Abstract
Mitochondrial permeability transition pore (mPTP) plays a central role in alterations of mitochondrial structure and function leading to neuronal injury relevant to aging and neurodegenerative diseases including Alzheimer’s disease (AD). mPTP putatively consists of the voltage-dependent anion channel (VDAC) and the adenine nucleotide translocator (ANT). Cyclophilin D (CypD) and reactive oxygen species (ROS) increase intra-cellular calcium and enhance the formation of mPTP that leads to neuronal cell death in AD. CypD-dependent mPTP can play a crucial role in ischemia/reperfusion injury. The interaction of amyloid beta peptide (Aβ) with CypD potentiates mitochondrial and neuronal perturbation. This interaction triggers the formation of mPTP, resulting in decreased mitochondrial membrane potential, impaired mitochondrial respiration function, increased oxidative stress, release of cytochrome c, and impaired axonal mitochondrial transport. Thus, the CypD-dependent mPTP is directly linked to the cellular and synaptic perturbations observed in the pathogenesis of AD. Designing small molecules to block this interaction would lessen the effects of Aβ neurotoxicity. This review summarizes the recent progress on mPTP and its potential therapeutic target for neurodegenerative diseases including AD.
Keywords: Amyloid β, Alzheimer’s disease, Cyclophilin D, Mitochondrial permeability transition pore, Neurodegeneration
1. Introduction
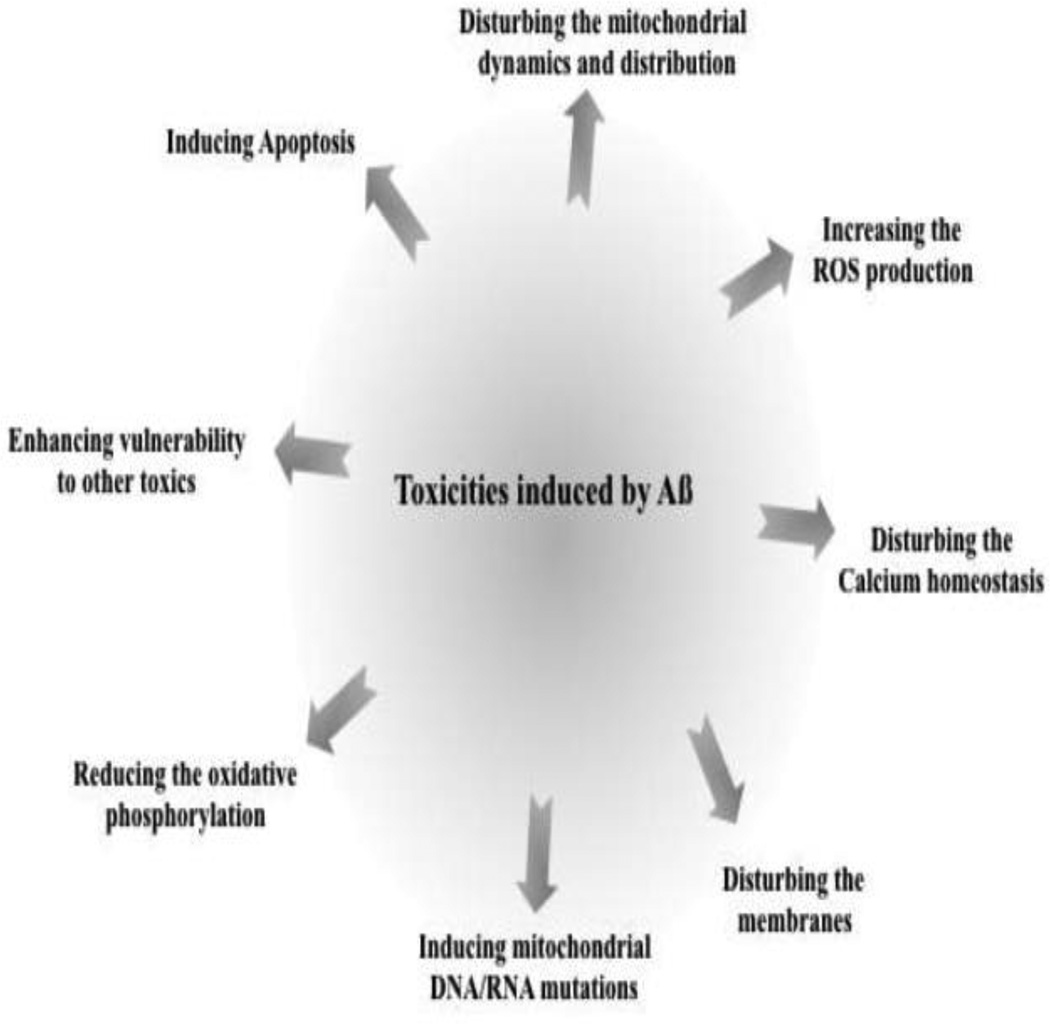
Alzheimer’s disease (AD) is a chronic neurodegenerative disease, predominantly affecting the elderly, for which only symptomatic treatments are currently available. There are two pathological features of AD: abnormal accumulations of amyloid beta peptide (Aβ) and phosphorylation of tau protein in the brain. Mitochondrial and synaptic dysfunction is an early pathological feature of AD brain [1–6]. Recent studies have highlighted the relation between mitochondrial Aβ accumulation and synaptic mitochondrial dysfunction. Known Aβ-related mitochondrial dysfunctions [7–9] include: excessive reactive oxygen species (ROS) production [10–13]; disrupted calcium homeostasis [14]; disturbance and distribution of mitochondrial dynamics, inducing mitochondrial DNA/RNA mutations [15], enhancing vulnerability to other toxicities, modifying the membranes, and reducing oxidative phosphorylation (Figure 1). These observations should provide a better understanding of the relationship between mitochondria and AD pathogenesis.
Figure 1. Toxicities induced by Aβ.
Aβ is known to cause neuronal toxicity by several mechanisms, including increased ROS production, induction of apoptosis, disturbing calcium homeostasis, and enhancing the vulnerability of neurons to other toxic substances, etc.
Progressive accumulation of mitochondrial Aβ in AD brain and in AD mouse models has been shown to induce mitochondrial malfunction [4, 16–19]. Cyclophilin D (CypD), a peptidyl-prolyl isomerase F, resides in the mitochondrial matrix and associates with the inner mitochondrial membrane during the mitochondrial membrane permeability transition [20]. CypD plays a central role in opening the mitochondrial membrane permeability transition pore (mPTP) that leads to cell death. The level of CypD was significantly elevated in neurons in AD-affected regions. Using surface Plasmon resonance with recombinant human CypD protein, Aβ binds to CypD during an in vitro protein-protein interaction. Indeed, this Aβ-CypD complex was found in Aβ-rich mitochondria from AD brain and transgenic AD mice [17, 21]. CypD deficiency (lacking Aβ binding partner) prevented Aβ-mediated mitochondrial and synaptic dysfunction [17, 21]. Although the precise role of Aβ in mitochondria is not yet defined, recent reports indicate that interaction of mitochondrial Aβ with mitochondrial proteins, Aβ binding alcohol dehydrogenase (ABAD) and CypD, exacerbates mitochondrial and neuronal stress in transgenic AD mouse models [16–18, 21].
Factors like the perturbation of intracellular calcium regulation, the release of pro-apoptotic factors, regulation in mitochondrial morphology, and ROS generation are often associated with mPTP formation. Increasing calcium concentration has been shown to increase ROS generation, decrease ATP production, and induce the release of apotogenic factors followed by swelling of the mitochondria [22–25]. In the absence of CypD, keystone molecules comprising the mPTP, and involved in Aβ-mediated mitochondrial, neuronal, and synaptic dysfunction are lessened [17, 21]. This knowledge has proven crucial to our understanding of Aβ toxicity and the pathogenesis of AD. Binding appropriate inhibitors to CypD even in the presence of Ca2+ leads to neuronal protection.
This review focuses on the molecular and cellular abnormalities that occur in the AD brain and discusses how these abnormalities result in synaptic dysfunction and cell death. Currently available therapeutic strategies for AD are highlighted, particularly those for mPTP prevention.
2. Mitochondria and mitochondrial permeability transition pore (mPTP)
Mitochondria are membranous enclosed organelles found in all eukaryotic cells; they play a vital role in cellular bioenergetics, thermogenesis, heme biosynthesis, lipid catabolism, calcium homeostasis, and other metabolic activities. Furthermore, mitochondria are exclusively poised to play an essential role in neuronal cell survival or death after central nervous system (CNS) injury because they are regulators of both energy metabolism and apoptotic pathways [26–28]. Therefore, structurally and functionally intact mitochondria are crucial for healthy cells. A mitochondrion contains outer and inner membranes composed of phospholipid bilayers and proteins. The two membranes, however, have different properties. The outer membrane is freely permeable to small molecules, such as ions and sugars, while the inner membrane does not contain porins and is highly impermeable to all molecules [29, 30]. Transporters, present in the inner mitochondrial membrane (IMM), allow the entry of specific substrates into the mitochondrial matrix. Hence, it provides mitochondrial matrix homeostasis by preventing the free exchange of substances between the matrix and cytosol. Two major transporters present in the IMM play an important role in calcium homeostasis [31–33]. Calcium ATPase helps in the uptake of calcium into mitochondria, whereas sodium calcium exchanger helps in the release of calcium into the cytosol from mitochondria. Under the conditions of calcium or phosphate overload and intracellular oxidative stress, mitochondria efflux calcium through mPTP by a transporter-independent process and thereby activates the apoptotic pathway as the mitochondria lose their calcium handling ability.
2.1. mPTP as a therapeutic target
The mitochondrial permeability transition is defined as the sudden increase in the permeability of the IMM to solutes with a molecular mass of less than 1,500 Da, which results in the loss of membrane potential (Δψ), mitochondrial swelling, and rupture of the outer mitochondrial membrane (OMM) [32, 34]. The molecular composition of the mPTP remains a puzzle in spite of extensive interest and thorough studies carried out over the last decades. The mitochondrial permeability transition is thought to occur after the opening of a mega channel that is known as the mPTP. Three major proteins are proposed to comprise the mPTP : the voltage-dependent anion channel (VDAC) present in the outer membrane, the adenine nucleotide translocator (ANT) located in the inner membrane, CypD found in the matrix, and other molecules [35, 36]. Under normal conditions, CypD resides in the mitochondrial matrix and the mPTP remains closed.
In the presence of factors acting as permeability transition inducers, CypD becomes associated with the IMM. This results in the formation of an ANT channel in the IMM, which in turn increases inner membrane permeability and opens the mPTP [36]. The channel, formed by VDAC in the OMM together with ANT, comprises a tunnel-like structure crossing the mitochondrial membranes, thus connects the mitochondrial matrix with the cytosol. [37, 38]. Studies in animal models have shown that CypD inhibitor Cyclosporine A (CsA), or its non-immunosuppressive analog N-methyl-Val-4-CsA, inhibits mPTP formation by blocking the interaction of CyPD with the ANT, and as a result the conformational change of ANT is blocked [39–42]. Bongkrekic acid and atractyloside, are two other modulators of the ANT and mPTP, which inhibit and induce the induction of the mPTP respectively [43–45].
However, the exact structure of the mPTP remains controversial. It is hypothesized that ANT from the IMM, VDAC from the OMM, the CsAbinding protein CypD from the matrix, and several other proteins come together to form the pore [24, 46–48]. In the recent investigation, genetic knockout studies challenge the validity of this model by showing that the mPTP still occurs in mitochondria that are deficient in ANT, VDAC and even CypD, although some properties of the mPTP are altered [49–52]. Additionally, a recent study suggests that phosphate carrier (PiC) in IMM is a potential constituent of the mPTP [53]. Leung and colleagues demonstrated that PiC could form a pore either by itself or in association with ANT, suggesting that PiC but not ANT is the necessary component of the mPTP. Also, the study shows that high concentrations of calcium alone can trigger PiC. Interestingly, CypD binding is also sufficient, but not necessarily an initiating step for PiC associated pore opening. These data may provide an explanation for the failure of VDAC or ANT ablation to prevent mPTP formation, whereas in CypD deficient mitochondria, mPTP induction requires greater calcium levels and is not completely blocked by CsA treatment.
2.2. Consequences of mPTP formation
The consequence of mPTP pore opening is that all small electrolytes equilibrate across the IMM, including cofactors and ions. This will not only lead to the disruption of metabolic gradients between the mitochondria and cytosol, including the release of accumulated calcium, but will also lead to osmotic swelling of mitochondria. The IMM no longer maintains a barrier to protons which leads to dissipation of the proton motive force. The resultant uncoupling of oxidative phosphorylation prevents mitochondria from generating ATP, leading to ATP depletion and increased generation of ROS. Mitochondrial swelling may rupture the OMM by releasing cytochrome c. In turn, cytochrome c initiates cellular apoptosis by activating pro-apoptotic factors. It can therefore be concluded that massive formation of mPTP under pathological conditions causes severe mitochondrial injury and cell death. Potential mPTP blockers include: the immune suppressant Cyclosporin A (CsA) [17, 54]; Sanglifehrin A (SfA) [55]; ADP [56]; a non-immunosuppressant derivative of CsA, N-methyl-Val-4-cyclosporin A (MeValCsA)[57] a non-immunosuppressive agent, NIM811, 2-aminoethoxydiphenyl borate (2-APB) [58]; and bongkrekic acid. Available evidence indicates that CypD is the most important initiating molecule for the mPTP, and that mPTP formation results in mitochondrial dysfunction, irreversible cell damage, and cell death.
2.3. Significance of mPTP in normal and disease states
AD [17], Parkinson’s disease (PD) [59, 60], amyotrophic lateral sclerosis (ALS) [61, 62], and Huntington’s disease (HD) [63] are the most common human adult-onset neurodegenerative diseases. In AD, involvement of the mPTP is evidenced by alterations in enzymes involved in oxidative phosphorylation, oxidative damage, and mitochondrial binding of Aβ and amyloid precursor protein (APP). Similarly, in PD, mutations in putative mitochondrial proteins have been identified and mitochondrial DNA mutations have been found in neurons in the substantia nigra. Moreover, changes in ALS occur in mitochondrial respiratory chain enzymes and mitochondrial cell death proteins. In our published studies [17], we demonstrated that mitochondria isolated from the hippocampus and temporal lobe of AD patients showed elevated CypD levels. Increased CypD expression is predominantly localized in neurons in these specific areas of AD patients [17]. Given the positive correlation of CypD expression to mPTP opening [17, 24, 62, 64], neurons with increased expression of CypD in AD-affected brain regions would be more susceptible to mPTP formation and the resultant consequences. Likewise, up-regulation of CypD expression in cortical mitochondria was seen in AD mice overexpressing APP and Aβ (APP mice). As expected, cortical mitochondria from APP mice demonstrate increased CypD translocation to the IMM and decreased mitochondrial calcium buffering capacity, suggesting that mitochondria enriched for Aβ environment are susceptible to mPTP formation, which is consistent with increased CypD expression [17, 21].
3. Role of the CypD-dependent mPTP in ischemia
CypD and calcium are well known for their role in the formation of the mPTP, mitochondrial permeability, and neuronal cell death by activation of apoptosis. However, the role of CypD in hypoxia-induced ischemic brain injury is not well understood. CypD-deficient mouse studies revealed that CypD-dependent mPTP opening plays a crucial role in ischemia/reperfusion injury affecting the heart [24, 65] and brain [66], suggesting that the CypD-dependent mPTP is involved in ischemia/reperfusion-induced cell death. Hence, CypD and other components of the mPTP are important targets for preventing cell damage. Ischemia/reperfusion injury is a very complex phenomenon, which involves several death mechanisms. Hence, ischemia/reperfusion injury can be improved by inhibiting apoptosis with caspase inhibitors [67–69], inhibiting necrosis with Nec1 [70], or blocking the Ask1 pathway [71]. In vitro cell culture model systems revealed that death mechanisms involve caspases, a Nec1 target, and Ask1. The Cyp D-dependent mPTP does not seem to overlap with each other. Different death mechanisms might operate in a sequential or parallel manner in the same cell. Inhibition of one mechanism might have a protective effect. Alternatively, different death mechanisms might act on different cells during ischemia/reperfusion injury and the dying cells might trigger the death process in other cells. It is also possible that different cell death mechanisms are activated by different ischemic conditions. For further studies of ischemia/reperfusion injury, mice that lack certain cell death mechanisms, such as CypD-deficient mice and Bax/Bak-deficient mice, would be useful tools. The CypD-dependent mPTP might also be involved in other diseases. Mitochondria isolated from the livers of neuromuscular disorder of mnd2 mutant mice ith mutation of the omi gene are more susceptible to the mPTP [72]. MND2 mice succumb to motor neuron disease [73], which might be caused by mPTP formation occurring at a lower threshold in neuronal mitochondria. Thus, future studies may unveil a role of the CypD-dependent mPTP in the pathogenesis of various diseases.
4.1. Role of the CypD-dependent mPTP in Aβ-mediated toxicity and oxidative stress
Significant evidence from recent studies shows that Aβ impairs mPTP function [5, 16, 18, 74, 75] by disrupting mitochondrial membrane potential, and increasing ROS generation, mitochondrial swelling, and cytochrome c release. We have demonstrated that CypD-deficient neurons are resistant to Aβ-impaired mitochondrial neuronal function. In fact, CypD-deficient transgenic mAPP mice overexpressing Aβ show significant improvement in mitochondrial and synaptic function as well as enhanced learning and memory compared to single mAPP mice [17, 21]. Potential mechanisms underlying mitochondrial perturbation in the presence of Aβ are triggering mPTP opening through enhancing CypD translocation to the inner membrane, thereby increasing mitochondrial ROS production and decreasing mitochondrial calcium buffering capacity. These data indicate that mPTP formation is augmented in the presence of Aβ.
Of note, Aβ can increase intracellular calcium and free radical levels, indirectly effecting mPTP [76]. As a result, this process in turn affects cellular damage primarily through induction of free radical generation and calcium dysregulation, leading to neuronal injury [77, 78]. The mPTP is strongly induced by calcium and free radicals and, contrarily, mPTP formation further aggravates oxidative stress and calcium perturbation. Hence, Aβ-mediated perturbation of neuronal calcium metabolism and ROS generation are possible mechanisms underlying Aβ-induced mPTP formation [77, 79], contributing to mitochondrial and neuronal degeneration. Furthermore, oxidative and other cellular stresses are strong inducers of CypD translocation to the IMM [24], and this translocation is a key factor that triggers mPTP opening and formation of Aβ-CypD complexes. Using surface Plasmon resonance with recombinant human CypD protein, Aβ binds to CypD during an in vitro protein-protein interaction. Indeed, this Aβ-CypD complex was found in Aβ-rich mitochondria from AD brain and transgenic AD mice [17, 21]. Additionally, a recent report using molecular docking experiments postulated that Aβ binds with ANT [80]. Both Aβ and oxidative stress have been shown to synergistically affect MPTP formation, which is critical for mitochondrial pathology and neuronal dysfunction in AD pathogenesis. We therefore propose that mPTP formation is a potential target for AD therapeutic strategies [80].
4.2. Reduction of CypD perpetuated changes in axonal mitochondrial dynamics and motility via Aβ-induction
To better understand the key role of CypD in mPTP function, it was decided to assess the effect of CypD on Aβ-induced axonal mitochondrial trafficking and synaptic damage. Findings revealed that the blockade of mPTP by CypD depletion rescues axonal mitochondrial trafficking and protects synapses from Aβ toxicity. Axonal mitochondria are distributed along axons [81] and decreased axonal mitochondrial density is a manifestation of disrupted mitochondrial trafficking. Significant differences are observed between cultured nonTg- and CypD-deficient hippocampal neurons after exposure to oligomeric Aβ (1–42). NonTg neurons revealed significantly decreased axonal mitochondrial density but CypD depletion protected axonal mitochondrial density from Aβ toxicity. Axonal mitochondrial density showed no significant changes in vehicle-treated nonTg neurons when compared to CypD-deficient eurons [81], suggesting no effect of CypD depletion on axonal mitochondrial distribution without Aβ insult. These results indicate that CypD depletion preserves the organization of axonal mitochondrial distribution following Aβ insult. Furthermore, neurons lacking CypD are resistant to Aβ-disrupted PKA/CREB signaling, as shown by increased PKA activity, phosphorylation of PKA catalytic subunit (PKA C), and CREB. CypD depletion rescues loss of synapses and improves synaptic activity [75]. Thus, CypD-dependent signaling pathway (PKA-CREB) is an important mechanism underlying Aβ- and oxidative stress-induce synaptic injury.
5. Current CypD inhibitors
CypD, an integral part of the mPTP, belongs to the cyclophilin family of peptidylprolyl cistransisomerases (PPIases) [20]. CypD displays an important role in the cell response to a variety of noxious stimuli, as it modulates the opening of the mPTP channel when it translocates to the IMM leading to eventual cell death [17]. A critical event in some forms of necrotic and apoptotic cell death is the opening of the mPTP [82, 83], the formation of which is widely thought to involve an interaction between the ANT and CypD [32]. To date, the most specific inhibitor of the mPTP is Cyclosporin A (CsA) [84], which acts by inhibiting the PPIase activity of CypD [85, 86]. CsA lacks clinical significance because of its immunosuppressive effect by inhibiting calcineurin (a calcium dependent protein phosphatase), and inability to pass through the bloodbrain barrier. However, several CsA derivatives have been developed, including N-Me-Ala-6-cyclosporin A and N-Me-Val-4-cyclosporin which lack the immunosuppressive effects but are still potent inhibitors of the PPIase activity of CypD, and thereby antagonize mPTP opening and apoptosis induction [87–89]. Sanglifehrin A (SfA) is also a recently developed potent inhibitor of the mPTP; although SfA does not prevent CyP-D binding to the ANT, it does inhibit its PPIase activity, preventing it from facilitating the conformational change of the ANT required for pore formation, thereby inhibiting apoptosis induction [55]. The SfA–CypD complex has no effect on the calcium-activated protein phosphatase, calcineurin [90]. All CsA derivatives lack significance as therapeutic molecules because of severe side effects including nephrotoxicity, neurotoxicity, and hepatotoxicity, and their poor permeability to the blood brain barrier. Azzolin et al., has developed a new class of drugs called antmanide (AA) from the fungus Amanita Phallioides for targeted inhibition of the CypD PPIase activity, leading to mPTP inhibition and cell protection from insults that cause pore opening [91]. AA lacks its inhibitory effects on mitochondria or cells derived from CypD null mice. AA inhibits mPTP formation in a CypD independent fashion, which requires two critical residues in the peptide ring Phe 6, 9. AA also exhibits an additive effect with ubiquinone 0, which inhibits mPTP opening in isolated hepatocytes. As a part of developing novel inhibitors of CypD PPIase activity, Guo, H., et al., has synthesized small molecule quinoxaline derivatives that inhibit mPTP opening [92]. ADP binding to the ANT causes a conformational change thereby inhibiting mPTP opening. Currently available CypD inhibitors lack clinical significance in AD; they are large molecules with high molecular weights, resulting in poor cell permeability and inability to cross the bloodbrain barrier. Hence there is a need for developing new small molecules that can overcome the above problems.
Conclusion
Several lines of evidence suggest that aging and age-related neurological diseases are predominantly associated with mitochondrial dysfunction. Given that mitochondrial and synaptic dysfunction is an early pathological feature of AD in the brain, targeting mitochondrial function may be a potential therapeutic strategy for early stages of AD treatment. Mitochondrial dysfunction leads to the increased generation of ROS, abnormal protein-protein interactions, and decreased mitochondrial ATP production. Increased production of ROS with accompanying compromised mitochondrial function results in damage to neurons following formation of the mPTP. Several other factors including increased intracellular calcium, Aβ, and CypD also play an important role in the formation of mPTP, which leads to mitochondrial and neuronal degeneration. Thus, inhibition of mPTP formation by blocking CypD is a rational target for potential therapeutic AD strategies. Because the currently available CypD inhibitors are large molecules with high molecular weights that have difficulty crossing the blood-brain barrier, and have low cell permeability, there is currently a needin development of small, drug–like, low-molecular weight compounds that inhibit CypD, thereby improving mitochondrial and neuronal function relevant to neurodegenerative diseases including AD (Figure 2).
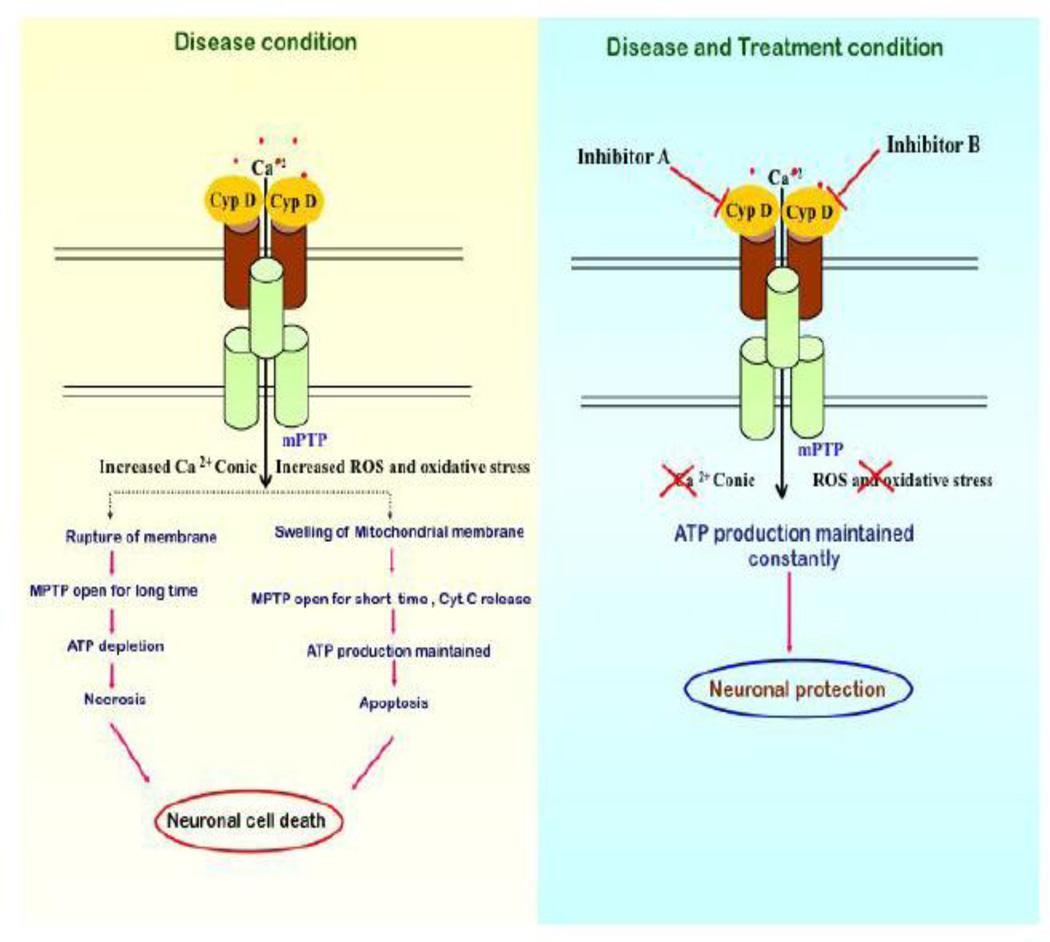
Figure 2. Formation of mPTP is triggered by two major noxious insults.
1. calcium overload in the mitochondrial matrix, and 2. oxidative stress, which triggers a conformational change in the ANT leading to the formation of mPTP along with VDAC. This is further facilitated by CypD. mPTP opening causes mitochondrial swelling, rupture of the outer mitochondrial membrane, which finally leads to the releases of a pro-apoptotic molecules such as cytochrome c into the cytosol. If the pore opens for longer period of time, loss of membrane potential occurs that leads to the depletion of ATP, which finally triggers necrotic oncosis. Mitochondria can maintain ATP levels if the mPTP opens for a short period of time, which triggers necrotic apoptosis. Both of these processes are major contributing factors for neuronal cell death in AD. CypD inhibitors have the potential to prevent the formation of mPTP and provide protection against neuronal cell death.
Highlights.
Mitochondrial permeability transition pore (mPTP) plays a central role in the development of Alzheimer’s disease (AD).
Cyclophilin D (CypD) and reactive oxygen species are also involved in neuronal cell death in AD through mPTP.
CypD-dependent mPTP is directly linked to the cellular and synaptic perturbations in AD.
mPTP as a potential therapeutic target for neurodegenerative diseases including AD.
Acknowledgements
This study is supported by grants from the National Institute of Aging (R37AG037319) and the National Institute of General Medical Science (R01GM095355).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Guo L, Yan SS. Synaptic Mitochondrial Pathology in Alzheimer's Disease. Antioxidants #x00026; redox signaling. 2011 doi: 10.1089/ars.2011.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2010;20(Suppl 2):S569–S578. doi: 10.3233/JAD-2010-100357. [DOI] [PubMed] [Google Scholar]
- 4.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends in molecular medicine. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nature medicine. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 7.Sergeant N, Wattez A, Galvan-valencia M, Ghestem A, David JP, Lemoine J, Sautiere PE, Dachary J, Mazat JP, Michalski JC, Velours J, Mena-Lopez R, Delacourte A. Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer's disease. Neuroscience. 2003;117:293–303. doi: 10.1016/s0306-4522(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 8.Tillement L, Lecanu L, Yao W, Greeson J, Papadopoulos V. The spirostenol (22R, 25R)-20alpha-spirost-5-en-3beta-yl hexanoate blocks mitochondrial uptake of Abeta in neuronal cells and prevents Abeta-induced impairment of mitochondrial function. Steroids. 2006;71:725–735. doi: 10.1016/j.steroids.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. The EMBO journal. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YP, Bushnell AF, Lee CM, Perlmutter LS, Wong SK. Beta-amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain research. 1996;738:196–204. doi: 10.1016/s0006-8993(96)00733-0. [DOI] [PubMed] [Google Scholar]
- 12.Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, Rydel RE. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant "apoptotic" pattern of gene induction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:7736–7745. doi: 10.1523/JNEUROSCI.17-20-07736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng B, Gong K, Niu Y, Liu L, Yan Y, Lu G, Zhang L, Hu M, Zhao N, Zhang X, Tang P, Gong Y. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: Implications for the treatment of Alzheimer's disease. Free radical biology & medicine. 2009;46:1362–1375. doi: 10.1016/j.freeradbiomed.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Tillement L, Lecanu L, Papadopoulos V. Alzheimer's disease: effects of beta-amyloid on mitochondria. Mitochondrion. 2011;11:13–21. doi: 10.1016/j.mito.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta neuropathologica. 2009;118:151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 16.Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 17.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nature medicine. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 19.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Human molecular genetics. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 20.Galat A, Metcalfe SM. Peptidylproline cis/trans isomerases. Progress in biophysics and molecular biology. 1995;63:67–118. doi: 10.1016/0079-6107(94)00009-x. [DOI] [PubMed] [Google Scholar]
- 21.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. The Journal of biological chemistry. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock TR, Carvalho AC, Jurkiewicz A, Frussa-Filho R, Smaili SS. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. Journal of neurochemistry. 2004;88:1220–1228. doi: 10.1046/j.1471-4159.2003.02250.x. [DOI] [PubMed] [Google Scholar]
- 24.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 25.Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer's disease: cyclophilin D and amyloid beta. Biochimica et biophysica acta. 2010;1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. Journal of neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- 27.Wieloch T. Mitochondrial involvement in acute neurodegeneration. IUBMB life. 2001;52:247–254. doi: 10.1080/15216540152846064. [DOI] [PubMed] [Google Scholar]
- 28.Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- 29.Kroner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Archives of biochemistry and biophysics. 1986;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths EJ. Mitochondrial calcium transport in the heart: physiological and pathological roles. Journal of molecular and cellular cardiology. 2009;46:789–803. doi: 10.1016/j.yjmcc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 31.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. The Biochemical journal. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 33.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochemical Society symposium. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 34.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochimica et biophysica acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? Journal of neuroscience research. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- 36.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis : an international journal on programmed cell death. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 37.Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS letters. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 38.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Molecular and cellular biochemistry. 1997;174:167–172. [PubMed] [Google Scholar]
- 39.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. The Journal of biological chemistry. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 40.Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. The Journal of biological chemistry. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 41.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. The Biochemical journal. 1994;302(Pt 2):321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broekemeier KM, Pfeiffer DR. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry. 1995;34:16440–16449. doi: 10.1021/bi00050a027. [DOI] [PubMed] [Google Scholar]
- 43.Brustovetsky N, Dubinsky JM. Dual responses of CNS mitochondria to elevated calcium. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:103–113. doi: 10.1523/JNEUROSCI.20-01-00103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. The Journal of biological chemistry. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. Journal of bioenergetics and biomembranes. 2004;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- 46.Connern CP, Halestrap AP. Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2+] Biochemistry. 1996;35:8172–8180. doi: 10.1021/bi9525177. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Shi Y, Tian C, Jiang C, Jin H, Chen J, Almasan A, Tang H, Chen Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pestana CR, Silva CH, Pardo-Andreu GL, Rodrigues FP, Santos AC, Uyemura SA, Curti C. Ca(2+) binding to c-state of adenine nucleotide translocase (ANT)-surrounding cardiolipins enhances (ANT)-Cys(56) relative mobility: a computational-based mitochondrial permeability transition study. Biochimica et biophysica acta. 2009;1787:176–182. doi: 10.1016/j.bbabio.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Bernardi P, Forte M. The mitochondrial permeability transition pore. Novartis Foundation symposium. 2007;287:157–164. discussion 164-159. [PubMed] [Google Scholar]
- 50.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Annals of the New York Academy of Sciences. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 51.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochimica et biophysica acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. The Journal of biological chemistry. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansson MJ, Persson T, Friberg H, Keep MF, Rees A, Wieloch T, Elmer E. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain research. 2003;960:99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- 55.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. The Journal of biological chemistry. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 56.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. The Journal of biological chemistry. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 57.Zenke G, Strittmatter U, Fuchs S, Quesniaux VF, Brinkmann V, Schuler W, Zurini M, Enz A, Billich A, Sanglier JJ, Fehr T. Sanglifehrin A, a novel cyclophilin-binding compound showing immunosuppressive activity with a new mechanism of action. Journal of immunology. 2001;166:7165–7171. doi: 10.4049/jimmunol.166.12.7165. [DOI] [PubMed] [Google Scholar]
- 58.Chinopoulos C, Starkov AA, Fiskum G. Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. The Journal of biological chemistry. 2003;278:27382–27389. doi: 10.1074/jbc.M303808200. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Molecular cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiology of disease. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Experimental neurology. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karlsson J, Fong KS, Hansson MJ, Elmer E, Csiszar K, Keep MF. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. Journal of neurosurgery. 2004;101:128–137. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- 63.Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. The Journal of biological chemistry. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 66.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng Y, Deshmukh M, D'Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. The Journal of clinical investigation. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu D, Bureau Y, McIntyre DC, Nicholson DW, Liston P, Zhu Y, Fong WG, Crocker SJ, Korneluk RG, Robertson GS. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:5026–5033. doi: 10.1523/JNEUROSCI.19-12-05026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature chemical biology. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe T, Otsu K, Takeda T, Yamaguchi O, Hikoso S, Kashiwase K, Higuchi Y, Taniike M, Nakai A, Matsumura Y, Nishida K, Ichijo H, Hori M. Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochemical and biophysical research communications. 2005;333:562–567. doi: 10.1016/j.bbrc.2005.05.151. [DOI] [PubMed] [Google Scholar]
- 72.Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, Alnemri ES. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- 73.Jones JM, Albin RL, Feldman EL, Simin K, Schuster TG, Dunnick WA, Collins JT, Chrisp CE, Taylor BA, Meisler MH. mnd2: a new mouse model of inherited motor neuron disease. Genomics. 1993;16:669–677. doi: 10.1006/geno.1993.1246. [DOI] [PubMed] [Google Scholar]
- 74.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, Yan SS. Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbadis.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, Perry G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer's disease. Free radical research. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chin JH, Tse FW, Harris K, Jhamandas JH. Beta-amyloid enhances intracellular calcium rises mediated by repeated activation of intracellular calcium stores and nicotinic receptors in acutely dissociated rat basal forebrain neurons. Brain cell biology. 2006;35:173–186. doi: 10.1007/s11068-007-9010-7. [DOI] [PubMed] [Google Scholar]
- 78.Schoneich C, Pogocki D, Hug GL, Bobrowski K. Free radical reactions of methionine in peptides: mechanisms relevant to beta-amyloid oxidation and Alzheimer's disease. Journal of the American Chemical Society. 2003;125:13700–13713. doi: 10.1021/ja036733b. [DOI] [PubMed] [Google Scholar]
- 79.Morais Cardoso S, Swerdlow RH, Oliveira CR. Induction of cytochrome c-mediated apoptosis by amyloid beta 25 – 35 requires functional mitochondria. Brain research. 2002;931:117–125. doi: 10.1016/s0006-8993(02)02256-4. [DOI] [PubMed] [Google Scholar]
- 80.Singh P, Suman S, Chandna S, Das TK. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer's disease. Bioinformation. 2009;3:440–445. doi: 10.6026/97320630003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo L, Du H, Yan S, Wu X, McKhann GM, Chen JX, Yan SS. Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons. PloS one. 2013;8:e54914. doi: 10.1371/journal.pone.0054914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nature reviews. Molecular cell biology. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 83.Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends in biochemical sciences. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 84.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. The Biochemical journal. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 85.Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, Ward JM, Crompton M. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. European journal of biochemistry / FEBS. 1996;238:166–172. doi: 10.1111/j.1432-1033.1996.0166q.x. [DOI] [PubMed] [Google Scholar]
- 86.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. The Biochemical journal. 1991;274(Pt 2):611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. The Biochemical journal. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. The Journal of biological chemistry. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 89.Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. The European journal of neuroscience. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 90.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 91.Azzolin L, Antolini N, Calderan A, Ruzza P, Sciacovelli M, Marin O, Mammi S, Bernardi P, Rasola A. Antamanide, a derivative of Amanita phalloides, is a novel inhibitor of the mitochondrial permeability transition pore. PloS one. 2011;6:e16280. doi: 10.1371/journal.pone.0016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo HX, Wang F, Yu KQ, Chen J, Bai DL, Chen KX, Shen X, Jiang HL. Novel cyclophilin D inhibitors derived from quinoxaline exhibit highly inhibitory activity against rat mitochondrial swelling and Ca2+ uptake/ release. Acta pharmacologica Sinica. 2005;26:1201–1211. doi: 10.1111/j.1745-7254.2005.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]