Abstract
Bone marrow stromal cells (BMSCs) have been used to treat acute graft-versus-host disease (GVHD) and other complications following allogeneic hematopoietic stem cell transplantation (SCT). We conducted a phase I trial using third party, early passage BMSCs for patients with steroid-refractory GVHD, tissue injury or marrow failure following SCT to investigate safety and efficacy. To identify mechanisms of BMSC immunomodulation and tissue repair, patients were serially monitored for plasma GVHD biomarkers, cytokines and lymphocyte phenotype. Ten subjects were infused a fixed dose of 2 × 106 BMSCs/kg intravenously weekly for 3 doses. There was no treatment related toxicity (primary endpoint). Eight subjects were evaluable for response at 4 weeks after the last infusion. Five of the seven patients with steroid-refractory acute GVHD achieved a complete response (CR), two of two patients with tissue injury (pneumomediastinum/pneumothorax) achieved resolution but there was no response in two subjects with delayed marrow failure. Rapid reductions in inflammatory cytokines were observed. Clinical responses correlated with a fall in biomarkers (Reg 3α, CK18, and Elafin) relevant for the site of GVHD or tissue injury. The GVHD complete responders survived significantly longer, had higher baseline absolute lymphocyte and central memory CD4 and CD8 counts. Cytokine changes also segregated with survival. These results confirm that BMSCs are associated with rapid clinical and biomarker responses in GVHD and tissue injury. However BMSCs were ineffective in patients with prolonged GVHD with lower lymphocyte counts, which suggest that effective GVHD control by BMSCs requires a relatively intact immune system.
Keywords: MSC, biomarkers, allogeneic stem cell transplantation, graft-versus-host disease
Introduction
BMSCs can readily be isolated and expanded from bone marrow aspirates. Their capacity to immunosuppress and promote tissue repair have led to clinical trials to explore their broad therapeutic potential. BMSCs are immunomodulatory, suppressing mixed lymphocyte reactions and attenuating alloresponses.1 Notably, BMSC infusions have shown promise in the treatment of complications following allogeneic stem cell transplantation (SCT). 2 First shown to successfully abrogate refractory acute graft versus host disease (GVHD) in 2004, investigators subsequently demonstrated that responders to BMSC infusions had improved overall survival.3, 4 Subsequent multicenter trials using BMSCs to treat steroid refractory acute GVHD have shown promise with complete abrogation of GVHD in some cases, associated with prolonged post-transplant survival. Favorable factors predictive for response include younger age, and GVHD occurring in the gut and liver. However, it is unclear whether therapeutic responses of acute GVHD to BMSC are due to immunosuppression or to the promotion of tissue repair of gut, and liver damaged by the alloimmune attack. Based on their capability to differentiate into specific cell types and promote tissue regeneration from damaged tissue progenitors, BMSCs have also been used to treat hemorrhagic cystitis, and pneumoperitoneum after SCT. 5, 6
While there is a consensus that BMSCs have some therapeutic effect in complications following SCT, there are many unanswered questions related to the mechanism underlying immune modulation and tissue repair, the optimum dose and schedule of infusions, and the impact of the method of manufacture on BMSC function. BMSCs are typically given in repeated infusions of about 2 × 106 cells per kilogram given a week apart. Whether the dose and schedule is optimal and whether BMSCs from different donors have distinct therapeutic characteristics are not known. The culture conditions and degree of expansion used for BMSC treatments vary widely and may influence the clinical outcomes obtained.
Advances in new diagnostic and therapeutic tools using GVHD-relevant biomarkers may provide the opportunity to more clearly define responses to BMSC therapy and identify individuals most likely to respond to this form of cellular therapy. 7 At the National Institutes of Health (NIH) Clinical Center, we developed a clinical grade BMSC cell bank from third party donors to explore the potential role of BMSC in treatment of post-transplant complications. In this phase I safety/efficacy study, BMSCs generated from healthy bone marrow donors were used to treat SCT recipients with steroid-refractory acute GVHD or other forms of organ damage. A fixed dose of 2× 106 BMSCs/kg was administered weekly for three doses to a single cohort of 10 subjects. To better define clinical responses and mechanism of BMSC action, patients were regularly monitored for biomarkers of GVHD and tissue damage, changes in circulating cytokines and lymphocyte phenotype.
Patients and Methods
Patients
Subjects, all allogeneic stem cell transplant recipients, provided written informed consent for the phase I study (clinicaltrials.gov identifier# NCT01633229) approved by the Institutional Review Board (IRB) at the National Heart, Lung and Blood Institute, NIH (protocol# 12-H-0010). From March through October 2012, 10 consecutive patients meeting protocol inclusion criteria were enrolled. Eligible indications fell into three broad categories: acute GVHD of gut or liver greater or equal to grade II, refractory to steroids, (defined as no response or worsening after at least 7 days treatment with methylprednisolone at a dose of 2mg/kg), tissue injury or bone marrow failure. Acute GVHD was defined using the NIH consensus definition inclusive of classic and persistent/recurrent/late onset, and was graded on the basis of severity of involvement of each target organ (Center for International Blood and Marrow Transplantation Research (CIBMTR) grading). Acute GVHD was biopsy confirmed and isolated skin GVHD was excluded. All patients were either steroid-refractory, defined as failure to respond to ≥ 2 mg/kg IV methylprednisolone after 7 days, or steroid-dependent requiring ≥ 1 mg/kg IV methylprednisolone for ≥ 7 days for control of GVHD. Other indications included poor graft function, lung injury, hepatic injury, and hemorrhagic cystitis. Poor graft function was defined as platelet or red cell transfusion dependence or absolute neutrophil count persisting < 500/μl for at least 14 days without other reversible or treatable causes in the presence of > 95% donor myeloid chimerism. Lung injury included diffuse alveolar hemorrhage (DAH), cryptogenic organizing pneumonia (COP), bronchiolitis obliterans syndrome (BOS), idiopathic interstitial pneumonia (IP), adult respiratory distress syndrome (ARDS) diagnosed by exclusion of active infection, and worsening pneumomediastinum or pneumothorax persisting after 7 days. Hepatic injury was defined as clinically diagnosed sinusoidal obstruction syndrome (SOS), with bilirubin > 2x the upper limit of the normal range (ULN) and transaminases > 3x ULN. Hemorrhagic cystitis required continuous bladder irrigation or requiring red cell or platelet transfusions at least intermittently for the past 7 days or unresponsive to antiviral treatment after 10 days. The primary and/or secondary indications for BMSC administration were defined at baseline.
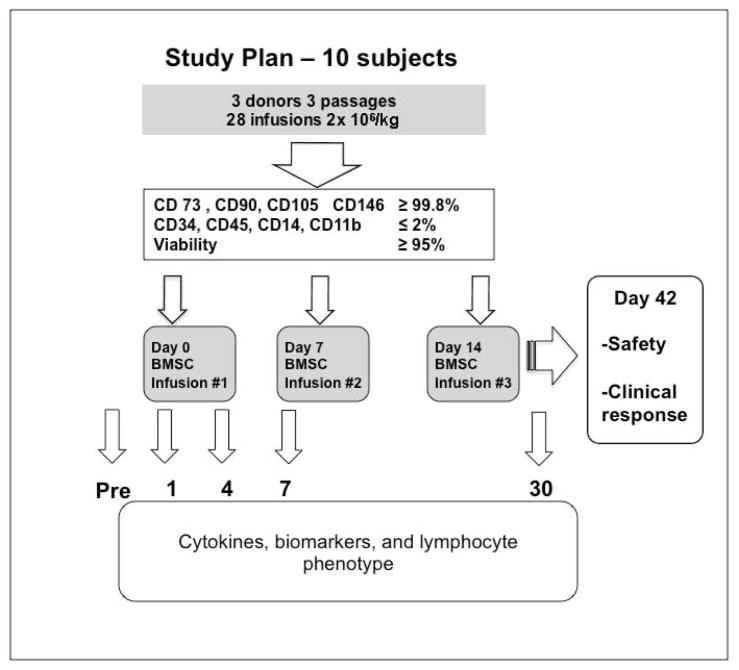
All patients were inpatient for the duration of treatment, observed during the entire infusion period and for at least 2 hours after infusion. We used the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, to analyze safety. The primary endpoint (and stopping rule) was the occurrence of any treatment related serious adverse event (SAE), defined as an SAE either probably or definitely attributed to the BMSC. Patients were assessed weekly for response to the primary indication according to criteria, for up to 3 weeks after the last infusion. Assessment including skin rash, nausea, anorexia, stool volumes and gastrointestinal bleeding amount, serum bilirubin level, liver function, and CT scans for tissue injury were recorded. Cytomegalovirus (CMV) surveillance was performed at least once weekly. For GVHD, a complete response (CR) was defined as a resolution of all reversible acute GVHD–associated manifestations, (Stage 0) in all organ systems and a partial response (PR) as improvement in at least one organ stage (but less than a CR) without worsening in any other organ stage. Responses to tissue injury were assessed by repeated imaging and organ specific responses such as resolution of hypoxia/ventilatory requirement (for pulmonary injury). Response criteria for marrow failure were improvements in blood counts and transfusion independence. The study schema is shown in Figure 1.
Figure 1.
Bone marrow mesenchymal stromal cells (BMSC) treatment plan.
Blood samples for biomarkers and lymphocyte phenotyping
Blood was collected for measurement of cytokine, biomarker levels and lymphocyte phenotypes pre-BMSC infusion (day 0), 1, 4, 7, 14, 21 and 28 days after the first infusion of BMSC. For cytokine and biomarker assays plasma was obtained after centrifugation of whole blood collected in EDTA-containing Vacutainer tubes (Becton-Dickinson). Samples were aliquoted without additives into cryovials and stored immediately at −80°C. For lymphocyte phenotyping mononuclear cells were separated from heparinized blood using ficoll-hypaque and cryopreserved in 5% DMSO in liquid nitrogen before batch testing. HLA-alloimmunization was tested prior to and immediately after completing the BMSC trial. All peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Ficoll-Hypaque, GE Healthcare), separated into aliquots, and cryopreserved in liquid nitrogen. As biomarker controls, plasma and PBMC samples from 6 GVHD free, uncomplicated patients day 26 to 112 (median day 38) post-myeloablative sibling matched allogeneic transplantation. There were no significant differences between the control and trial patients with respect to days post SCT and age (p=0.28 and p=0.13)
BMSC preparation and administration
The Cell Processing Section of the Department of Transfusion Medicine at the Clinical Center, NIH has developed a procedure for collecting, expanding and cryopreserving clinical grade BMSC under an FDA Drug Master File (DMF), (IRB approved protocol # 10-CC-0053).8 Samples of human bone marrow donated from consenting healthy donors were used to isolate and expand BMSC. Briefly, approximately 12 ml of marrow was aspirated and diluted by adding BMSC Culture Media, alpha MEM (Lonza, Walkersville, MD) with 20% fetal bovine 1serum (Hyclone Laboratories, Inc., Logan UT). A single-cell suspension was prepared and the BMSC were initially cultured at a density of 2 to 3 ×105 cell per cm2 in T-75 flasks (passage 1) (Corning, NY). The primary cultures were harvested with recombinant trypsin (Try-PLE Express Invitrogen, CA) when more than half of the colonies with at least 50 cells were 70%–80% confluent. The harvested passage 1 cells were plated into 2-layer cell factories (Cell Factory, easy fill 2-trays, Nunc A/S Roskilde, Denmark) at a density of 2,500 to 4,000 cells per cm2. These BMSCs were harvested when 70% confluent after 5 or 6 days. The passage 2 and 3 cells were seeded, cultured and harvested in the same manor in 10-layer cell factories (Cell Factory, easy fill 10-trays, Nunc A/S Roskilde, Denmark). Final product (passage 4) was cryopreserved in FEP cryobags (AFC Kryosure VP-20F, Gaithersburg, MD) at a concentration of 100×106 viable cells in 20 ml of solution made up for 5% DMSO, 6% pentastarch and 4% human serum albumin in Plasma-Lyte A (Baxter Healthcare Corporation, Deerfield, IL) using a controlled rate freezer (Kryosave, Integra, Planer plc, Sunbury-on-Thames, UK) and stored in the vapor phase of a liquid nitrogen tank under continuous monitoring. All BMSC products administered to patients met lot release criteria.8 The cryopreserved BMSCs were thawed, diluted with 20 ml of Plasma-Lyte A. Post-thaw the BMSCs were counted manually and their viability was assessed by trypan blue staining. Cells were administered within 4 hours through a central line weekly at a targeted dose of 2× 106 viable cells per kilogram for a total of 3 doses.
Luminex assays
Measurement of all cytokines was performed by an immune-bead-based multiplex assay (Luminex) according to the manufacturer’s instructions. Panels of capture antibody-coated beads and labeled detection antibodies were purchased from Affymetrix Inc. Assay sensitivity varied from 0.1 to 12.44 pg/mL, depending on the analytes. The Luminex panel includes factors of growth, angiogenesis, homing, inflammatory, GVHD biomarkers, and chemokines as detailed in Supplemental Table 1.
ELISA assays
Elafin and tumor necrosis factor receptor 1 (TNFR1) antibody pairs were purchased from R&D Systems (DuoSet) and measurements were performed according to the manufacturer’s protocol with a plasma dilution of 1:25 and 1:20. Regenerating islet-derived 3-α (REG 3α) ELISA kit was purchased from MBL International (Ab-Match Assembly Human PAP1 kit and Ab-Match Universal kit), and measurements were according to the manufacturer’s protocol with a plasma dilution of 1:10 to 20. Cytokeratin fragment 18 ELISA kit was purchased from Peviva AB (M30 Apoptosense ELISA) and measurements were performed according to the manufacturer’s protocol with a plasma dilution of 1:25. Samples and standards were run in duplicate, absorbance was measured at 450 nm determined using a reader of Perkin Elmer Co. The absorbance data were analyzed using Excel. REG 3α and TNFR1 were significantly higher in patients 2, 3, 4, 6, 10 who mainly manifested gastrointestinal GVHD than the 6 controls (p=0.027 and p=0.008). CK18 was significantly higher in patients 1, 2, 4, 5, 6, 7, and 8 who developed liver GVHD or tissue injury (p=0.044) and Elafin was higher in patients 1, 3, 6, 8 who developed acute or chronic skin GVHD than the 6 controls (p=0.027, Figure S1).
Flow cytometry
Protocol-specified immunophenotypic analyses were performed. Cells were stained with fluorochrome-conjugated monoclonal antibodies as detailed in Supplemental Table 2. T regulatory cells (Treg) were defined as CD3+CD4+FoxP3+CD25med-highCD127low and thymus-derived natural T-reg (nTreg) were also Helios+. CD4 (and CD 8) central memory cells were defined as CD3+CD4+CD197+CD27+CD45RO+ and CD3+CD8+CD197+CD27+CD45RO+. Acquisition and analysis were performed with the use of the Fortessa flow cytometer (BD Biosciences) and FlowJo Version 8.8.6 software (TreeStar).
Statistical Analysis
Two-way hierarchical cluster analysis was performed on log-transformed cytokine levels of patients’ plasma samples using Ward’s method (JMP version 10.0.0, SAS Institute, Cary, NC). Cell plot graphs were made based on the hierarchical cluster analysis to show the changes of selected cytokines/biomarkers in an order of time points post BMSC infusion. Student t-test was used to compare the characteristics between the controls and the patients who received the BMSC therapy. It was also used to compare the expression of cytokine and biomarker profiles among the BMSC trial patients and the controls, and the expression of cytokines, biomarkers, and T cell phenotyping markers between patients with GVHD who achieved CR after BMSC therapy or not. Differences were considered to be significant at P less than .05. All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Prism Software Inc., San Diego, CA).
Results
Patient Characteristics
Six males and 4 females with a median age of 38 years were enrolled in the study. Primary diseases included 5 patients with acute leukemia (3 AML and 2 ALL), myelodysplastic syndrome (1), severe aplastic anemia (1), diffuse large B cell lymphoma (1), chronic granulomatous disease (1), and cutaneous T cell lymphoma (1). The primary indication was acute GVHD in 9 patients and tissue injury in one. Secondary indications were marrow failure in two and tissue injury in one. The organs affected by acute GVHD were GI in patients 2, 3, 4, 6, 9 and 10; liver in patients 2, 4, 5, 6, and 7 and skin in patients 3, 6, and 8. Additionally, patient 1 had extensive sclerotic skin chronic GVHD. The indication for BMSC in the two remaining patients was pneumothorax, pneumomediastinum, pneumoperitoneum, pneumatosis coli (patient 1) and idiopathic pulmonary syndrome and pneumomediastinum (patient 7). Six out of the 10 patients received myeloablative conditioning regimen, versus 4 with non-myeloablative regimens. The median time from transplant or donor lymphocyte infusion to the first BMSC infusion and the onset of symptoms (acute GVHD or tissue injury) was 58.5 days (range 24–1521) and 26 days (range 15–1511) respectively. Patient characteristics are shown in Table 1.
Table 1.
Patients characteristics
| UPN | Age/Sex | Primary indication |
Secondary indication |
Primary disease |
Transplant Type |
Days post SCT/DLI on starting MSC trial |
Days of onset of symptoms post SCT |
Primary indication response |
Secondary indication response |
Response duration (days) |
New CMV rectivation |
Days to death or survival |
Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23M | pneumoperitoneum, pneumomediastinum, severe extensive cGVHD | None | SAA | MRD/non-MAC | 1521 | 1511 | Complete | N/A | 190 | no | 190 | Death MOF |
| 2 | 22M | Upper and lower GI GVHD(G3), liver GVHD (2) | BM Failure | ALL | MRD/MAC | 58 | 30 | Complete | No response | 133 | yes | >401 | Alive |
| 3 | 56M | Lower GI GVHD G4 | None | DLBCL | MRD/non-MAC | 139/45 | 115/21 | No response | N/A | N/A | no | 108 | Death Relapse |
| 4 | 48M | Upper and Lower GI GVHD G3 | BM Failure | ALL | MRD/MAC | 59 | 15 | Complete | No response | 66 | no | >320 | Alive |
| 5 | 53F | Liver GVHD G4 | None | AML | MRD/MAC | 127 | 89 | Partial | N/A | N/A | no | 17 | Death, MOF, Sepsis |
| 6 | 49F | Upper and lower GVHD (G2) | None | MDS-RCMD transformed from SAA | MRD/MAC | 30 | 16 | Complete | N/A | >300 | no | >300 | Alive |
| 7 | 22M | Liver GVHD G3 | IPS and pneumomediastinum | AML | MRD/MAC | 51 | 22 | Complete | Complete | >293 | yes | >293 | Alive |
| 8 | 20M | GI GVHD G3 and skin GVHD G4 | None | CGD | MUD/non-MAC | 350/253 | 145/48 | Partial | N/A | N/A | no | 49 | Death Sepsis |
| 9 | 28F | Lowcr GI GVHD G2 | None | AML | MRD/MAC | 24 | 17 | Complete | N/A | >222 | no | >222 | Alive |
| 10 | 71F | Lower GI GVMD, G3 | None | CTCL | MUD/non-MAC | 240 | 126 | N/A | N/A | N/A | N/A | 12 | Death Hospice |
BMSC Product Characteristics
BMSCs derived from 4 healthy volunteers provided 28 infusions given to the 10 subjects. The healthy volunteers were a 27.2-year old African American male, a 21.7-year old White male, a 23.7-year old White female and a 27.6-year old African American male. The second and third infusions were derived from the same donor as the first infusion. All infusions achieved the target dose of 2 × 106 BMSC/kg recipient weight. Nine subjects received all three planned infusions but subject 10 opted for hospice in the first week after her first MSC infusion and withdrew from the study. BMSCs were characterized by high expression (>99.8%) of CD 73, CD90, CD105 and CD146, low expression (<2%) of CD34, CD45, CD14, CD11b, and with viability ≥95%.
Safety
The study met the primary objective of safety. Protocol defined treatment related serious adverse event (SAE) was encountered by none of the subjects. Infusional reactions were seen in only one subject (grade 1 pruritus). There was one death while on study (gram negative bacterial sepsis). There were 7 serious adverse events bacterial infection (5), gastrointestinal bleeding bleeding (1) and a mucus plug in the respiratory tract (1). In addition, there were 13 non-serious AEs: grade 1 (7), grade 2 (4) and grade 3 or 4 (2). None of these adverse events were attributable to the BMSC product. The HLA antibody screen remained negative prior to and immediately after completing the BMSC trial. New CMV reactivation without the development of CMV organ diseases occurred in patients 2 and 7, controlled by preemptive antiviral therapy.
Response and relationship to biomarkers
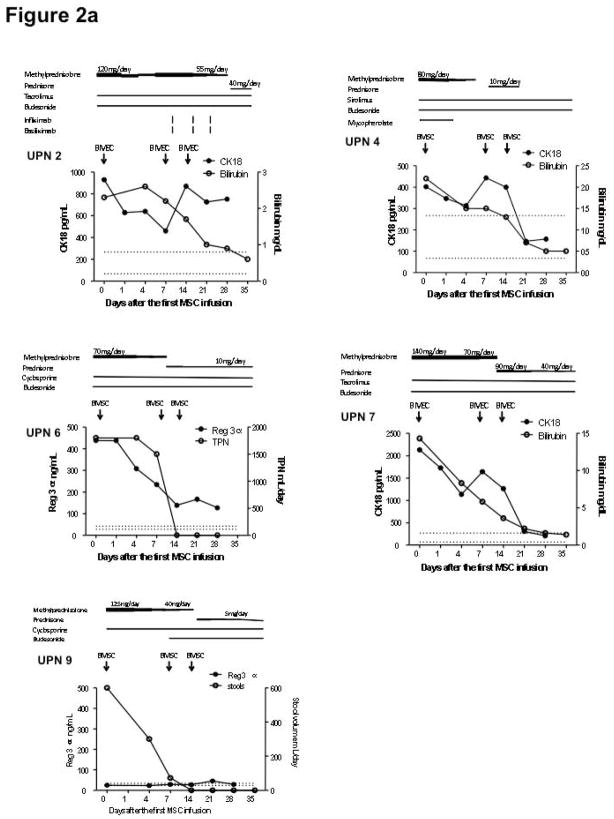
10 subjects were treated for primary or secondary indications of acute GVHD (9), tissue injury (2) or marrow failure (2). Eight were evaluable for response at day 42 (one withdrawal and one death while on study). Of the 7 evaluable subjects with acute GVHD, 5 were complete responders and 2 were non-responders. In addition, both subjects with tissue injury were GVHD.7, 9, 10 Figure 2a shows the course of 5 patients with steroid-refractory acute GVHD, who complete responders and both subjects with marrow failure were non-responders (Figure 2). To correlate clinical observations with biomarkers of response to BMSC therapy, we monitored relevant acute GVHD biomarkers: Reg 3α: (GI GVHD), CK18: (liver GVHD), and Elafin: (skin GVHD) while CK18 (epithelial apoptosis) was used as a dual marker of tissue injury and achieved CR after receiving BMSC treatment. Patients 2, 4, and 7 demonstrated improved liver function associated with decreased CK18 levels. Patient 6 improved symptoms of anoxia and nausea, resumed diet and discontinued total parenteral nutrition (TPN) with notable decrease in Reg 3α. Patient 9 had similar levels of Reg 3α to the controls throughout the BMSC trial. This patient had a significant reduction in stool output and improved abdominal pain prior to entering the trial and subsequently achieved CR after 3 doses of BMSC. Two of the 6 patients who achieved CR had recurrent acute GVHD on days 66 and 133 after initiating the BMSC treatment. This phase 1 protocol did not permit retreatment with BMSC.
Figure 2.
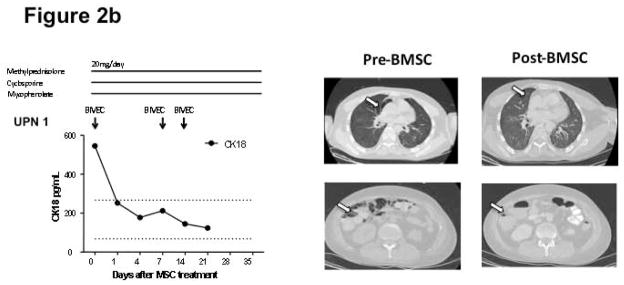
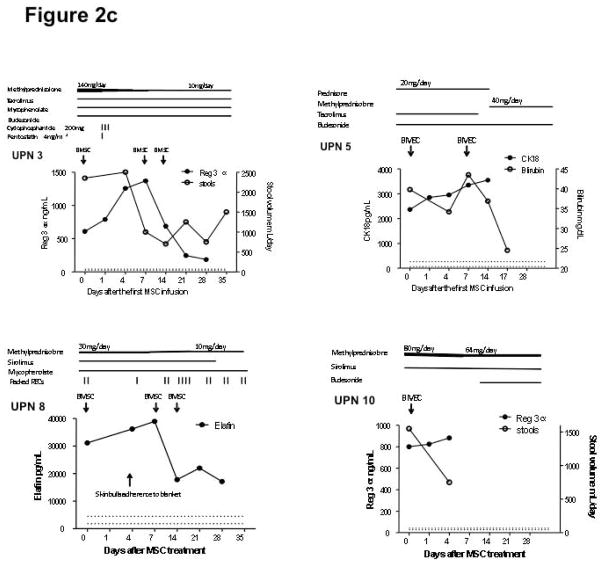
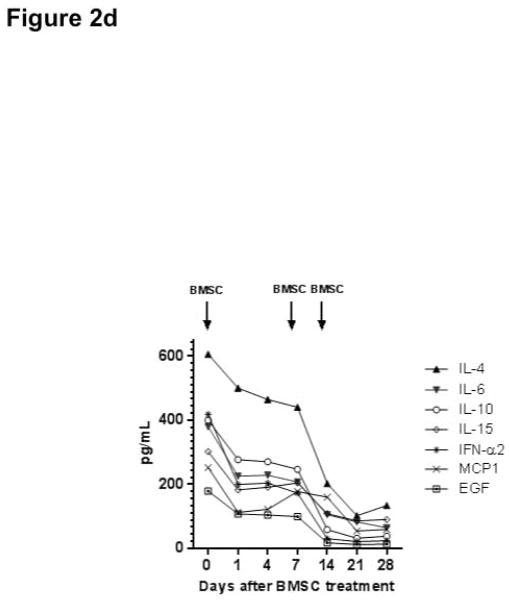
Responses to bone marrow mesenchymal stromal cell (BMSC) therapy. The dosages of tacrolimus and sirolimus were adjusted to a target level 10–15 ng/mL. The dose of cyclosporine was adjusted to a target level of 100–200 mcg/L. The dose of budesonide was 3 mg three times daily and mycophenolate 1000 mg twice daily. Figure 2a shows that the 5 patients with steroid-refractory acute GVHD achieved complete remission after receiving BMSC treatment. Figure 2b shows patient 1 achieved complete resolution of tissue injury after BMSC treatment. Arrows indicate air-filled areas resolving after MSC infusion. Figure 2c shows the 4 patients who did not achieve a response after BMSC treatment. Abbreviation: REG 3α, Regenerating islet-derived 3-α, CK18, Cytokeratin fragment 18, TPN: total parenteral nutrition. Figure 2d shows the rapid changes in cytokines in a responding subject (UPN#6)
Figure 2b demonstrates a complete resolution of diffuse pneumothorax, pneumomediastinum, pneumoperitoneum, pneumatosis coli in patient 1 together with declining levels of CK18. Figure 2c describes 4 patients with PR or NR after BMSC treatment. Patient 3 had a significant decrease in Reg 3α, but continued to have diarrhea at the end of the trial. Patient 5 had partial improvement on liver function, but succumbed to sepsis and multiple organ failure on day 17, which correlated with persistent high levels of CK18. Patient 8 with refractory gastrointestinal hemorrhage and late onset acute skin GVHD achieved notable decrease in bloody stools, but continued to require 2.5 units RBCs/week. The skin lesion on this patient’s back, from grade 4 acute skin GVHD, was further injured on day 4 due to adherence to a blanket which required surgical skin debridement. His skin lesion was improved following dose 2 and 3 BMSC infusions, along with the improvement on the levels of Elafin. Patient 10 opted for hospice care on day 4 and died on day 12 after the first dose of BMSC.
All patients with GVHD had received additional immunosuppressive agents in association with oral or intravenous steroid treatment for GVHD at the trial entry, and were refractory to high dose steroids. Five patients received one other agent, 3 received 2 other agents one received three other agents and one received four other agents (see fig 2). Immunosuppressive therapy was tapered in 8 patients. The immunosuppressive dose was unchanged in patient 1 (cGVHD), and patient 5 required increasing corticosteroids therapy and died on day 17 after the first BMSC infusion. Patient 4 required additional treatment with infliximab and basiliximab following the development of pneumatosis of the cecal wall after receiving 1 or 2 mg/kg of methylprednisone for more than 4 weeks. This bowel wall pneumotosis occurred on day 9 after the first BMSC infusion and was completely resolved on day 29. BMSC infusions had no impact on the two patients with bone marrow failure.
For responders with GVHD, cytokine responses were rapid, within 24 hours of the first BMSC infusion. Figure 2d shows a representative subject (UPN 6).
The comparison of GVHD complete responders with non-responders is summarized in Table 2. Complete responders tended to be younger, have less severe and early onset diseases and higher numbers of absolute lymphocytes, and CD4 and CD8 central memory cells at trial entry. However, this difference was not statistically significant (p>0.05). Throughout the period of observation GVHD complete responders continued to have higher numbers of absolute lymphocytes, CD4 and CD8 central memory cells, but again these changes were not statistically different from non-responders (p>0.05, Figure S2).
Table 2.
Comparison of patients with GVHD complete responder (CR) vs non-CR
| GVHD CR (5) | GVHD non-CR (4) | |
|---|---|---|
| Age | 28 (22–49) | 37 (20–71) |
| GVHD grade I–II | 2 | 0 |
| III–IV | 3 | 4 |
| Days post SCT | 51 (24–59) | 190 (127–350) |
| Lymphocyte count at entry (/μL) | 190 (80–530) | 115 (30–210) |
| CD4 central memory cells (%CD4) | 15.7 (0.41–18) | 8.27 (0.36–12) |
| CD8 central memory cells (% CD8) | 2.79 (0.2–11) | 1.73 (0.56–3.66) |
| Survival (days) | >300 (>222–>401) | 33 (12–108) |
Survival
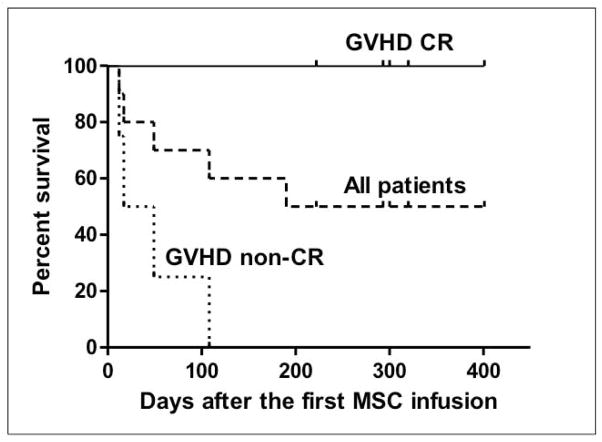
Actuarial survival is shown in Figure 3. Five out of 6 complete responders were alive with a median follow-up of 300 days (range 222–401) from the first BMSC infusion and 344 days (range 246–459) from the SCT. Three other evaluable patients who achieved PR or NR died at a median of 49 days (range 17–108) after the first BMSC infusion. The causes of death were multiple organ failure and sepsis (patients 5 and 8). Multiple organ failure and severe malnutrition on day 190 after entering the BMSC trial despite response to pneumomediastinum (patient 1). Patient 3 died from relapsed CTCL, and patient 10 opted for hospice care.
Figure 3.
Survival in 10 patients who received bone marrow mesenchymal stromal cells (BMSC). Upper lane: 5 subjects with GVHD who achieved complete remission (patients # 6,7,2,4,9); middle lane: all 10 patients; lower lane: 4 subjects with GVHD who did not achieve a complete remission (patients # 5,8,1,3,10)
Cytokine profiles
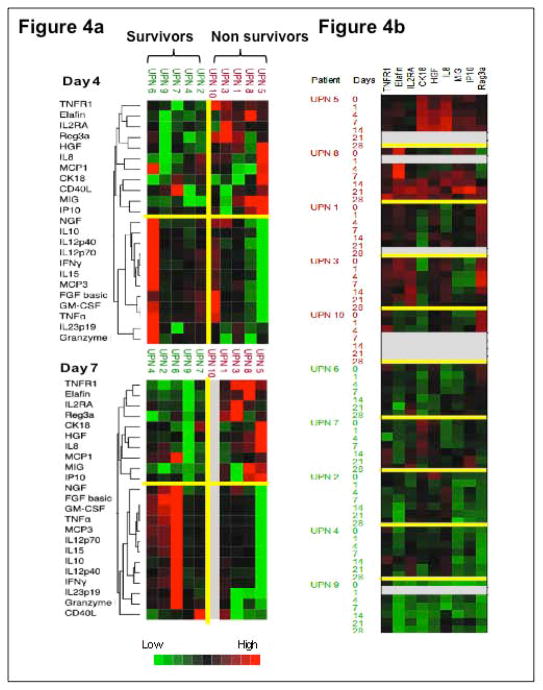
Figure 4 summarizes the cytokine/biomarker profiles of 10 patients who received BMSC treatment. Among all the time points when plasma samples were collected, a panel of cytokines and GVHD biomarkers was able to cluster patients into 2 distinct groups on day 4 and 7 after entering the BMSC trial (Figure 4a). The first group included patients 6, 9, 7, 4, 2 who survived at the time of censored and the second group included patients 10, 3, 1, 8, 5 who died within 12 to 190 days after entering the BMSC trial. In general, the levels of TNFR1, Elafin, IL2RA, Reg3α, HGF, IL-8, MCP1, CK18, CD40L, MIG, and IP10 in the first group were lower than those of the second group; while the levels of NGF, IL-10, IL-12p40, IL-12p70, IFN-γ, IL-15, MCP3, FGF basic, GM-CSF, TNFα, IL-23p19, and Granzyme B were higher than the second group, as shown in the color scale (Figure 4a). Furthermore, when we made a cell plot with all the time points together (Figure 4b), we found the levels of TNFR1, Elafin, IL2RA, CK18, IL-8, MIG, IP10, and Reg3α were generally up-regulated in a group of patients 5, 8, 1, 3, 10, which was associated with 100% mortality and down-regulated in a second group of patients 2, 4, 6, 7, 9 which was associated with 100% survival (p<0.05), consistent with Figure 4a.
Figure 4.
Cytokine/biomarker profiles in 10 patients who received bone marrow mesenchymal stromal cells (BMSC) treatment. Figure 4a shows the cytokine profiles on days 4 and 7 after the first dose of BMSC infusion. Two-way hierarchical cluster analysis was performed on log-transformed cytokine levels of patients’ samples using Ward’s method. The dendrogram on the left side of the figure classifies 2 clusters according to the proximities of cytokines. Two groups of patients (the dead or the alive) were separated based on their cytokine profiles. Figure 4b shows the cell plot of selected cytokines at all time points. The upper panel includes patients 5, 8, 1, 3, and 10 who showed up-regulated cytokines and GVHD biomarkers who had early mortality; the lower panel incudes patients 6, 7, 2, 4, and 9 who had prolonged survival showed down-regulated cytokines and GVHD biomarkers. Color scales represent cytokine levels (red indicates high levels; green, low levels). Gray indicates that the sample was not assayed for that protein. Color characters beside the plot represent patients’ survival status (red indicates the dead; green, the alive) and days post BMSC infusion.
Lymphocyte phenotyping
There was no difference in the total T regulatory population between patients who responded the BMSC treatment or not (data not shown). CD4 and CD8 central memory cells were persistently higher in patients with GVHD who achieved CR after BMSC treatment than patients who did not, but this was not statically significant (p>0.05, Figure S2)
Discussion
Following allogeneic SCT for hematological malignancies relapse of the disease and transplant related mortality (predominantly from GVHD) contribute equally as causes of treatment failure. Despite a progressive fall in TRM, organ failure and steroid refractory GVHD account for a significant proportion of transplant-related deaths. Steroid refractory GVHD has been reported to have a survival rate of only 17% at 2 years.11 The dual properties of BMSC to suppress alloresponses and the promote tissue repair make them promising and novel therapeutic options for a broad spectrum of post SCT complications. To explore the therapeutic potential of a new BMSC product and understand the mechanism of action we conducted a comprehensive analysis of patients with steroid-refractory GVHD and tissue injury treated with third-party BMSC infusions after allo-SCT. There is some data that early passage BMSC are more effective at controlling GVHD than later passages.12 For this reason the BMSC generated at the NIH Clinical Center were expanded for only three passages. The infusion schedule of doses of 2×106 BMSC/kg weekly followed previously used schedules effective for controlling GVHD. We chose to evaluate BMSC infusions in patients with post transplant complications after SCT (including steroid-refractory GVHD) that are associated with a high mortality. To better define severity of organ damage at onset and after BMSC infusion we used a series of biomarkers and cytokine profiles in order to temporally relate BMSC infusion with response. We also measured inflammatory markers and lymphocyte phenotype to identify immune changes induced by BMSC.
Consistent with the tolerogenic nature of third party BMSC in the SCT setting 13 we found BMSC infusion was well tolerated without acute infusional side effects or activation of HLA alloimmunization. There is a theoretical risk that BMSC could induce immunosuppression and promote viral reactivation. However only two patients reactivated CMV during the study period, a figure comparable to the incidence in a historical cohort of patients affected by steroid-refractory GVHD and treated with conventional immunosuppression. 14, 15 Despite the fact that many of the patients treated on this phase 1 study had poor performance status and multiple complications, we observed clinically significant responses in GVHD and in one patient whose primary indication for BMSC was tissue injury. It was notable that clinical responders showed a prompt fall within days of the first infusion in biomarkers of GVHD and tissue injury, strongly associating BMSC infusion with the response. Furthermore responders to BMSC had a sustained benefit from infusion with a significantly superior survival median survival (344 days) compared with non-responders (49 days). These results are consistent with a previous study which reported significantly lower one year TRM in complete responders to BMSC therapy compared to partial and non-responders (37% versus 72%). 3 Two of our 7 patients achieved complete recovery from organ perforations after BMSC infusions consistent with other reports of tissue repair induced by BMSC. 6 In contrast to other more positive studies related to engraftment 16, BMSC had no apparent effect on bone marrow failure as we did not detect any BMSC-associated alterations regarding growth factor expression and blood count recovery in patients with bone marrow failure. However the small size and hererogeneity of the study group precludes any definitive conclusions about the efficacy of BMSC in this context.
To better understand the mechanism of action of BMSC we carried out extensive profiling of a variety of cytokines GVHD biomarkers and lymphocyte phenotyping. Notably biomarkers for tissue damage (CK18) and gut GVHD (Reg3a) consistently fell towards normal levels in responders. The cytokine array strongly correlated with disease activity after BMSC infusions in our study with rapid falls in plasma cytokines in responders. Immune alterations in the T-cell, B-cell, and NK-cell compartments of steroid-refractory GVHD patients up to 6 months have been reported recently by Jitschin et al. 1 Our findings taken together with those of Jitschin et al suggest that initial benefit from BMSC is related to an anti-inflammatory effect of BMSC with rapid increase in growth factors, which may promote tissue repair. Subsequently lymphocyte subsets changes and a rise in T regs 1 may provide more prolonged protection against GVHD. It should also be pointed out that we currently have no comparable data on weekly fluctuations in biomarkers in patients with or without GVHD not receiving BMSC. Further studies involving comprehensive biomarker analysis at regular timepoints post transplant are planned to define the patterns encountered associated with variations in post-transplant events and better interpret the effect of therapeutic interventions.
Although the study group was small, we identified distinct differences between GVHD responders and non responders following BMSC infusion. As previously reported, responders tend to be younger. We noted that responders were more likely to be treated early after transplant, had less severe GVHD grade and higher lymphocyte counts including central memory CD4 and CD8 cells. These findings suggest that BMSC may be more effective when the T cell immunity is more intact and less damaged by prolonged immunosuppression possibly because the therapeutic effect of BMSC may require lymphocyte intermediaries to provide the most beneficial tissue repair milieu. The distinct plasma cytokine and acute GVHD biomarker values, especially by day 7 following first BMSC infusion, readily segregated patients into responder and non-responder groups. GVHD survivors had lower expression levels of the acute GVHD biomarkers TNFR1, Elafin, IL2RA, Reg3α, HGF, IL-8, CK18, MIG, with a significantly higher pattern of expression of growth factors and immonomodulatory markers notably NGF, GM-CSF, FGF basic, IL-10, IL-12p40, IL-12p70, IFN-γ, IL-15. These patterns point to a dual mechanism of anti-inflammatory processes associated with growth factors promoting tissue repair.
Many questions concerning the optimization of BMSC to treat post transplant complications remain unanswered. While we followed a standard treatment schedule for BMSC infusion there is little to guide the selection of an optimum dose and timing of infusions. In future, biomarker studies may guide the timing of infusions and reinfusion of BMSC to prevent recurrence of GVHD for example. Our study further strengthens the importance of biomarkers and cytokine arrays to determine disease severity and predict and follow responses to BMSC in patients with acute GVHD. In future clinical trials with BMSC infusions, such monitoring could identify likely responders and non-responders providing opportunities for alternative treatment for better outcome. Finally, this study does not address the possibility that individual BMSC batches may have distinctly different therapeutic effects. To address this we are using expression arrays of BMSC to better define potency.17
In summary these results confirm that BMSC induce rapid clinical responses and biomarker normalization in patients with steroid-refractory GVHD and tissue injury. BMSC appeared ineffective in patients with more aggressive GVHD with lower lymphocyte counts. A relatively intact immune system with higher absolute lymphocyte counts and favorable cytokine and T cell phenotype patterns may be required for effective GVHD control by BMSC. Our study is limited by its small sample size; both the clinical findings and biomarker changes need to be confirmed in larger sample studies.
Supplementary Material
Figure S1. Comparison of GVHD biomarkers in relevant patients prior to bone marrow mesenchymal stromal cells (BMSC) infusion with the 6 controls, excluding patient 9 who had similar GVHD levels to controls throughout the trial. Regenerating islet-derived 3-α (REG 3α) and tumor necrosis factor receptor 1 (TNFR1) were used to compare patients 2, 3, 4, 6, 10 who mainly manifested gastrointestinal GVHD and the 6 controls (p=0.027 and p=0.008); Cytokeratin fragment 18 (CK18) was used to compare patients 1, 2, 4, 5, 6, 7, and 8 who developed liver GVHD or tissue injury (p=0.044) and the controls; Elafin was used to compare patients 1, 3, 6, 8 who developed acute or chronic skin GVHD (p=0.027) and the 6 controls.
Figure S2. Frequency of absolute lymphocyte count (ALC), CD4 and CD 8 central memory cells in patients with refractory GVHD who achieved complete remission (CR) or not (non-CR).
Acknowledgments
This study was supported in part by the Bone Marrow Stromal Cell Transplant Center. National Institute of Dental and Craniofacial Research, NIH
Footnotes
Conflict-of-interest disclosure: The authors declare no financial conflicts of interests.
Author Contributions:
F Yin, M Battiwalla and S Ito: Conception and design, Collection and assembly of data, Data analysis and interpretation, manuscript writing.
X Feng: Collection of data, analysis and interpretation, F Chinian: provision of study materials collection of data; JJ Melenhorst: Data analysis and interpretation, manuscript writing. E Koklanaris: collection of data, provision of study materials, conception and design, M Sabatino and D Stroncek: conception and design, collection of data, provision of study materials, manuscript writing; L Samsel: Data collection, analysis and interpretation; J Klotz provision of study materials and patient care, manuscript writing; N Hensel: provision of study materials, administrative support; P Robey: Administrative support, Manuscript writing; A Barrett: Conception and design, manuscript writing, final approval of manuscript.
References
- 1.Jitschin R, Mougiakakos D, Von Bahr L, et al. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem-cell transplantation. Stem Cells. 2013 doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 2.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 5.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and Safety of Ex-vivo Cultured Adult Human Mesenchymal Stem Cells (Prochymal(TM)) in Pediatric Patients with Severe Refractory Acute Graft-Versus-Host Disease in a Compassionate Use study. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 7.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–594. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatino M, Ren J, David-Ocampo V, et al. The establishment of a bank of stored clinical bone marrow stromal cell products. J Transl Med. 2013;10:23. doi: 10.1186/1479-5876-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris AC, Ferrara JL, Braun TM, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2011;119:2960–2963. doi: 10.1182/blood-2011-10-387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–3860. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westin JR, Saliba RM, De Lima M, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol. 2011;2011:601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Bahr L, Sundberg B, Lonnies L, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18:557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Sundin M, Barrett AJ, Ringden O, et al. HSCT recipients have specific tolerance to MSC but not to the MSC donor. J Immunother. 2009;32:755–764. doi: 10.1097/CJI.0b013e3181ab1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchini G, Dander E, Pavan F, et al. Mesenchymal stromal cells do not increase the risk of viral reactivation nor the severity of viral events in recipients of allogeneic stem cell transplantation. Stem Cells Int. 2012;2012:690236. doi: 10.1155/2012/690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan R, Chakrabarti S, Walsh T, et al. Improved survival in steroid-refractory acute graft versus host disease after non-myeloablative allogeneic transplantation using a daclizumab-based strategy with comprehensive infection prophylaxis. Br J Haematol. 2004;124:777–786. doi: 10.1111/j.1365-2141.2004.04856.x. [DOI] [PubMed] [Google Scholar]
- 16.Macmillan ML, Blazar BR, DeFor TE, et al. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant. 2009;43:447–454. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Jin P, Sabatino M, et al. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy. 2011;13:661–674. doi: 10.3109/14653249.2010.548379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of GVHD biomarkers in relevant patients prior to bone marrow mesenchymal stromal cells (BMSC) infusion with the 6 controls, excluding patient 9 who had similar GVHD levels to controls throughout the trial. Regenerating islet-derived 3-α (REG 3α) and tumor necrosis factor receptor 1 (TNFR1) were used to compare patients 2, 3, 4, 6, 10 who mainly manifested gastrointestinal GVHD and the 6 controls (p=0.027 and p=0.008); Cytokeratin fragment 18 (CK18) was used to compare patients 1, 2, 4, 5, 6, 7, and 8 who developed liver GVHD or tissue injury (p=0.044) and the controls; Elafin was used to compare patients 1, 3, 6, 8 who developed acute or chronic skin GVHD (p=0.027) and the 6 controls.
Figure S2. Frequency of absolute lymphocyte count (ALC), CD4 and CD 8 central memory cells in patients with refractory GVHD who achieved complete remission (CR) or not (non-CR).