Abstract
Background
How drug adverse events (AEs) are communicated in the United States may mislead consumers and result in low adherence. Requiring written information to include numeric AE-likelihood information might lessen these effects, but providing numbers may disadvantage less skilled populations.
Objective
To determine risk comprehension and willingness to use a medication when presented with numeric or non-numeric AE-likelihood information across age, numeracy, and cholesterol-lowering-drug-usage groups.
Design
In a cross-sectional internet survey (N=905; American Life Panel, 5/15/08–6/18/08), respondents were presented with a hypothetical prescription medication for high cholesterol. AE likelihoods were described using one of six formats (non-numeric: Consumer-Medication-Information (CMI)-like list, risk labels; numeric: percentage, frequency, risk-labels-plus-percentage, risk-labels-plus-frequency). Main outcome measures were risk comprehension (recoded to indicate presence/absence of risk overestimation and underestimation), willingness to use the medication (7-point scale; not likely=0, very likely=6), and main reason for willingness (chosen from eight predefined reasons).
Results
Individuals given non-numeric information were more likely to overestimate risk, less willing to take the medication, and gave different reasons than those provided numeric information across numeracy and age groups (e.g., among less numerate: 69% and 18% overestimated risks in non-numeric and numeric formats, respectively; among more numerate: these same proportions were 66% and 6%). Less numerate middle-aged and older adults, however, showed less influence of numeric format on willingness to take the medication.
Limitations
It is unclear whether differences are clinically meaningful although some differences are large.
Conclusions
Providing numeric AE-likelihood information (compared to non-numeric) is likely to increase risk comprehension across numeracy and age levels. Its effects on uptake and adherence of prescribed drugs should be similar across the population, except perhaps in older, less numerate individuals.
Keywords: risk comprehension, risk communication, pharmaceutical decision making, adherence, numeracy, aging, informed decision making, statins
INTRODUCTION
Patients are often reluctant to initiate new treatment, and one reason may be fear of adverse events (AEs)1 that are not well understood. Studies have shown that physicians disclose less information about possible AEs2–4 than the full disclosure that patients say they prefer.5 Pharmacists also do not provide counseling in the majority of cases.6 As a result, pharmacist-provided written drug information is an important source of information for patients.7,8 Three types of written information exist, two of which are regulated and approved by the Food and Drug Administration (Medication Guides and patient package inserts; each is required only for some medications). The third and most common written information – consumer medication information (CMI) – is provided to patients with their prescribed medication (about 94% of the time in a recent study) but is not currently FDA-reviewed or FDA-approved.9 CMIs are developed by commercial vendors (not pharmaceutical companies) and then sold to pharmacies.9 The information generally includes a list of serious or frequent AEs; serious AEs are sometimes augmented with qualitative labels of their low likelihood (e.g., “rare”; called risk labels from here on). CMIs for a popular statin, for example, describe a list of AEs including nausea, stomach upset, and “very rare” muscle pain and liver problems. In the present paper, these descriptive AE lists are called CMI lists.
In other consumer domains such as purchases of homes and lottery tickets, numeric information (mortgage rates and likelihoods of winning) is provided to better inform choices. Prescription drugs are also probabilistic in terms of their AEs and benefits, but probabilities are rarely provided even though their provision has been found to influence patient understanding and willingness to take medications.10,11 On average, individuals tend to overestimate risk likelihood when provided only with risk labels (e.g., “common,” “rare”)12–17 or CMI-like lists18 compared to when they are provided numeric information. For example, the European Commission proposed that “common” be used to describe AEs that occur for 1%–10% of the user population. But the lay public generally interprets “common” to mean the AE will affect 45%–50% of users, and experienced physicians interpret it as affecting roughly 25%.10 To the best of our knowledge, no studies have compared CMI lists with descriptive risk labels only.
Based on this prior research, it appears that probabilistic information about AEs and benefits should be provided. However, both the lay public and physicians have trouble with numeric health information19–23 and consumers cannot always pick out correct numbers from simple tables or graphs.24 As a result, providing numeric information about medications may pose problems, particularly in less numerate populations (numeracy is defined as the ability to understand and use mathematical and probabilistic concepts; it is measured with a math test). 24,25 For example, Schwartz et al.26 found that only 5.8% of their least numerate respondents could accurately assess risk-reduction data versus 40% of the most numerate. Less numerate individuals also tend to remember numeric risks less well and to perceive risks to be greater than the highly numerate across a variety of domains.27–30 In the present context, lower comprehension, less recall, and higher risk perceptions may cause less numerate individuals to be less likely to take medications, particularly with provision of numeric AE information rather than risk labels or CMI lists. Consistent with this reasoning, policy makers and others have questioned whether less numerate populations can “handle” numeric information.31 No studies, however, have tested the effects of numeric vs non-numeric AE-likelihood information in populations varying in numeracy.
Potential problems with numeric information may be further exacerbated in older age. Compared to younger adults, older adults tend to process information (especially numeric information) less efficiently.32,33 These age differences may be important because older adults take the vast majority of medications. Of adults 60+ years, 88% used at least one drug in the past month compared to 48% of 20–59 year olds,34 but older adults and their physicians do not communicate well about medications35 so that written information may be particularly important. Little is known about age differences in the effects of numeric vs non-numeric AE-likelihood information on medication perceptions and use.
We examine individuals who report taking a medication (similar to the hypothetical one we pose) and those who do not. Both populations are important due to issues with medication adherence as well as uptake.1 The effects of format are not expected to differ based on medication usage because they are thought to have a domain-general influence on how the mind processes information. It is possible, however, that individuals taking a similar medication may be immune to format effects because they have already committed to taking the medication. As a result, they may have fixed beliefs about the low risks of such medications and may have already maximized their willingness to take similar medications.
The present study examines how different formats for presenting likelihood information about AEs (CMI lists vs risk labels, non-numeric vs numeric formats) influence comprehension of AE likelihood and reported willingness to use a drug. The paper extends previous research to examine these effects in a single study so that ready comparisons can be made across formats, numeracy levels, adult age, and use of a similar medication. We hypothesized that:
-
H1
Presenting only non-numeric AE information (vs presenting numeric information) will result in more risk overestimation and less willingness to take the drug on average. This finding would replicate prior studies.
-
H2
Based on speculation that less numerate individuals may not be able to “handle” numeric risk information, numeric AE formats will reduce risk comprehension and willingness to take a drug vs non-numeric formats in less numerate populations compared to the highly numerate.
-
H3
Numeric AE formats will be particularly problematic compared to non-numeric formats and result in lower comprehension and willingness to use the drug among less numerate older adults who do not process numeric information as well as other groups due to age-related inefficiencies in cognitive processes.32,33
-
H4
Providing only non-numeric information will result in different main reasons for willingness-to-take-a-drug responses compared to providing numeric AE information. In particular, reasons in the non-numeric group are expected to focus more on side effects being more likely and especially on the lowest likelihood most serious AE whereas those in the numeric group should reflect the AEs being less important.
The overarching purpose of this study is to identify formats for presenting medication risk information that will help guide diverse patients towards making more informed decisions.
METHODS
From 5/15/08–6/18/08, we conducted a randomized experiment over the Internet in the American Life Panel (ALP; www.rand.org/labor/roybalfd/american_life.html). ALP respondents are paid $20 for each half-hour interview.
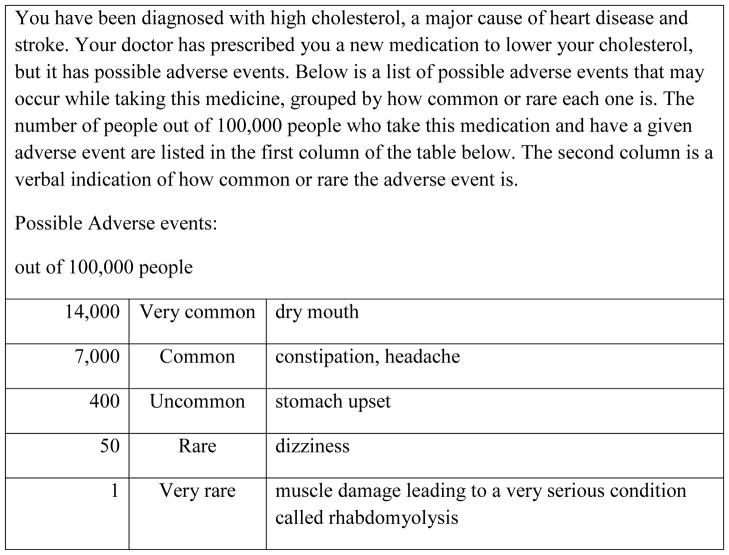
Respondents were told “Imagine you have been diagnosed with high cholesterol, a major cause of heart disease and stroke. Your doctor has prescribed you a new medication to lower your cholesterol, but it has possible side effects. Below is a list of possible side effects that may occur while taking this medicine.” They then were given risk information about medication AEs in one of six formats, randomly assigned. AE information was hypothetical but modified from statin CMIs available from a pharmacy and the Physicians’ Desk Reference. The scenario was designed to include one serious rare AE and other less serious common AEs to provide respondents with a relatively realistic scenario. The CMI format stated that adverse events were: “dry mouth, constipation, headache, upset stomach, or dizziness. This medicine may very rarely cause muscle damage that can lead to a very serious condition called rhabdomyolysis.” In the remaining five formats, adverse events were placed in a table in the same order as the CMI list with accompanying information about AE likelihood (see Figure 1 for an example in the Frequency+Risk label condition and Table 1 for a description of all conditions). Risk-label descriptors were based on recommendations for descriptive labels given by the European Commission (e.g., 14%=very common).15
Figure 1.
Frequency-plus-Risk-label example
Table 1.
Description of six likelihood formats – In each case (except Consumer Medication Information (CMI)), a table similar to that in Figure 1 was provided. Differences from Figure 1 are noted under the format description
| Format type | Format | Format description |
|---|---|---|
| Non-numeric | CMI-like list (CMI) | Instead of the table, the CMI list included this text: “Possible Side Effects: dry mouth, constipation, headache, stomach upset, or dizziness. This medicine may very rarely cause muscle damage that can lead to a very serious condition called rhabdomyolysis.” No numeric information was provided. |
| Non-numeric | Risk-label only | The table included Risk labels for likelihoods of all AEs (e.g., very common, rare). No numeric information was provided. |
| Numeric | Percent only | The table included Percentages in place of frequencies (e.g., 14%, 0.05%). No risk labels were provided. |
| Numeric | Frequency only | The table included frequencies (e.g., 14,000, 50 – out of 100,000 people). No risk labels were provided. |
| Numeric | Percent+Risk label | The table included risk labels and included Percentages instead of frequencies. |
| Numeric | Frequency+Risk label | This is Figure 1. Risk labels and frequencies were provided. |
For willingness to take the drug, respondents answered the question “How likely is it that you will take this drug?” on a 7-point scale (0=not likely; 6=very likely). They then chose the most important reason for this response: a) most of the adverse events are not very serious; b) any serious adverse events are very unlikely, c) prefer to avoid taking medications and will do something else, d) there are too many possible adverse events, e) a lot of people will experience at least one of the adverse events, and I don’t want to be one of them, f) the very serious muscle damage, g) other, and h) none of the above. For risk comprehension, respondents were asked to estimate the likelihood of an upset stomach, either as a percentage (for respondents in the two percentage formats) or as a frequency (in all remaining formats). Respondents were also asked a risk-comprehension question for one additional side effect (dizziness) on a verbal scale (1=very common to 5=very rare) to examine whether the effects of numeric vs non-numeric format were similar between verbatim comprehension assessed on the numeric scale and gist comprehension assessed on the verbal scale.23 Dizziness results were largely the same as those reported for upset stomach and are available from the first author.
Respondents reported whether they regularly took a prescription medication to lower cholesterol. Finally, numeracy was measured using a new 8-item scale reduced from 18 items obtained from existing numeracy scales. The new scale was developed across two large, independent samples that varied widely in age and educational level. It had excellent psychometric properties based on a Rasch analysis and good predictive abilities relative to existing scales, supporting its predictive validity.36 The primary usefulness of the new scale is that it can be used in a wide range of subject populations, allowing for a clearer understanding of how numeracy influences the decision process across the lifespan.
The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Analyses
Using SPSSv18, predictors were mean-centered. Risk comprehension and willingness to take the drug (both as continuous variables) were compared first between CMI and risk-label formats using analyses of variance (ANOVAs). CMI and risk-label formats were then combined as non-numeric with the remaining formats grouped as numeric. Analyses comparing the four numeric formats are beyond the scope of the present paper and will be reported elsewhere (Peters, Tusler, Sinayev, & Fraenkel, in preparation).
Risk-comprehension responses then were coded as the presence vs absence of overestimation and underestimation or as the correct response of 400. Logistic analyses examined proportions of respondents who overestimated and underestimated risks by numeric/non-numeric format, numeracy, age, and whether they reported taking a cholesterol-lowering medication. Regression analyses of willingness to take the drug compared between formats, numeracy, age, and medication usage. All interactions of format, numeracy, age, and medication usage were included in logistic and regression analyses. Logistic analyses also were conducted of each main reason for reported willingness to take the drug; main-reason analyses were conducted separately among those who reported taking vs not taking a cholesterol-lowering drug.
RESULTS
The response rate was 78.9%. Non-respondents did not differ from respondents in education or gender but were slightly younger (49.6 years vs. 51.3 years, p=.04). Five responders provided incomplete data and were not included in analyses. Respondents were 60% female (mean/median age=54.8/56 years; median income=$60,000 – $74,999; median education=12 where 12=Associate degree and 13=Bachelor’s degree; 91% Caucasian, 6.0% Black, 1.4% Asian, 0.7% Native American, and 1.1% reported Other). Compared to the 2010 U.S. Census, the sample is older, better educated, and higher in income (see Table 2). 39% reported regularly taking prescription medication to lower cholesterol. The final sample was comprised of 905 respondents who answered all questions (n=155, 140, 155, 167, 142, and 146, respectively, in CMI, Risk-label, percentage, frequency, percentage+Risk-label, and frequency+Risk-label conditions). We compare responses in CMI vs Risk-label formats as well as in non-numeric formats (n=295) vs numeric formats (n=610). Respondents assigned to CMI vs Risk-label formats and non-numeric vs numeric formats did not differ significantly by numeracy, current use of cholesterol-lowering drug, age, education, household income, gender, or ethnicity (see Table 3).
Table 2.
Characteristics of the sample and of the U.S. Census
| Characteristic | Sample | 2010 Census |
|---|---|---|
| Age | ||
| 18–34 | 8.7% | 30.7% |
| 35–64 | 68.2% | 52.7% |
| 65–89 | 23.1% | 16.6% |
| Education | ||
| Less than high school | 1.3% | 14.6% |
| High school diploma | 14.7% | 28.5% |
| Some college/vocational school | 36.6% | 31.1% |
| College graduate or more | 47.3% | 25.8% |
| Household income | ||
| Less than $20,000 | 8.9% | 18.9% |
| $20,000 to $39,999 | 18.5% | 21.5% |
| $40,000 to $59,999 | 20.7% | 17.4% |
| $60,000 or more | 51.9% | 42.1% |
Table 3.
Comparison of experimental conditions. No significant differences were observed between numeric-format and non-numeric-format groups or between CMI and label-only groups. P values ranged from .2 to .9.
| Non-numeric Formats | ||||
|---|---|---|---|---|
| Numeric Formats (n=610) | Non-Numeric Formats (n=295) | CMI List (n=155) | Label Only (n=140) | |
| Gender (% female) | 59.0% | 62.4% | 61.9% | 62.9% |
| Mean Age in Years (SD) | 54.9 (13.6) | 54.5 (12.9) | 54.3 (12.6) | 54.8 (13.3) |
| Education (% Associate degree or less)* | 51.6% | 54.6% | 57.4% | 51.4% |
| Mean Household Income (SD) (11 = $40,000 to $49,999, 12 = $50,000 to $59,999)** | 11.5 (3.0) | 11.5 (3.2) | 11.3 (3.4) | 11.7 (3.1) |
| Use of Statins (% Yes) | 39.5% | 39.3% | 36.8% | 42.1% |
| Ethnicity (% White) | 91.5% | 89.5% | 89.7% | 89.3% |
| Mean Numeracy (SD) *** | 4.1 (1.8) | 4.1 (1.8) | 4.0 (1.8) | 4.2 (1.7) |
We also compared mean values for the more detailed education categories collected. These original education categories ranged from 1 = less than first grade to 16 = doctorate degree. Respective means (SD) in the four columns above were 11.7 (2.1), 11.7 (2.1), 11.6 (2.1), and 11.8 (2.0). No significant differences emerged.
Income categories range from 1, less than $5,000, to 14, more than $75,000. Income groupings in Table 2 are aggregates of the more detailed categories that are reported here.
The numeracy scale consists of 8 items. The distribution covered the full possible range of 0–8 and did not vary significantly from a normal distribution. The percentage of respondents receiving each possible score is as follows: 0 correct = 2%, 1 = 4%, 2 = 14%, 3 = 18%, 4 = 20%, 5 = 17%, 6 = 13%, 7 = 8%, 8 correct = 3%.
On the comprehension items, percentage responses were transformed into frequencies out of 100,000 for analysis purposes. One response greater than 100,000 was deleted on the stomach-upset question. Other than the correct response of 400, no respondent provided an estimate between 301 and 449.The continuous numeracy measure was used in inferential analyses; a median split categorized respondents as more and less numerate for descriptive purposes only. Respondents were grouped by age (18–39, 40–59, and 60–89 years).
Comprehension of Risks
CMI vs Risk label comparison
Results of an ANOVA indicated that individuals given the CMI format estimated greater risk likelihoods than those in the risk-label format, F(1, 292)=4.7, p=.03 (median risk estimates were 3,000 and 900, respectively, compared to the correct response of 400, which was also the median response in the four numeric formats). Based on logistic regression, the proportions of CMI and risk-label respondents who overestimated risks did not differ significantly (70% and 65%, respectively, p=.24, model χ2 (df = 15) = 22.6, p =.09, Nagelkerke R2 = .10) nor did those who underestimated risks (30% and 35%, respectively, p=.24, model χ2 = 22.6(15), p =.09, Nagelkerke R2 = .10). No CMI or risk-label respondents estimated the correct risk response of 400.
Non-numeric vs numeric comparison
Logistic-regression results indicated a format effect, with individuals in non-numeric formats overestimating risks substantially more than those provided numeric formats (68% and 12%, respectively, Wald χ2 (df=1)=144.0, p<.001, model χ2 (df = 15) = 328.1, p < .001, Nagelkerke R2 = .43). Less numerate individuals overestimated risk more than the more numerate (34% and 26%, respectively, Wald χ2 (df=1)=10.9, p<.001). The numeracy difference was seen only in the numeric formats (Figure 2; Wald χ2 (df=1)=8.0, p=.005). In addition, greater risk overestimation by the less numerate emerged more from individuals already on cholesterol-lowering medications (among those on medication, 41% and 25%, of the less and more numerate, respectively, overestimated risk, whereas among those not on medication, 32% and 25% overestimated risk, Wald χ2 (df=1)=4.5, p=.03). No other significant differences emerged, including no support for the hypothesized age effect.
Figure 2.
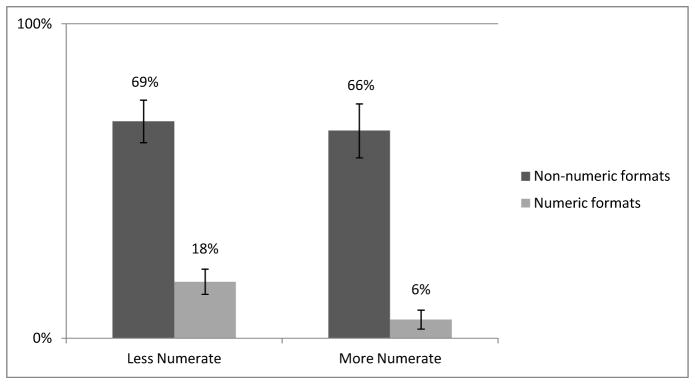
Proportion of respondents who overestimated the risk of stomach upset by format and numeracy. Error bars indicate 95% confidence intervals.
Risk underestimation was less prevalent than overestimation. Similar to overestimation results, respondents in non-numeric formats underestimated risks more than those provided numeric formats (32% and 7%, respectively, Wald χ2 (df=1)=68.0, p<.001, model χ2 (df = 15) = 129.7, p < .001, Nagelkerke R2 = .24). Older adults underestimated risks more often than did middle-aged or younger adults (17%, 14%, and 12%, respectively, in the three age groups, Wald χ2 (df=1)=5.7, p=.02). The simple effect of numeracy did not attain significance, and it had little effect on underestimations among those on cholesterol-lowering drugs (16% and 14% of the less and more numerate underestimated the risk); numeracy did, however, have a small effect on those who were not on cholesterol-lowering drugs (interaction Wald χ2 (df=1)=7.2, p=.007; 17% and 12%, respectively of the less and more numerate underestimated the risk likelihood). Two significant three-way interactions existed. Results of a three-way interaction between age, numeracy, and being on a cholesterol-lowering drug suggested that older, less numerate respondents who were on a drug underestimated most often (23% of them) whereas middle-aged, less numerate respondents on a drug underestimated the least often (6%; interaction Wald χ2 (df=1)=6.3, p=.01); the proportions of respondents not on the drug who underestimated did not differ as much between the age and numeracy groups (range=11%–19%). In addition, the significant three-way interaction between numeracy, numeric format, and age revealed that less numerate older adults underestimated more often in the numeric format (15%) compared to other groups provided a numeric format (range=2%–6%; interaction Wald χ2 (df=1)=4.1, p=.04); underestimations in the non-numeric-format groups were more frequent than in numeric-format groups and the proportions of underestimations was similar across age and numeracy groups (range=29%–34%).
Willingness to take the drug
Being provided a non-numeric compared to a numeric format produced both greater underestimation and greater overestimation, begging the question of whether underestimating risks might increase willingness to use the drug and/or overestimating risks might decrease it. Across all respondents, those estimating higher vs lower risks reported less willingness to take the drug, r=−.13, p<.001. Examination of this same correlation among only those respondents who overestimated risks produced a similar result (r=−.13, p=.03), but risk estimations were unrelated to willingness to take the drug among those respondents who underestimated risk (r=.00, p=.997). In fact, the mean willingness to take the drug was lower among those who underestimated risks compared to those who gave the correct response of 400 (mean willingness was 3.6, 4.3, and 3.6, respectively, among respondents who underestimated, gave the correct response, and overestimated risk likelihood).
CMI vs Risk label comparison
ANOVA results revealed that CMI and risk-label respondents did not differ significantly in their willingness to take the drug (2.4 and 2.6, respectively).
Non-numeric vs numeric comparison
Respondents on cholesterol-lowering medication reported greater willingness to take the described drug than those not on medication (mean willingness = 3.3 and 2.8, respectively, b=.33, t(1,889)=3.8, p<.001; overall ANOVA model F(15,889)=7.2, p<.001, R2=.11). Independent of this effect, individuals given numeric-format AE information were more willing to take the drug than those provided a non-numeric format (means=3.2 and 2.5, respectively, b=0.35, t(889)=4.1, p<.001). More numerate respondents were more willing to use the drug than the less numerate (mean willingness=3.0 and 2.7, respectively, b=0.10, t(889)=2.1, p=.04), but the two-way interaction of numeracy with numeric vs nonnumeric format was nonsignificant. In addition, younger adults were more willing to take the drug than older adults (mean willingness=3.2, 2.9, and 2.5 for the respective age groupings of 18–39, 40–59, and 60–89 years; b=−0.45, t(889)= −3.8, p<.001).
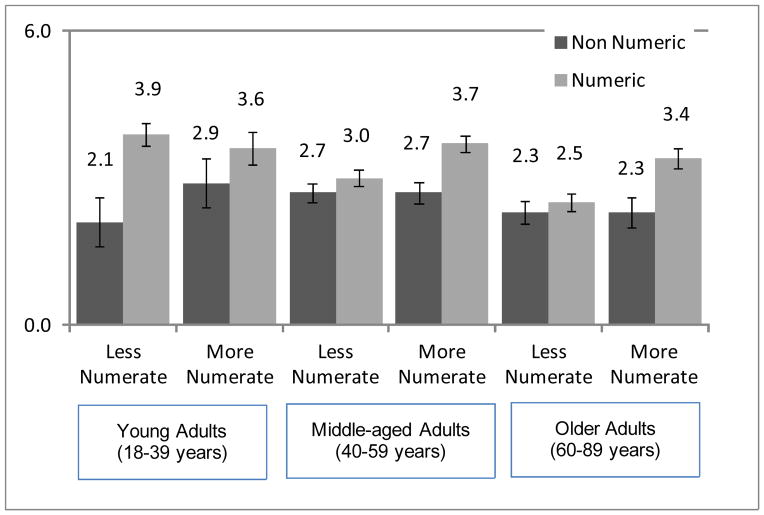
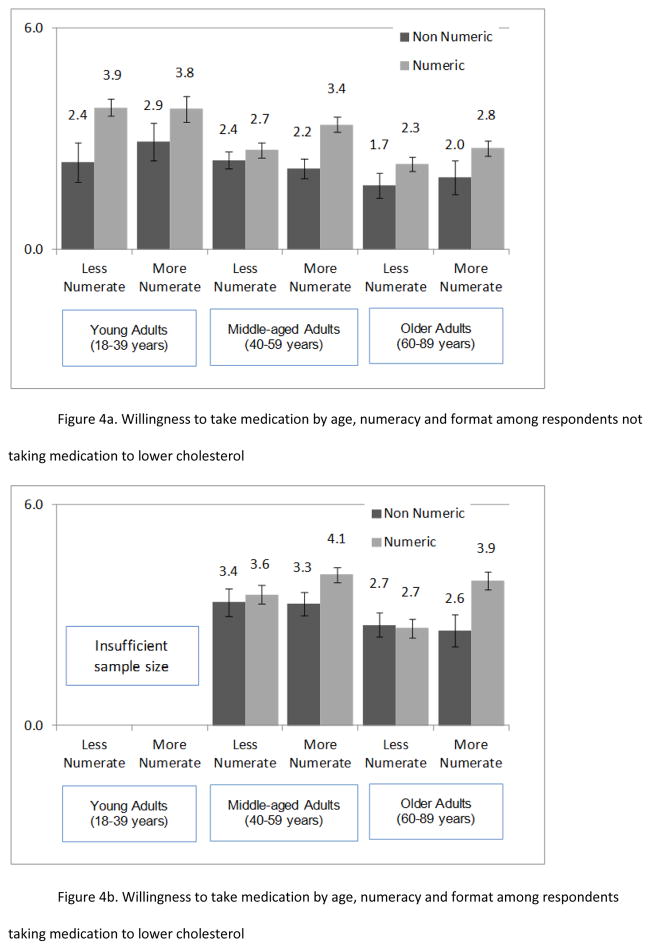
The interaction between format and numeracy was modified by age group such that less numerate middle-aged and older adults showed less influence of numeric format (Figure 3; three-way interaction b=0.21, t(889)=3.3, p=.001). This effect was modified by whether the respondent reported use of cholesterol-lowering drugs (Figure 4a and 4b; four-way interaction b=.15, t(889)=2.4, p=.02). Any beneficial effect of numeric format disappeared among less numerate older adults already on a similar medication. For the interested reader and to better understand this four-way interaction of format, numeracy, age, and medication usage, six regressions of willingness were conducted by age group and medication usage. These more detailed results complemented the main analyses described above and are reported in web-only materials.
Figure 3.
Mean willingness to take the medication by format (non-numeric, numeric), age group, and numeracy. Error bars indicate +/− 1 standard error of the mean.
Figure 4.
Figure 4a and 4b. Mean willingness to take the medication by format, numeracy, age, and medication usage a) among respondents who were not taking medication to lower cholesterol and b) among respondents who were taking medication to lower cholesterol. Error bars indicate +/− 1 standard error of the mean.
Reasons for willingness to take the drug
Table 4 includes the proportions of people overall and by numeric vs non-numeric format stating each of eight reasons for their willingness to take the drug. We compared responses in non-numeric formats (where risk estimates were more likely to be exaggerated) with those in numeric formats and examined interactions with numeracy and age. Because respondents not taking cholesterol-lowering medications reported lower willingness to take the drug compared to those who were on such medications and because format influenced risk overestimation and willingness to use the drug, we analyzed reasons for taking the drug separately in two medication-usage groups and focused on significant results with respect to format.
Table 4.
Proportion of people overall and in non-numeric and numeric formats who state a most important reason for their answer about how likely they are to take the drug. Ordering of reasons was based on proportion of responses in the CMI format (the third column).
| Non-numeric formats | Numeric formats | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-numeric formats | Numeric formats | CMI | Risk label | Frequency | Frequency_Risk label | Percent | Percent_Risk label | |
| Very serious muscle damage | 17 | 10 | 24 | 10 | 15 | 5 | 10 | 9 |
| Serious side effects not likely | 18 | 26 | 21 | 16 | 22 | 30 | 25 | 30 |
| Prefer to avoid meds | 20 | 15 | 19 | 21 | 16 | 16 | 18 | 11 |
| Side effects not serious | 16 | 25 | 11 | 21 | 28 | 18 | 25 | 28 |
| Too many side effects | 13 | 8 | 9 | 17 | 8 | 8 | 8 | 8 |
| You don’t want to experience side effect | 3 | 6 | 3 | 4 | 5 | 9 | 6 | 4 |
| Other | 10 | 7 | 10 | 10 | 6 | 10 | 6 | 7 |
| None | 2 | 3 | 3 | 1 | 1 | 4 | 3 | 2 |
| 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
Among those not using a similar medication, non-numeric-format respondents were more likely than numeric-format respondents to choose as their most important reason “The very serious muscle damage” (17% vs. 9%, b=−.38, p=.01) whereas numeric-format respondents were more likely than non-numeric-format ones to state that “Most of the side effects are not very serious” (24% vs. 12%, b=.45, p=.001). Format also interacted with age such that older adults were more likely to state in the numeric vs non-numeric condition that “A lot of people will experience at least one of the side effects, and you don’t want to be one of them,” 9% and 0%, respectively) whereas condition did not matter as much to younger (7% and 9%) or middle-aged adults (5% and 1%, interaction b=1.3, p=.02). Except for this age interaction, neither age nor numeracy interacted with format to predict reasons although each was sometimes a significant predictor itself. Tables 5 and 6 indicate significant predictors of each reason among respondents, respectively, not using and using cholesterol-lowering medication.
Table 5.
Logistic-regression results for each “most important reason” (Non-standardized coefficients b (Standard Error (SE)) among those respondents not using cholesterol-lowering medication (n = 548). Significant predictors are in bold for emphasis.
| Very serious muscle damage B (SE) |
Prefer to avoid medications B (SE) |
Serious side effects not likely B (SE) |
Side effects not serious B (SE) |
Too many side effects B (SE) |
You don’t want to experience side effect B (SE) |
|
|---|---|---|---|---|---|---|
| Constant | −2.05 (.15) | −1.19 (.11) | −1.48 (.12) | −1.60 (.14) | −2.30 (.17) | −3.75 (.50) |
| Numeric Format | −.38 (.15) | −.10 (.11) | .20 (.12) | .45 (.14) | −.28 (.17) | .89 (.50) |
| Numeracy | −.06 (.08) | .03 (.06) | .12 (.07) | −.17 (.08) | −.17 (.09) | −.04 (.32) |
| Age | .43 (.21) | .16 (.16) | −.03 (.18) | −.56 (.21) | .35 (.24) | −1.03 (.58) |
| Numeric Format * Numeracy | .01 (.08) | −.01 (.06) | .06 (.07) | .07 (.08) | −.04 (.09) | .01 (.32) |
| Numeric Format * Age | −.07 (.21) | .12 (.16) | −.30 (.18) | .00 (.21) | −.09 (.24) | 1.33 (.58) |
| Numeracy * Age | .01 (.08) | −.10 (.09) | .02 (.10) | −.08 (.11) | .04 (.12) | .16 (.34) |
| Numeric Format * Numeracy * Age | −.21 (.11) | .14 (.09) | .06 (.10) | .02 (.11) | −.06 (.12) | .18 (.34) |
| Nagelkerke R2=.06, χ2 (df=7) = 16.7, p=.02 | Nagelkerke R2=.02, χ2 (df=7) = 6.8, p=.45 | Nagelkerke R2=.04, χ2 (df=7) = 12.8, p=.08 | Nagelkerke R2=.08, χ2 (df=7) = 28.8, p=.00 | Nagelkerke R2=.04, χ2 (df=7) = 10.2, p=.18 | Nagelkerke R2=.09, χ2 (df=7) = 16.6, p=.02 |
Table 6.
Logistic-regression results for each “most important reason” (Non-standardized coefficients b (Standard Error (SE)) among those respondents using cholesterol-lowering medication (n = 357). Significant predictors are in bold for emphasis.
| Very serious muscle damage B (SE) |
Prefer to avoid medications B (SE) |
Serious side effects not likely B (SE) |
Side effects not serious B (SE) |
Too many side effects B (SE) |
You don’t want to experience side effect B (SE) |
|
|---|---|---|---|---|---|---|
| Constant | −1.92 (.24) | −2.40 (.28) | −1.13 (.20) | −.97 (.18) | −2.57 (.30) | −3.62 (.57) |
| Numeric Format | −.09 (.24) | −.64 (.28) | .39 (.20) | .14 (.18) | −.42 (.30) | −.64 (.57) |
| Numeracy | .02 (.13) | −.10 (.14) | .07 (.10) | −.11 (.09) | −.15 (.15) | .47 (.24) |
| Age | .07 (.32) | −.38 (.41) | .23 (.26) | −.46 (.26) | .60 (.37) | .87 (.65) |
| Numeric Format * Numeracy | −.14 (.13) | .14 (.14) | .01 (.10) | .02 (.09) | −.08 (.15) | .30 (.24) |
| Numeric Format * Age | −.21 (.32) | .39 (.41) | −.17 (.26) | −.05 (.26) | −.19 (.37) | .88 (.65) |
| Numeracy * Age | −.24 (.17) | −.10 (.20) | .07 (.14) | .03 (.13) | .22 (.19) | −.37 (.28) |
| Numeric Format * Numeracy * Age | .13 (.17) | −.22 (.20) | .12 (.14) | .04 (.13) | −.19 (.19) | −.50 (.28) |
| Nagelkerke R2=.04, χ2 (df=7) = 7.1, p=.42 | Nagelkerke R2=.07, χ2 (df=7) = 9.8, p=.20 | Nagelkerke R2=.05, χ2 (df=7) = 12.6, p=.08 | Nagelkerke R2=.03, χ2 (df=7) = 6.8, p=.45 | Nagelkerke R2=.07, χ2 (df=7) = 11.5, p=.12 | Nagelkerke R2=.08, χ2 (df=7) = 10.4, p=.17 |
Among those respondents using cholesterol-lowering drugs, numeric-format respondents were more likely than non-numeric-format respondents to state that “Most of the side effects are not very likely” (33% vs. 22%) and they were less likely to state that “You have high cholesterol, but prefer to avoid taking medications, and will do something else to lower your cholesterol” (5% vs. 11%). Again, neither age nor numeracy interacted with format to predict reasons although numeracy was a significant predictor in one case.
GENERAL DISCUSSION
In this study, risk comprehension was assessed by whether respondents gave the correct numeric risk response. Such a correct response should have been (and was) more likely in the numeric formats (81% and 0% of numeric- and nonnumeric-format respondents, respectively, gave the correct response). Whether or not the numeric risk was understood correctly, we found that the numeric risk perceptions mattered. Specifically, respondents who estimated higher risk likelihoods were also less willing to take the medication. The possible concern that underestimating risk to a significant degree would lead respondents to be particularly willing to take medications was not supported by the data. Not being sufficiently aware of possible adverse events did not appear to encourage willingness to take the medication as much as overestimating risks appeared to discourage it. This finding, combined with the small correlation between higher risk estimates and lower willingness to take the drug (r=−.13, p<.001), highlights the fact that risk perception (as assessed by these numeric risk estimates), while important, is not the only factor that underlies willingness. Other factors that were not the focus of our experimental format manipulation may also play a role, including a lack of ability and experience necessary to make precise numeric AE estimates (although the non-numeric AE estimates for dizziness demonstrated similar effects), dread of the AE, perceptions of AE likelihood in comparison to other AEs or to the medication benefit, and perceptions that AEs can be monitored and/or mitigated.
The primary experimental focus of the present paper – presenting possible AEs in numeric versus non-numeric formats – also mattered. Risk comprehension and perception, willingness to take a medication, and reasons for this willingness changed depending on the format used to describe AEs. First, with respect to providing AE information in a CMI list (as is currently done in the U.S.) versus with non-numeric descriptive risk labels, CMI respondents perceived greater risk, on average; the two groups did not differ in their average willingness to take the drug. Thus, one step the FDA could take to reduce risk perceptions closer to actual levels is to provide such descriptive risk labels. However, similar proportions of respondents in both groups overestimated risk, and further results indicate that this non-numeric solution is inadequate.
Second, respondents provided numbers were, of course, more likely to report back correct numeric risks compared to respondents who did not get the numbers. Two findings are nevertheless important. A sizeable minority of numeric-format respondents overestimated risk as if they could not accurately locate and report numeric information from tables; this was particularly true among the less numerate (see Figure 2). These data are consistent with prior research that demonstrates lower comprehension and greater risk perceptions in this group when numbers are provided.22–30 The results highlight the need to understand which numeric formats work best relative to the goals of a given communication.
Third, the size of this numeracy effect, however, was small relative to the large differences between numeric and non-numeric formats. Consistent with our Hypothesis 1 and previous research, non-numeric formats (CMI and risk labels) led respondents to overestimate risk likelihood more and to be less willing to take the drug compared to numeric formats. These results were similar regardless of reported medication usage. It may be that non-numeric-format AE lists are perceived as more ambiguous than numeric formats and result in greater risk aversion to the medication.37–39 Providing risk-likelihood information in numeric formats may allow instead for comparisons between various AEs and other risks that put into perspective the risks posed by the medication.
This reasoning is consistent with our Hypothesis 4 and the factors most important to numeric-format respondents – 50% indicated that most AEs were not serious or not likely compared to 30% of non-numeric-format respondents (Table 4). As expected, non-numeric-format respondents were more likely than numeric-format respondents to consider the rare, serious AE as the most important factor in their response, if they were not on a cholesterol-lowering medication. If they were on a similar medication, non-numeric format versus numeric-format respondents were more likely to say that they prefer to avoid medications. Providing numbers may have encouraged a greater focus on the less serious AEs and/or their overall likelihood as opposed to focusing on the large number of possible AEs and very serious AE. Because risk comprehension is a basic building block of good decisions and less accurate risk comprehension (and particularly risk overestimation) was associated with lower willingness to take the medication, these findings may have important clinical implications. Individuals provided only non-numeric information may be less likely to uptake and adhere to prescribed medications (relative to those provided numeric AEs) because they understand risks less well and overestimate them more.
Prior research had not examined age and numeracy differences in response to numeric vs non-numeric AE formats. Speculation existed that providing numeric information could harm risk comprehension among less numerate populations.31 This supposition (encapsulated in our Hypothesis 2) was not supported by the current results. Both more and less numerate respondents were less likely to overestimate risks and were more willing to take the prescribed medication when provided numeric versus non-numeric information. In partial support of Hypothesis 2, the beneficial effects of numeric information on risk overestimation were somewhat larger among the highly numerate. Responses of less numerate individuals to numeric information emphasize the need to provide comprehensible and usable numeric information to consumers across numeracy levels.
At first glance and in partial support of our Hypothesis 3, it appears that the benefits of numeric information, however, may not extend past younger adulthood. Middle-aged and older respondents who were less numerate demonstrated smaller differences in reported willingness to use the drug in response to numeric versus non-numeric AE information (particularly among respondents who reported taking a cholesterol-lowering medication). When provided numeric rather than non-numeric information, older adults not on a cholesterol-lowering medication unexpectedly focused more on the quantity of people who would experience an AE and not wanting to be one of them. This set of results may signal a problem and suggest that provision of numeric drug information could prove detrimental to uptake and adherence in older populations.33,40 They also demonstrate that individuals already on a similar medication are not immune to format effects, and it is possible that a change to numeric AE communications, even among experienced users, could affect adherence. However, the data may reflect more informed decisions. Risk-overestimation results revealed that all age and numeracy groups were less likely to overestimate risks in the numeric compared to non-numeric formats. Inconsistent with our Hypothesis 3, age played no significant role in these overestimations. Thus, although some groups experience more difficulties with numeric information, risk overestimation in the nonnumeric formats dominates that in the numeric formats for every age-by-numeracy group.
One caveat is that the descriptive results by age and numeracy shown in Figures 3–4 were based on a median split of numeracy scores. Due to sample size considerations in individual subgroups, we did not break down the descriptive results further. The inferential results were based on continuous numeracy scores, and the current descriptive results beg the question of what happens to willingness to use a drug among particularly low numerate older adults where it is plausible that providing numbers could have a detrimental effect on uptake and/or adherence. This population is important for more targeted future studies due to the potential for health disparities that could emerge due to their lower numeracy combined with age increases in the use of prescription drugs.
Strengths of this study include strong internal validity due to the experimental design. External validity was aided by a diverse sample of respondents and high response rate. The study was US-centric although its results are likely applicable to other countries. Because this study used a hypothetical situation, it is possible that respondents may behave differently if actually prescribed medication. We do not know whether the format differences found are clinically meaningful although some differences were large (e.g., risk overestimation in non-numeric vs numeric formats). In addition, recruitment through an internet panel meant that respondents, while diverse in age and numeracy, were not representative of the U.S. population and likely had more computer skills than the average consumer; they may have differed in other ways as well. We do not know, for example, whether prior experience with a medication AE may have influenced perceptions. Experience may increase risk perceptions, especially among the less numerate, based on studies demonstrating that the less numerate are more likely to use nonnumeric and often emotional sources of information in decisions compared to the highly numerate and prior experience with an AE seems likely to have such an emotional impact.22 On the other hand, others have found that patients who have previously experienced AEs are more willing to accept the risk of toxicity compared to patients who have not experienced AEs41, suggesting that patients’ anticipated effects of AEs on quality of life might be worse than their actual effects. We also did not examine provision of benefit information, an equally important topic; provision of numeric benefits is likely to reduce willingness to take drugs across age and numeracy groups because, otherwise, patients may assume that the drug always works.42 Finally, this is a single study. Risk-comprehension and willingness-to-use results comparing numeric and non-numeric formats are likely robust given prior similar results. Less well studied, however, are results with respect to numeracy and age. These are important topics that deserve further study, particularly among less numerate older adults – a population that is both vulnerable and among the primary users of prescription drugs.
The FDA currently does not mandate provision of numeric information about medications, although research consistently points towards its benefits.15,43,44 The current results point towards the need for FDA to develop a strategic plan to confront the complexities of providing risk information including the consideration of information formats (numeric vs nonnumeric) and of patient factors (e.g., numeracy, age).
Patients are playing an increasingly active role in medical decisions;45,46 this requires them to interpret, process, and apply medical information to assess potential risks and benefits of their options. In these cases, effective risk communication can be defined as messages that help an individual make informed choices aligned with personal values that they are less likely to regret in the future.11,47 The question remains whether effective risk communication is possible without the provision of the best numeric information available.
Based on current and past results, a recommendation can be made to provide numeric information concerning AE likelihoods. This study revealed that providing numeric likelihood information for AEs is likely to generate improved risk comprehension and greater adherence to taking prescribed medication overall, which in turn may lead to better health outcomes.
Supplementary Material
Acknowledgments
Financial support for this study was provided by National Science Foundation SES-1047757 and 1155924 to Dr. Peters. Dr. Fraenkel was supported by the NIAMS K24 AR060231-01. Data collection was supported by the National Institute on Aging, Grant Numbers R01AG20717 and P30 AG024962. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Main findings were presented at the 2012 annual conference for the Society for Medical Decision Making.
Ellen Peters had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported by National Science Foundation SES-1047757 and -1155924 to Dr. Peters. Dr. Fraenkel was supported by the NIAMS K24 AR060231-01. Data collection was supported by the National Institute on Aging, Grant Numbers R01AG20717 and P30 AG024962. All views expressed in this paper are those of the authors alone. No author has any relevant financial interests in this manuscript. We thank the editors and anonymous reviewers for helpful comments.
References
- 1.World Health Organization. [Accessed August 22, 2012.];Adherence to long-term therapies: evidence for action. http://www.who.int/chp/knowledge/publications/adherence_report/en/. Published 2003.
- 2.Katz JN, Daltroy LH, Brennan TA, Liang MH. Informed consent and the prescription of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1992;35(11):1257–1263. doi: 10.1002/art.1780351103. [DOI] [PubMed] [Google Scholar]
- 3.Sleath B, Tulsky JA, Peck BM, Thorpe J. Provider-patient communication about antidepressants among veterans with mental health conditions. Am J Geriatr Pharmacother. 2007;5(1):9–17. doi: 10.1016/j.amjopharm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. doi: 10.1001/archinte.166.17.1855. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler DK, Mosier MC, Buenaver M, Okuyemi K. How much information about adverse effects of medication do patients want from physicians? Arch Intern Med. 2001;161(5):706–713. doi: 10.1001/archinte.161.5.706. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect. 2004;7(3):235–245. doi: 10.1111/j.1369-7625.2004.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrank WH, Avorn J. Educating patients about their medications: the potential and limitations of written drug information. Health Aff. 2007;26(3):731–740. doi: 10.1377/hlthaff.26.3.731. [DOI] [PubMed] [Google Scholar]
- 8.Winterstein AG, Linden S, Lee AE, Fernandez EM, Kimberlin CL. Evaluation of consumer medication information dispensed in retail pharmacies. Arch Intern Med. 2010;170(15):1317–1324. doi: 10.1001/archinternmed.2010.263. [DOI] [PubMed] [Google Scholar]
- 9.Ostrove N. Background and overview of the CMI, PPI, Medication Guide Programs. Presented at: Food and Drug Administration Risk Communication Advisory Committee Meeting; February 26, 2009; Washington, DC. [Google Scholar]
- 10.Berry DC. Informing people about the risks and benefits of medicines: implications for the safe and effective use of medicinal products. Curr Drug Saf. 2006;1:121–126. doi: 10.2174/157488606775252638. [DOI] [PubMed] [Google Scholar]
- 11.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27(5):696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 12.Berry DC, Knapp P, Raynor DK. Provision of information about drug side-effects to patients. Lancet. 2002;359(9309):853–854. doi: 10.1016/s0140-6736(02)07923-0. [DOI] [PubMed] [Google Scholar]
- 13.Berry DC, Raynor DK, Knapp P. Communicating risk of medication side effects: an empirical evaluation of EU recommended terminology. Psychol Health Med. 2003;8(3):251–263. [Google Scholar]
- 14.Berry DC, Raynor DK, Knapp P, Bersellini E. Patients’ understanding of risk associated with medication use: impact of European Commission Guidelines and other risk scales. Drug Saf. 2003;26:1–11. doi: 10.2165/00002018-200326010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Berry D, Raynor T, Knapp P, Bersellini E. Over the counter medicines and the need for immediate action: a further evaluation of European Commission recommended wordings for communicating risk. Patient Educ Couns. 2004;53(2):129–134. doi: 10.1016/S0738-3991(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 16.Steiner MJ, Dalebout S, Condon S, Dominik R, Trussell J. Understanding risk: a randomized controlled trial of communicating contraceptive effectiveness. Am J Obstet Gynecol. 2003;102(4):709–717. doi: 10.1016/s0029-7844(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 17.Knapp P, Raynor DK, Berry DC. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Qual Saf Health Care. 2004;13(3):176–180. doi: 10.1136/qshc.2003.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young S, Oppenheimer DM. Effects of communication strategy on personal risk perception and treatment adherence intentions. Psychol Health Medicine. 2009;14(4):430–442. doi: 10.1080/13548500902890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson BL, Obrecht NA, Chapman GB, Driscoll DA, Schulkin J. Physicians’ communication of Down syndrome screening test results: the influence of physician numeracy. Genet Med. 2011;13(8):744–749. doi: 10.1097/GIM.0b013e31821a370f. [DOI] [PubMed] [Google Scholar]
- 20.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 21.Sheridan SL, Pignone M. Numeracy and the medical student’s ability to interpret data. Eff Clin Pract. 2002;5(1):35–40. [PubMed] [Google Scholar]
- 22.Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17(5):407–413. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 23.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135(6):943–973. doi: 10.1037/a0017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters E, Dieckmann N, Dixon A, Hibbard JH, Mertz CK. Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2):169–190. doi: 10.1177/10775587070640020301. [DOI] [PubMed] [Google Scholar]
- 25.Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff. 2007;26(3):741–748. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Inter Med. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Dieckmann NF, Slovic P, Peters E. The use of narrative evidence and explicit likelihood by decisionmakers varying in numeracy. Risk Anal. 2009;29(10):1473–1488. doi: 10.1111/j.1539-6924.2009.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns WJ, Peters E, Slovic P. Risk perception and the economic crisis: a longitudinal study of the trajectory of perceived risk. Risk Anal. 2012;32(4):659–677. doi: 10.1111/j.1539-6924.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 29.Lipkus IM, Peters E, Kimmick G, Liotcheva V, Marcom P. Breast cancer patients’ treatment expectations after exposure to the decision aid program, adjuvant online: the influence of numeracy. Med Decis Making. 2010;30(4):464–473. doi: 10.1177/0272989X09360371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Retamero R, Galesic M. Using plausible group sizes to communicate information about medical risks. Patient Educ Couns. 2011;84(2):245–250. doi: 10.1016/j.pec.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PH. Questioning the quantitative imperative decision aids, prevention, and the ethics of disclosure. Hastings Cent Rep. 2011;41(2):30–39. doi: 10.1353/hcr.2011.0029. [DOI] [PubMed] [Google Scholar]
- 32.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- 33.Peters E, Hess TM, Västfjäll D, Auman C. Adult age differences in dual information processes: implications for the role of affective and deliberative processes in older adults’ decision making. Perspect Psychol Sci. 2007;2(1):1–23. doi: 10.1111/j.1745-6916.2007.00025.x. [DOI] [PubMed] [Google Scholar]
- 34.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief. 2010;42:1–8. [PubMed] [Google Scholar]
- 35.Wilson IB, Schoen C, Neuman P, et al. Physician-patient communication about prescription medication nonadherence: a 50-state study of America’s seniors. J Gen Intern Med. 2007;22(1):6–12. doi: 10.1007/s11606-006-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weller J, Dieckmann NF, Tusler M, Mertz CK, Burns W, Peters E. Development and testing of an abbreviated numeracy scale: a Rasch Analysis approach. J Behav Decis Mak. doi: 10.1002/bdm.1751. Advance online publication. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dieckmann NF, Peters E, Gregory R, Tusler M. Making sense of uncertainty: advantages and disadvantages of providing an evaluative structure. J Risk Res. In press. [Google Scholar]
- 38.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Politi MC, Han PKJ, Col NF. Communicating the uncertainty of harms and benefits of medical interventions. Med Decis Making. 2007;27(5):681–695. doi: 10.1177/0272989X07307270. [DOI] [PubMed] [Google Scholar]
- 40.Peters E, Bruine de Bruin W. Aging and decision skills. In: Dhami MK, Schlottmann A, Waldmann M, editors. Judgment and Decision Making as a Skill: Learning, Development, and Evolution. Cambridge, United Kingdom: Cambridge University Press; 2012. pp. 113–139. [Google Scholar]
- 41.Fraenkel L, Bogardus S, Concato J, Felson D. Unwillingness of rheumatoid arthritis patients to risk adverse effects. Rheumatology(Oxford) 2002;41(3):253–61. doi: 10.1093/rheumatology/41.3.253. [DOI] [PubMed] [Google Scholar]
- 42.Woloshin S, Schwartz LM, Welch HG. The value of benefit data in direct-to-consumer drug ads. Health Affairs. 2004;W4:234–245. doi: 10.1377/hlthaff.w4.234. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz LM, Woloshin S, Welch GH. The drug facts box: providing consumers with simple tabular data on drug benefit and harm. Med Decis Making. 2007;27(5):655–662. doi: 10.1177/0272989X07306786. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz LM, Woloshin S, Welch GH. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516–W93. doi: 10.7326/0003-4819-150-8-200904210-00106. [DOI] [PubMed] [Google Scholar]
- 45.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 46.Joosten E, DeFuentes-Merillas L, de Weert G, Sensky T, van der Staak C, de Jong C. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 47.Edwards A, Elwyn G. How should effectiveness of risk communication to aid patients’ decisions be judged?: A review of the literature. Med Decis Making. 1999;19(4):428–434. doi: 10.1177/0272989X9901900411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.