Abstract
Increases in rates of individual leaf photosynthesis (P n) are critical for future increases of rice yields. A previous study, using introgression lines derived from a cross between indica cultivar Habataki, with one of the highest recorded values of P n, and the Japanese elite cultivar Koshihikari, identified four QTLs (qCAR4, qCAR5, qCAR8, and qCAR11) that affect P n. The present study examined the combined effect of qCAR4 and qCAR8 on P n in the genetic background of Koshihikari. The pyramided near-isogenic line NIL(qCAR4+qCAR8) showed higher P n than both NIL(qCAR4) and NIL(qCAR8), equivalent to that of Habataki despite being due to only two out of the four QTLs. The high P n of NIL(qCAR4+qCAR8) may be attributable to the high leaf nitrogen content, which may have been inherited from NIL(qCAR4), to the large hydraulic conductance due to the large root surface area from NIL(qCAR4), and to the high hydraulic conductivity from NIL(qCAR8). It might be also attributable to high mesophyll conductance, which may have been inherited from NIL(qCAR4). The induction of mesophyll conductance and the high leaf nitrogen content and high hydraulic conductivity could not be explained in isolation from the Koshihikari background. These results suggest that QTL pyramiding is a useful approach in rice breeding aimed at increasing P n.
Key words: Hydraulic conductance, leaf nitrogen content, Oryza sativa, photosynthesis, quantitative trait locus, stomatal conductance.
Introduction
Increasing the rates of leaf photosynthesis (P n) should increase the yield potential of rice (Oryza sativa L.), since P n affects dry matter production via photosynthesis within the canopy (Long et al., 2006; Murchie et al., 2009). The use of natural genetic variation in photosynthesis within species can be an effective strategy for crop improvement (Flood et al., 2011). Wide variations in P n among rice cultivars have been shown in a number of studies (Murata, 1961; Takano and Tsunoda, 1971; Cook and Evans, 1983; Yeo et al., 1994; Osada, 1995; Xu et al., 1997; Masumoto et al., 2004; Kanemura et al., 2007; Jahn et al., 2011). However, most of the natural genetic resources have yet to be tapped.
Quantitative genetics is useful in assessing the genetic factors underlying the variation in photosynthesis and in designing breeding programmes (Flood et al., 2011). The complete genome sequence of rice and many DNA markers are already available (IRGSP, 2005). Several advanced populations, including backcrossed inbred lines and chromosome segment substitution lines, have been developed to facilitate the genetic analysis of rice (Yamamoto et al., 2009; Fukuoka et al., 2010). Recent improvements in the quantitation of photosynthesis have reduced measurement times while maintaining accuracy in the field (Long and Bernacchi, 2003). These advances facilitate the identification of quantitative trait loci (QTLs) and isolation of the underlying genes. In recent studies, several QTLs for P n have been identified in rice (Teng et al., 2004; Hu et al., 2009; Takai et al., 2010; Gu et al., 2012), and one gene has been isolated (Takai et al., 2013).
The combination of multiple QTLs—QTL pyramiding—offers a straightforward and useful way for improving target traits in rice (Wang et al., 2012). To evaluate the precise effects of the combination of QTLs, it is necessary to develop near-isogenic lines (NILs), which carry a single target QTL in a unique genetic background to eliminate background noise, and to cross these NILs (Ashikari and Matsuoka, 2006; Hospital, 2009). However, there has been limited effort so far to develop NILs for rice P n (Gu et al., 2012) and no attempt to evaluate the effect of pyramiding QTLs on P n.
It is widely acknowledged that P n is closely related to leaf nitrogen content (LNC) in rice, because large amounts of N are invested in ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the primary CO2-fixation enzyme (Cook and Evans, 1983; Makino et al., 1992). Wide varietal differences in LNC have been observed even at the same rate of N application (Kanemura et al., 2007; Hirasawa et al., 2010). P n is also affected by the diffusion of CO2 from the atmosphere to the chloroplasts. Varietal differences in stomatal conductance (g s) have been observed even at a small vapour pressure deficit (Ohsumi et al., 2008; Hirasawa et al., 2010). Since g s decreases as leaf water potential decreases, the hydraulic conductance from roots to leaves (C p), which controls the water balance in plants, would affect the value of g s (Hirasawa et al., 1989; Hirasawa and Ishihara, 1992; Brodribb et al., 2007). Mesophyll conductance (g m), with respect to the diffusion of CO2 from the intercellular airspace to the chloroplasts, might also be important to improving rice P n (Makino, 2011). Recent studies report genetic differences in g m among Oryza species (Scafaro et al., 2011) and rice lines (Adachi et al., 2013). In addition to Rubisco content, LNC is associated with also g s and g m (Makino et al., 1988; Ishihara, 1995; Li et al., 2009). To clarify factors underlying the differences in P n among rice cultivars and lines, the influences of differences in LNC should be considered carefully.
The P n of young, newly expanded leaves ranges between ~20 and ~30 μmol CO2 m–2 s–1 among rice cultivars at an ambient CO2 concentration of 370–400 μmol mol–1 under light-saturating and unstressed conditions (Kanemura et al., 2007; Hirasawa et al., 2010; Jahn et al., 2011). The high-yielding indica cultivar Habataki has one of the highest recorded rates of P n among rice cultivars, at 30–33 μmol m–2 s–1 (Asanuma et al., 2008). In contrast, Koshihikari, the most popular cultivar in Japan, has a relatively low P n of 25–28 μmol m–2 s–1.
In previous studies using introgression lines derived from a cross between Koshihikari and Habataki, this study group identified four QTLs (qCAR4 on chromosome 4, qCAR5 on chromosome 5, qCAR8 on chromosome 8, and qCAR11 on chromosome 11), Habataki alleles of which increased P n (Sueyoshi et al., 2009; Adachi et al., 2011). The present work developed a pyramided line, NIL(qCAR4+qCAR8), by crossing NIL(qCAR4) and NIL(qCAR8), each of which has a single chromosome segment from Habataki substituted in the genetic background of Koshihikari, and quantified the effects on the rate of leaf photosynthesis and on processes related to photosynthesis.
Materials and methods
Plant materials
Four QTL-NILs carrying a single chromosome segment from Habataki in the genetic background of Koshihikari were developed (Fig. 1; Sueyoshi et al., 2009; Adachi et al., 2011). NIL(qCAR4), which carries a chromosome segment from Habataki on the long arm of chromosome 4, was crossed with NIL(qCAR8), with a segment from the short arm of chromosome 8, and plants homozygous for Habataki in both regions were selected, to create the line NIL(qCAR4+qCAR8). F2 progeny were used in field experiments and F3 progeny were used in pot experiments.
Fig. 1.
Graphical genotypes of Koshihikari and the quantitative trait loci near-isogenic lines. Black bars indicate regions homozygous for Habataki alleles. Values in boxes indicate lengths of substituted regions.
Cultivation of rice plants
Plants were grown at the university farm (35° 40′ N 139° 28′ E). In the field experiment, seedlings at the fourth-leaf stage were transplanted into the paddy field (alluvial clay loam) at a density of 22.2 hills m–2 (30×15cm), with one plant per hill. As a basal dressing, manure was applied at 15 t ha–1 and inorganic fertilizer was applied at 30kg N, 60kg P2O5, and 60kg K2O ha–1. One-third of the total N was applied as nitrogen sulphate, one-third as LP-50 elution-controlled urea (Chisso Asahi Fertilizer, Tokyo, Japan), and one-third as LPS-100 elution-controlled urea. No topdressing was applied.
In the pot experiment, plants were grown outdoors in 12-l pots filled with a 1:1 (v/v) mixture of paddy soil and upland soil (diluvial volcanic ash) at a density of three hills per pot, three plants per hill. Basal fertilizer was applied at 1.0g N, 1.0g P2O5, and 1.0g K2O per pot, and additional N was applied at 0.5g per pot at booting stage when the flag leaves had fully expanded. To examine the relationship between P n and LNC, different amounts of N were applied to Koshihikari and Habataki at booting stage: at 0.25, 0.5, 1.0 or 2.0g to Koshihikari and 0, 0.25 or 0.5g to Habataki.
Plants were also grown in 3-l pots in a growth chamber (Koitotron, Koito Manufacturing, Tokyo, Japan) under a 12/12 light/dark cycle (28/23 °C, relative humidity 60/80%, and a photosynthetic photon flux density (PPFD) at the top of the canopy of 1000 μmol m–2 s–1). Basal fertilizer was applied at 0.5g N, 0.5g P2O5, and 0.5g K2O per pot. No topdressing was applied.
Gas exchange measurements
Leaf gas exchange was measured with a portable gas-exchange system (LI-6400, LI-COR, Lincoln, NE, USA); flag leaves were measured at the full heading stage. The CO2 assimilation rate (A 370) and stomatal conductance (g s) were measured at an ambient CO2 concentration of 370 μmol mol–1, a PPFD of 2000 μmol m–2 s–1, a leaf-to-air vapour pressure difference of 1.3–1.6 kPa, and an air temperature of 30 °C. The CO2 assimilation rate at an intercellular CO2 concentration (C i) of 280 μmol mol–1 (A 280) was also measured by changing the ambient CO2 concentration. Plants were examined from 08:30 to 11:00, when the photosynthetic rate was close to the daily maximum (Hirasawa and Ishihara, 1992; Ishihara, 1995). Measurements were made at the full heading stage. The measurement for each leaf was conducted once a day and was repeated on day 2 or 3 and the mean of measurements calculated. Between five and seven leaves were used for each replicate.
Determination of nitrogen content and dry matter weight
The leaves were collected immediately after completion of the gas exchange measurements and were stored at –80 °C. The area of an 80-mm-long segment cut from the centre of the leaf was measured with a leaf area meter (AAM-9; Hayashi Denko, Tokyo, Japan). The segments were dried at 80 °C for 24h to determine the nitrogen content with a CN analyser (MT700 Mark II, Yanako, Kyoto, Japan) and the dry matter weight.
Determination of hydraulic conductance and conductivity
The hydraulic conductance of the plants grown in 3-l pots, from the soil through the roots to the leaves (C p, 10–8 m3 s–1 MPa–1) was calculated as U w/(Ψs–Ψl) (Hirasawa and Ishihara, 1991), where U w (10–8 m3 s–1) is the water uptake rate of the whole plant, Ψs (MPa) is the water potential of the soil immediately outside the root, and Ψl (MPa) is the average water potential of the uppermost three leaves. Since plants were grown under submerged conditions and the water potential of the soil solution was high compared with Ψl and was kept constant, Ψs could be regarded as 0. Measurements were made in a controlled-environment chamber (air temperature 28 °C, air vapour pressure deficit 1.5 kPa, PPFD at the top leaves 1000 μmol m–2 s–1). U w was determined from the rate of weight loss of the pot over 20min after a steady state had been reached. To prevent evaporation from the surface of the pot, the top was covered with polystyrene foam and the gap between the foam and the stem was sealed with oil clay. After measurement of U w, Ψl of the uppermost three leaves was measured in a pressure chamber (model 3005, Soil Moisture Equipment, Santa Barbara, CA, USA). Measurements were conducted under the steady-state condition where the transpiration rate is equal to U w. It is reported that the transpiration rate and g s do not influence hydraulic conductance when the transpiration rate is high (Fiscus, 1975; Hirasawa and Ishihara, 1991; Stiller et al., 2003). The U w per leaf area was sufficiently high (>2.0 mmol m–2 s–1) to eliminate the effect of the difference in water uptake rate on C p. After roots had been washed gently in water, the root surface area of the total root system (S r) was measured with an image analyser (Win-Rhizo REG V 2004 b, Regent, PQ, Canada). For comparing root hydraulic conductivity, the hydraulic conductivity of a plant (L p, 10–8 m s–1 MPa–1), defined as hydraulic conductance per root surface area (Steudle and Peterson, 1998).
Results
Leaf photosynthesis
The CO2 assimilation rate at an ambient CO2 concentration of 370 μmol mol–1 (A 370) in the four NILs were significantly higher than that in Koshihikari—by 23% in NIL(qCAR4), 11% in NIL(qCAR5), 17% in NIL(qCAR8), and 9% in NIL(qCAR11)—and significantly smaller than that in Habataki—7, 21, 12, and 13%, respectively (Fig. 2). In the field experiment, A 370 in NIL(qCAR4+qCAR8) was higher than in both NIL(qCAR4) and NIL(qCAR8) (Table 1), indicating that the combination of the two QTLs additively increased leaf photosynthesis. Interestingly, A 370 in NIL(qCAR4+qCAR8) was comparable to that in Habataki despite being due to only two out of the four QTLs. Similar results were obtained in the pot experiments.
Fig. 2.
Comparison of CO2 assimilation rates at a photosynthetic photon flux density of 2000 μmol m–1 s–1 and an ambient CO2 concentration of 370 μmol mol–1 (A 370) of Koshihikari, Habataki, and the quantitative trait loci near-isogenic lines in the paddy field. Measurements for each panels were conducted in different field plots. Data are mean±SD (n=3–5). Values above bars are percentages relative to Koshihikari. Different letters above bars indicate significant differences (P<0.05, Tukey’s test).
Table 1.
CO2 assimilation rate at a PPFD of 2000 μmol m–1 s–1 and an ambient CO2 concentration of 370 μmol mol–1, leaf nitrogen content, dry matter weight per leaf area, and stomatal conductance of plants grown in the field and in 12-l potsValues are means±SD (n=3–6). Different superscript letters indicate significant differences between rice lines (P<0.05, Tukey’s test). Values in parentheses are percentages relative to Koshihikari. A 370, CO2 assimilation rate at an ambient CO2 concentration of 370 μmol mol-1; g s, stomatal conductance; LMA, dry matter weight per leaf area; LNC, leaf nitrogen content.
| A 370 (μmol CO2 m–2 s–1) | LNC (g m–2) | LMA (g m–2) | g s (mol H2O m–2 s–1) | ||
|---|---|---|---|---|---|
| Paddy field | Koshihikari | 23.5±1.1c | 1.46±0.15b | 58.4±4.1b | 0.66±0.06c |
| NIL(qCAR4) | 29.0±0.1 a,b (123) | 1.85±0.06a (127) | 66.7±1.8a (114) | 0.82±0.07 b,c (124) | |
| NIL(qCAR8) | 27.4±1.4b (117) | 1.59±0.15 a,b (109) | 59.1±1.2b (101) | 0.85±0.06 b,c (129) | |
| NIL(qCAR4+qCAR8) | 31.0±1.2a (132) | 1.81±0.17a (124) | 65.2±0.4a (112) | 1.01±0.05b (152) | |
| Habataki | 31.3±0.9a (133) | 1.85±0.07a (126) | 57.6±0.5b (99) | 1.82±0.12a (274) | |
| Pots | Koshihikari | 22.5±1.5d | 1.35±0.06d | 55.8±1.1b | 0.55±0.05d |
| NIL(qCAR4) | 28.8±0.8b (129) | 1.67±0.11 b,c (124) | 63.6±2.8a (115) | 0.82±0.03b (151) | |
| NIL(qCAR8) | 26.1±0.8c (117) | 1.54±0.06c (115) | 57.4±1.1b (104) | 0.70±0.02c (128) | |
| NIL(qCAR4+qCAR8) | 31.0±0.5a (139) | 1.91±0.12a (142) | 64.2±3.1a (116) | 0.87±0.04b (159) | |
| Habataki | 31.5±1.8a (141) | 1.80±0.06 a,b (133) | 53.8±1.1b (97) | 1.81±0.15a (331) |
Leaf nitrogen content
In the field experiment, LNC was significantly higher in NIL(qCAR4) (by 27%) than in Koshihikari, but LNC in NIL(qCAR8) was similar to that in Koshihikari (Table 1). LNC in NIL(qCAR4+qCAR8) was higher than that in Koshihikari (by 24%) and similar to those in NIL(qCAR4) and Habataki. In the pot experiment, not only NIL(qCAR4) but also NIL(qCAR8) showed higher LNC than Koshihikari. LNC in NIL(qCAR4+qCAR8) was even higher than that in NIL(qCAR4) (Table 1).
Dry matter weight per leaf area
In the field experiment, dry matter weight per leaf area (LMA) in Habataki was similar to that in Koshihikari (Table 1). LMA was significantly higher in NIL(qCAR4) (by 14%) than in Koshihikari, but LMA in NIL(qCAR8) was similar to that in Koshihikari. LMA in NIL(qCAR4+qCAR8) was higher than that in Koshihikari (by 12%) and similar to that in NIL(qCAR4). Similar results were obtained in the pot experiment.
Stomatal conductance and hydraulic conductance
In the field experiment, g s was 24% higher in NIL(qCAR4) and 29% higher in NIL(qCAR8) than in Koshihikari (Table 1). It was 52% higher in NIL(qCAR4+qCAR8) than in Koshihikari and was higher than those in both NIL(qCAR4) and NIL(qCAR8), but still lower than that in Habataki. Similar results were obtained in the pot experiment.
C p was significantly higher in Habataki than in Koshihikari (Fig. 3A). It was significantly higher in NIL(qCAR4) (by 43%) and NIL(qCAR8) (by 40%) than in Koshihikari. It was 84% higher in NIL(qCAR4+qCAR8) than in Koshihikari, and was higher in NIL(qCAR4+qCAR8) than in NIL(qCAR4) (P=0.07) and NIL(qCAR8) (P=0.05), but still lower than in Habataki.
Fig. 3.
(A) Hydraulic conductance from roots to leaves (C p), (B) root surface area (S r), and (C) hydraulic conductivity (L p) of plants grown in 3-l pots. C p and S r are expressed on a per stem basis. Values are mean±SD (n=4). Values above bars are percentages relative to Koshihikari. Different letters above bars indicate significant differences (P<0.05, Tukey’s test).
C p is related to S r and L p (Steudle and Peterson, 1998). The high value of S r in Habataki contributed to the high C p in Habataki (Fig. 3A, B). S r in NIL(qCAR8) was similar to that in Koshihikari, while that in NIL(qCAR4) was higher (although not significantly) than that in Koshihikari (Fig. 3B). S r in NIL(qCAR4+qCAR8) was higher than that in Koshihikari and similar to that in NIL(qCAR4). L p in Habataki was lower than that in Koshihikari (Fig. 3C). L p in NIL(qCAR4) was similar to that in Koshihikari, while L p in NIL(qCAR8) was higher (although not significantly) than that in Koshihikari. L p in NIL(qCAR4+qCAR8) was higher than that in Koshihikari and similar to that in NIL(qCAR8). These results indicate that the elevated C p in NIL(qCAR4+qCAR8) resulted from simultaneous increases of S r, which may have been inherited from NIL(qCAR4), and L p, from NIL(qCAR8).
Relationship between P n and LNC
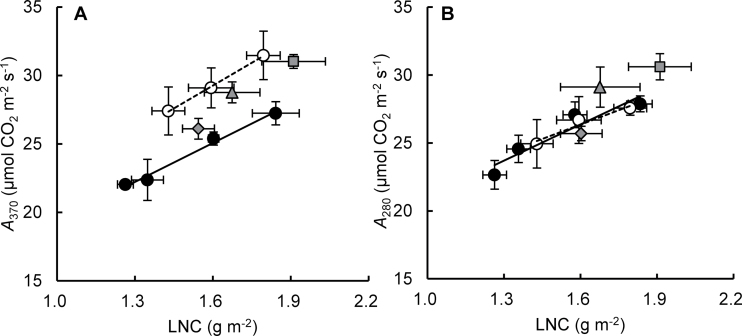
A 370 was always higher in Habataki than in Koshihikari at a given LNC and it increased with increases in LNC in both cultivars (Fig. 4A). A 370 was also higher in NIL(qCAR4), NIL(qCAR8), and NIL(qCAR4+qCAR8) than in Koshihikari at the same LNC. It is possible to estimate photosynthetic activity without considering the effect of g s by measuring P n at identical C i (von Caemmerer and Farquhar, 1981). There was no difference in A 280 between Koshihikari and Habataki at all levels of LNC examined (Fig. 4B). This indicates that the difference in A 370 was entirely due to the differences in LNC and g s between Koshihikari and Habataki (Supplementary Fig. S1 available at JXB online). A 280 in NIL(qCAR8) was similar to that in Koshihikari at the same LNC, while A 280 in NIL(qCAR4) and NIL(qCAR4+qCAR8) was higher than that in Koshihikari at given values of LNC. This indicates that factors other than LNC and g s are related to the high A 370 in both NIL(qCAR4) and NIL(qCAR4+qCAR8).
Fig. 4.
Relationships between leaf N content (LNC) and (A) CO2 assimilation rate at an ambient CO2 concentration of 370 μmol mol–1 (A 370) and (B) an intercellular CO2 concentration of 280 μmol mol–1 (A 280) in flag leaves of Koshihikari (filled circles), Habataki (open circles), NIL(qCAR4) (triangles), NIL(qCAR8) (diamonds), and NIL(qCAR4+qCAR8) (squares) grown in 12-l pots. The solid and broken lines indicate the regression lines for Koshihikari and Habataki, respectively. Values are mean±SD (n=4–6).
Discussion
Pyramiding of qCAR4 and qCAR8, which are QTLs for leaf photosynthesis, additively increased P n in rice. Similar approaches have yielded additive increases in number of grains (Ashikari et al., 2005; Ando et al., 2008), grain yield (Ohsumi et al., 2011; Wang et al., 2012), grain quality (Wang et al., 2012), plant height (Wang et al., 2012), and heading date (Shibaya et al., 2011; Wang et al., 2012). This is the first report to show that QTL pyramiding is effective at improving rice leaf photosynthesis.
Habataki has one of the highest recorded values of P n among rice cultivars, and at least four QTLs for leaf photosynthesis are associated with the difference in P n between Koshihikari and Habataki (Asanuma et al., 2008; Sueyoshi et al., 2009; Adachi et al., 2011). Because A 370 in NIL(qCAR4+qCAR8), with two of the four QTLs, was the same as in Habataki, with all four QTLs (Table 1), the possible reasons for the high A 370 need to be discussed from both genetic and physiological viewpoints.
Possible reasons for the high rate of leaf photosynthesis in the pyramided line
LNC strongly affects P n because it is closely related to the content of Rubisco (Makino et al., 1992). A difference in Rubisco content is a key factor in varietal differences in the capacity for leaf photosynthesis in rice (Hubbart et al., 2007; Hirasawa et al., 2010). In addition, the diffusion of CO2 from the atmosphere to the chloroplasts, which is regulated by g s and g m, is another important determinant (Ohsumi et al., 2008; Hirasawa et al., 2010; Makino, 2011). The high P n of Habataki might result from the high LNC, due to its elevated capacity for N accumulation, and from the high g s, due to the large hydraulic conductance of plants, in turn due to the large root surface area compared with Koshihikari (Adachi et al., 2011).
In the field experiment, the higher LNC in NIL(qCAR4+qCAR8) would have contributed to the higher A 370 than that in Koshihikari (Table 1). The high LNC may have been inherited from NIL(qCAR4), because the values of LNC in these two NILs were similar and reached the level of Habataki. In a preliminary study, NIL(qCAR5) and NIL(qCAR11) also showed higher LNC than Koshihikari (data not shown). This result suggests that at least three QTLs are involved in the difference in LNC between Koshihikari and Habataki. The large increase in LNC in NIL(qCAR4+qCAR8) and NIL(qCAR4) cannot be explained in isolation from the Koshihikari background: that is, there might be other QTLs, the Koshihikari alleles of which increase LNC, or genetic interactions between qCAR4 and other unknown QTLs. The detailed genetic mechanisms should be examined in future research. In the pot experiment, LNC in NIL(qCAR8) was also higher than that in Koshihikari. This suggests that qCAR8 is also associated with LNC under some growing conditions. The interaction between genes related to photosynthesis and the environment would be another subject to study.
The high g s in NIL(qCAR4+qCAR8) may also have contributed to the higher A 370 than in Koshihikari (Table 1). g s was additively increased by the combination of qCAR4 and qCAR8. Although g s in Habataki was significantly higher than that in NIL(qCAR4+qCAR8), a greater g s might not increase photosynthesis in rice further (Hirasawa et al., 1988), as Habataki had a much greater g s but no greater A 370. The critical water potential for stomatal closure is very much higher in rice than in other crop plants (Hirasawa, 1999). The value of g s in Koshihikari was already decreased by the reduction in leaf water potential because of its low C p even when the vapour pressure deficit was as low as ~1.5MPa, whereas the higher g s in Habataki was supported by the maintenance of higher leaf water potential through the higher hydraulic conductance (Adachi et al., 2011). This is clear because water-stress-relaxation treatments increased A 370 and g s in Koshihikari leaves to the same level as in Habataki at similar LNC but had no effect in Habataki leaves (Adachi et al., 2011). This means that rice g s is affected markedly by C p (Taylaran et al., 2011). Because the NILs in this research were derived from the cross between Koshihikari and Habataki and the measurement conditions of both A 370 and C p were exactly the same as in Adachi et al. (2011) and Taylaran et al. (2011), the connection between C p and g s may be the case with the rice lines in this research. The combination of NIL(qCAR4) and NIL(qCAR8) may have resulted in the higher C p in NIL(qCAR4+qCAR8) than in Koshihikari (Fig. 3A) and thus in the high g s. The even greater C p in Habataki might thus explain the far larger g s than that of NIL(qCAR4+qCAR8). These assumptions should be tested by further water-stress-relaxation experiments.
The high C p in NIL(qCAR4) is attributable to the high S r, whereas the high C p in NIL(qCAR8) is attributable to the high L p (Fig. 3B, C). The higher C p in NIL(qCAR4+qCAR8) seems to result from the combination of both. This result also suggests that it is possible to improve S r and L p simultaneously in rice breeding. C p was lower in NIL(qCAR4) than in Habataki on account of its smaller S r. This suggests that other QTLs are associated with the difference in S r between Koshihikari and Habataki. Although L p in Habataki was somewhat lower than that in Koshihikari, L p in NIL(qCAR8) was even higher than that in Koshihikari, which seems to be a result of the combination of the Habataki chromosome segment with the Koshihikari background. L p is controlled by the activity of aquaporins, the water-channel proteins of the cell membrane (Murai-Hatano et al., 2008; Sakurai-Ishikawa et al., 2011), but no open reading frame encoding an aquaporin is found in the region of qCAR8 (http://rapdb.dna.affrc.go.jp/). Isolating genes underlying qCAR4 and qCAR8 will provide useful information on how S r and L p are controlled in rice plants.
In addition to the high LNC and g s in NIL(qCAR4+qCAR8), another factor may be associated with the high A 370 in NIL(qCAR4+qCAR8) and might be inherited from NIL(qCAR4) (Fig. 4). With reference to the processes that control photosynthesis as mentioned above, this factor is likely to be g m. Generally, leaves with high LMA show high g m owing to the high total chloroplast surface area exposed to intercellular airspaces per leaf area (Hanba et al., 1999; Terashima et al., 2001; Montpied et al., 2009). LMA was significantly higher in NIL(qCAR4) and NIL(qCAR4+qCAR8) than in Koshihikari (Table 1). This result supports the possibility that the high g m of each NIL is associated with the high value of A 370. The result that LMA in Habataki did not differ from that in Koshihikari suggests that the high LMA in NIL(qCAR4) and NIL(qCAR4+qCAR8) is a result of the combination of the Habataki chromosome segment with the Koshihikari background. It is known that LMA is also related to LNC (Niinemets et al., 1999). However, the correlation coefficient between these two parameters in this study was not high (0.53 for paddy and 0.47 for pots), suggesting that the LNC was primarily controlled by factors other than LMA. This study cannot rule out the possibility that differences in the Michaelis–Menten constant, the maximum carboxylation rate, and the activation state of Rubisco are associated with the difference in A 370 (Ishikawa et al., 2011), although these traits seem to be similar among rice cultivars (Makino et al., 1987). Further studies should clarify which traits are related to the high A 370 in NIL(qCAR4) and NIL(qCAR4+qCAR8).
The flowering time of NIL(qCAR8) and NIL(qCAR4+qCAR8) was 7 days earlier than that of Koshihikari, while that of NIL(qCAR4) was similar to that of Koshihikari. This indicates that the genetic region of qCAR8 includes genes associated with flowering time. The causal link between flowering and photosynthesis is unknown at this time and should be examined in future research.
Potential for further enhancement of photosynthesis beyond Habataki
Despite having only two out of the four QTLs, NIL(qCAR4+qCAR8) had the same A 370 as Habataki. This phenomenon may be explained by the combined effect of the two Habataki chromosome segments in the Koshihikari genetic background. The process of photosynthesis is complex and is controlled by many genes (Shi et al., 2005). Its improvement might be a challenging task (Flood et al., 2011). However, the current results suggest that it is possible to increase P n to the highest level known by introducing a small number of genes from donor plants if the right combinations of cultivars are selected. It might be possible to develop a rice with a P n even higher than that of Habataki if the additional QTLs for P n on chromosomes 5 and 11 (Fig. 2) were stacked into NIL(qCAR4+qCAR8). To test this hypothesis, this study group is developing rice lines that carry multiple QTLs for P n in the genetic background of Koshihikari.
Conclusion
The P n of NIL(qCAR4+qCAR8) was as high as that of Habataki, even though the NIL had only two of the four known QTLs for P n. The high values of LNC, S r, and possibly g m found in NIL(qCAR4) and of L p in NIL(qCAR8) combined in NIL(qCAR4+qCAR8) to increase P n. The increases in some of these traits cannot be explained in isolation from the Koshihikari genetic background. These results suggest that QTL pyramiding is a powerful approach in breeding of rice for increased P n, and it should be possible to develop rice with even higher capacity for photosynthesis by stacking other QTLs into NIL(qCAR4+qCAR8).
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Relationships between leaf N content and stomatal conductance in flag leaves.
Acknowledgements
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (nos 23658014 and 25252007) and from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, QTL-1002).
References
- Adachi S, Nakae T, Uchida M, et al. 2013. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. Journal of Experimental Botany 64, 1061–1072 [DOI] [PubMed] [Google Scholar]
- Adachi S, Tsuru Y, Nito N, Murata K, Yamamoto T, Ebitani T, Ookawa T, Hirasawa T. 2011. Identification and characterization of genomic regions on chromosomes 4 and 8 that control the rate of photosynthesis in rice leaves. Journal of Experimental Botany 62, 1927–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Yamamoto T, Shimizu T, Ma XF, Shomura A, Takeuchi Y, Lin SY, Yano M. 2008. Genetic dissection and pyramiding of quantitative traits for panicle architecture by using chromosomal segment substitution lines in rice. Theoretical and Applied Genetics 116, 881–890 [DOI] [PubMed] [Google Scholar]
- Asanuma S, Nito N, Ookawa T, Hirasawa T. 2008. Yield, dry matter production and ecophysiological characteristics of rice cultivar, Habataki compared with cv. Sasanishiki. Japanese Journal of Crop Science 77, 474–480 [Google Scholar]
- Ashikari M, Matsuoka M. 2006. Identification, isolation and pyramiding of quantitative trait loci for rice breeding. Trends in Plant Science 11, 344–350 [DOI] [PubMed] [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. 2005. Cytokinin oxidase regulates rice grain production. Science 309, 741–745 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M, Evans L. 1983. Some physiological aspects of the domestication and improvement of rice (Oryza spp.). Field Crops Research 6, 219–238 [Google Scholar]
- Fiscus EL. 1975. The interaction between osmotic- and pressure-induced water flow in plant roots. Plant Physiology 55, 917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood PJ, Harbinson J, Aarts MGM. 2011. Natural genetic variation in plant photosynthesis. Trends in Plant Science 16, 327–335 [DOI] [PubMed] [Google Scholar]
- Fukuoka S, Nonoue Y, Yano M. 2010. Germplasm enhancement by developing advanced plant materials from diverse rice accessions. Breeding Science 60, 509–517 [Google Scholar]
- Gu J, Yin X, Struik PC, Stomph TJ, Wang H. 2012. Using chromosome introgression lines to map quantitative trait loci for photosynthesis parameters in rice (Oryza sativa L.) leaves under drought and well-watered field conditions. Journal of Experimental Botany 63, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Miyazawa SI, Terashima I. 1999. The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Functional Ecology 13, 632–639 [Google Scholar]
- Hirasawa T. 1999. Physiological characterization of the rice plant for tolerance of water deficit. In: Ito O, O’Tool J, Hardy B, eds, Genetic improvement of rice for water-limited environments. Los Baños, Philippines: International Rice Research Institute, pp 89–98 [Google Scholar]
- Hirasawa T, Iida Y, Ishihara K. 1988. Effect of leaf water potential and air humidity on photosynthetic rate and diffusive conductance in rice plants. Japanese Journal of Crop Science 57, 112–118 [Google Scholar]
- Hirasawa T, Iida Y, Ishihara K. 1989. Dominant factors in reduction of photosynthetic rate affected by air humidity and leaf water potential in rice plants. Japanese Journal of Crop Science 58, 383–389 [Google Scholar]
- Hirasawa T, Ishihara K. 1991. On resistance to water transport in crop plants for estimating water uptake ability under intense transpiration. Japanese Journal of Crop Science 60, 174–183 [Google Scholar]
- Hirasawa T, Ishihara K. 1992. The relationship between resistance to water transport and the midday depression of photosynthetic rate in rice plants. In: Murata N, ed, Research in photosynthesis , vol. IV Dordrecht, Netherlands: Kluwer Academic Publishers, pp 283–286 [Google Scholar]
- Hirasawa T, Ozawa S, Taylaran RD, Ookawa T. 2010. Varietal differences in photosynthetic rates in rice plants, with special reference to the nitrogen content of leaves. Plant Production Science 13, 53–57 [Google Scholar]
- Hospital F. 2009. Challenges for effective marker-assisted selection in plants. Genetica 136, 303–310 [DOI] [PubMed] [Google Scholar]
- Hu SP, Zhou Y, Zhang L, Zhu XD, Li L, Luo LJ, Liu GL, Zhou QM. 2009. Correlation and quantitative trait loci analyses of total chlorophyll content and photosynthetic rate of rice (Oryza sativa) under water stress and well-watered conditions. Journal of Integrative Plant Biology 51, 879–888 [DOI] [PubMed] [Google Scholar]
- Hubbart S, Peng S, Horton P, Chen Y, Murchie EH. 2007. Trends in leaf photosynthesis in historical rice varieties developed in the Philippines since 1966. Journal of Experimental Botany 58, 3429–3438 [DOI] [PubMed] [Google Scholar]
- IRGSP (International Rice Genome Sequencing Project). 2005. The map-based sequence of the rice genome. Nature 436, 793–800 [DOI] [PubMed] [Google Scholar]
- Ishihara K. 1995. Leaf structure and photosynthesis. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H, eds, Science of the rice plant 2. Physiology. Tokyo: Food and Agriculture Policy Research Center, pp 491–511 [Google Scholar]
- Ishikawa C, Hatanaka T, Misoo S, Miyake C, Fukayama H. 2011. Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiology 156, 1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn CE, Mckay JK, Mauleon R, Stephens J, McNally KL, Bush DR, Leung H, Leach JE. 2011. Genetic variation in biomass traits among 20 diverse rice varieties. Plant Physiology 155, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura T, Homma K, Ohsumi A, Shiraiwa T, Horie T. 2007. Evaluation of genotypic variation in leaf photosynthetic rate and its associated factors by using rice diversity research set of germplasm. Photosynthesis Research 94, 23–30 [DOI] [PubMed] [Google Scholar]
- Li Y, Gao YX, Xu XM, Shen QR, Guo SW. 2009. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. Journal of Experimental Botany 60, 2351–2360 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54, 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant, Cell and Environment 29, 315–330 [DOI] [PubMed] [Google Scholar]
- Makino A. 2011. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiology 155, 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. 1987. Variations in the contents and kinetic properties of ribulose-1,5-bisphosphate carboxylases among rice species. Plant and Cell Physiology 28, 799–804 [Google Scholar]
- Makino A, Mae T, Ohira K. 1988. Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase and the relationship to photosynthetic gas exchange. Planta 174, 30–38 [DOI] [PubMed] [Google Scholar]
- Makino A, Sakashita H, Hidema J, Mae T, Ojima K, Osmond B. 1992. Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance. Plant Physiology 100, 1737–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto C, Ishii T, Kataoka S, Hatanaka T, Uchida N. 2004. Enhancement of rice leaf photosynthesis by crossing between cultivated rice, Oryza sativa, and wild rice species, Oryza rufipogon . Plant Production Science 7, 252–259 [Google Scholar]
- Montpied P, Granier A, Dreyer E. 2009. Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. Journal of Experimental Botany 60, 2407–2418 [DOI] [PubMed] [Google Scholar]
- Murai-Hatano M, Kuwagata T, Sakurai J, Nonami H, Ahamed A, Nagasuga K, Matsunami T, Fukushi K, Maeshima M, Okada M. 2008. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant and Cell Physiology 49, 1294–1305 [DOI] [PubMed] [Google Scholar]
- Murata Y. 1961. Studies on the photosynthesis of rice plants and its culture significance. Bulletin of the National Institute of Agricultural Sciences D9, 1–169 [Google Scholar]
- Murchie EH, Pinto M, Horton P. 2009. Agriculture and the new challenges for photosynthesis research. New Phytologist 181, 532–552 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Tenhunen J, Canta N, Chaves M, Faria T, Pereira J, Reynolds J. 1999. Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Global Change Biology 5, 455–470 [Google Scholar]
- Ohsumi A, Hamasaki A, Nakagawa H, Homma K, Horie T, Shiraiwa T. 2008. Response of leaf photosynthesis to vapor pressure difference in rice (Oryza sativa L) varieties in relation to stomatal and leaf internal conductance. Plant Production Science 11, 184–191 [Google Scholar]
- Ohsumi A, Takai T, Ida M, Yamamoto T, Arai-Sanoh Y, Yano M, Ando T, Kondo M. 2011. Evaluation of yield performance in rice near-isogenic lines with increased spikelet number. Field Crops Research 120, 68–75 [Google Scholar]
- Osada A. 1995. Photosynthesis and respiration in relation to nitrogen responsiveness. In: Matuso T, Kumazawa K, Ishii R, Ishihara K, Hirata H, eds, Science of the rice plant 2. Physiology. Tokyo: Food and Agriculture Policy Research Center, pp 696–703 [Google Scholar]
- Sakurai-Ishikawa J, Murai-Hatano M, Hayashi H, Ahamed A, Fukushi K, Matsumoto T, Kitagawa Y. 2011. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell and Environment 34, 1150–1163 [DOI] [PubMed] [Google Scholar]
- Scafaro AP, von Caemmerer S, Evans JR, Atwell BJ. 2011. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant, Cell and Environment 34, 1999–2008 [DOI] [PubMed] [Google Scholar]
- Shi T, Bibby TS, Jiang L, Irwin AJ, Falkowski PG. 2005. Protein interactions limit the rate of evolution of photosynthetic genes in cyanobacteria. Molecular Biology and Evolution 22, 2179–2189 [DOI] [PubMed] [Google Scholar]
- Shibaya T, Nonoue Y, Ono N, Yamanouchi U, Hori K, Yano M. 2011. Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theoretical and Applied Genetics 123, 1133–1143 [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49, 775–788 [Google Scholar]
- Stiller V, Lafitte HR, Sperry JS. 2003Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiology 132, 1698–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Asanuma S, Tsuru Y, Murata K, Ebitani T, Ookawa T, Hirasawa T. 2009. Estimation and characterization of a quantitative trait locus on chromosome 11 for leaf photosynthetic rate in paddy rice. Japanese Journal of Crop Science 78 (Suppl. 1 ), 216–217 [Google Scholar]
- Takai T, Adachi S, Taguchi-Shiobara F, et al. 2013. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Scientific Reports 3, 2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Kondo M, Yano M, Yamamoto T. 2010. A quantitative trait locus for chlorophyll content and its association with leaf photosynthesis in rice. Rice 3, 172–180 [Google Scholar]
- Takano Y, Tsunoda S. 1971. Curvilinear regression of the leaf photosynthetic rate on leaf nitrogen content among strains of Oryza species. Japanese Journal of Breeding 21, 69–76 [Google Scholar]
- Taylaran RD, Adachi S, Ookawa T, Usuda H, Hirasawa T. 2011. Hydraulic conductance as well as nitrogen accumulation plays a role in the higher rate of leaf photosynthesis of the most productive variety of rice in Japan. Journal of Experimental Botany 62, 4067–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Qian Q, Zeng D, Kunihiro Y, Fujimoto K, Huang D, Zhu L. 2004. QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica 135, 1–7 [Google Scholar]
- Terashima I, Miyazawa SI, Hanba YT. 2001. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research 114, 93–105 [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 [DOI] [PubMed] [Google Scholar]
- Wang P, Xing Y, Li Z, Yu S. 2012. Improving rice yield and quality by QTL pyramiding. Molecular Breeding 29, 903–913 [Google Scholar]
- Xu YF, Ookawa T, Ishihara K. 1997. Analysis of the photosynthetic characteristics of the high-yielding rice cultivar Takanari. Japanese Journal of Crop Science 66, 616–623 [Google Scholar]
- Yamamoto T, Yonemaru J, Yano M. 2009. Towards the understanding of complex traits in rice: substantially or superficially? DNA Research 16, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo ME, Yeo AR, Flowers TJ. 1994. Photosynthesis and photorespiration in the genus Oryza . Journal of Experimental Botany 45, 553–560 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.