Abstract
Monocytes are mononuclear circulating phagocytes that originate in the bone marrow and give rise to macrophages in peripheral tissue. For decades, our understanding of monocyte lineage was bound to a stepwise model that favored an inverse relationship between cellular proliferation and differentiation. Sophisticated molecular and surgical cell tracking tools have transformed our thinking about monocyte topo-ontogeny and function. Here, we discuss how recent studies focusing on progenitor proliferation and differentiation, monocyte mobilization and recruitment, and macrophage differentiation and proliferation are reshaping knowledge of monocyte lineage in steady state and disease.
Keywords: monocyte, hematopoiesis, macrophage, proliferation, atherosclerosis
INTRODUCTION
For years, monocytes were viewed simply as transitional cells, fated to give rise to either macrophages or dendritic cells depending on tissue context. With the discovery of functionally heterogeneous monocyte subsets in several species, it is clear that monocyte participation in the immune response extends beyond differentiation. Several populations of increasingly restricted myeloid and monocyte progenitors have been identified in the bone marrow. Adding to the complexity, recent evidence points to embryonic, rather than hematopoietic, origins for macrophages in many tissues. This finding has led to the realization that macrophage renewal often relies on local proliferation rather than monocyte recruitment. Our own research demonstrates that monocyte-derived macrophages also self-renew via mechanisms that are distinct from those facilitating monocyte generation and recruitment. Here, we discuss the latest contributions to our understanding of monocyte-macrophage lineage commitment in homeostasis and during inflammation. We will focus on the monocyte-macrophage lineage in cardiovascular disease, the major cause of death worldwide [1] but will also draw from other literature to provide broader context. A graphical representation of our discussion is found in Figure 1.
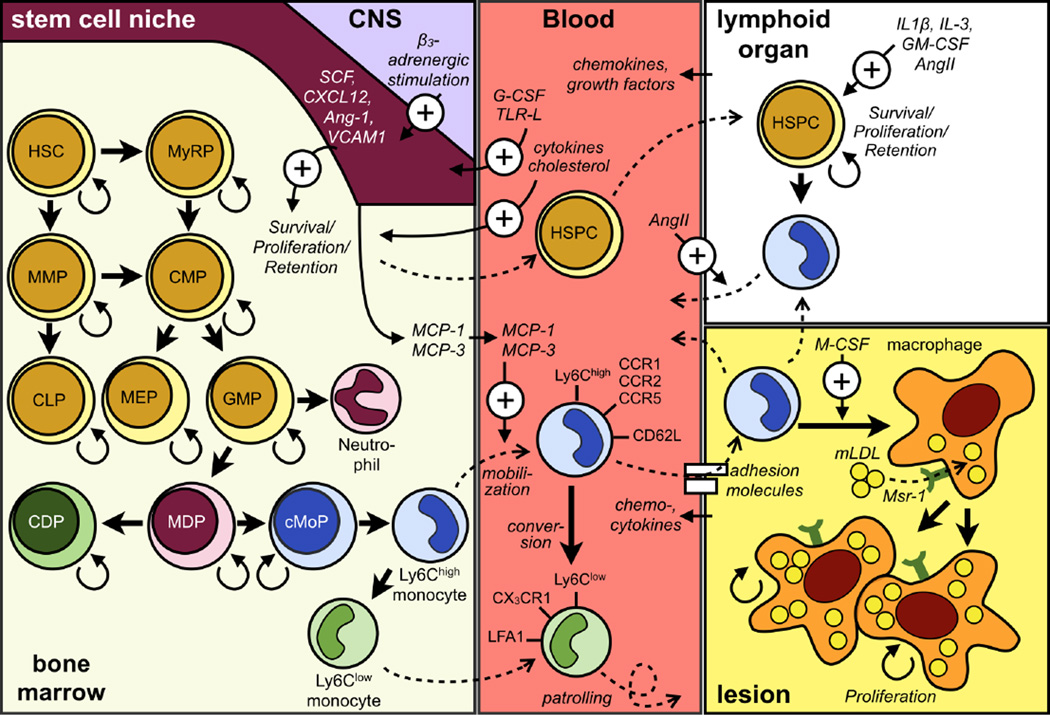
Figure 1.
The topo-ontogenic relationships in the monocyte lineage. The figure shows the development of monocytes in the bone marrow and extramedullary sites from hematopoietic precursors, their circulation in the blood, and differentiation and proliferation in tissue.
PROGENITOR PROLIFERATION AND DIFFERENTIATION
In the healthy adult, monocytes arise through the proliferation and differentiation of hematopoietic stem and progenitor cells (HSPC) in the bone marrow. The sequential differentiation that links the multipotent, self-renewing hematopoietic stem cell (HSC) to the “terminally-differentiated” and “non-renewing” monocyte involves a series of distinct intermediate populations that can be characterized phenotypically by the expression of multiple surface markers such as Sca-1, IL7Rα, c-Kit, the SLAM family markers (CD150, CD229, CD48, CD244), CD34, CD16/32, CD115, and CD135, among others. In general, as HSPC progress through multipotent hematopoietic progenitors (MPP), common myeloid progenitors (CMP), granulocyte and macrophage progenitors (GMP), macrophage and dendritic cell progenitors (MDP), and common monocyte progenitors (cMoP) – in short, as they become more restricted toward the monocyte lineage – they lose self-renewing capacity and multipotency [2–5]. A recent study challenged this stepwise model, however, by identifying myeloid-restricted progenitors with long-term repopulating activity (MyRP) within the HSC compartment. Using single-cell transplantation systems, the authors show that HSC can differentiate directly into MyRP via asymmetric division. The event bypasses HSC’s progression through specific differentiation stages while retaining their self-renewing capacity [6]. Identifying the MyRP not only highlights an elegant nuance in the hematopoietic system’s plasticity, reorienting its structure from hierarchical to networked, but also provides a molecular rationale for the rapid generation of myeloid cells in response to inflammatory stimuli.
Lineage-commitment occurs under tight transcriptional control. Among the transcription factors that shape hematopoiesis, PU.1 is the master regulator of myeloid and lymphoid differentiation. PU.1−/− mice either lack or have significantly reduced numbers of B and T cells, monocytes, neutrophils and macrophages [7, 8], and PU.1-deficient HSC are defective in generating CMP and CLP but not MEP. The transcription factor is important throughout the myeloid differentiation cascade because targeted deletion of PU.1 in the downstream progenitors CMP/GMP impairs the appearance of mature CD11b+ cells [9]. Transcription factors other than PU.1 also contribute, and decisions between particular lineages require transcription factor co-operation. Among the most important factors that promote monocyte production are C/EBPα, which facilitates the CMP to GMP transition [10]; interferon regulatory factor 8 (IRF8), which interacts with PU.1 to favor monocyte/macrophage over granulocyte differentiation [11, 12]; Krüppel-like factor 4 (KLF4), which is essential to the generation of inflammatory Ly-6Chigh monocytes [13, 14]; and the orphan nuclear hormone receptor NR4A1/Nur77 which generates Ly6Clow monocytes [15, 16].
If transcription factors guide HSPC proliferation and differentiation intrinsically, then growth factors are the environmental, or extrinsic, instructors [17, 18]. For example, M-CSF, GM-CSF, G-CSF, and IL-3 are four growth factors that promote granulocyte/macrophage differentiation. M-CSF is most important to monocyte production in the steady state because M-CSF (or its receptor) deficiency causes a near-absence of monocytes, and because MDP, the committed monocyte precursors, are defined by surface M-CSF receptor (CD115) expression. In accordance with the idea that hematopoiesis bypasses stepwise differentiation, recent studies have shown that M-CSF is systemically elevated during inflammation and induces PU.1 in HSC, leading to myeloid cell differentiation [19].
Unlike M-CSF, the β-chain growth factors, including GM-CSF and IL-3, are dispensable to monocyte production because their deficiency has little to no effect on circulating monocyte numbers in the steady state. That said, GM-CSF and IL-3 are important to inflammation-borne emergency myeloid cell production [20] in either the bone marrow or, as will be discussed below, extramedullary sites. That monocyte production utilizes different growth factors during inflammation raises questions about whether and how emergency and steady state hematopoiesis differ. The panoply of cytokines, chemokines, toll-like receptor ligands, and other mediators that are undetectable in the steady state but dominate during inflammation may profoundly affect hematopoiesis at virtually every stage. HSPC can expand in the context of some, but not all bacterial, viral and fungal infections [21], but expansion may be accompanied by reduced differentiation capacity [22]. Depending on the context, interferon (IFN) α and γ can stimulate HSC proliferation but can also exhaust cells’ repopulation capacities. Likewise, TLRs, which are expressed on HSPC, stimulate proliferation but decrease repopulation capacity and induce myeloid skewing [23]. These examples highlight the importance of examining hematopoiesis not as a singular immutable process but rather as a highly adaptable series of context-dependent interactions.
Homeostasis of HSC and restricted progenitors is further regulated by neighboring cells that form the stem cell niche. HSC are heterogenous in terms of cycling frequency and location in the bone marrow niche. More quiescent HSC found in the endosteal bone marrow niche relocate to perivascular niches when activated [2, 24, 25]. Niches comprise different cell types such as osteoblasts, fibroblasts, macrophages, endothelial cells, and perivascular stromal and mesenchymal stem cells with distinct and complementary functions. Stromal cell derived factor-1 (SDF1/CXCL12), for example, is produced by osteoblasts, endothelial cells, and different perivascular stromal cells; deletion of the CXCL12 receptor CXCR4 in the adult mouse depletes HSC in the bone marrow [26]. The effects of deleting CXCL12 depend on the cell source. Targeted deletion of CXCL12 from nestin-negative mesenchymal progenitor cells decreases the frequency, quiescence, and repopulation capacity of HSC; deletion from endothelial cells has only modest effects; deletion from osteoblasts primarily affects CLP maintenance; and deletion from osteoprogenitors, perivascular reticular, and sinusoidal stromal cells induces HSPC mobilization into the blood and spleen [27, 28]. Additionally, HSC are maintained in the bone marrow niche by stem cell factor (SCF), adhesion molecules, nestin-positive mesenchymal stem cells, and sialoadhesin (also known as siglec-1 or CD169)-expressing macrophages[29–32]. The effects of multiple other niche factors on the maintenance, retention, and differentiation of HSPC is under intense investigation.
Like the neighboring cells that constitute the bone marrow niche, the nervous system is an important contributor to HSPC retention and mobilization. In the steady state, for example, the central circadian clock promotes HSPC mobilization via β3-adrenergic receptor signaling and CXCL12 downregulation [33–35]. In pathological conditions such as atherosclerosis [36], myocardial infarction [37], and cancer [38], HSPC mobilization increases acutely and progressively. Although the mechanisms that promote HSPC mobilization during pathology are incompletely understood, HSPC release after MI involves β3-adrenergic receptor signaling and CXCL12 downregulation [39], suggesting that other nervous system stimuli – perhaps related to stress or pain [40] – converge on this pathway. Aside from its granulopoietic functions, G-CSF is perhaps best known for its capacity to promote HSPC mobilization. Recent work has shown that peripheral sympathetic nerve neurons, which express the G-CSF receptor, increase norepinephrine availability in response to G-CSF [41]. Taken together, these studies establish the bone marrow as the principal site of monocyte production and the medullary niche in particular as the primary controller of monocyte progenitor proliferation, differentiation, retention, and mobilization. In the next section, we detail the mechanisms of monocyte mobilization and the consequences of both monocyte and HSPC mobilization.
MONOCYTE MOBILIZATION AND RECRUITMENT
Niche reconstruction, HSPC quality control, and immunosurveillance have been proposed as the major drivers of HSPC mobilization [42–44]. HSPC depart their niches, the argument goes, to facilitate niche maintenance and growth. The process, which may involve reciprocal anabolic and catabolic functions of the various cellular and acellular niche constitutents, requires that HSPC temporarily depart their home. Selecting for the most fit HSPC is a collateral advantage of niche maintenance, and, in turn, competitively finding the “best” bone marrow niche will presumably select only the most fit HSPC. After all, HSPC are required to repopulate the entire leukocyte repertoire; they are essential to life, so it makes sense that the organism would have methods for selecting optimal HSPC. Circulating HSPC – that is, mobilized HSPC – may also participate in immunosurveillance by surveying tissue and giving rise to their progeny if necessary.
Like niche and HSPC selection and immunosurveillance, extramedullary hematopoiesis is driven by HSPC mobilization. Studies have shown that mobilized HSPC instigate extramedullary hematopoiesis in chronic diseases, such as atherosclerosis and cancer, and in acute inflammatory conditions, such as myocardial infarction [36–39]. In other words, increased HSPC mobilization during disease seeds secondary lymphoid organs such as the spleen. It is unclear to what extent establishing extramedullary hematopoiesis depends on increased mobilization versus HSPC retention in extramedullary sites, though evidence points to both processes playing a role. In atherosclerosis, lipid-laden splenic macrophages secrete IL-23 that, via IL-17 production, generates G-CSF, a known liberating factor [45]. Once liberated, HSPC may be retained in extramedullary sites such as the spleen via Angiotensin-II-dependent control of S1P signaling [46]. The seeded cells can then respond to growth factors such as GM-CSF and IL-3 [36] and inflammatory cytokines such as IL-1β [37], which may directly or indirectly guide HSPC proliferation and differentiation into progeny. The growth factors’ cellular sources and corresponding molecular triggers may involve the recently identified innate response activator B cell [47]. Additional research is needed to better understand the cellular and molecular components that constitute the extramedullary niche.
Extramedullary niches in atherosclerosis, cancer, and after myocardial infarction preferentially generate myeloid cells (particularly Ly-6Chigh monocytes and neutrophils) largely to the exclusion of other leukocytes, despite the plethora of upstream progenitors that can be readily identified in extramedullary sites. The reasons for this myeloid bias, which increases with age in the bone marrow [48], are not entirely clear but may be related to either the dominance of myeloid-generating growth factors (GM-CSF) in extramedullary sites or the preferential expansion of a specific HSPC subset that can withstand the harsh extramedullary environment and escape phagocytosis by red pulp macrophages and Kuppfer cells. Regardless of how extramedullary hematopoiesis is established, it may occur during inflammation because the bone marrow outsources production of highly inflammatory and proteolytic cells to distance them from the bone marrow in order to preserve its homeostatic role as the housekeeping leukocyte production site. Given the sites where extramedullary hematopoiesis can occur (spleen, liver), experimental verification of this hypothesis is challenging. However, that myeloid cells produced in extramedullary sites do exit, circulate, and accumulate in diseased or injured tissue counters the argument that extramedullary hematopoiesis is a collateral artifact of HSPC mobilization.
Extramedullary monocytopoiesis notwithstanding, the bone marrow remains the primary monocyte-producing location. Mobilizing monocytes to exit the bone marrow depends on the chemokine receptor CCR2 and the corresponding ligands MCP-1 and MCP-3, which are produced by mesenchymal stem cells and released in the vicinity of the lumen [49–53] or, in some contexts, by B cells in the periphery [54]. Monocytes release into the blood does not occur at a constant rate, even in the steady state, but is controlled in part by circadian rhythms [35, 55, 56]. A recent study has shown that peripheral circadian clocks are expressed in monocytes and control their cyclical fluctuations in the blood [57]. Bmal1, which is one of the molecular clock’s core transcription factors, fluctuates in Ly-6Chigh monocytes and their descendant macrophages. As Bmal1 expression rises, the number of circulating Ly-6Chigh monocytes decreases, possibly because Bmal1 represses MCP-1 expression in macrophages, thus attenuating monocyte mobilization from the bone marrow.
Steady state mouse blood contains a nearly equal mixture of two monocyte subsets that differ in many ways but are defined most often by differential expression of the surface glycoprotein Ly-6C (Reviewed and discussed in [4, 58–62]). The blood contains Ly-6Chigh monocytes (Ly-6C is sometimes called Gr-1, but the two are not interchangeable because Gr-1 also recognizes the neutrophil marker Ly-6G) which are also CCR2high; and Ly-6Clow monocytes, which do not express CCR2 but do express high levels of the fractalkine receptor CX3CR1. Ly-6Chigh monocytes are short lived, circulate through tissue where they participate in antigen transport [63], convert to Ly-6Clow monocytes [64, 65], accumulate in inflammatory sites, and differentiate to macrophages or dendritic cells. Ly-6Clow monocytes are Ly-6Chigh-derived, circulate for longer periods, and patrol the vasculature [66]. Accumulating evidence indicates that Ly-6Clow monocytes do not differentiate to macrophages but remain in the circulation where they participate in endothelial cell disposal [67, 68].
During inflammation, as the bone marrow mobilizes a large number of Ly-6Chigh monocytes and extramedullary sites release their reservoirs of Ly-6Chigh monocytes, Ly-6Chigh monocytosis frequently ensues [69–71]. Consequently, the blood accrues a large number of inflammatory cells that can infiltrate inflammatory tissue. Implicit in this observation is the idea that increased numbers of circulating inflammatory cells correspond to increased numbers of accumulating cells in affected tissue. This raises the possibility that blocking heightened monocyte production in the bone marrow and spleen will attenuate disease and suggests that monocyte numbers should be considered potential biomarkers. In support of the latter point, clinical studies have consistently implicated leukocytosis as an independent risk factor for cardiovascular disease, although neutrophils, rather than monocytes, are more consistently associated (reviewed in [59, 72]).
One caveat worth mentioning as caution against linear correspondence between blood and tissue monocytes concerns the vast literature on adhesion, integrin, and chemokine-dependent cell accumulation [73]. In atherosclerosis, endothelial cells produce surface and secreted factors that attract monocytes; monocytes penetrate the growing lesion or wound because they are permitted to do so, not necessarily because more of them circulate. While this does not negate the contribution of monocytosis – if the endothelium expresses the requisite compendium of factors that permit monocytes to enter, then heightened monocyte concentration in the circulation may stochastically augment entrance – the fact that certain chemokine axes are essential to generating macrophage-rich atherosclerotic lesions highlights the importance of monocyte entry.
In recent years, increased interest in monocyte heterogeneity has led to more focus on chemokines’ role in promoting influx of specific monocyte subsets. Although Ly-6Chigh monocytes express Ccr2 at high levels and preferentially accumulate in atherosclerotic lesions [69, 70], other chemokines also participate. Complete blockade of CCR2, CCR5, and CX3CR1, for example, can almost abolish atherosclerosis [74–76], although CCR1 and CCR5, but not CCR2 or CX3CR1, have also been suggested as crucial chemokines that drive monocyte recruitment [77]. The precise contributions of specific chemokine receptors may require additional work to reach consensus. Variables such as animal model, diet, stage of disease, and location in the aorta may significantly impact monocyte recruitment. Nonetheless, there is general agreement that monocytes do migrate to atherosclerotic lesions and may be doing so continuously throughout the development, progression, and perhaps even regression of atherosclerosis [78–85]. What happens to them afterwards is the topic of the next section.
MACROPHAGE DIFFERENTIATION AND PROLIFERATION
In 1968 Ralph van Furth and Zanvil Cohn proposed that bone marrow progenitors give rise to circulating monocytes, which then differentiate to tissue macrophages [86]. The suggestion that not all tissue macrophages are monocyte-derived dates back to at least 1984 [87], but only recently have state-of-the-art lineage tracing technologies dramatically transformed our understanding of monocyte-macrophage topo-ontogenic (those dealing with tissue location and cellular origin) relationships. In many tissues, resident macrophages originate prenatally and independently of c-myb, hematopoietic stem cells, and their progeny [88]. Microglia in the brain, for example, derive from primitive yolk sac macrophages that arise prior to the establishment of definitive hematopoiesis [89]. Similarly, Langerhans cells derive from embryonic epidermal progenitors of the fetal liver and yolk sac [90]. Several studies have now confirmed the presence of prenatal tissue resident macrophage populations in the lung, peritoneum, liver, pancreas, kidney, and spleen that renew in adulthood independent of circulating monocytes [4, 65, 91]. Less clear, however, is the function of these monocyte-independent tissue macrophages, though it likely aligns with host tissue function [92]: adipose tissue macrophages control adaptive thermogenesis, for example, which is a well-known property of fat [93].
If monocytes continuously replenish tissue macrophages, as in the 1968 model, then macrophages may not need to self-renew. On the other hand, if macrophages arise independently of monocytes, they must do so either through self-renewal or through the proliferation of local progenitors. Although the existence of local tissue progenitors has been demonstrated [94], it is difficult to discern between circulating mobilized progenitors that can generate extramedullary hematopoiesis during inflammation and truly local progenitors that are independent of the bone marrow. Without detailed molecular and fate mapping studies we cannot rule out the possibility that those few colony forming units are the result of blood contamination or transient HSPC accumulation. Although bone marrow-independent, long-term repopulating tissue macrophage progenitors may indeed exist, recent data favor the idea that mature macrophages – as macrophages – proliferate. Microglia in the brain, epidermal Langerhans cells (LC), pleural macrophages, and resident lung macrophages maintain their monocyte-independent numbers through local proliferation [65, 89–91, 95].
These convincing observations present several important questions that merit further discussion. First, it is not clear how many times a single mature macrophage can successfully navigate the entire mitotic cycle. One possibility is that a macrophage proliferates no more than a few times, perhaps two or three, before succumbing to apoptosis. It may be that any macrophage has an equal chance to proliferate. Alternatively, small populations of multi-proliferative macrophages, akin to tissue progenitors, may be particularly adept at repopulating the tissue macrophage pool. Because current CFU protocols can only generate a few colonies from non-lymphoid steady state tissue, this latter scenario is less likely. That said, it is possible that the conditions for growing tissue macrophage progenitors in vitro are not yet optimal.
Another question is whether the molecular mechanisms that orchestrate tissue macrophage renewal converge on similar pathways or whether they are tissue-specific and molecularly divergent. Whereas repopulating tissue resident lung macrophages relies on M-CSF-and GM-CSF and is independent of IL-4 [91], replenishing macrophages during intestinal inflammation not only depends on IL-4 but may be independent of M-CSF under some conditions [96]. This apparent discrepancy may reflect differences that arise in inflammation; nevertheless, it suggests more than one route to macrophage proliferation.
Even if macrophages that can be replenished by monocytes do not need to self-renew locally, they may still do so. During the development of atherosclerosis, monocytes infiltrate the endothelium at sites of low and oscillatory shear stress and settle in the intima. Upon infiltration, monocytes can differentiate to macrophages, ingest lipoproteins, and develop into foam cells, which are major contributors to the bulk of the atherosclerotic lesion and primary culprits of the disease’s complications (reviewed in [59, 61]). Though macrophage proliferation in human, rabbit, and murine atherosclerotic lesions was identified some time ago [97–104] the phenomenon has been frequently ignored or overlooked, especially in recent years, likely because its importance to atherosclerotic development has been obscured by a number of factors. First, established atherosclerotic lesions, regardless of species, contain only a small fraction of cells in cell cycle (~1–2%), which may lead to the perception that proliferation is rare. Cell cycle analysis, however, involves markers such as PCNA or Ki67, that are expressed during specific phases of mitosis. If the lesion is relatively inactive, harboring long-lived macrophages, then a few Ki67 cells are likely insignificant to the overall pathology. If a lesion is a highly bustling hub of cellular turnover, on the other hand, then a few proliferating cells identified at any one point in time may be the tip of the iceberg: perhaps cells are continuously cycling through mitosis. As a point of comparison, the percentage of cells in mitosis in many tumors, which are known to be sites of intense cellular proliferation, is also ~2% [105]. The cells’ phenotype and ontogeny are a second factor fueling the uncertainty about the importance of macrophage proliferation in atherosclerotic lesions. In the 1990s, phenotypic analysis was much more limited, and it was not possible to determine whether a Ki-67+ cell was indeed a mature macrophage. Moreover, experimental approaches using BrdU incorporation to track proliferation could not discriminate between current (that is, local) or prior proliferation. More recently, sophisticated phenotypic protocols and surgical approaches, such as parabiosis, have provided the tools to address these issues. Third, the many studies on monocytes’ essential role in the development of atherosclerosis might have overshadowed macrophage proliferation as an important mechanism. Consequently, the study of macrophage proliferation in atherosclerosis fell to the wayside.
We have recently taken up the challenge of elucidating the relative importance of monocyte influx and macrophage proliferation in atherosclerosis [106]. By combining approaches such as parabiosis, sustained BrdU delivery in osmotic pumps, clodronate-based depletion of circulating monocytes, irradiation and generation of mixed chimeras, among others, we concluded that macrophage turnover in established lesions is rapid and largely dependent on local macrophage proliferation. Monocyte-derived macrophages enter mitosis because they recognize components of the lesional environment, possibly modified lipoproteins [107–110]. This insight distinguishes lesional macrophage proliferation from HSPC proliferation during hyperlipidemia. HSPC proliferate in mice that lack APOE, ABCA1, or ABCG1 because the defect in reverse cholesterol transport forms lipid rafts and promotes common β chain surface expression, thus rendering the cells susceptible to GM-CSF and IL-3-driven proliferation [111, 112]. In contrast, lesional macrophage proliferation is independent of the common β chain and can occur even in ApoE-expressing cells. This observation about lesional macrophage proliferation also contrasts with dendritic cell proliferation in the nascent aorta, which, like HSPC proliferation, relies on the common β chain [113]. Instead, intimal macrophage self-renewal in established atherosclerosis depends in part on the type 1 scavenger receptor class A (Msr1). We demonstrated this by generating mixed chimeric mice and evaluating proliferation rates of wild type and Msr1-deficient macrophages in the same animal. Msr1−/− macrophages proliferated less frequently than wild type (Msr1+/+) macrophages. In support of this, Msr1-mediated internalization of lysophosphatidylcholine, a phospholipid component of ox-LDL, induces proliferation in peritoneal macrophages in vitro [114].
The importance of Msr1 to atherosclerosis has been controversial. Although Msr1, which is expressed on lesional macrophages, recognizes and internalizes oxidized lipoproteins [115–118], its deletion or overexpression have yielded conflicting results that led to conclusions that Msr1 augments lesion size [119, 120], attenuates lesion size [121], or has no effect on lesion size [122–124]. If the current body of evidence using mice backcrossed at least 10 generations to the C57BL/6 strain is consistent with the hypothesis that Msr1 does not contribute to lesion size, and if Msr1 contributes to frequent macrophage proliferation – then what is the consequence of macrophage proliferation? The simple answer may be that macrophage proliferation increases the number of pathogenic cells. Proliferating cells may be more inflammatory and more susceptible to apoptosis, thus contributing not only to lesion bulk but to lesional vulnerability, a function that has been attributed to Msr-1 [125]. By this thinking, macrophage proliferation is harmful and should be targeted therapeutically. Yet macrophage proliferation can also be conceptualized as atheroprotective: macrophages, overwhelmed by the high lipid content in their environment, might escape apoptosis by shuttling ingested cholesterol to the plasma membrane where it is needed during cell division. It is also possible that Msr1-dependent lipid internalization and cell proliferation foster a less, rather than more, inflammatory cell phenotype, which would be consistent with studies showing a negative correlation between foam cell formation and inflammation [126]. Msr1 may be involved in many processes, but its contributions to cellular proliferation and effects on lesion growth and stability remain to be determined.
Like the consequences of proliferation, the mechanism by which Msr1 promotes macrophage proliferation remains a puzzle. Does Msr1 stimulate proliferation directly via an intracellular signaling cascade [127, 128] or indirectly by facilitating ligand internalization [117, 129]? What are the natural ligands? Do other growth factors and scavenger receptors contribute [130]? For example, CD36, a class B scavenger receptor implicated in the development of necrotic lesions in advanced atherosclerosis, is an archetypal pattern-recognition receptor uniquely positioned, together with TLR4 and TLR6, to prime and activate the NLRP3 inflammasome [131]. While CD36 promotes vascular smooth muscle cell proliferation and neointimal hyperplasia, its role in regulating macrophage proliferation requires more study.
Additional questions that need to be addressed are whether, and if so how, macrophage proliferation influences cell function. Macrophages are frequently grouped according to the ‘M1–M2’ paradigm. According to the concept, macrophages undergo either classical M1 or alternative M2 activation, but other types of activation have been described [132–137]. M1 macrophages are activated by microbial components such as lipopolysaccharide (LPS) or IFNγ and express increased levels of inflammatory cytokines, and reactive nitrogen and oxygen intermediates. M2 macrophages are associated with resolution of inflammation, tissue remodeling, wound healing, and immune regulation. They are highly phagocytic; express scavenging, mannose and galactose receptors; and produce ornithine and polyamines. The phenotypic profile of macrophages in many inflammatory contexts is poorly understood but is likely influenced by both lineage commitment and response to microenvironment. Our own observations in experimental atherosclerosis suggest lesional macrophages display a mixed profile of M1–M2 polarization (data not shown). Proliferating macrophages are, by definition, metabolically active and will likely differ from non-proliferating cells. Future studies will locate proliferating macrophages on the M1/M2 continuum.
Arguably, of all the questions generated by the recognition that local macrophage proliferation is important to atherosclerosis, none are more intriguing than those related to the role of monocytes. If local proliferation dominates macrophage accumulation in atherosclerosis, what are monocytes good for? For one, our data confirm that lesional macrophages are Ly-6Chigh monocyte-derived because circulating non-proliferating Ly-6Chigh monocytes infiltrate the lesion and differentiate to proliferating macrophages. The conclusion that macrophages self-renew was based on the observation that, in a parabiosis setting, macrophage chimerism is low. Interestingly, however, monocyte chimerism in the aorta and blood equilibrated shortly after establishment of parabiosis, which is consistent with studies showing that monocyte influx is continuous [84]. By implication, even though monocytes accumulate continuously, only a few of them differentiate to macrophages. This may explain why in some studies targeting and tracking monocyte influx in established atherosclerosis had little if any effect on lesion size [138–142]. As for the rest of the accumulating monocytes, their fate is unclear, although it is likely that monocytes either succumb to apoptosis shortly after influx or exit the lesion altogether. If they exit, is the brief sojourn into the lesion consequential to atherosclerosis? More specifically, we might ask whether monocyte activity in the lesion is atheroprotective (removal of lipids from the vessel wall?), atherogenic (release of cytokines, activation of macrophages?), or both. Indeed, the relevance of leukocyte exit to the development and regression of atherosclerosis is a timely topic that requires further exploration [143–146].
As for Ly-6Clow monocytes in atherosclerosis, their role remains even more uncertain than that of Ly-6Chigh monocytes. Nur77-deficient mice, which lack Ly-6Clow monocytes, develop aggravated atherosclerosis [147, 148]. These observations have been challenged [149], but nevertheless suggest that Ly-6Clow monocytes are protective. As mentioned earlier, however, Ly-6Clow monocytes enter tissue poorly and represent only a small fraction of infiltrating cells in atherosclerosis. Major questions moving forward, then, will be whether Ly-6Clow monocytes indeed protect against atherosclerosis, or whether the effect of Nur77 is independent of Ly-6Clow monocyte absence. If protection is Ly-6Clow monocyte-dependent, does it occur in the circulation via monocytes’ patrolling behavior or direclty in the lesion?
Understanding the balance between monocyte influx and macrophage proliferation may also help clarify the role of inflammation in atherothrombosis, which is now being tested clinically [150, 151]. A possible therapeutic strategy may be to attenuate monocyte accumulation by targeting either monocyte production or the relevant chemokines that guide monocyte entry [152]. The problem with this approach is specificity. If monocytes participate importantly in immunity, inhibiting their production or migration will compromise the host’s ability to respond to injury and infection. An alternative therapeutic strategy may be to target macrophage proliferation locally. The challenge here is different. In order to attenuate or augment local macrophage proliferation it will be necessary to target the lesion while sparing other sites. If the mechanisms promoting lesional macrophage proliferation are unique to the lesional microenvironment, then perhaps specificity can be achieved by exploiting the responsible molecular pathways. But if lesional macrophage proliferation mobilizes common molecular pathways, then other strategies, such as the delivery of lesion-tropic nanoparticles, will need to be considered. Beyond delivery, it will be important to consider whether events known to augment atherosclerosis, such as myocardial infarction [39], warrant inhibiting monocyte entry or macrophage proliferation.
CONCLUDING REMARKS
Cellular proliferation is a major mechanism by which HSPC give rise to monocytes. Monocytes produced in the bone marrow mobilize and enter the general circulation. During diseases such as atherosclerosis, these non-proliferating cells accumulate in the intima, differentiate to macrophages, and proliferate once again. In this respect, the monocyte lineage comes full circle: from proliferating progenitors to proliferating progeny. Our topo-ontogenic understanding of monocyte lineage has grown substantially since the discovery of monocytes in the 1920s, yet the nuances of leukocyte flux and differentiation are as fascinating as ever.
REFERENCES
- 1.Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Noncommunicable Diseases. 2011 [Google Scholar]
- 2.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 8.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS, Lekstrom-Himes JA, Akashi K, Tenen DG. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21:853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimura H, Nagamura-Inoue T, Tamura T, Ozato K. IFN consensus sequence binding protein/IFN regulatory factor-8 guides bone marrow progenitor cells toward the macrophage lineage. J Immunol. 2002;169:1261–1269. doi: 10.4049/jimmunol.169.3.1261. [DOI] [PubMed] [Google Scholar]
- 12.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121:1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alder JK, Georgantas RW, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metcalf D, Nicola NA. The clonal proliferation of normal mouse hematopoietic cells: enhancement and suppression by colony-stimulating factor combinations. Blood. 1992;79:2861–2866. [PubMed] [Google Scholar]
- 19.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 21.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA, Rice S, Rahme LG, Carlesso N. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064–4076. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Front Immunol. 2013;4:204. doi: 10.3389/fimmu.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013 doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludin A, Itkin T, Gur-Cohen S, Mildner A, Shezen E, Golan K, Kollet O, Kalinkovich A, Porat Z, D’Uva G, Schajnovitz A, Voronov E, Brenner DA, Apte RN, Jung S, Lapidot T. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 33.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 34.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Druzd D, de Juan A, Scheiermann C. Circadian rhythms in leukocyte trafficking. Semin Immunopathol. 2013 doi: 10.1007/s00281-013-0414-4. [DOI] [PubMed] [Google Scholar]
- 36.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas D, Bruns I, Battista M, Mendez-Ferrer S, Magnon C, Kunisaki Y, Frenette PS. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119:3962–3965. doi: 10.1182/blood-2011-07-367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31. Has our knowledge matured? Ann N Y Acad Sci. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y, Kohler R, Marinelli B, Gorbatov R, Wojtkiewicz G, Panizzi P, Mino-Kenudson M, Forghani R, Figueiredo JL, Chen JW, Xavier R, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38:296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 50.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 53.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilgendorf I, Swirski FK. Making a difference: monocyte heterogeneity in cardiovascular disease. Curr Atheroscler Rep. 2012;14:450–459. doi: 10.1007/s11883-012-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 2010;67:2685–2693. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 65.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 67.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 73.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 74.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 75.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 77.Soehnlein O, Drechsler M, Doring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gomez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 79.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim CJ, Khoo JC, Gillotte-Taylor K, Li A, Palinski W, Glass CK, Steinberg D. Polymerase chain reaction-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tumor necrosis factor-alpha and interleukin-1 beta. Arterioscler Thromb Vasc Biol. 2000;20:1976–1982. doi: 10.1161/01.atv.20.8.1976. [DOI] [PubMed] [Google Scholar]
- 83.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–2155. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 89.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 99.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol. 1993;142:1787–1793. [PMC free article] [PubMed] [Google Scholar]
- 100.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol. 1995;147:668–677. [PMC free article] [PubMed] [Google Scholar]
- 101.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes. 2004;53:3217–3225. doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- 102.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 103.Lutgens E, Daemen M, Kockx M, Doevendans P, Hofker M, Havekes L, Wellens H, de Muinck ED. Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999;99:276–283. doi: 10.1161/01.cir.99.2.276. [DOI] [PubMed] [Google Scholar]
- 104.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 105.Eldridge SR, Goldsworthy SM. Cell proliferation rates in common cancer target tissues of B6C3F1 mice and F344 rats: effects of age gender, and choice of marker. Fundam Appl Toxicol. 1996;32:159–167. doi: 10.1006/faat.1996.0119. [DOI] [PubMed] [Google Scholar]
- 106.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martens JS, Reiner NE, Herrera-Velit P, Steinbrecher UP. Phosphatidylinositol 3-kinase is involved in the induction of macrophage growth by oxidized low density lipoprotein. J Biol Chem. 1998;273:4915–4920. doi: 10.1074/jbc.273.9.4915. [DOI] [PubMed] [Google Scholar]
- 108.Hamilton JA, Myers D, Jessup W, Cochrane F, Byrne R, Whitty G, Moss S. Oxidized LDL can induce macrophage survival, DNA synthesis, and enhanced proliferative response to CSF-1 and GM-CSF. Arterioscler Thromb Vasc Biol. 1999;19:98–105. doi: 10.1161/01.atv.19.1.98. [DOI] [PubMed] [Google Scholar]
- 109.Hamilton JA, Jessup W, Brown AJ, Whitty G. Enhancement of macrophage survival and DNA synthesis by oxidized-low-density-lipoprotein (LDL)-derived lipids and by aggregates of lightly oxidized LDL. Biochem J. 2001;355:207–214. doi: 10.1042/0264-6021:3550207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamilton JA. Nondisposable materials, chronic inflammation, and adjuvant action. J Leukoc Biol. 2003;73:702–712. doi: 10.1189/jlb.0103037. [DOI] [PubMed] [Google Scholar]
- 111.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakai M, Miyazaki A, Hakamata H, Kodama T, Suzuki H, Kobori S, Shichiri M, Horiuchi S. The scavenger receptor serves as a route for internalization of lysophosphatidylcholine in oxidized low density lipoprotein-induced macrophage proliferation. J Biol Chem. 1996;271:27346–27352. doi: 10.1074/jbc.271.44.27346. [DOI] [PubMed] [Google Scholar]
- 115.Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Hiltunen MO, Turunen M, Herttuala SY, Kodama T, Gordon S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 116.Teupser D, Stein O, Burkhardt R, Nebendahl K, Stein Y, Thiery J. Scavenger receptor activity is increased in macrophages from rabbits with low atherosclerotic response: studies in normocholesterolemic high and low atherosclerotic response rabbits. Arterioscler Thromb Vasc Biol. 1999;19:1299–1305. doi: 10.1161/01.atv.19.5.1299. [DOI] [PubMed] [Google Scholar]
- 117.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 118.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- 120.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 121.Whitman SC, Rateri DL, Szilvassy SJ, Cornicelli JA, Daugherty A. Macrophage-specific expression of class A scavenger receptors in LDL receptor(−/−) mice decreases atherosclerosis and changes spleen morphology. J Lipid Res. 2002;43:1201–1208. [PubMed] [Google Scholar]
- 122.Herijgers N, de Winther MP, Van Eck M, Havekes LM, Hofker MH, Hoogerbrugge PM, Van Berkel TJ. Effect of human scavenger receptor class A overexpression in bone marrow-derived cells on lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knockout mice. J Lipid Res. 2000;41:1402–1409. [PubMed] [Google Scholar]
- 123.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Winther MP, Gijbels MJ, van Dijk KW, van Gorp PJ, suzuki H, Kodama T, Frants RR, Havekes LM, Hofker MH. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 125.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AHJ, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coller SP, Paulnock DM. Signaling pathways initiated in macrophages after engagement of type A scavenger receptors. J Leukoc Biol. 2001;70:142–148. [PubMed] [Google Scholar]
- 129.Kim WS, Ordija CM, Freeman MW. Activation of signaling pathways by putative scavenger receptor class A (SR-A) ligands requires CD14 but not SR-A. Biochem Biophys Res Commun. 2003;310:542–549. doi: 10.1016/j.bbrc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 130.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 131.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 133.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 134.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 137.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calin MV, Manduteanu I, Dragomir E, Dragan E, Nicolae M, Gan AM, Simionescu M. Effect of depletion of monocytes/macrophages on early aortic valve lesion in experimental hyperlipidemia. Cell Tissue Res. 2009;336:237–248. doi: 10.1007/s00441-009-0765-2. [DOI] [PubMed] [Google Scholar]
- 140.Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol. 2005;25:1014–1019. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 141.Aiello RJ, Perry BD, Bourassa PA, Robertson A, Weng W, Knight DR, Smith AH, Frederick KS, Kalgutkar A, Gladue RP. CCR2 receptor blockade alters blood monocyte subpopulations but does not affect atherosclerotic lesions in apoE(−/−) mice. Atherosclerosis. 2010;208:370–375. doi: 10.1016/j.atherosclerosis.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 142.Ye D, Zhao Y, Hildebrand RB, Singaraja RR, Hayden MR, Van Berkel TJ, Van Eck M. The dynamics of macrophage infiltration into the arterial wall during atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Am J Pathol. 2011;178:413–422. doi: 10.1016/j.ajpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O’Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 149.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 151.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 152.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]