Introduction
MicroRNAs (miRNAs) are single-stranded noncoding RNAs that regulate gene expression by partially base pairing with target mRNAs and either inhibiting their translation or promoting their degradation [1]. Recently, a heterozygous mutation c.57 C>U in the seed region of miR-184 (MIRN184 [MIM 613146]) located on15q25.1 was found in the affected members of the large Northern Irish family with rare familial syndrome of anterior polar cataracts and keratoconus [MIM 613146.0001] [2]. Same mutation was later identified in a large family with EDICT syndrome, an autosomal dominant syndromal anterior segment dysgenesis characterized by endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning [MIM 614303][3]. Here we describe third occurrence of the same mutation in the members of five-generation family originated from Galicia, Spain with congenital cataracts and variable corneal abnormalities which include both non-ectatic corneal thinning and severe early onset keratoconus. We suggest that ophthalmologists should consider testing for this mutation in patients presenting with similar symptoms.
Materials and Methods
Research Subjects
A 9 year old boy (proband) was referred to one of the authors (YSR) for progressive loss of vision in both eyes. Later his parents and maternal and paternal grandmothers were examined. This study was conducted in concordance with the provisions of the Declaration of Helsinki and Institutional Review Board (IRB) approval. Written informed consent was obtained from all subjects.
Clinical Diagnosis
Complete ophthalmological evaluation was performed by a cornea trained ophthalmologist (YSR). A diagnosis of keratoconus was made if there was corneal stromal thinning on slit-lamp evaluation, scissoring on retinoscopy, and an AB/RAX pattern on videokeratography [4], accompanied by marked corneal thinning on OCT evaluation device (Optovue Inc., Fremont, CA). A diagnosis of generalized thinning was made if there was thinning demonstrated on OCT but no clinical or videokeratography signs of keratoconus.
DNA isolation
Genomic DNA was extracted from immortalized with Epstein-Barr virus lymphoblastoid cell lines was extracted using NucleoSpin Tissue kit (Macherey-Nagel Inc., Bethlehem, PA) according to the manufacturer’s protocol.
Sequencing
Stem-loop region of MIR184 gene was amplified by PCR as described [2] and sequenced in both directions using Sanger DNA Sequencing services by Genewiz, Inc. (La Jolla, CA).
Results
Evaluation of the family members
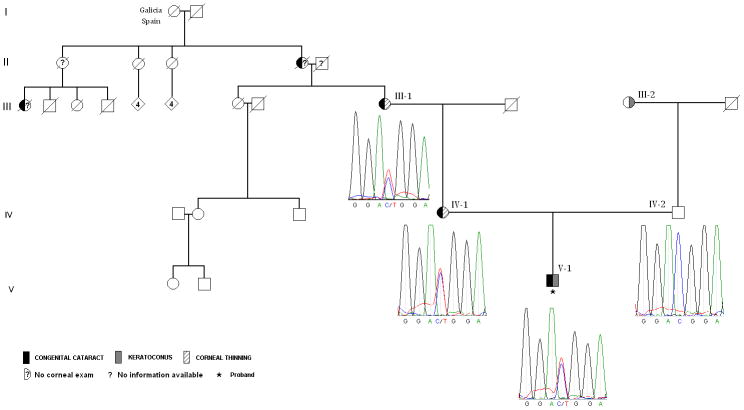
Clinical findings of the family members are summarized in Table 1 and family structure id presented in Figure 2. Congenital cataracts and keratoconus were diagnosed in the proband; whereas proband’s mother, maternal grandmother, and maternal great-grandmother were previously diagnosed with congenital cataracts show extreme corneal thinning. The great-grandmother was born in the city of La Estrada in Galicia, Spain. She had three sisters, none of whom have been diagnosed with cataracts. All other relatives on the maternal line (still residing in Spain) have not been diagnosed with either lens or corneal abnormalities. The paternal side of the family is described by mixed ethnicity which includes Eastern European and German roots.
Table 1.
Clinical features of the examined family members of the Galician family.
| Subject | Age | Right eye | Left eye |
|---|---|---|---|
| III-1 | 83 | Not available | Congenital cataract; Uniform corneal thinning |
| III-2 | 74 | Age-related late onset cataract | Age-related late onset cataract Late onset keratoconus |
| IV-1 | 47 | Congenital cataract Uniform corneal thinning |
Congenital cataract Uniform corneal thinning |
| IV-2 | 48 | Normal | Normal |
| V-1 | 10 | Congenital posterior cataract Keratoconus |
Congenital posterior cataract Keratoconus |
Figure 2.
Family structure with the results of sequencing analysis identifying c.57 C>U mutation in MIR184 gene.
Identification of the MIR184 c.57 C>U mutation in affected members of the family
To test for the MIR184 c.57 C>U mutation we amplified and sequenced 300 bp of the MIR184 gene overlapping the position of the mutation in a proband and both of his parents. We observed this mutation in a heterozygous state in the proband and his mother (Figure 2, individuals IV-1 and V-1). A homozygous wild type C allele was identified in proband’s father (Figure 2, individual IV-2). Same mutation was identified in the subsequently collected and sequenced DNA of the proband’s maternal grandmother (Figure 2, individual III-1).
Discussion
The combination of the clinical signs of congenital cataracts with childhood bilateral keratoconus in the proband in the family we report here is very rare and shares a significant similarity to the clinical features described in the affected members of the Northern Irish family [5] (Table 2). In contrast, maternal relatives of the proband share a combination of the congenital cataracts with diffuse stromal thinning (but no keratoconus) similar to the corneal defect described in the affected members of the EDICT family [6]. Based on clinical similarity between these three families, two of which carry the MIR184 c.57 C>U mutation, we decided to perform molecular testing for this mutation using the proband’s DNA sample. We identified c.57 C>U mutation in the heterozygous state in all maternally related affected members of the family including proband, his mother and maternal grandmother. The mutation was not present in the father of the proband.
Table 2.
Families with corneal and lens abnormalities and MIR184 c.57C>T mutation.
| # | Description | Ethnicity | Inheritance | Corneal Defects | Iris Defect | Lens Defect |
|---|---|---|---|---|---|---|
| 1 | Keratoconus with cataract | Northern Irish | Autosomal Dominant | Keratoconus | None | Congenital polar cataract |
| 2 | EDICT | ? | Autosomal Dominant | Corneal endothelial dystrophy; Uniform corneal thinning with uniform steepening | Hypoplasia | Congenital cataract |
| 3 | Keratoconus and corneal thinning with cataract | Galician | Autosomal Dominant | Keratoconus; Extreme corneal thinning with uniform steepening | None | Congenital posterior cataract |
It is quite common that the same mutation may lead to different clinical manifestations (phenotypes) in different individuals [7]. This is the case with c.57 C>U mutation which already has been documented with a significant heterogeneity of ocular abnormalities across different families (Table 2). This observation led to an extensive discussion in an effort to explain this phenotypic variability by potential phenotype misclassification or additional genomic variants [8], [9], [10, 11]. In the Galician family there is also a clear heterogeneity of corneal defect between a proband and his maternal female relatives (keratoconus vs. corneal thinning, Figure 1), and of lens defect between a proband and Northern Irish and EDICT families (posterior vs. anterior cataracts). This genetic heterogeneity can be explained by modification by individual’s genomic variant (s) millions of which we carry in our genome [12]. Some of these ‘modifiers’ may have been introduced many thousands years ago and some - recently. Such recently acquired genetic ‘modifier (s)’ may have been inherited by a proband from his paternal grandmother (Figure 2, individual III-2) who was diagnosed with keratoconus at the age of 65.
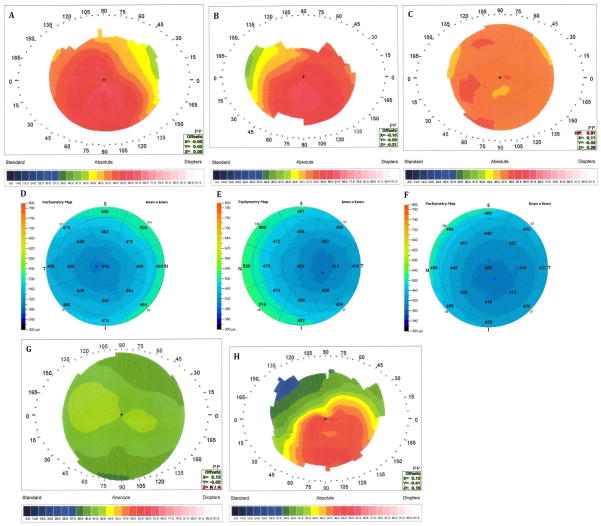
Figure 1.
Videokeratography and OCT measurements of the proband and additional family members. A. Proband (V-1), OD, videokeratography map; B. Proband (V-1), OS, videokeratography map; C. Mother of the proband (IV-1), OS, videokeratography map; D. Proband (V-1), OD, OCT pachymetry; E. Proband (V-1), OS, OCT pachymetry; F. Mother of the proband (IV-1), OS, OCT pachymetry; G. Father of the proband (IV-2), OS, videokeratography map; H. Paternal grandmother of the proband (III-2), OS, videokeratography map.
In conclusion, we suggest that the third independent occurrence of the MIR184 c.57 C>U mutation presented here supports genetic testing for this variant in patients with familiar corneal and lens abnormalities.
Acknowledgments
We thank all family members for participation. This work was supported by National Eye Institute grant NEI 09052, the Skirball Foundation for Molecular Ophthalmology, and the Eye Defects Research Foundation Inc.
Footnotes
MIR 184 mutation in corneal abnormalities with cataracts
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Dostie J, Mourelatos Z, Yang M, Sharma A, Dreyfuss G. Numerous microRNPs in neuronal cells containing novel microRNAs. Rna. 2003;9(2):180–186. doi: 10.1261/rna.2141503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, Willoughby CE. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am J Hum Genet. 2011;89(5):628–633. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff BW, Riazuddin SA, Gottsch JD. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53 (1):348–353. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11(5):371–379. doi: 10.3928/1081-597X-19950901-14. [DOI] [PubMed] [Google Scholar]
- 5.Hughes AE, Dash DP, Jackson AJ, Frazer DG, Silvestri G. Familial keratoconus with cataract: linkage to the long arm of chromosome 15 and exclusion of candidate genes. Invest Ophthalmol Vis Sci. 2003;44(12):5063–5066. doi: 10.1167/iovs.03-0399. [DOI] [PubMed] [Google Scholar]
- 6.Akpek EK, Jun AS, Goodman DF, Green WR, Gottsch JD. Clinical and ultrastructural features of a novel hereditary anterior segment dysgenesis. Ophthalmology. 2002;109(3):513–519. doi: 10.1016/s0161-6420(01)00975-7. [DOI] [PubMed] [Google Scholar]
- 7.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Iliff BW, Riazuddin SA, Gottsch JD. Documenting the corneal phenotype associated with the MIR184 c. 57C>T mutation. Am J Hum Genet. 2012;90 (5):934. doi: 10.1016/j.ajhg.2012.01.019. author reply 934–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AEBD, Campbell M, Simpson DA, Willoughby CE. Response to Iliff et al. Am J Hum Genet. 2012;90:934–935. [Google Scholar]
- 10.Giasin O, Khan RS, Khan K. Corneal thinning phenotypes: an alternative perspective. Invest Ophthalmol Vis Sci. 2012;53(2):1048. doi: 10.1167/iovs.11-9372. reply 1048. [DOI] [PubMed] [Google Scholar]
- 11.Iliff BWRS, Gottsch JD. Author response: corneal thinning phenotypes--an alternative perspective. Invest Ophthal Vis Sci. 2012;53:1048. doi: 10.1167/iovs.11-9372. [DOI] [PubMed] [Google Scholar]
- 12.McKernan KJ, Peckham HE, Costa GL, McLaughlin SF, Fu Y, Tsung EF, Clouser CR, Duncan C, Ichikawa JK, Lee CC, et al. Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome research. 2009;19(9):1527–1541. doi: 10.1101/gr.091868.109. [DOI] [PMC free article] [PubMed] [Google Scholar]