Abstract
Purpose
Several studies have identified relationships between weight loss and adipokine levels; however none have looked at the combined effect of aerobic exercise training with consumption of a low, or high glycemic diet. We examined the effects of 12-weeks of aerobic exercise combined with either a low- (GI: ~40, LoGIX) or high-glycemic (GI: ~80, HiGIX) diet on plasma leptin and adiponectin (total and high molecular weight - HMW) in 27 older obese adults (age 65±0.5 years; BMI 34.5±0.7 kg/m2).
Methods
Insulin sensitivity was calculated from an oral glucose tolerance test (ISIOGTT). Fasting HMW adiponectin and leptin were quantified from plasma samples obtained prior to the ISIOGTT. Glucose and insulin measures were obtained before and every 30 min during the test. Dual-energy X-ray absorptiometry and computerized tomography was used to determine body composition and to quantify subcutaneous and visceral abdominal adiposity, respectively.
Results
Fasting leptin was significantly decreased in both groups (LoGIX, pre: 33.8±4.7, post: 19.2±4.5; HiGIX, pre: 27.9±4.2, post: 11.9±2.2 ng/ml, P=0.004), and HMW adiponectin was significantly increased (LoGIX, pre: 1606.9±34.6, post: 3502.3±57; HiGIX, pre: 3704.8±38.1, post: 4284.3±52.8 pg/ml; P=0.003) following the 12-week intervention. Total body fat was reduced after both interventions, and visceral fat mass was inversely correlated with HMW adiponectin, while subcutaneous fat correlated with leptin.
Conclusions
The data suggest that exercise training, independent of dietary GI, favorably alters HMW adiponectin and leptin secretion, and that a reduction in visceral fat mass is a key factor regulating HMW adiponectin in older obese persons.
Keywords: adiponectin, leptin, aerobic exercise, glycemic diet
INTRODUCTION
Adipose tissue plays an active role in regulating energy homeostasis and this is partly achieved through secretion of various adipokines including leptin, adiponectin, resistin and visfatin (1, 14). Lower adiponectin levels in obesity are associated with chronic inflammation, endothelial dysfunction, enhanced oxidative stress and insulin resistance. More specifically, high molecular weight (HMW) adiponectin is the biologically active isoform that significantly correlates with insulin sensitivity and has been linked to inflammation. Recently, Kirwan et al (15) showed that a low glycemic index diet combined with exercise augments insulin sensitivity. Further, we have previously shown that aerobic exercise training combined with a low glycemic diet alleviates inflammation in older obese persons (13). However, the mechanistic link between inflammation and insulin sensitivity, and the role of glycemic index diets in combination with exercise is not known. Adiponectin may be a candidate for the aforementioned connection as it has been shown to regulate both lipid and glucose metabolism in skeletal muscle (18). Moreover, elevated leptin levels are also associated with obesity and insulin sensitivity and exert adverse pleiotropic effects on several organ systems including the heart, kidneys and sympathetic nervous system. Adiposopathy, a term that describes pathogenic adipocytes is characterized by adipocyte hypertrophy and adverse endocrine consequences that lead to the development of type 2 diabetes, hypertension and cardiovascular disease (1, 2).
Several studies have shown that both moderate and high intensity acute and short-term exercise (including one bout of exercise) alone improves insulin resistance (16, 31). Recently, we reported that 7-consecutive days of aerobic exercise improve insulin sensitivity, fat oxidation, and HMW adiponectin in obese individuals independent of changes in body weight (12, 31). Further, we found that insulin sensitivity was related to changes in adipokine secretion, specifically leptin and HMW adiponectin. Although there are data on the combined effects of diet and exercise on changes in leptin and improvements in insulin sensitivity, there is a paucity of data with respect to changes in HMW adiponectin. Previously, we found that 12 weeks of exercise training decreased plasma leptin (32), and altered the adiponectin multimer ratio, favoring an increase in HMW oligomer ratio as compared to middle molecular and low molecular weight isoforms (25) in obese humans. Further, we showed that older insulin resistant adults on a low-glycemic diet reduced pro-inflammatory cytokine secretion as compared to those on a high-glycemic diet (13). Cytokines that are known to target adipose tissue are also produced and secreted from adipocytes, inducing disruption in adipose tissue metabolism and function (1, 2, 8). Thus if a low-glycemic diet can attenuate cytokine production, a low-glycemic diet may alter adipose tissue function.
Although there are data to show that obesity is associated with abnormal leptin and adioponectin levels, there is a gap in the literature regarding the effects of a low glycemic diet combined with exercise on adiponectin and leptin levels in older obese insulin resistant adults. A eucaloric low glycemic diet has been shown to lower total fat mass and serum leptin levels in both overweight non-diabetic men (4), and insulin resistant subjects (40). In the Health Professional's Follow-up Study, a low dietary GI was associated with higher total plasma adiponectin concentrations (28). Furthermore, an 8-week hypocaloric diet increased circulating adiponectin in obese subjects (6). However, none of the previous studies examined the combined effects of aerobic exercise and a low glycemic diet on adipokines.
Thus the purpose of this study was to determine the effects of a low-glycemic diet and aerobic exercise intervention on changes in HMW adiponectin and leptin in older obese, insulin resistant adults. We hypothesized that: a) the effects of a low-glycemic diet plus exercise intervention would elicit greater improvements in circulating HMW adiponectin and leptin, and reduce insulin resistance compared to a high glycemic diet and exercise intervention; and b) increased insulin sensitivity would, be at least partially related to an increase in circulating HMW adiponectin concentrations.
METHODS
Subjects
Thirty-four older obese previously sedentary adults (age range 60-75, average age 65±0.5 years; BMI 34.5±0.7 kg/m2) were recruited from the local community to undergo a 12-week exercise training and diet intervention. Participants in this study were part of a larger randomized clinical trial and some of the data from these subjects were reported in related studies (13, 30). All volunteers underwent a medical history, physical exam, oral glucose tolerance test (OGTT), and complete blood profile (lipid profile, and hepatic/renal/hematological function tests). Individuals with heart, kidney, liver, thyroid, intestinal, and pulmonary diseases, or those taking medications known to affect the outcome variables were excluded. Screening also excluded those with any contraindications to physical activity highlighted during a resting twelve-lead electrocardiogram and a sub-maximal exercise stress test. Female subjects were postmenopausal and based upon self-report were not using hormone replacement therapy. Prior physical activity levels were recorded using the Minnesota Leisure Time Physical Activity (MLTPA) questionnaire (34); volunteers were deemed sedentary if their leisure time activity was below 300 kcal/day. Subjects were required to be weight stable for at least the previous six months. Individual total caloric requirements were determined by measurement of basal metabolic rate and application of a standard activity factor of 1.2, which is consistent with a sedentary lifestyle (30, 36). The study was approved by the Cleveland Clinic Institutional Review Board and all subjects provided signed informed written consent in accordance with guidelines for the protection of human subjects.
Intervention
Subjects were randomized to one of two groups; either low GI diet plus exercise (LoGIX), or high GI diet plus exercise (HiGIX). All volunteers undertook sixty minutes of aerobic exercise five days per week for 12-weeks. Exercise intensity was targeted at ~85% of the maximum heart rate obtained during an incremental maximal aerobic exercise test (VO2max test). Every session was fully supervised by an exercise physiologist. All meals for the 12-week intervention were provided to participants on a daily basis. Diets were designed by a registered dietitian and based on measured resting metabolic rate and activity as previously described (13, 30). Participants were in negative energy balance relative to the exercise session only; otherwise diets were isocaloric to the subjects’ individual requirements. The dietary macronutrient composition (including fiber) was matched between groups; however the diets were calculated so that the LoGIX subjects received a diet with an average GI of 40 units, while HiGIX subjects consumed foods with an average GI of 80 units as previously described (30). The primary difference between the two diets was the carbohydrate provided in order to achieve the glycemic index and load. An example of food substitution includes: white rice (HiGIX) versus barley or quinoa (LoGIX). Adherence to the diet was determined via daily food container weigh backs, plus a weekly counseling session with the study dietitian. Dietary analysis was performed using Nutritionist Pro software (Axxya Systems, Stafford, TX).
Body Composition
Height and body weight were measured by standard techniques (26). Whole body adiposity (fat mass [FM] and fat-free mass [FFM]) was measured by dual-energy X-ray absorptiometry (model iDXA; GE Healthcare - Lunar, Madison, WI). Computerized tomography scanning was used to quantify subcutaneous (both deep and superficial) and visceral abdominal adiposity with a SOMOTOM Sensation 16 Scanner (Siemens Medical Solutions, Malvern, PA), as previously described (26) .
Aerobic Fitness
An incremental, graded treadmill exercise test was used to determine maximal oxygen consumption (VO2max; Jaeger Oxycon Pro, Viasys, Yorba Linda, CA). Maximal heart rate recorded during this test was used to prescribe the correct exercise intensity during training as previously described (26). VO2max tests were also performed at weeks four and eight in order to maintain the appropriate exercise intensity corresponding to changes in participants’ aerobic fitness. To control for the acute effects of exercise, pre-intervention VO2max tests were conducted >48 h prior to metabolic measures.
Inpatient Control Period
Pre- and post-intervention assessments of body composition, aerobic fitness, glucose tolerance, and adipokines were performed during a 3-d in-patient stay in the Clinical Research Unit. During this period, participants received a weight maintenance eucaloric diet (total kcal/d = resting metabolic rate × 1.2; 55% carbohydrate, 28% fat, 17% protein) as previously described (30).
Oral Glucose Tolerance Test
A 75 g oral glucose tolerance test (OGTT) was performed after an overnight fast, pre- and post-intervention. Fasting baseline samples were drawn to determine initial glucose and insulin concentrations. Following baseline draws, a 75 g glucose drink was ingested within a 10-minute period. Blood samples were drawn at 30, 60, 90, 120 and 180 minutes after ingestion. Plasma glucose was determined immediately on a YSI 2300 STAT Plus analyzer (Yellow Springs, OH). The samples were stored at −80°C for subsequent substrate analysis. Plasma insulin was determined via radioimmunoassay (Millipore, Billerica, MA). Insulin sensitivity was determined using the Matsuda index (ISIOGTT) (23).
Adipokine Analyses
Baseline blood samples were collected after an overnight fast prior to the OGTT. All blood samples were measured in duplicate, and each participant's pre- and post-intervention samples were batch-analyzed. Plasma leptin, and total and HMW adiponectin were analyzed via ELISA (Millipore, Billerica, MA).
Statistics
Between-group (LoGIX vs. HiGIX) comparisons were analyzed using two-way (group × time) repeated measures ANOVA. Baseline values for each variable were compared between groups using student t-tests. Bivariate correlation analyses were used to identify relationships between variables. Statistical significance was accepted when P<0.05. Analyses were carried out using StatView for Windows 5.0.1 (SAS Institute, NC), and all data are expressed as mean ± S.E.M.
RESULTS
Subjects
Twenty-seven individuals (LoGIX, N=12, 6 female/6 male; HiGIX, N=15, 7 female/8 male) completed the 12-week intervention. Seven subjects withdrew from the study for personal reasons and/or inability to comply with the diet and adherence to the exercise program.
Diet and Exercise
Dietary analysis shows that diets for both groups were matched with respect to macronutrient composition, including fiber, with GI units of 40.1±0.2 and 80.1±0.5 units, respectively (Table 1). The glycemic responses to the study diets were confirmed and were reported in a separate publication (31). Diet and exercise compliance was high (~97%). Exercise was performed at 83.2±0.5% of HRmax, and following the study there was an increase in VO2max in both groups (LoGIX, pre: 39.3±1.6, post: 44.4±3.4; HiGIX, pre: 39.6±1.4, post: 45.7±1.8 ml.kgFFM−1.min-1, P=0.0003).
Table 1.
Composition of the study diets
| Study Diet | LoGIX | HiGIX |
|---|---|---|
| GI, a.u. | 40.1±0.2 | 80.2±0.5* |
| GL, a.u. | 101.9±6.0 | 218.2±13.9* |
| EI, kcal·d−1 | 1789±104 | 1885±111 |
| Carbohydrate, g·d−1 | 251.4±15 | 264.4±58 |
| Carbohydrate, % kJ | 56.2±0.1 | 56.0±0.6 |
| Fat, g·d−1 | 57.4±3.1 | 60.0±3.4 |
| Fat, % kJ | 32.1±0.3 | 31.8±0.3 |
| Protein, g·d−1 | 78.0±4.7 | 80.6±4.7 |
| Protein, % kJ | 17.5±0.4 | 17.1±0.1 |
| Fiber, g·d−1 | 28.9±1.6 | 28.1±1.8 |
Data represents mean ± SEM. GI = glycemic index; GL = glycemic load; a.u. = arbitrary units; EI = energy intake.
Significant group differences are represented by, P<0.05.
Body Composition
Subject groups were well matched for BMI prior to the onset of the intervention (P>0.05). Post-study, both groups significantly reduced body weight, and there was no difference between the groups in the amount of weight loss achieved (Table 2). Whole body fat mass was markedly reduced in both groups as was total abdominal, subcutaneous, and visceral fat mass (Table 2).
Table 2.
Subject Characteristics
| Subject | LoGIX (n=9) | HiGIX (n=15) | ANOVA | |||
|---|---|---|---|---|---|---|
| Characteristics | PRE | POST | PRE | POST | time | Time*trial |
| Sex | 4f, 5m | - | 7f, 8m | - | - | - |
| Age, y | 66±1 | - | 64±1 | - | - | - |
| Weight, kg | 95.6±5.2 | 87.7±4.4 | 97.8±4.5 | 90.4±3.5 | 0.01 | 0.70 |
| BMI, kg/m2 | 34.4±1.3 | 31.0 ±1.2 | 34.8±1.3 | 31.9±1.2 | 0.01 | 0.86 |
| FM, kg | 44.1±2.5 | 36.8±2.3 | 42.2±2.4 | 35.1±2.6 | 0.009 | 0.96 |
| FFM, kg | 51.5±3.5 | 50.8±3.4 | 56.9±3.5 | 55.3±3.3 | 0.89 | 0.96 |
| Total AB, cm2 | 601.9±39.1 | 503.9±37.9 | 563.1±39.2 | 463±42.7 | <0.0001 | 0.42 |
| Total SQ, cm2 | 489.0±43.1 | 426.1±45.0 | 443.7±34.2 | 384.9±35.3 | <0.0001 | 0.67 |
| Deep SQ, cm2 | 249.8±29.7 | 192.6±17.8 | 214.6±19.6 | 167.7±15.5 | <0.0001 | 0.80 |
| Superficial SQ, cm2 | 239.2±25.1 | 233.5±30.8 | 229.0±18.9 | 210.1±24.5 | 0.15 | 0.44 |
| Total VF, cm2 | 112.9±19.2 | 77.8±15.2 | 119.4±22.7 | 78.0±16.4 | <0.0001 | 0.46 |
| FPG, mg/dl | 113.5±6.1 | 98.2±3.7 | 100.5±2.7 | 92.7±2.3 | 0.002 | 0.15 |
| FPI, μU/ml | 29.6±9.6 | 13.7±1.8 | 17.6±4.1 | 9.6±1.9 | 0.009 | 0.36 |
| Matsuda Index | 1.8±0.3 | 3.5±0.4 | 2.5±0.5 | 4.7±0.8 | <0.0001 | 0.43 |
| VO2max(ml/kg FFM/min) | 39.3±1.6 | 44.4±3.4 | 39.6±1.4 | 45.7±1.8 | 0.0003 | 0.72 |
| Total Adiponectin (νg/ml) | 29.3±3.7 | 28.3±3.7 | 35.2±6.1 | 33.7±7.5 | 0.75 | 0.95 |
Data represents mean ± S.E.M.; BMI = body mass index; FM = fat mass; FFM = fat-free mass; FPG = fasting plasma glucose; FPI = fasting plasma insulin, AB = abdominal, SQ = subcutaneous fat, VF = visceral fat.
Blood Biochemistry
The lifestyle intervention reduced fasting plasma glucose and insulin in both the LoGIX and HiGIX groups (Table 2; all P<0.05). Further, there was a significant improvement in insulin sensitivity in both groups as determined by the Matsuda index (LoGIX, pre: 1.8±0.3, post: 3.5±0.4; HiGIX, pre: 2.5±0.5, post: 4.7±0.8, P<0.0001)
Adipokine measures
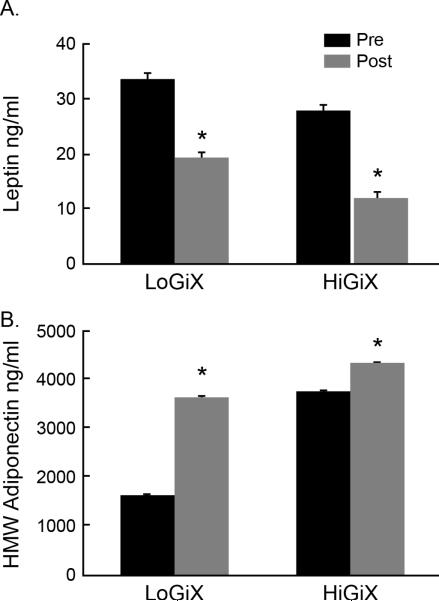
Fasting plasma leptin was significantly decreased in both groups (LoGIX, Δ14.6±4.6; HiGIX, Δ16.0±3.2 ng/ml, P=0.004; Fig. 1A), and HMW adiponectin was significantly increased (LoGIX, Δ1895.4±45.9; HiGIX, Δ579.2±45.5 pg/ml; P=0.003; Fig. 1B) following the 12-week intervention. There was no change in total adiponectin for either group (P=0.75). The HMW adiponectin to leptin ratio was significantly increased following the intervention (LoGIX, pre: 0.06±0.03, post: 0.46±0.08; HiGIX, pre: 0.2±0.05, post: 1.2±0.1; pre, P=0.04; Fig 1C) with no difference between groups.
Figure 1.
Changes in adipokines following 12 weeks of aerobic exercise and either a low-(LoGIX) or high- (HiGIX) glycemic diet. Plasma leptin (A) significantly decreased while HMW adiponectin (B) increased independent of dietary GI following 12 weeks of intervention. There were no differences between groups. * P<0.05
Regression Analysis
There was an inverse correlation between changes in leptin and HMW adiponectin following the intervention (r=-0.46, P=0.02). Further, there was a strong positive correlation between total adiponectin and HMW adiponectin (r=0.62, P=0.003). Although there was no significant change in total adiponectin, the increase in HMW isoform indicates a shift in isomer distribution that favors post-translational processing of the active form of the protein. There was no association between pre-HMW levels and visceral fat (r=0.025, P=0.9); however following the intervention the HMW form of the protein was inversely correlated with visceral fat (r=-0.48, P=0.01; Fig 2A). Further, the improvement in insulin sensitivity correlated with the change in body weight (r=-0.57, P=0.005), specifically the change in total fat mass (r =-0.63, P=0.001) and the change in total abdominal fat (r=-0.42, P=0.04). As expected changes in total abdominal fat were related to changes in both visceral and subcutaneous fat (r=0.57, P=0.003; r=0.84, P<0.001, respectively).
Figure 2.
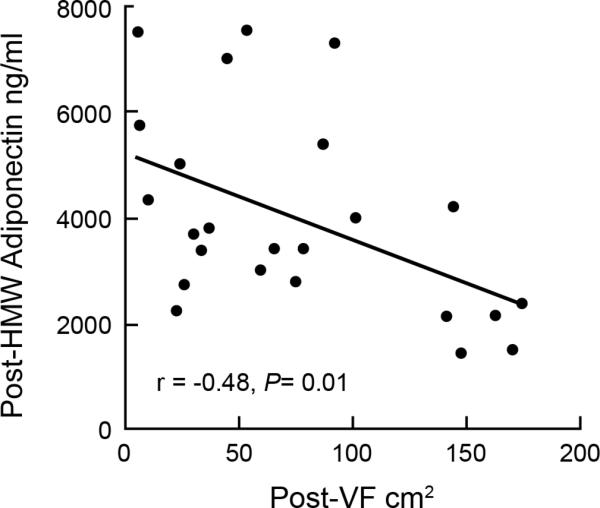
Relationship between HMW adiponectin and visceral fat post-intervention.
DISCUSSION
The primary finding from this study is that 12 weeks of aerobic exercise combined with weight loss favorably alters adipokine secretion independent of the dietary GI, contrary to the original hypothesis that a low-glycemic diet would elicit changes as compared to a high-glycemic diet. HMW adiponectin is inversely associated with metabolic syndrome, insulin resistance and blood lipids (11, 39). We recently reported that 12 weeks of aerobic exercise training combined with either a low or high glycemic diet improved peripheral and hepatic insulin resistance and severity of metabolic syndrome (22, 30), and that short term aerobic exercise increased circulating HMW adiponectin and reduced circulating leptin (12). Herein, we demonstrate that regular exercise training, independent of diet, not only increases HMW adiponectin, but also induces changes in HMW adiponectin that are related to change in visceral fat and changes in leptin.
It has been suggested that circulating HMW adiponectin may be influenced by the magnitude of weight loss and correlates with body mass (6, 17); however we found no associations between HMW adiponectin and weight loss. This suggests that it is not the magnitude of weight loss, but rather the location from which the fat is reduced, or rather the change in fat distribution, that regulates leptin and adiponectin concentrations (17, 33). Evidence suggests that accumulation of intra-abdominal visceral fat can lead to dysfunctional adipocytes resulting in metabolic disease (1, 2, 8). We report that participants in this study significantly reduced total abdominal fat, subcutaneous and visceral fat, and that changes in adipose tissue distribution are related to changes in adipokine secretion. Recently, Kishida et al. (17) and Tamei et al. (33) reported that visceral fat was inversely related to plasma HMW adiponectin in obese people. Our findings concur with these reports suggesting that HMW adiponectin concentrations are dependent upon visceral fat mass. Additionally, we found a direct correlation between leptin and subcutaneous fat. While both adipose tissue depots produce and secrete leptin and adiponectin, subcutaneous adipose tissue has been shown to be the major site of leptin secretion, and strong correlations between leptin secretion and subcutaneous adipocyte cell size and secretion rate have been found (35). Moreover, there are consistent observations that enlarged adipocytes are independent markers of insulin resistance (21, 27), and it has been shown that adipocyte cell size decreases with aerobic exercise training and diet-induced weight loss (38). We measured changes in body weight, total abdominal fat mass (CT scan), as well as total body fat mass (DEXA), and showed that these changes correlated with changes in insulin sensitivity. Previous data suggests that modification of dietary carbohydrate intake can reduce both systemic levels and adipose tissue production of inflammatory cytokines (3, 10, 37); thus we hypothesized that those randomized LoGIX group would have greater improvements in adiopokine regulation than those in the HiGIX intervention
However, herein we report that 12 weeks of exercise training, independent of dietary composition, leads to normalization of adipokine secretion and insulin sensitivity, possibly due to a decrease in adipocyte cell size. While diet may influence cytokine release from adipose tissue, the present data suggests there is no dietary influence on adipokine secretion. The variance in protein secretion is likely due to the different cell sources within adipose tissue; cytokines being released from non-fat tissue whereas leptin and adiponectin are secreted from adipocytes. We also found that changes in HMW adiponectin were related to the change in leptin. In human obesity these two adipokines are reciprocally related; circulating adiponectin is depressed while leptin is elevated (14). Further, the adiponectin to leptin ratio is known to correlate with insulin resistance, atherogenesis, and metabolic syndrome (7, 29), and is therefore likely to reflect the overall balance of adipocyte function and health. Bays et al. (2) recently coined the term “adiposopathy” to describe abnormal endocrine function in obesity, and suggested that the adiponectin/leptin index can provide an indication of the health of the adipocyte. Adiposopathy can be translated as representing “sick fat”, and this term emphasizes that adipose tissue has as much of a pathogenic potential to cause ill health as the pathologic dysfunction of other body tissues. Morphologically, adiposopathy manifests as enlarged adipocytes with a concomitant increase in visceral and/or ectopic fat deposition. Physiologically, adiposopathy is characterized by adverse endocrine and immune function, including aberrant adipokine and cytokine production, which can contribute to chronic diseases such as type 2 diabetes and cardiovascular disease (2). Our results show that the increase in HMW adiponectinto-leptin ratio following exercise and weight loss is independent of dietary GI and that there is an inverse correlation between the changes in HMW adiponectin and leptin. Thus, it can be surmised that following 12 weeks of aerobic exercise with moderate weight loss, adipocyte health and function is enhanced and adipocyte size is likely reduced resulting in the favorable changes in adipokine secretion.
Both groups showed favorable improvements in adipokine secretion and insulin sensitivity independent of diet; thus it appears from these data that dietary GI did not influence the regulation of leptin and adiponectin in this older obese population. However, improvements in diabetes, cardiovascular disease, and obesity-related parameters have been documented in people consuming low glycemic foods (5, 19, 20). Recently, low glycemic diets alone have been shown to modestly increase adiponectin and reduce leptin (24), whereas other studies have shown that these changes are independent of dietary composition (9). The diets that were used in the current study were matched for percent composition of macronutrients in the diet, as well as fiber, with the only variable being the GI of the carbohydrates. Therefore, the contrary findings in this study as compared to others may be due to the fact that the only dietary difference was the quality of the carbohydrate suggesting that postprandial blood glucose concentrations may not be the driving factor in adipokine regulation.
In conclusion, our results show that quantitative change in fat mass, following exercise and weight loss appears to be the more important factor regulating adipokine production and secretion in older obese persons. We found no additional benefit of including a low glycemic diet with exercise on insulin sensitivity or plasma adipokine concentrations. Further, the favorable changes in HMW adiponectin and leptin coupled to the changes in fat mass and insulin sensitivity suggest an improvement in adipocyte health and function. Adipocytes are of crucial importance, buffering the daily influx of dietary fat while regulating secretion of factors that act in an endocrine as well as autocrine/paracrine manner. Our data provide further evidence that the relationship between adiposity and adipokine production can be regulated by exercise, and these favorable improvements in adipocyte function can improve health in the older obese.
ACKNOWLEDEMENTS
The authors wish to thank the research volunteers for their outstanding dedication and effort, and the nursing staff of the Clinical Research Unit, and the staff and students who helped with the implementation of the study and data collection. We also acknowledge our clinical research coordinator, Julianne Filion B.S.N., R.N., for her excellent nursing and organizational assistance.
This research was supported by NIH grant RO1 AG12834 (JPK), and was supported in part by the NIH National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio. KRK and JMH were supported by NIH grants T32 DK007319 and T32 HL007887, respectively.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Contributions of each author:
Karen R. Kelly: Data collection, data analysis and interpretation, writing the manuscript Sankar D. Navaneethan: Data analysis and interpretation, and writing the manuscript Thomas P.J. Solomon: Data collection, analysis and interpretation, writing the manuscript Jacob M. Haus: Data collection, analysis and interpretation, writing the manuscript
Marc Cook: Data collection and writing the manuscript
Hope Barkoukis: Design of the study, data collection and interpretation, writing the manuscript
John P. Kirwan: Design of the study, data collection, analysis and interpretation, writing the manuscript
The authors report no conflict of interest.
REFERENCES
- 1.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 2.Bays HE, Gonzalez-Campoy JM, Henry RR, Bergman DA, Kitabchi AE, Schorr AB, Rodbard HW. Is adiposopathy (sick fat) an endocrine disease? Int J Clin Pract. 2008;62:1474–1483. doi: 10.1111/j.1742-1241.2008.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity. 2009;17:1664–1670. doi: 10.1038/oby.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouche C, Rizkalla SW, Luo J, Vidal H, Veronese A, Pacher N, Fouquet C, Lang V, Slama G. Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care. 2002;25:822–828. doi: 10.2337/diacare.25.5.822. [DOI] [PubMed] [Google Scholar]
- 5.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. The Journal of clinical endocrinology and metabolism. 2010;95:911–919. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- 7.Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B, Flyvbjerg A, Walker M, Hojlund K, Nolan JJ, Savage DB. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Heggen E, Klemsdal TO, Haugen F, Holme I, Tonstad S. Effect of a Low-Fat Versus a Low-Gycemic-Load Diet on Inflammatory Biomarker and Adipokine Concentrations. Metab Syndr Relat Disord. 2012;10:437–442. doi: 10.1089/met.2012.0012. [DOI] [PubMed] [Google Scholar]
- 10.Kallio P, Kolehmainen M, Laaksonen DE, Pulkkinen L, Atalay M, Mykkanen H, Uusitupa M, Poutanen K, Niskanen L. Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. The American journal of clinical nutrition. 2008;87:1497–1503. doi: 10.1093/ajcn/87.5.1497. [DOI] [PubMed] [Google Scholar]
- 11.Kawada T, Hasegawa M. Predictive ability of serum high-molecular-weight adiponectin in combination with serum insulin and serum C-reactive protein for the presence of metabolic syndrome. Ann Hum Biol. 2012;39:108–112. doi: 10.3109/03014460.2011.652170. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TP, Kalinski MI, Kirwan JP. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Medicine and science in sports and exercise. 2012;44:69–74. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 13.Kelly KR, Haus JM, Solomon TP, Patrick-Melin AJ, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 15.Kirwan JP, Barkoukis H, Brooks LM, Marchetti CM, Stetzer BP, Gonzalez F. Exercise training and dietary glycemic load may have synergistic effects on insulin resistance in older obese adults. Annals of nutrition & metabolism. 2009;55:326–333. doi: 10.1159/000248991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151–156. doi: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishida K, Kim KK, Funahashi T, Matsuzawa Y, Kang HC, Shimomura I. Relationships between circulating adiponectin levels and fat distribution in obese subjects. J Atheroscler Thromb. 2011;18:592–595. doi: 10.5551/jat.7625. [DOI] [PubMed] [Google Scholar]
- 18.Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig DS. Clinical update: the low-glycaemic-index diet. Lancet. 2007;369:890–892. doi: 10.1016/S0140-6736(07)60427-9. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA : the journal of the American Medical Association. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 21.Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 22.Malin SK, Niemi N, Solomon TP, Haus JM, Kelly KR, Filion J, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Exercise Training with Weight Loss and either a High- or Low-Glycemic Index Diet Reduces Metabolic Syndrome Severity in Older Adults. Annals of nutrition & metabolism. 2012;61:135–141. doi: 10.1159/000342084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 26.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olefsky JM. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977;100:1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- 28.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–1028. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 29.Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 30.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. The American journal of clinical nutrition. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90:1222–1229. doi: 10.3945/ajcn.2009.28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol. 2008;104:1313–1319. doi: 10.1152/japplphysiol.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamei N, Ogawa T, Ishida H, Ando Y, Nitta K. Relationship of high-molecular-weight adiponectin levels to visceral fat accumulation in hemodialysis patients. Intern Med. 2010;49:299–305. doi: 10.2169/internalmedicine.49.2905. [DOI] [PubMed] [Google Scholar]
- 34.Taylor HL, Jacobs J,DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chron Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 35.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 36.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Bjorck I, Brighenti F, Brown R, Brynes A, Casiraghi MC, Cazaubiel M, Dahlqvist L, Delport E, Denyer GS, Erba D, Frost G, Granfeldt Y, Hampton S, Hart VA, Hatonen KA, Henry CJ, Hertzler S, Hull S, Jerling J, Johnston KL, Lightowler H, Mann N, Morgan L, Panlasigui LN, Pelkman C, Perry T, Pfeiffer AF, Pieters M, Ramdath DD, Ramsingh RT, Robert SD, Robinson C, Sarkkinen E, Scazzina F, Sison DC, Sloth B, Staniforth J, Tapola N, Valsta LM, Verkooijen I, Weickert MO, Weseler AR, Wilkie P, Zhang J. Measuring the glycemic index of foods: interlaboratory study. The American journal of clinical nutrition. 2008;87:247S–257S. doi: 10.1093/ajcn/87.1.247S. [DOI] [PubMed] [Google Scholar]
- 38.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. International journal of obesity. 2006;30:1211–1216. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]
- 39.Yu D, Yu Z, Sun Q, Sun L, Li H, Song J, Mi M, Wu H, Lu L, Liu C, Zhang G, Hu FB, Lin X. Effects of body fat on the associations of high-molecular-weight adiponectin, leptin and soluble leptin receptor with metabolic syndrome in Chinese. PLoS One. 2011;6:e16818. doi: 10.1371/journal.pone.0016818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Lanza E, Ross AC, Albert PS, Colburn NH, Rovine MJ, Bagshaw D, Ulbrecht JS, Hartman TJ. A high-legume low-glycemic index diet reduces fasting plasma leptin in middle-aged insulin-resistant and -sensitive men. Eur J Clin Nutr. 2011;65:415–418. doi: 10.1038/ejcn.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]