bedside, return to the operating room, and extended length of stay. The patient had a drain placed postoperatively, with 80 mL of total output over the two days before airway compromise occurred. Notably, this patient carried all identified independent risk factors for increased drain output (age ≥ 50, multilevel procedure, and history of smoking). This case also highlights that even with a drain in place (it was still in at the time of the complication), the serious complication of neck hematoma with airway compromise can still occur. Only 1 of 151 patients experienced this complication (0.7%), which is consistent with previously reported incidences of this postoperative complication (0.2% to 1.9%).20
Limitations of this study include its retrospective nature and differences in practice of surgeons. Neither surgical technique nor criteria for drain removal was fully standardized across the sample. As mentioned previously, when drains were removed, any residual volume in the drain reservoir may not have been recorded. However, drains were generally removed when there was minimal output, so significant drainage should not have been missed. By performing this study at a single institution, hospital-related confounding variables should have been reasonably controlled. In addition, while this study describes factors predicting increased drain output, it is still not known what level of drain output can be directly linked to increased risk of clinical complications.
Overall, this study is the first of its kind to identify factors associated with increased drain output after ACDF. While the decision to use a drain after ACDF is at the surgeon’s discretion, the results of this study suggest that younger patients without a smoking history who are undergoing a single-level ACDF are less likely to have increased drain output. Drains may not be necessary in this population, although further research is needed to support this conclusion. Conversely, patients over the age of 50 with smoking history undergoing a multilevel ACDF are more likely to have an increased amount of drainage following the procedure and may benefit more from drain placement. However, as we unfortunately found in one of our patients, a drain does not eliminate the risk of serious complications from the development of a postoperative hematoma.
Supplementary Material
Key Points.
Surgical drains are typically placed after ACDF to reduce the risk of neck hematoma, yet there are no guidelines for identifying which patients most benefit from drain placement.
Using consecutive patients at a single academic institution (all of whom had surgical drains placed), multivariate analysis was conducted to identify factors independently associated with increased drain output, with increased drain output defined as drain output ≥ 50th percentile (30mL).
This study found that patients with age ≥ 50 years, those undergoing ACDF at two or more levels, and those with a history of smoking are more likely to have increased drain output.
This is the first study of its kind identifying these factors, and patients with the above-identified factors associated with increased drain output may benefit most from surgical drain placement after ACDF.
Even with drain placement after ACDF, neck hematoma leading to airway compromise can still occur.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000141. This project was approved by the Yale University Institutional Review Board.
The Manuscript submitted does not contain information about medical device(s)/drug(s). National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000141 funds were received to support this work. Relevant financial activities outside the submitted work: grant, consultancy and expert testimony.
Footnotes
Level of Evidence: 3
The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
No conflicts of interest are reported.
Contributor Information
Bryce A. Basques, Yale University School of Medicine
Daniel D. Bohl, Yale University School of Medicine
Nicholas S. Golinvaux, Yale University School of Medicine
Alem Yacob, Yale University School of Medicine
Arya G. Varthi, Yale University School of Medicine
Jonathan N. Grauer, Yale University School of Medicine
References
- 1.Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine. 2005;30:2138–44. doi: 10.1097/01.brs.0000180479.63092.17. [DOI] [PubMed] [Google Scholar]
- 2.Klein GR, Vaccaro AR, Albert TJ. Health outcome assessment before and after anterior cervical discectomy and fusion for radiculopathy: a prospective analysis. Spine. 2000;25:801–3. doi: 10.1097/00007632-200004010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Parker MJ, Livingstone V, Clifton R, et al. Closed suction surgical wound drainage after orthopaedic surgery. The Cochrane database of systematic reviews. 2007:CD001825. doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou XD, Li J, Xiong Y, et al. Do we really need closed-suction drainage in total hip arthroplasty? A meta-analysis. International orthopaedics. 2013 doi: 10.1007/s00264-013-2053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter MA, Keating EM, Faris PM. Closed wound drainage in total hip or total knee replacement. A prospective, randomized study. The Journal of bone and joint surgery. 1994;76:35–8. doi: 10.2106/00004623-199401000-00005. American volume. [DOI] [PubMed] [Google Scholar]
- 6.Beer KJ, Lombardi AV, Jr, Mallory TH, et al. The efficacy of suction drains after routine total joint arthroplasty. The Journal of bone and joint surgery. 1991;73:584–7. American volume. [PubMed] [Google Scholar]
- 7.Hadden WA, McFarlane AG. A comparative study of closed-wound suction drainage vs. no drainage in total hip arthroplasty. The Journal of arthroplasty. 1990;5 (Suppl):S21–4. doi: 10.1016/s0883-5403(08)80021-6. [DOI] [PubMed] [Google Scholar]
- 8.Reilly TJ, Gradisar IA, Jr, Pakan W, et al. The use of postoperative suction drainage in total knee arthroplasty. Clinical orthopaedics and related research. 1986:238–42. [PubMed] [Google Scholar]
- 9.Lang GJ, Richardson M, Bosse MJ, et al. Efficacy of surgical wound drainage in orthopaedic trauma patients: a randomized prospective trial. Journal of orthopaedic trauma. 1998;12:348–50. doi: 10.1097/00005131-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Cobb JP. Why use drains? The Journal of bone and joint surgery. 1990;72:993–5. doi: 10.1302/0301-620X.72B6.2246304. British volume. [DOI] [PubMed] [Google Scholar]
- 11.Kanayama M, Oha F, Togawa D, et al. Is closed-suction drainage necessary for single-level lumbar decompression? : review of 560 cases. Clinical orthopaedics and related research. 2010;468:2690–4. doi: 10.1007/s11999-010-1235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scuderi GJ, Brusovanik GV, Fitzhenry LN, et al. Is wound drainage necessary after lumbar spinal fusion surgery? Medical science monitor: international medical journal of experimental and clinical research. 2005;11:CR64–6. [PubMed] [Google Scholar]
- 13.Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine. 2004;29:1066–8. doi: 10.1097/00007632-200405150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Payne DH, Fischgrund JS, Herkowitz HN, et al. Efficacy of closed wound suction drainage after single-level lumbar laminectomy. Journal of spinal disorders. 1996;9:401–3. [PubMed] [Google Scholar]
- 15.Blank J, Flynn JM, Bronson W, et al. The use of postoperative subcutaneous closed suction drainage after posterior spinal fusion in adolescents with idiopathic scoliosis. Journal of spinal disorders & techniques. 2003;16:508–12. doi: 10.1097/00024720-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Diab M, Smucny M, Dormans JP, et al. Use and outcomes of wound drain in spinal fusion for adolescent idiopathic scoliosis. Spine. 2012;37:966–73. doi: 10.1097/BRS.0b013e31823bbf0b. [DOI] [PubMed] [Google Scholar]
- 17.Rao SB, Vasquez G, Harrop J, et al. Risk factors for surgical site infections following spinal fusion procedures: a case-control study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:686–92. doi: 10.1093/cid/cir506. [DOI] [PubMed] [Google Scholar]
- 18.Walid MS, Abbara M, Tolaymat A, et al. The role of drains in lumbar spine fusion. World neurosurgery. 2012;77:564–8. doi: 10.1016/j.wneu.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 19.Sethi R, Tandon MS, Ganjoo P. Neck hematoma causing acute airway and hemodynamic compromise after anterior cervical spine surgery. Journal of neurosurgical anesthesiology. 2008;20:69–70. doi: 10.1097/ANA.0b013e318157f749. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo MA, Aidlen JP, Daniels AH, et al. Airway compromise due to wound hematoma following anterior cervical spine surgery. The open orthopaedics journal. 2012;6:108–13. doi: 10.2174/1874325001206010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn JE, Graziano GP. Airway compromise as a result of retropharyngeal hematoma following cervical spine injury. Journal of spinal disorders. 1991;4:264–9. [PubMed] [Google Scholar]
- 22.Samraj K, Gurusamy KS. Wound drains following thyroid surgery. The Cochrane database of systematic reviews. 2007:CD006099. doi: 10.1002/14651858.CD006099.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabaqchali MA, Hanson JM, Proud G. Drains for thyroidectomy/parathyroidectomy: fact or fiction? Annals of the Royal College of Surgeons of England. 1999;81:302–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Shandilya M, Kieran S, Walshe P, et al. Cervical haematoma after thyroid surgery: management and prevention. Irish medical journal. 2006;99:266–8. [PubMed] [Google Scholar]
- 25.Choi HK, Law S, Chu KM, et al. The value of neck drain in esophageal surgery: a randomized trial. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus/ISDE. 1998;11:40–2. [PubMed] [Google Scholar]
- 26.Yeom JS, Buchowski JM, Shen HX, et al. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: a retrospective matched pair analysis. Spine. 2008;33:E543–7. doi: 10.1097/BRS.0b013e31817c6c9b. [DOI] [PubMed] [Google Scholar]
- 27.Cho SK, Yi JS, Park MS, et al. Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery. The Journal of bone and joint surgery. 2012;94:1952–8. doi: 10.2106/JBJS.K.00632. American volume. [DOI] [PubMed] [Google Scholar]
- 28.Amiri AR, Fouyas IP, Cro S, et al. Postoperative spinal epidural hematoma (SEH): incidence, risk factors, onset, and management. The spine journal: official journal of the North American Spine Society. 2013;13:134–40. doi: 10.1016/j.spinee.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Sokolowski MJ, Garvey TA, Perl J, 2nd, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine. 2008;33:108–13. doi: 10.1097/BRS.0b013e31815e39af. [DOI] [PubMed] [Google Scholar]
- 30.Kou J, Fischgrund J, Biddinger A, et al. Risk factors for spinal epidural hematoma after spinal surgery. Spine. 2002;27:1670–3. doi: 10.1097/00007632-200208010-00016. [DOI] [PubMed] [Google Scholar]
- 31.Gosain A, DiPietro LA. Aging and wound healing. World journal of surgery. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 32.Moller AM, Pedersen T, Villebro N, et al. Effect of smoking on early complications after elective orthopaedic surgery. The Journal of bone and joint surgery. 2003;85:178–81. doi: 10.1302/0301-620x.85b2.13717. British volume. [DOI] [PubMed] [Google Scholar]
- 33.Morton RP, Mak V, Moss D, et al. Risk of bleeding after thyroid surgery: matched pairs analysis. The Journal of laryngology and otology. 2012;126:285–8. doi: 10.1017/S0022215111001460. [DOI] [PubMed] [Google Scholar]
- 34.Demars SM, Harsha WJ, Crawford JV. The effects of smoking on the rate of postoperative hemorrhage after tonsillectomy and uvulopalatopharyngoplasty. Archives of otolaryngology--head & neck surgery. 2008;134:811–4. doi: 10.1001/archotol.134.8.811. [DOI] [PubMed] [Google Scholar]
- 35.Moller AM, Villebro N, Pedersen T, et al. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114–7. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.