Abstract
Objective
The platelet glycoprotein (GP) Ib-IX receptor is a well characterized adhesion receptor supporting hemostasis and thrombosis via interactions with von Willebrand factor (VWF). We examine the GPIb-IX/VWF axis in murine polymicrobial sepsis, as modeled by cecal ligation and puncture (CLP).
Approach and Results
Genetic absence of the GPIb-IX ligand, VWF, prolongs survival following CLP, but absence of the receptor, GP Ib-IX, does not. Since absence of either VWF or GP Ib-IX significantly impairs hemostasis and thrombosis we sought to define additional GP Ib-IX-dependent pathways impacting survival in the CLP model. We document the absence of GPIb-IX leads to reduced platelet-neutrophil and platelet-monocyte interactions. Twenty four hours following CLP, absence of GP Ib-IX coincides with an alteration in cytokine levels, such as TNFα secreted by monocytes, and increased Mac-1 expression by neutrophils.
Conclusions
In contrast, to the well-characterized pro-inflammatory properties of platelets, we describe in the CLP model an anti-inflammatory property associated with platelet GP Ib-IX. Thus, a single platelet receptor displays a dual modulatory role in both the thrombotic and inflammatory pathways associated with polymicrobial sepsis. In sharing leucine rich motifs with toll-like receptors, platelet GPIb-IX can be considered a multi-functional participant in hemostasis, thrombosis, and the inflammatory cascade. The results highlight a dynamic role for platelets in systemic inflammation and add to the complex pathophysiologic events that occur during the dysregulated coagulation and inflammation associated with sepsis.
Keywords: platelet glycoprotein Ib-IX, sepsis, cytokine, monocyte, neutrophil
Introduction
The well-established role of circulating blood platelets is to support hemostasis and thrombosis. Platelets constantly survey the vasculature and upon recognition of vessel injury aggregate to form a platelet-rich hemostatic plug and limit blood loss.1, 2 The ability of platelets to aggregate also contributes to cardiovascular diseases as the platelet-rich thrombus obstructs blood flow.3, 4 More recently, platelet function beyond hemostasis and thrombosis is being recognized with relevance to disease pathways associated with inflammation and cancer.5–7 Relative to inflammation, platelets affect a wide range of immune functions including: leukocyte migration, release of pro-inflammatory mediators, release of antimicrobial proteins, promotion of NET formation, and expression of toll-like receptors.8–13 Thus, platelets are an active extension of the immune system most likely reflecting the ancestral origins of the mammalian platelet to the thrombocyte of lower vertebrates.14–17
Sepsis is a dynamic syndrome bridging a number of seemingly distinct pathophysiologic events, most notably inflammation and coagulation.18 Indeed, the complexity of sepsis pathophysiology has made it one of the most difficult clinical conditions to model in animals.19 However, given the complexity and potential interrelationships of different pathways, animal models are indispensable and have been valuable to characterize the role of specific genes and pathophysiologic processes in the progression of the syndrome.19 For the current study, we utilized mice deficient in platelet GPIb-IX with well characterized defects in hemostasis and thrombosis mimicking the human disorder, the Bernard-Soulier syndrome.20, 21 These mice lack the platelet adhesion receptor for VWF, an essential ligand for tethering platelets to damaged or altered endothelial surfaces.22 Though the best characterized function of GP Ib-IX is binding to VWF, GP Ib-IX has a diverse array of other ligands.23 One such ligand is the leukocyte integrin Mac-1 (CD11b/CD18) which plays an integral role in promoting the migration of immune cells into extravascular tissues.24, 25 Although there has been limited data addressing the in vivo consequences of this interaction, it establishes a potential involvement for GP Ib-IX in the process of inflammation.
Utilizing a model of GPIb-IX dysfunction (hIL-4R/Ibα) we present studies to determine the physiologic consequence of platelet GP Ib-IX in a mouse model of dysregulated inflammation, the cecal ligation and puncture (CLP) model.19, 26 Mice devoid of VWF have improved survival following CLP while mice deficient in the VWF receptor, GP Ib-IX, do not have improved survival. We identify GPIb-IX contributions to a platelet/neutrophil and platelet/monocyte axis with significant consequences to the innate immune response as evidenced by cytokine levels and increased Mac-1 expression. Our results illustrate a platelet interface between coagulation and inflammation involving the GP Ib-IX complex.
Materials and Methods
Materials and methods are available in the online-only data supplement.
Results
The platelet GP Ib-IX/VWF axis in CLP
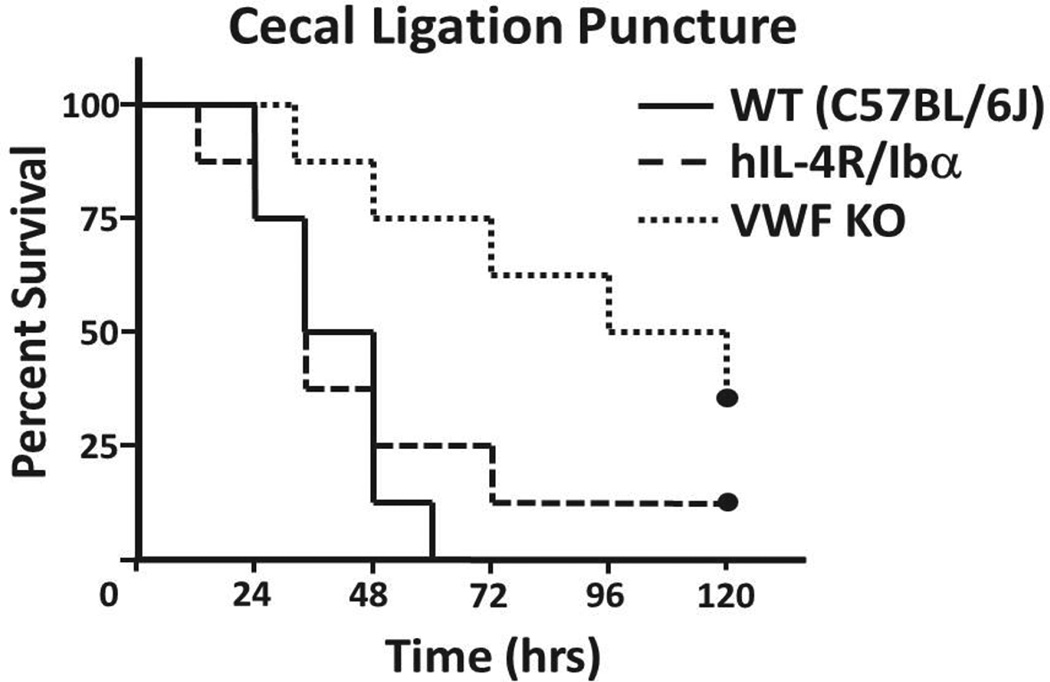
Previously, VWF knockout (KO) mice have been reported to have prolonged survival after a cecal ligation and puncture (CLP) surgery to induce severe sepsis.27 Since VWF serves as the primary ligand for the platelet GPIb-IX receptor, we hypothesized the absence of the ligand binding subunit (GP Ibα) of the receptor would improve septic survival in a similar manner. The CLP procedure was performed on male cohorts of wild-type (C57BL/6J), VWF KO, and hIL-4R/Ibα mice (Figure 1). Interestingly, survival of hIL-4R/Ibα mice following CLP was not statistically different from the wild-type group, whereas survival for VWF KO mice was improved. Thus, eliminating the ligand portion of the GP Ib-IX/VWF axis improves survival in this model, while eliminating the receptor portion of the axis was not beneficial. Twenty-four hours following CLP, all strains displayed an approximate 50% reduction in circulating platelet counts, typical of the consumptive coagulopathy associated with septic shock (not shown).
Figure 1. Kaplan-Meier survival curves following CLP.
Severe sepsis induced by CLP generated varying mortalities observed over a 5 day period. All wild-types succumbed to septic burden prior to the 72 hour mark. Deletion of the gene encoding VWF (VWF KO) significantly alleviated septic burden as the rate of mortality was reduced with several mice surviving past 5 days (p = 0.003). Conversely, ablation of a functional platelet GP Ib-IX axis (hIL-4R/Ibα) resulted in no discernible differences in survival when compared to wild-type (p = 0.614). N = 8 for each group. P values were determined by a Logrank analysis.
The platelet GPIb-IX/neutrophil and monocyte axes
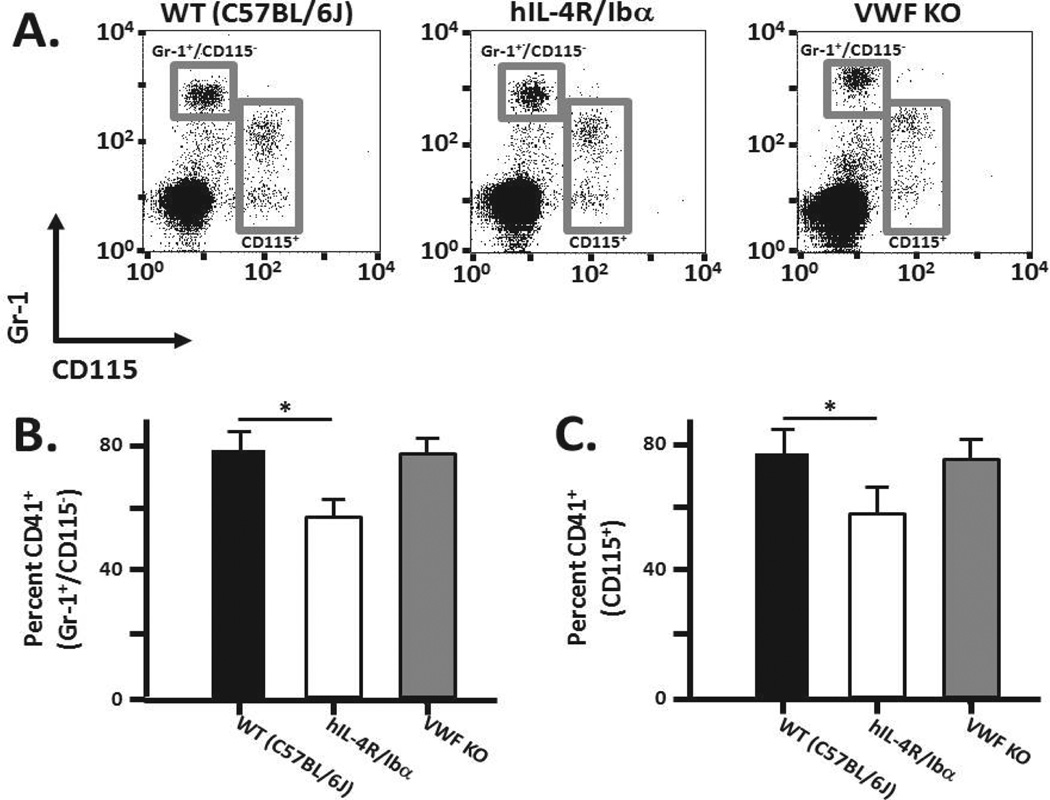
The leukocyte integrin Mac-1 (CD11b/CD18) has been reported to be a counter-receptor for GPIbα.24, 25 To further understand the role of GPIb-IX in potentially influencing outcome in murine CLP, we assessed whether disrupting GPIb-IX altered the extent of platelet association with neutrophils and monocytes. Whole blood collected from wild-type, hIL-4R/Ibα, and VWF KO mice was stained using a platelet-specific marker (CD41), a neutrophil/granulocyte marker (Gr-1), and a monocyte/macrophage marker (CD115). The number of platelet-neutrophil/monocyte interactions was analyzed via flow cytometry. The surface marker Gr-1 is an indicator of granulocytes, with neutrophils being the most predominant granulocyte. Therefore Gr-1 positive staining is interpreted loosely as a marker for the neutrophil population.
Figure 2A displays representative flow cytometry profiles identifying the Gr-1+/CD115− population that was further analyzed for CD41+ signals (Figure 2B). Following blood collection, approximately 80% of wild-type Gr-1+/CD115− events were also CD41+ positive with no statistical difference between wild-type and VWF KO samples (p = 0.54). However, a statistically significant reduced number of CD41+ events within the Gr-1+/CD115− gate was observed comparing wild-type and hIL-4R/Ibα samples (p = 0.0008). Focusing on the monocyte population (Figure 2A,C), a statistically significant reduction of CD41+ events within the CD115+ gate was also observed comparing wild-type and hIL-4R/Ibα samples (p = 0.003), but no difference between wild-type and VWF KO samples (p = 0.244). These preliminary findings demonstrate a reduction of CD41+ (platelet events) within neutrophil and monocyte populations in the hIL-4R/Ibα strain with no dependence on the presence or absence of VWF.
Figure 2. Platelet-neutrophil/monocyte interactions.
Whole blood was collected from wild-type, hIL-4R/Ibα and VWF KO mice under static conditions. (A) Representative flow analysis of samples identifying the neutrophil population (Gr-1+/CD115−) within wild-type, hIL-4R/Ibα or VWF KO blood is shown. Gating exclusively onto a Gr-1+/CD115− population (upper left gray box in panel A) allowed for a second gating of associated CD41+ (platelet) events within the neutrophil gate. (B) The CD41+/Gr-1+/CD115− population analyzed as a percent of total Gr-1+/CD115− events illustrates platelet-neutrophil interaction based on GP Ib-IX expression. Wild-type and VWF KO express endogenous GP Ib-IX and are indiscernible from one another (p = 0.54) but both exhibit significantly higher platelet-neutrophil levels compared to hIL-4R/Ibα (p < 0.001). This establishes a GP Ib-IX role in augmenting platelet binding to neutrophils. (C) Utilizing CD115+ fluorescence to gate exclusively onto monocytes (lower right gray box in panel A) the GP Ib-IX dependent association with platelets is presented. Comparison of the sample groups showed the wild-type and VWF KO strains to be indistinguishable (p = 0.24). Blood from hIL-4R/Ibα mice had significantly lower platelet-monocyte associations relative to wild-type (p = 0.003). This indicates a significant GPIb-IX dependent modulation of platelet binding to monocytes in the circulation. N = 6 for each group.
To further support the hIL-4R/Ibα findings, an alternative analysis was performed whereby CD41+ events were first identified in whole blood and then further gated for Gr-1+/CD115− cells and CD115+ cells (Supplemental Figure I). Only 2.5 percent of CD41+ events were present in the Gr-1+/CD115− population in wild-type samples and this was reduced to 0.5 percent analyzing blood from the hIL-4R/Ibα strain. A similar reduction within the CD115+ population was also observed using hIL-4R/Ibα blood. No statistically significant difference was observed in comparing wild-type to VWF KO samples. Thus, wild-type and VWF KO blood contained a significantly higher percentage of the platelet population associated with neutrophils and monocytes when compared to hIL-4R/Ibα blood samples further validating that platelet GP Ib-IX contributes to a platelet interaction with these leukocyte subclasses.
The platelet GPIb-IX/neutrophil and monocyte axes following CLP
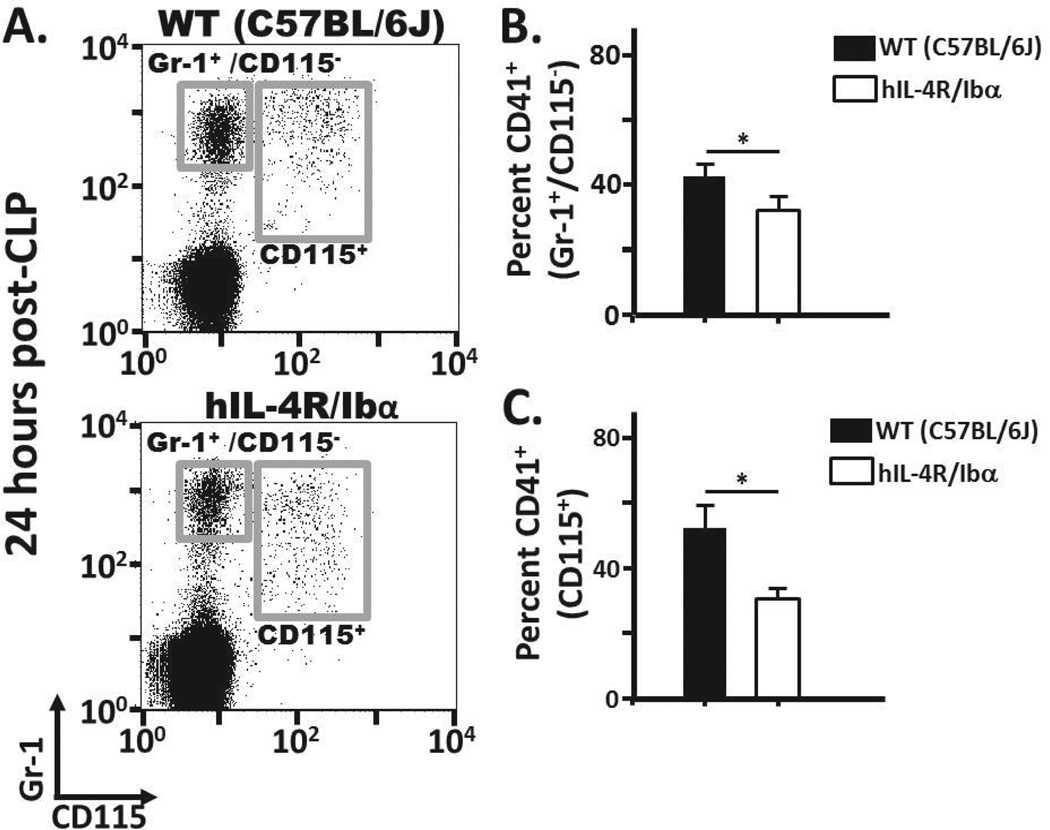
The induction of severe sepsis significantly alters the ability to maintain homeostasis of both the inflammatory and hemostatic pathways. This global dysregulation of both physiologic mechanisms could significantly alter the ability of platelets and leukocytes to interact as membrane receptor expression is rapidly modified via cellular activation events. In order to investigate the GP Ib-IX dependent platelet-neutrophil/monocyte axis under septic-like conditions, we conducted the same flow cytometry analysis as described above with blood collected from wild-type and hIL-4R/Ibα mice 24 hours-post CLP (Figure 3A). Overall, CD41+ events within the Gr-1+/CD115− and CD115+ population for both wild-type and hIL-4R/Ibα blood samples were reduced following CLP compared to sham treated controls (Figure 3B,C). hIL-4R/Ibα samples still displayed reduced platelet/neutrophil and platelet/monocyte interactions compared to WT samples following CLP, similar to that observed under normal conditions. In addition, we observed in both WT and hIL-4R/Ibα samples a positive shift in monocyte (CD115+) Gr-1 populations following CLP (comparing Figures 2 and 3).
Figure 3. Platelet/neutrophil and platelet/monocyte interactions following CLP.
(A) Twenty four hours after CLP, whole blood from wild-type and hIL-4R/Ibα mice was analyzed via flow cytometry to determine GP Ib-IX mediated platelet-neutrophil or platelet/monocyte events. (B) The percentage of neutrophils bound by platelets is reduced following CLP in both animal strains relative to static conditions. (C) A similar trend was observed in the monocyte gate with an overall reduction in platelet associated events following CLP. Despite the overall reduction in of platelet associated events in both gates, wild type samples did retain elevated interactions with platelets for both the neutrophil (p = 0.04) and monocyte (p = 0.05) populations. N = 6 (Normal) N = 11 (24 hrs post-CLP).
CLP-Induced TNFα Secretion
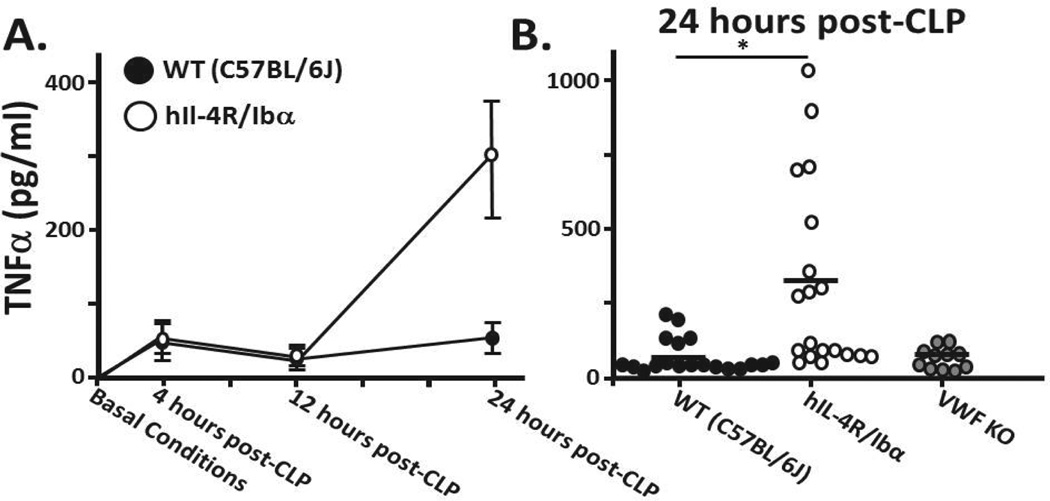
Platelets have been shown to be capable of influencing monocyte/macrophage secretion of TNFα.28 Having established the loss of GPIb-IX reduces the number of platelet-monocyte interactions we further hypothesized GPIb-IX absence would cause an alteration in monocyte/macrophage secretion during sepsis. Prior to CLP, serum levels of TNFα were undetectable in wild-type, hIL-4R/Ibα, or VWF KO strains (Figure 4A). TNFα was detectable at 4 and 12 hrs following CLP with no difference between wild type and hIL-4R/Ibα serums (Figure 4A). However, at 24 hrs post-CLP TNFα levels in hIL-4R/Ibα serum were, on average, significantly higher than serum levels from wild-type or VWF KO mice (Figure 4A,B).
Figure 4. TNFα levels following CLP.
(A) Serum was collected from mice under basal conditions as well as 4, 12 and 24 hours post CLP. Under non-septic (normal) conditions, TNFα is undetectable in serum. At 4 and 12 hours wild type and hIL-4R/Ibα samples exhibit indistinguishable serum TNFα levels. A marked difference is observed at 24 hours following sepsis induction. (B) TNFα levels of individual mice 24 hours post-CLP show on average significantly elevated TNFα levels in those mice lacking GP Ib-IX expression (p = 0.005). Comparison of wild-type and VWF KO show no distinction between serum TNFαlevels (p = 0.933). A horizontal bar represents the overall mean. N = 19 (WT); N = 19 (hIL-4R/Ibα); N = 11 (VWF KO).
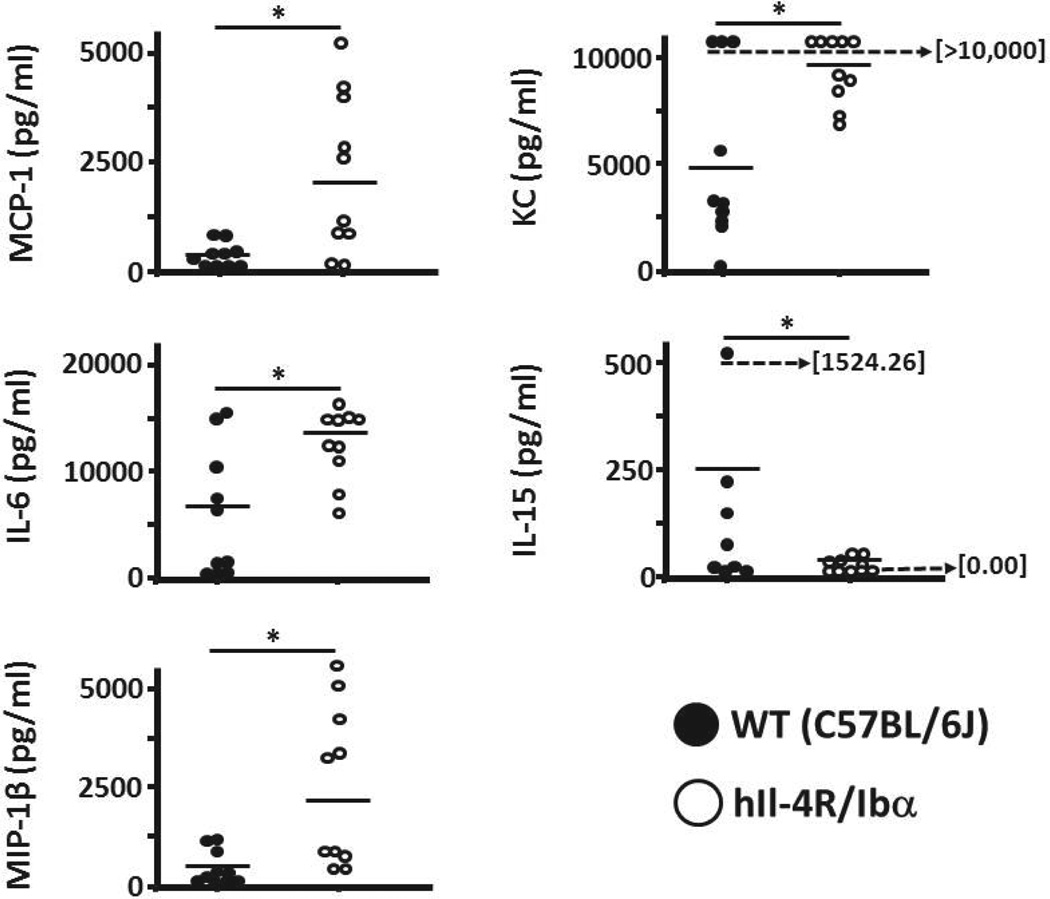
After observing the effect of GPIb-IX on modifying TNFα serum levels post-CLP, we investigated potential GPIb-IX influence on the secretion of other inflammatory mediators. Utilizing a Multiplex platform we found significant differences in several cytokines and chemokines 24 hours after CLP induction. A representative sampling revealed increase in hIL-4R/Ibα serum for MCP-1, IL-6, MIP-1β, and KC while showing a reduction in IL-15 concentration (Figure 5). Interestingly, these effectors are primarily secreted by the monocyte and macrophage population of cells. Cytokines associated with neutrophils did not reveal significant differences between WT and hIL-4R/Ibα samples (not shown). These findings provide evidence the platelet modifies the secretion pattern of monocyte lineage cells in a GPIb-IX dependent manner.
Figure 5. Inflammatory cytokine and chemokine levels 24 hours post-CLP.
Serum levels of 25 different inflammatory mediators were assessed following CLP surgery. Serum collected from mice was analyzed on an individual basis. A total of six different analytes (including TNFα, not shown) secreted primarily by cells of monocyte/macrophage lineage had significantly different concentrations when comparing wild-type and hIL-4R/Ibα serum. Four of the five analytes showed elevated levels in the hIL-4R/Ibα samples; MIP-1β (p = 0.0078), MCP-1 (p = 0.0129), KC (p = 0.0054) and IL-6 (p = 0.0085). Serum levels of IL-15 was reversed with the wild-type strain exhibiting higher serum levels (p = 0.0006). A horizontal bar indicates overall mean. N = 10 for each group.
Mac-1 Expression Following CLP
Mac-1 is upregulated on the surface of neutrophils and monocytes during inflammation. Reserve pools are stored within vesicles that readily fuse with the plasma membrane upon leukocyte activation.29 After tethering and rolling on the inflamed endothelium, the leukocyte must firmly adhere to the vessel wall before initiating a slow crawl to endothelial junctions before extravasation occurs.30–32 Thus, Mac-1 expression serves to indicate the migratory potential and activation state of the leukocyte.
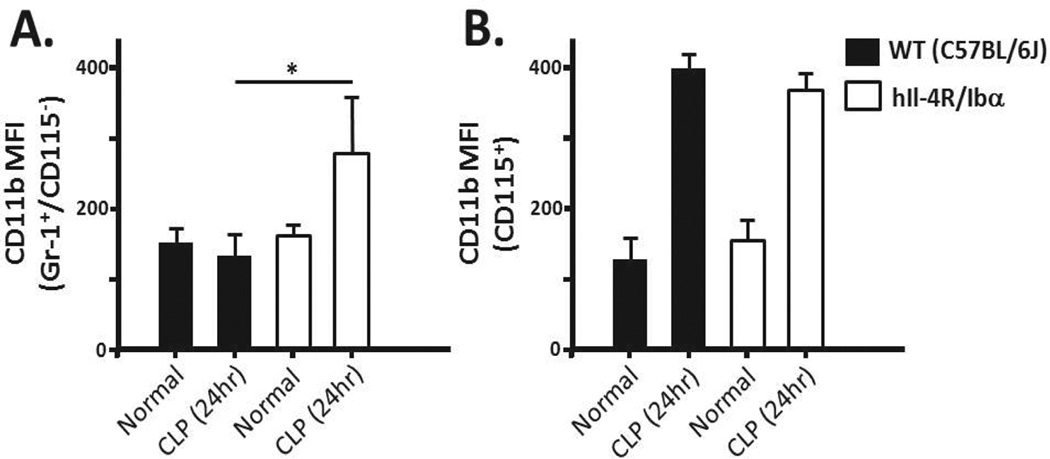
Whole blood was collected as previously described and stained for neutrophils and monocytes. In addition, a marker for CD11b was added to identify Mac-1 (CD11b/CD18) expression. Under basal conditions the Mac-1 expression by neutrophils does not differ between wild-type and hIL-4R/Ibα samples (Fig 6A). Furthermore, no significant difference is observed within the monocyte population (Fig 6B). Mac-1 expression was dramatically upregulated from basal conditions within the monocyte population for both WT and hIL-4R/Ibα samples collected 24 hours post-CLP indicating that GPIb-IX dysfunction did not directly lead to increased monocyte Mac-1 levels (Fig 6B). Interestingly, an increase in Mac-1 expression was observed in the hIL-4R/Ibα neutrophil population following CLP (Fig 6A). These results demonstrate GP Ib-IX attenuates neutrophil Mac-1 expression during systemic inflammation.
Figure 6. Mac-1 expression changes 24 hours post-CLP.
Blood isolated from wild-type (WT) and hIL-4R/Ibα mice under normal conditions or following CLP was stained using Gr-1 (neutrophils), CD115 (monocytes) and CD11b (Mac-1). Expression of Mac-1 for both the (A) neutrophil and (B) monocyte populations did not differ between strains in the absence of CLP (normal). Twenty four hrs following CLP, hIL-4R/Ibα display increased surface Mac-1 staining on the surface of neutrophils (p = 0.0348). Monocyte Mac-1 did not differ between strains 24 hours post-CLP. N = 6 for Normal samples and N = 11 for CLP samples.
Discussion
Platelets express receptors important in immunology, such as TLR4, and this likely reflects the common origin for leukocytes and platelets, the thrombocyte, which functions in lower vertebrates in both hemostasis and inflammation.10, 17, 33, 34 A common structural motif - the leucine rich repeat (LRR) - is present in each subunit of the GP Ib-IX complex and is a unifying motif for each of the 13 members of the TLR family.35, 36 Thus, a participatory role for GP Ib-IX in both hemostasis and immune function could reflect an ancestral link between innate pattern recognition receptors and receptors dedicated to hemostasis.17 More importantly, defining a GPIb-IX dependent role in immune regulation complements its well characterized role in hemostasis and thrombosis and represents a significant advancement in defining the complex pathophysiologic response and sequelae of events associated with septic shock and death.
Though sepsis is primarily characterized as systemic inflammation, it is also a state of hemostatic dysregulation.18 Indeed, the dysregulation of inflammation, coagulation, and the overlapping influence of each is a major challenge in accurately defining the septic syndrome. The platelet itself has been implicated as being an extension of the immune system due to its ability to elicit a number of inflammatory functions.16, 33, 37 Platelet adhesion to leukocytes has been speculated to form an interface where cross-talk between the cellular mediators of inflammation and coagulation occurs.38 The platelet’s ability to bind neutrophils and monocytes, in particular, has been implicated as a critical factor in generating vascular inflammation.39, 40 More challenging has been to define the importance of cellular activation at the level of the platelet, neutrophil, or monocyte for whether the interaction facilitates a proinflammatory or anti-inflammatory state. While under some conditions the platelet contributes to a proinflammatory state, our experiments identify a previously unidentified anti-inflammatory influence where an abrogated GP Ib-IX dependent platelet interaction with monocytes results in elevated serum cytokine levels and increased Mac-1 expression by neutrophils. Twenty four hours following CLP the majority of monocyte-derived cytokines in the hIL-4R/Ibα mouse are significantly elevated (Figure 5). This increase is reported from a single time point (24 hrs) in a dynamic process. How this impacts the complex pathophysiology of sepsis and outcome is still unclear. However, the results are similar to a recent report of platelet depletion causing elevated serum levels of TNFα and IL-6 in models of endotoxemia and E. coli induced sepsis.41
Our initial findings that VWF KO displayed improved survival following CLP suggested to us, that if the platelet/VWF thrombotic axis was aiding the improved survival, mice deficient in GP Ib-IX would have a similar improved outcome. Since this was not the case (Figure 1) we sought to explore possible mechanisms where platelet GP Ib-IX might influence inflammation. We do acknowledge, the improved survival in VWF KO mice could be unrelated to VWF’s role in thrombosis and could reflect altered endothelial cell function, Weibel-Palade body formation, or even P-selectin expression. In fact, the concurrent dysregulated coagulation and inflammation of in vivo sepsis does complicate a simple explanation for why VWF KO mice survive long and animals with dysfunctional GP Ib-IX die earlier. Despite an overwhelming inflammatory response in the hIL-4R/Ibα model, GP Ib-IX deficiency does drastically reduce the ability to generate occlusive thrombi. In contrast, wild-type mice have an intact GP Ib-IX complex participating in thrombosis, disseminated intravascular coagulation, and presumably organ failure during sepsis. Thus, while GP Ib-IX deficiency may protect against thrombosis, it subsequently contributes to excessive inflammation complicating the interpretation of survival as the single outcome.
Flow cytometry identified a GP Ib-IX dependent interaction for platelets with neutrophils and monocytes under normal physiologic conditions and following CLP. This interaction most likely reflects the known GP Ib-IX/Mac-1 axis.25, 42 However, even in the absence of GPIb-IX, there still exists platelet/neutrophil and platelet/monocyte populations. While the GP Ib-IX relevance in platelet/neutrophil and platelet/monocyte interaction seems modest, the effects on cytokine release and Mac-1 expression 24 hrs following CLP seems robust. The molecular basis of this interaction is unknown but could be dependent upon the activation state of the platelet with exposure of potential counter-receptors, such as P-selectin. A caveat yet to be addressed in this analysis is whether the CD41+ population is composed of platelets or platelet-derived microparticles also linked to various aspects of platelet-mediated inflammation.43
A variety of animal models have been developed to understand the molecular and genetic mechanisms of sepsis.19, 44 The goal of each is to reproduce the pathophysiology observed in septic patients. The more simplistic approaches are the administration of exogenous toxins, such as lipopolysaccharide (LPS) or viable pathogens. Both are relatively simple, reproducible, and restricted to TLR4 pathways (LPS) or mechanisms of host response to pathogens. We have recently reported a prolonged survival following LPS administration in the absence of functional GPIb-IX.45 This improved outcome most likely reflects the predominant effects of thrombosis occurring in the LPS model and the importance of GPIb-IX in pathologic thrombus formation. The CLP model differs from LPS-induced endotoxemia by using the host’s enteric bacteria to initiate the peritonitis followed by bacteria in the bloodstream, activation of an inflammatory response, and septic shock.26 Furthermore, reports indicate variation in both thrombus formation and cytokine secretion when comparing LPS administration to CLP.46, 47 Thus, major differences in key pathways exist when interpreting LPS experimental outcomes to CLP outcomes.48 With respect to the GP Ib-IX/VWF axis, absence of ligand (VWF) is beneficial in CLP while absence of the receptor (GPIb-IX) is not. We conclude platelet GPIb-IX dependent suppression of the inflammatory response is beneficial in CLP and less significant in the LPS model of endotoxemia.
Defining the importance of platelet GP Ib-IX to inflammation and thrombosis identifies opportunities to specifically target platelet function. Indeed, differentially targeting platelet function in inflammation and thrombosis to fit a specific pathophysiologic event or improve a clinical outcome would be a future goal. Recognizing an expanded functional influence for platelet GP Ib-IX in these processes is another piece to add to the complex pathophysiologic events of inflammation.
Supplementary Material
Significance.
The intrinsic “sticky” nature of platelets is relevant in the pathophysiology for a number of seemingly unrelated diseases. However, major gaps in our understanding of platelet function in these other disease states do exist. Fortunately, the well-characterized mechanisms of hemostasis and thrombosis are providing new mechanistic insights into the relevance of platelets in these other diseases. Using a mouse model of polymicrobial sepsis, we provide data illustrating a modulatory role for platelet GP Ib-IX during systemic inflammation. Well characterized as a major platelet adhesion receptor, the studies illustrate a dynamic platelet/neutrophil and platelet/monocyte axis that is platelet GP Ib-IX receptor dependent. The consequences of this interaction are changes in the cytokine storm and upregulation of the neutrophil Mac-1 following induction of sepsis. Understanding the platelet’s modulatory role in the immune response is an important advancement in the pathophysiology of sepsis.
Acknowledgments
The authors are thankful to Dr. Phil Mayeux (Department of Pharmacology & Toxicology, UAMS) for assistance in the cecal ligation and puncture model.
Sources of funding: This work was supported by the NHLBI RO1HL50545 (J.W.), an American Heart Association Pre-doctoral Award (A.C.), and Core facilities funded by IDeA award P20 GM103425.
Abbreviations
- GP
Glycoprotein
- WT
Wild Type
- VWF
Von Willebrand Factor
- hIL-4R
Human Interleukin 4 Receptor
- CLP
Cecal Ligation and Puncture
- TNF
Tumor Necrosis Factor
- MCP-1
Monocyte Chemotactic Protein-1
- IL-6
Interleukin 6
- MIP-1β
Macrophage Inflammatory Protein 1β
- TLR
Toll-like receptor
- KC
Keratinocyte Derived Chemokine
- IL-15
Interleukin 15
- Mac-1
Macrophage-1 Antigen
- CD
Cluster of Differentiation
- LPS
Lipopolysaccharide
Footnotes
Disclosure: None.
References
- 1.Cimmino G, Golino P. Platelet biology and receptor pathways. J Cardiovasc Transl Res. 2013;6:299–309. doi: 10.1007/s12265-012-9445-9. [DOI] [PubMed] [Google Scholar]
- 2.Clemetson KJ. Platelets and primary haemostasis. Thromb Res. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 5.Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 6.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 7.Ware J, Corken A, Khetpal R. Platelet function beyond hemostasis and thrombosis. Curr Opin Hematol. 2013 doi: 10.1097/MOH.0b013e32836344d3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornerup KN, Salmon GP, Pitchford SC, Liu WL, Page CP. Circulating plateletneutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol. 2010;109:758–767. doi: 10.1152/japplphysiol.01086.2009. [DOI] [PubMed] [Google Scholar]
- 9.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemetson KJ. Platelets and pathogens. Cell Mol Life Sci. 2010;67:495–498. doi: 10.1007/s00018-009-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 12.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 13.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14:785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Yang F, Dunn S, Gross AK, Smyth SS. Platelets as immune mediators: their role in host defense responses and sepsis. Thromb Res. 2011;127:184–188. doi: 10.1016/j.thromres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petaja J. Inflammation and coagulation. An overview. Thromb Res. 2011;127(Suppl 2):S34–S37. doi: 10.1016/S0049-3848(10)70153-5. [DOI] [PubMed] [Google Scholar]
- 16.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 17.Levin J. The evolution of mammalian platelets. In: Michelson AD, editor. Platelets. 3rd ed. New York: Elsevier Inc; 2013. pp. 3–25. [Google Scholar]
- 18.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci USA. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 22.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Clemetson KJ. A short history of platelet glycoprotein Ib complex. Thromb Haemost. 2007;98:63–68. [PubMed] [Google Scholar]
- 24.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zago AC, Simon DI, Wang Y, Sakuma M, Chen Z, Croce K, Ustinov V, Shi C, Martinez Filho EE. The importance of the interaction between leukocyte integrin Mac-1 and platelet glycoprotein Ibαfor leukocyte recruitment by platelets and for the inflammatory response to vascular injury. Arq Bras Cardiol. 2008;90:54–63. doi: 10.1590/s0066-782x2008000100009. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 27.Lerolle N, Dunois-Larde C, Badirou I, Motto DG, Hill G, Bruneval P, Diehl JL, Denis CV, Baruch D. von Willebrand factor is a major determinant of ADAMTS-13 decrease during mouse sepsis induced by cecum ligation and puncture. J Thromb Haemost. 2009;7:843–850. doi: 10.1111/j.1538-7836.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 28.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, Moita LF, Schurer S, Traver D, Ruiz P, Vazquez-Padron RI, Ley K, Reiser J, Gupta V. Small Molecule-Mediated Activation of the Integrin CD11b/CD18 Reduces Inflammatory Disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Mao D, Lu S, Tong C, Zhang Y, Long M. Distinct binding affinities of Mac-1 and LFA-1 in neutrophil activation. J Immunol. 2013;190:4371–4381. doi: 10.4049/jimmunol.1201374. [DOI] [PubMed] [Google Scholar]
- 32.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 33.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 34.Berthet J, Damien P, Hamzeh-Cognasse H, Pozzetto B, Garraud O, Cognasse F. Toll-like receptor 4 signal transduction in platelets: novel pathways. Br J Haematol. 2010;151:89–92. doi: 10.1111/j.1365-2141.2010.08292.x. [DOI] [PubMed] [Google Scholar]
- 35.Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC. Glycoprotein Ib-IX-V. Int J Biochem Cell Biol. 2003;35:1170–1174. doi: 10.1016/s1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 36.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 38.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131:191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 41.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun. 2013;4:2657–2668. doi: 10.1038/ncomms3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibαvia the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 43.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010 doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 45.Yin H, Stojanovic A, Xu W, Corken A, Zakharov A, Qian F, Pavlovic A, Krbanjevic A, Lyubimov AV, Wang ZJ, Ware J, Du X. The role of platelet glycoprotein Ib-IX and effect of an inhibitor of GPIb-IX function in endotoxemia-induced microvascular thrombosis and thrombocytopenia. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.302339. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorgersen EB, Hellerud BC, Nielsen EW, Barratt-Due A, Fure H, Lindstad JK, Pharo A, Fosse E, Tonnessen TI, Johansen HT, Castellheim A, Mollnes TE. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 2010;24:712–722. doi: 10.1096/fj.09-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel KN, Soubra SH, Lam FW, Rodriguez MA, Rumbaut RE. Polymicrobial sepsis and endotoxemia promote microvascular thrombosis via distinct mechanisms. J Thromb Haemost. 2010;8:1403–1409. doi: 10.1111/j.1538-7836.2010.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.