Abstract
Objectives
Lymphopenia is a common consequence of chemoradiation therapy yet is seldom addressed clinically. This study was conducted to determine if patients with locally advanced pancreatic cancer (LAPC) treated with definitive chemoradiation develop significant lymphopenia and if this affects clinical outcomes.
Methods
A retrospective analysis of patients with LAPC treated with chemoradiation at a single institution from 1997 to 2011 was performed. Total lymphocyte counts (TLCs) were recorded at baseline and then monthly during and after chemoradiation. The correlation between treatment-induced lymphopenia, established prognostic factors, and overall survival was analyzed using univariate Cox regression analysis. Important factors identified by univariate analysis were selected as covariates to construct a multivariate proportional hazards model for survival.
Results
A total of 101 patients met eligibility criteria. TLCs were normal in 86% before chemoradiation. The mean reduction in TLC per patient was 50.6% (SD, 40.6%) 2 months after starting chemoradiation (P< 0.00001), and 46% had TLC< 500 cells/mm3. Patients with TLC < 500 cells/mm3 2 months after starting chemoradiation had inferior median survival (8.7 vs. 13.3mo, P= 0.03) and PFS (4.9 vs. 9.0mo, P = 0.15). Multivariate analysis revealed TLC< 500 cells/mm3 to be an independent predictor of inferior survival (HR= 2.879, P= 0.001) along with baseline serum albumin (HR= 3.584, P = 0.0002), BUN (HR = 1.060, P= 0.02), platelet count (HR= 1.004, P = 0.005), and radiation planning target volume (HR= 1.003, P= 0.0006).
Conclusions
Severe treatment-related lymphopenia occurs frequently after chemoradiation for LAPC and is an independent predictor of inferior survival.
Keywords: pancreatic adenocarcinoma, locally advanced, lymphopenia, chemoradiation, survival
Many clinical investigations have suggested that the immune system and circulating lymphocyte populations play an integral role in oncologic outcomes.1 Recent studies have demonstrated that the presence of tumor-infiltrating lymphocytes correlates with improved prognosis and that immune surveillance can eliminate cancer before it becomes clinically apparent.2–4 Total lymphocyte count (TLC) seems to correlate with survival in multiple cancer types. For example, among patients with advanced melanoma who were treated with ipilimumab, median survivals of 11.9 versus 1.4 months were observed in patients with lymphocyte counts >1000 versus <1000 cells/µL, respectively.5 Likewise, lymphocyte counts independently predicted for survival in patients with metastatic renal cell carcinoma who received subcutaneous treatment with interleukin-26 as well as in patients with brain metastases from breast adenocarcinoma.7
Mounting evidence indicates that the immune system strongly influences the natural history of pancreatic cancer. The ability of pancreatic tumor cells to escape host immune surveillance by causing immunosuppression through expression of the apoptosis-inducing molecule Fas ligand,8 defective signaling through Fas,9 and secretion of transforming growth factor-β10 is thought to contribute to the poor prognosis of this disease.11 Corroborating this idea, circulating lymphocyte counts are lower in patients with pancreatic cancer than in those with benign pancreatic disease.12 In addition, patients with locally advanced or metastatic pancreatic cancer (stages III and IV) have lower lymphocyte counts than patients with resectable tumors (stages I and II).12 Low baseline and preoperative lymphocyte counts are associated with decreased survival in pancreatic cancer patients, and a reduced number of CD8+ -lymphocyte infiltrates in pancreatic tumor specimens correlates with worse prognosis.12–14
Until recently, however, little attention has been paid to the lymphopenia that frequently results from therapy for pancreatic cancer. Up to 50% of patients develop grade I to II lymphopenia and 20% to 40% develop grade III to IV lymphopenia after chemoradiation in prospective trials for pancreatic cancer.15,16 Recent work has demonstrated that treatment-induced lymphopenia is associated with survival in patients receiving adjuvant chemoradiation for resected pancreatic adenocarcinoma and in those treated with definitive chemoradiation for high-grade gliomas.17,18 Herein we investigate whether treatment-related lymphopenia is associated with overall and progression-free survival (PFS) in patients with locally advanced pancreatic cancer (LAPC) who receive definitive chemoradiotherapy.
MATERIALS AND METHODS
Patient Selection
This study was a retrospective chart review study approved by the Johns Hopkins Institutional Review Board. The records of all pancreatic cancer patients treated at our institution from 1997 through 2011 were reviewed to identify patients with LAPC who received definitive chemoradiation therapy. A diagnosis of locally advanced disease was based on review of cross-sectional imaging by an attending surgeon or anatomic findings at the time of surgical exploration rendering the tumor unresectable. Specific features defining unresectability included tumor encasement of the celiac axis and/or superior mesenteric artery or loss of patency of the superior mesenteric-portal vein confluence. Additional inclusion criteria included: (1) age 18 years or above, (2) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, (3) biopsy-confirmed pancreatic ductal adenocarcinoma, (4) definitive chemoradiation therapy administered at our institution, and (5) baseline and follow-up laboratory values measured at our institution and available in the electronic medical record. Patients were excluded if they went on to have successful tumor resection with curative intent or if they had evidence of distant metastatic disease before chemoradiation.
Data Collection
Demographic, baseline disease, and treatment characteristics were obtained from the electronic medical record for each patient. In addition, data were gathered regarding potential prognostic factors identified in previously published reports.19–29 Patient-specific variables included age, sex, race, ECOG performance status, and baseline laboratory values [CA19-9, blood urea nitrogen (BUN), albumin, aspartate aminotransferase, alkaline phosphatase, hematocrit, platelet count, white blood cell count, and TLC]. Tumor-specific variables included location within the pancreas, maximum tumor diameter, and histologic grade. Treatment parameters consisted of radiation dose, fractionation, planning target volume (PTV; defined as the volume of tissue receiving ≥95% of the prescribed radiation dose, consisting of the gross tumor volume plus a 1.5 to 2 cm margin to account for breathing motion and setup error), concurrent chemotherapy type, treatment breaks, and whether or not induction and/or maintenance chemotherapy was given in addition to chemoradiation. Data from complete blood counts (CBCs; including hematocrit, platelet, leukocyte, and lymphocyte values) were recorded at monthly intervals after the start of chemoradiation. If no CBC was available at the exact monthly interval of interest, the CBC drawn closest to the desired date was used with the constraint that it must have been drawn within 2 weeks of the exact monthly interval of interest. If no CBC was available within 2 weeks of the exact monthly interval of interest, no hematologic data were recorded for that time point. The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 were used to categorize patients as having severe lymphopenia (grade III to IV; <500 cells/mm3) or milder lymphopenia (grade 0 to II; ≥500 cells/mm3) at the first time point after completion of chemoradiation (2 mo CBC).
Statistical Analysis
Demographic, baseline, and treatment characteristics were summarized using descriptive statistics. The Fisher exact test was used to compare proportions between groups. The Mann-Whitney U test was used to compare medians between groups. The Welch t test and the paired t test were used to compare 2 means between and within groups, respectively. The primary outcomes of interest were survival and PFS. Survival and PFS were calculated from the start date of chemoradiation to the date of death and the date of first radiographic progression or death, respectively. Survival was censored if the subject remained alive at the date of last follow-up, and PFS was censored if the subject remained alive without progression at the date of last follow-up. Survival and PFS probabilities were estimated using Kaplan-Meier statistics.30 Univariate Cox regression analyses were used to assess for an association between potential prognostic factors and survival. Factors identified as statistically significant on univariate analysis along with factors of accepted clinical importance (age, performance status, histologic grade, baseline laboratory values, radiation dose received, and induction chemotherapy) were selected as covariates to construct a multivariate proportional hazards regression model for survival.31 This model was used to estimate the hazard ratio (HR) for death attributable to each covariate using backward elimination. All P-values were reported as 2-sided, and the a priori level of significance was set at P≤0.05. Analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patients
In total, 104 patients met inclusion criteria; however, 3 patients were excluded due to subsequent surgical resection of their pancreatic tumors. Consequently, 101 patients who received definitive chemoradiation for unresectable LAPC were included in the analysis. Demographic, baseline, and treatment characteristics are summarized in Table 1. There were no significant differences in these characteristics between patients who did and did not experience severe lymphopenia at 2 months postinitiation of chemoradiation (all P > 0.05), except for baseline platelet count, which was lower on average in patients who experienced severe lymphopenia, though still well within the normal range (median 203,000/µL vs. 248,000/µL; P = 0.03) (Table 1).
TABLE 1.
Demographic, Baseline, and Treatment Characteristics for the Entire Cohort (n = 101) and Broken Down by Total Lymphocyte Count <500 or ≥500 cells/mm3 2 Months After Starting Chemoradiation
| Groups Patients (n = 101) |
Patients With Lymphocyte Counts <500 cells/mm3 at 2 mo (n = 46) |
Patients With Lymphocyte Counts ≥500 cells/mm3 at 2 mo (n = 55) |
P | |
|---|---|---|---|---|
| Demographic data | ||||
| Age [median (IQR)] | 62 (55–69) | 62 (54–74) | 62 (57–69) | 0.87 |
| Age ≥65 y [N (%)] | 37 (37) | 18 (39) | 19 (35) | 0.68 |
| Sex: male [N (%)] | 57 (56) | 28 (61) | 29 (53) | 0.43 |
| Race: white [N (%)] | 76 (75) | 37 (80) | 39 (71) | 0.36 |
| ECOG performance status ≥1 [N (%)] | 44 (44) | 23 (50) | 21 (38) | 0.31 |
| Baseline tumor data | ||||
| Tumor location [no. head of pancreas (%)] | 76 (75) | 33 (72) | 43 (78) | 0.49 |
| Tumor size (cm) [median (IQR)] | 3.6 (2.9–4.7) | 4.0 (3.0–5.0) | 3.5 (2.6–4.5) | 0.57 |
| Histologic grade | ||||
| No. well differentiated (%) | 3 (3) | 0 (0) | 3 (5) | 0.25 |
| No. moderately differentiated (%) | 10 (10) | 7 (15) | 3 (5) | 0.18 |
| No. poorly differentiated (%) | 20 (20) | 8 (17) | 12 (22) | 0.62 |
| No. adenocarcinoma not otherwise specified (%) | 68 (67) | 31 (67) | 37 (67) | 0.99 |
| Baseline laboratory data | ||||
| Lymphocyte count in normal range (≥1000) [N (%)] | 87 (86) | 39 (85) | 48 (87) | 0.78 |
| Albumin [median (IQR)] | 4.0 (3.7–4.2) | 4.1 (3.7–4.2) | 4.0 (3.7–4.3) | 0.42 |
| Alkaline phosphatase [median (IQR)] | 99 (76–180) | 99 (82–185) | 99 (75–177) | 0.87 |
| AST [median (IQR)] | 26 (19–38) | 26 (19–35) | 27 (19–38) | 0.91 |
| BUN [median (IQR)] | 13 (10–17) | 12 (9–16) | 13 (10–18) | 0.17 |
| CA19-9 [median (IQR)] | 184 (46–716) | 200 (83–877) | 139 (42–384) | 0.37 |
| Hematocrit [median (IQR)] | 36.9 (33.6–39.4) | 36.7 (34.1–38.9) | 37.6 (33.5–39.8) | 0.82 |
| Platelet count [median in thousands (IQR)] | 228 (181–297) | 203 (155–276) | 248 (198–315) | 0.03 |
| Leukocyte count [median (IQR)] | 6780 (5315–8295) | 6305 (5180–7845) | 7240 (5505–8835) | 0.23 |
| Treatment data | ||||
| Time from diagnosis to chemoradiation initiation (mo) [median (IQR)] | 1.6 (1.1–2.5) | 1.8 (1.1–2.7) | 1.6 (1.0–2.4) | 0.68 |
| Concurrent chemotherapy | ||||
| 5-fluorouracil [N (%)] | 77 (76) | 34 (74) | 43 (78) | 0.65 |
| Gemcitabine [N (%)] | 22 (22) | 12 (26) | 10 (18) | 0.47 |
| Other agent [N (%)] | 2 (2) | 0 (0) | 2 (4) | 0.50 |
| Maintenance chemotherapy | 53 (52) | 26 (57) | 27 (49) | 0.55 |
| Radiation dose received (cGy) [median (IQR)] | 5040 (5000–5040) | 5040 (5000–5040) | 5040 (5040-5040) | 0.52 |
| Radiation daily fraction size (cGy) [median (IQR)] | 180 (180–200) | 180 (180–200) | 180 (180–190) | 0.83 |
| Radiation technique [no. IMRT (%)] | 44 (44) | 22 (48) | 22 (40) | 0.55 |
| Radiation treatment break required [N (%)*] | 9 (9) | 3 (7) | 6 (11) | 0.50 |
The right-most column shows P-values for statistical comparison of each characteristic between patients with lymphocyte counts <500 (n = 46) and ≥500 cells/mm3 (n = 55) at 2 months postinitiation of chemoradiation.
Includes patients who stopped chemoradiation therapy early before completion of the initigroupsy prescribed dose.
AST indicates aspartate aminotransferase; BUN, blood urea nitrogen; CA19-9, carbohydrate antigen 19-9; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity-modulated radiation therapy; IQR, interquartile range.
Eighty-six patients (85.1%) received definitive chemoradiation as their first antineoplastic therapy, whereas 15 patients (14.9%) received induction chemotherapy before chemoradiation. These latter 15 patients were included because after induction chemotherapy TLCs among these patients at baseline (within 1 mo before starting chemoradiation) were not significantly different from those of the 86 patients who received immediate chemoradiation. The 15 patients receiving induction chemotherapy underwent a median of 2 cycles [interquartile range (IQR), 2 to 4] of gemcitabine-based chemotherapy; the median time elapsed from the last dose of induction chemotherapy to baseline measurement of TLC before starting chemoradiation was 1.02 months (IQR, 0.61 to 1.33). Chemotherapy administered concurrently with radiation consisted primarily of 5-fluorouracil (5-FU)/capecitabine-based therapy (76%) or gemcitabine-based therapy (22%). At baseline, patients receiving immediate chemoradiation had a mean TLC of 1540 cells/mm3 (SD, 655) compared with 1554 cells/mm3 (SD, 415) for those who had received induction chemotherapy (P = 0.76). Furthermore, the proportion of patients with a normal TLC (≥1000 cells/mm3) after induction chemotherapy [13 of 15 patients (87%)] was nearly identical to the proportion of patients with a normal TLC in the immediate chemoradiation group [74 of 86 patients (86%)] (P = 0.99). Subsequently, receipt of induction chemotherapy was not found to affect either survival [HR = 1.120; 95% confidence intervals (CI), 0.602–2.083; P = 0.72] or PFS (HR = 1.324; 95% CI, 0.705–2.486; P = 0.38); likewise, the time elapsed between diagnosis and the start of chemoradiation did not influence survival (HR = 1.081; 95% CI, 0.939–1.244; P = 0.28) or PFS (HR = 1.062; 95% CI, 0.912–1.236; P = 0.44).
Lymphopenia
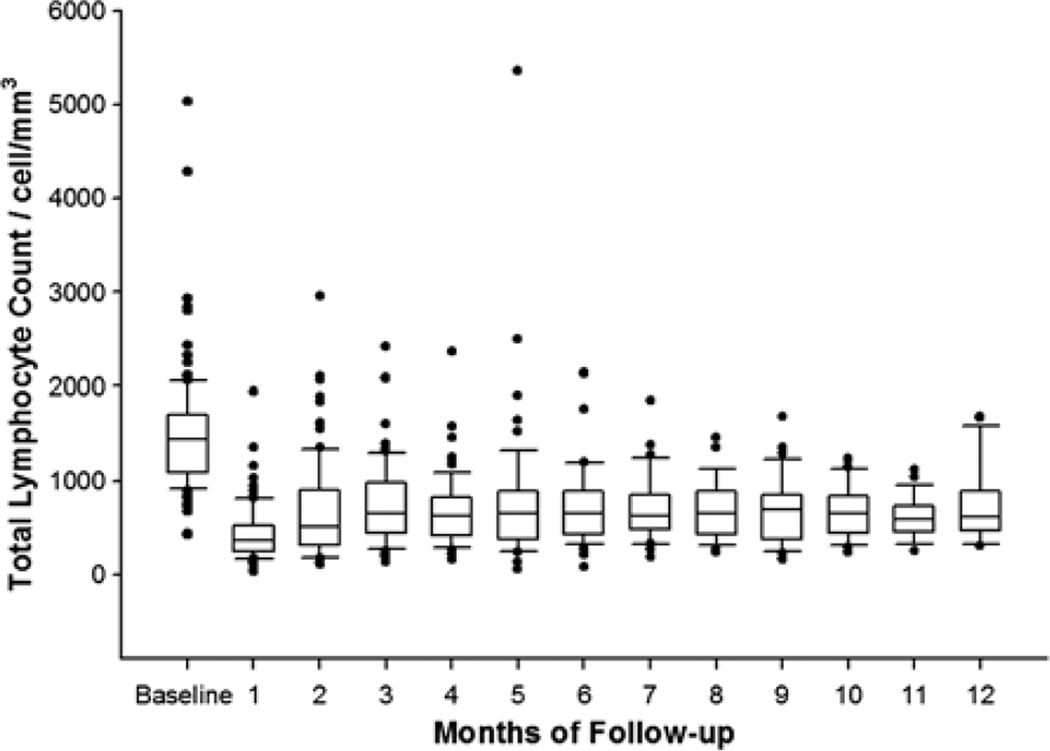
Eighty-seven patients (86%) had a normal baseline TLC (defined as ≥1000 cells/mm3 according to the NCI CTCAE) before initiation of chemoradiation. Mean TLC for the entire cohort decreased from 1508 cells/mm3 (SD, 613) at baseline to 662 cells/mm3 (SD, 497) 2 months after starting chemoradiation (P << 0.00001, Welch t test) and remained low for at least 12 months after initiation of chemoradiation (Fig. 1). Per patient, the mean reduction in TLC was 50.6% (SD, 40.6%; P << 0.00001, paired t test). Among patients who received induction chemotherapy, 13/15 (87%) had normal TLCs (≥1000 cells/mm3) after induction chemotherapy. Mean baseline TLC among these patients was 1554 cells/mm3 (SD, 415) before starting chemoradiation after induction chemotherapy.
FIGURE 1.
Total lymphocyte counts over time. Baseline corresponds to prechemoradiation, whereas months of follow-up are measured from the chemoradiation start date.
The NCI CTCAE threshold for grade III to IV lymphopenia was used to divide the 101 patients in the cohort into a high TLC group (≥500 cells/mm3; n = 55) and a low TLC group (< 500 cells/mm3; n = 46) 2 months after starting chemoradiation. Given the retrospective nature of this study, the 2-month time point from which these TLCs were derived was, by necessity, an approximate time point. The median time elapsed from the start of chemoradiation therapy to drawing of blood for the 2-month TLC was 2.07 months (IQR, 1.81 to 2.27); the median deviation in timing of the 2-month TLC from exactly 2 months after starting chemoradiation was 8.32 days (IQR, 3.23 to 11.44). The median time elapsed from the completion of chemoradiation therapy to drawing of blood for the 2-month TLC was 0.85 months (IQR, 0.36 to 1.05). Two patients did not have TLCs available at the 2-month time point, so TLCs at 1 month after beginning chemoradiation were used as surrogates in these 2 cases.
Survival
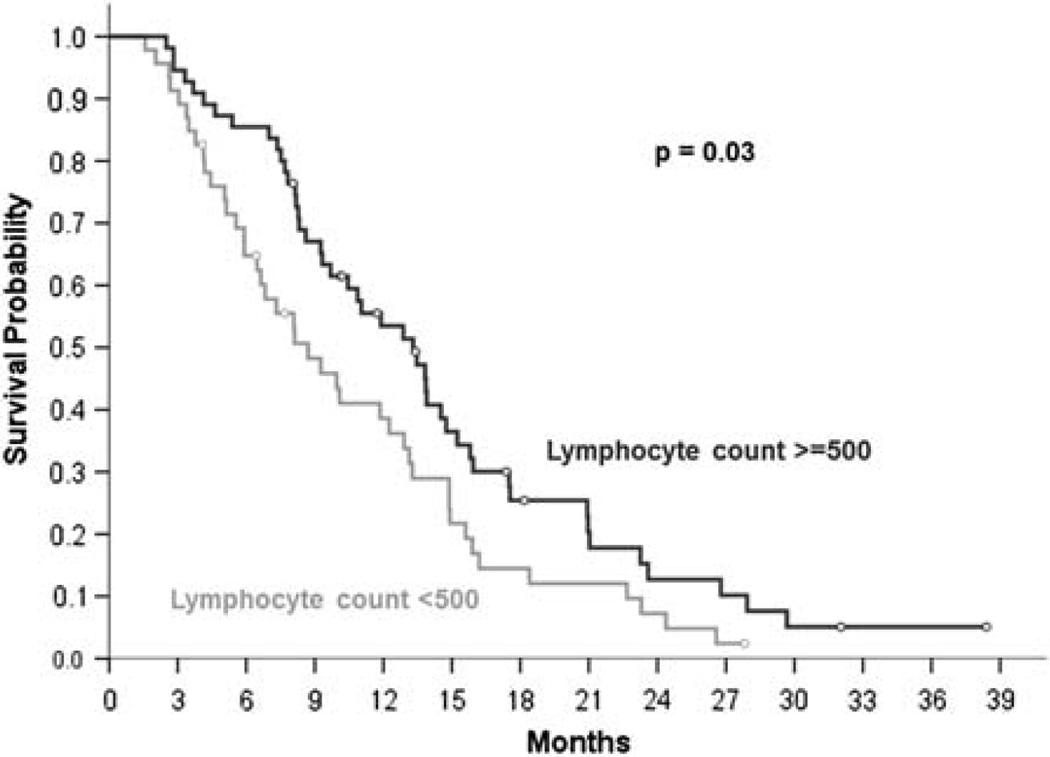
Median follow-up was 10.1 months (IQR, 6.4 to 15.3) from the start of chemoradiation in all patients and 11.7 months (IQR, 8.1 to 18.2) for patients remaining alive at last follow-up. The median survival for the entire cohort (n = 101) was 11.0 months (95% CI, 7.8–14.3), and the median PFS was 8.4 months (95% CI, 6.7–10.2). The median survival for patients in the high TLC group was 13.3 months (95% CI, 10.3–16.3) versus 8.7 months (95% CI, 5.5–11.9) for patients in the low TLC group (P = 0.03, log-rank test; Fig. 2). Median PFS for patients in the high TLC group was 9.0 months (95% CI, 7.3–10.7) from the start of chemoradiation compared with 4.9 months (95% CI, 2.3–7.3) for patients in the low TLC group (P = 0.15, log-rank test). Only 2 of the 88 deaths (2.3%) in the cohort were due to infection, 1 each in the high and low TLC groups; the remaining deaths resulted from progression of pancreatic cancer.
FIGURE 2.
Kaplan-Meier plot of survival stratified by total lymphocyte count <500 (gray curve) versus ≥500 (black curve) cells/mm3 2 months after starting chemoradiation.
Univariate and multivariate associations between potential prognostic factors and survival for this cohort of 101 patients are presented in Table 2. Seven factors were significantly associated with survival on univariate analysis: age, baseline BUN, baseline albumin, baseline alkaline phosphatase, baseline platelet count, TLC 2 months after starting chemoradiation, and PTV (see Table 2 for HRs, 95% CIs, and P-values associated with each factor). Neither baseline TLC as a continuous variable (HR = 1.000; 95% CI, 1.000-1.000; P = 0.81) nor having a baseline TLC in the abnormal (< 1000 cells/mm3) versus normal (≥1000 cells/mm3) range (HR = 0.875; 95% CI, 0.460–1.667; P = 0.69) was associated with survival. The type of chemotherapy administered during radiation was not associated with significantly different TLCs 2 months after starting chemoradiation (median 560 cells/mm3, IQR = 330 to 920 for 5-FU vs. median 585 cells/mm3, IQR = 430 to 945 for gemcitabine; P = 0.46), nor was the concurrent chemotherapy regimen significantly associated with survival (HR = 0.952; 95% CI, 0.569–1.594; P = 0.85). The 7 significant factors on univariate analysis listed above along with factors considered clinically important (age, ECOG performance status, baseline lymphocyte count, and whether induction chemotherapy was received) were used to construct the multivariate proportional hazards model for survival. Backward elimination yielded the following 5 factors remaining in the model, listed in order of HR magnitude (Table 2): baseline albumin (HR = 3.584; 95% CI, 1.835–6.993; P = 0.0002), TLC < 500 cells/mm3 2 months after beginning chemoradiation (HR = 2.879; 95% CI, 1.531–5.415; P = 0.001), baseline BUN (HR = 1.060; 95% CI, 1.010–1.112; P = 0.02), baseline platelet count (HR = 1.004; 95% CI, 1.001–1.007; P = 0.005), and larger PTV (HR = 1.003; 95% CI, 1.001–1.004; P = 0.0006). Therefore, survival for this cohort of patients with LAPC undergoing definitive chemoradiation seems to be independently predicted primarily by baseline plasma albumin levels and TLC 2 months after beginning chemoradiation, whereas small but significant independent contributions are additionally made by baseline BUN, baseline platelet count, and PTV magnitude.
TABLE 2.
Univariate and Multivariate Associations Between Patient Characteristics and Survival
| Characteristics | Hazard Ratio (95% CI) |
P |
|---|---|---|
| Univariate associations | ||
| Age: ≥65 vs. <65 y | 1.164 (0.752–1.802) | 0.50 |
| Age: continuous | 1.019 (1.000–1.039) | 0.05 |
| Sex: female vs. male | 0.978 (0.640–1.495) | 0.92 |
| Race: white vs. other | 0.860 (0.525–1.409) | 0.86 |
| ECOG: ≥1 vs. 0 | 1.210 (0.790–1.851) | 0.29 |
| Tumor location: head/uncinate vs. body/tail | 1.506 (0.910–2.490) | 0.11 |
| Histologic grade: poorly differentiated vs. other | 1.125 (0.662–1.911) | 0.67 |
| Tumor diameter: maximum cm | 1.061 (0.945–1.191) | 0.32 |
| Baseline CA19-9: continuous | 1.000 (1.000-1.000) | 0.19 |
| Baseline AST: continuous | 1.001 (0.999–1.004) | 0.33 |
| Baseline BUN: continuous | 1.044 (1.008–1.081) | 0.02 |
| Baseline albumin: continuous | 1.706 (1.285–2.262) | 0.0002 |
| Baseline alkaline phosphatase: continuous | 1.001 (1.000–1.002) | 0.05 |
| Baseline hematocrit: continuous | 1.007 (0.978–1.037) | 0.63 |
| Baseline platelet count: continuous | 1.002 (1.000–1.004) | 0.05 |
| Baseline white blood cell count: continuous | 1.000 (1.000-1.000) | 0.17 |
| Baseline lymphocyte count: continuous | 1.000 (1.000-1.000) | 0.81 |
| Baseline lymphocyte count: <1000 vs. ≥1000* | 0.875 (0.460–1.667) | 0.69 |
| Lymphocyte count at 2 mo: <500 vs. ≥500† | 1.591 (1.040–2.433) | 0.03 |
| Induction chemotherapy before chemoradiation: yes vs. no | 1.120 (0.602–2.083) | 0.72 |
| Concurrent chemotherapy type: 5-FU vs. gemcitabine based | 0.952 (0.569–1.594) | 0.85 |
| Radiation dose received: continuous | 1.000 (1.000-1.000) | 0.27 |
| PTV: continuous‡ | 1.001 (1.000–1.002) | 0.01 |
| Radiation technique: IMRT vs. 3-D conformal | 0.849 (0.554–1.303) | 0.45 |
| Time from diagnosis to chemoradiation initiation: continuous | 1.081 (0.939–1.244) | 0.28 |
| Treatment break required during chemoradiation§ yes vs. no | 1.799 (0.889–3.639) | 0.10 |
| Multivariate associations | ||
| Baseline albumin: continuous | 3.584 (1.835–6.993) | 0.0002 |
| Lymph count at 2 mo: <500 vs. ≥500† | 2.879 (1.531–5.415) | 0.001 |
| Baseline BUN: continuous | 1.060 (1.010–1.112) | 0.02 |
| Baseline platelet count: continuous | 1.004 (1.001–1.007) | 0.005 |
| PTV: continuous‡ | 1.003 (1.001–1.004) | 0.0006 |
Pretreatment lymphocyte count is dichotomized at 1000 (per the National Cancer Institute Common Terminology Criteria for Adverse Events threshold for abnormal vs. normal lymphocyte counts).
Lymphocyte count at 2 months is dichotomized at 500 (per the National Cancer Institute Common Terminology Criteria for Adverse Events threshold for grade III to IV treatment-induced lymphopenia).
PTV is defined as the total volume of tissue (including tumor plus a 1.5 to 2 cm margin to account for breathing motion and setup error) receiving ≥95% of the prescribed radiation dose.
Includes patients who stopped chemoradiation therapy early before completion of the initigroupsy prescribed dose.
3-D indicates 3-dimensional; 5-FU, 5-fluorouracil; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CA19-9, carbohydrate antigen 19-9; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity-modulated radiation therapy; PTV, planning target volume.
DISCUSSION
Chemoradiation is a standard treatment option for LAPC in the United States.32–35 To our knowledge, this study is the first to examine whether treatment-related lymphopenia influences disease outcomes in this patient population. Our results demonstrate that patients undergoing definitive chemoradiation for LAPC commonly experience a substantial reduction in TLC, with nearly half of all patients developing severe (NCI grade III to IV) lymphopenia at 2 months after starting therapy despite having normal baseline TLCs in the vast majority of cases. This treatment-related lymphopenia persists throughout a prolonged period of follow-up with no signs to indicate an eventual return to baseline (Fig. 1). Moreover, the development of severe lymphopenia at 2 months after the initiation of chemoradiation independently predicts for PFS and overall survival among patients with LAPC. This study, therefore, brings to light a novel prognostic factor in this patient population that merits further study and is potentially targetable through straightforward clinical interventions.
The importance of the immune system in pancreatic cancer is the subject of considerable clinical interest, as evidenced by the registration of >50 vaccine trials including pancreatic cancer patients on the NIH clinical trials Web site as of 2011.36 Although other studies have highlighted baseline and preoperative lymphocyte counts as prognostic factors for patients with pancreatic adenocarcinoma,12,13 treatment-related lymphopenia may also be clinically important. Although baseline lymphopenia may reflect poor baseline health status, this does not seem to be the case for treatment-related lymphopenia. In our study, patients who developed grade III to IV lymphopenia after chemoradiation were indistinguishable at baseline from the group of patients that did not (Table 1). This observation suggests that treatment-related lymphopenia is not due to inherent differences in patient health status. Second, if lymphopenia is a consequence of cancer treatment, it follows that treatment-related lymphopenia may be prevented or treated with appropriate adjustments in therapy. Addressing treatment-related lymphopenia could improve the survival of patients with LAPC.
The sizeable drop in TLCs observed in this study after chemoradiation is similar in magnitude and duration to the sustained lymphocyte count reductions documented in previous reports of patients with high-grade gliomas treated with radiation, temozolomide, and glucocorticoids18 and in patients with resected pancreatic adenocarcinoma receiving adjuvant 5-FU or gemcitabine-based chemoradiation.17 Treatment-related lymphopenia also occurs after radiation treatment for resectable breast cancer and squamous cell carcinoma of the head and neck, with long-term follow-up studies demonstrating persistent lymphopenia for up to 15 years after radiation therapy.37,38 Given these similar findings among tumors located in different anatomic sites, of diverse stages, and treated with different chemotherapy regimens or no chemotherapy at all, it is plausible that as radiation therapy is the common denominator among the above regimens, it may play a prominent role in lymphocyte depletion. Such a notion is not altogether surprising. Lymphocytes and lymphocyte stem cells exhibit high sensitivity to ionizing radiation in vitro, with doses as low as 1 Gy producing approximately a natural log decrement in cell survival as well as a significant reduction in survival time.39–43 This effect has been borne out in vivo among lymphocytes in the blood of human patients by studies examining the utility of extracorporeal irradiation of the circulating blood as an immune suppressant before renal transplant.44 Investigators attached β-radiation and γ-radiation emitters to dialysis units and irradiated the patient’s circulating blood as it was passing through. After median transit doses of only 14 to 57 rads (0.14 to 0.57 Gy) over an average of 160 hours of continuous extracorporeal irradiation, all patients developed a 40% to 50% reduction in lymphocyte concentration that persisted during the following 200 days of observation. Similar observations of postirradiation lymphocyte depletion have been made in patients treated with external beam thoracic radiation and oral brachytherapy,45 as well as in animal models where small radioactive strips were implanted in the abdominal cavity.46,47 Thus, radiation seems to have both an immediate and sustained effect on circulating lymphocytes that corresponds well to our clinical observations of TLC over time after chemoradiation.
Although lymphopenia has, as noted above, been well documented as a possible consequence of radiation therapy, few have examined the prognostic implications of treatment-related lymphopenia or consider it an important factor in the day-to-day clinical management of cancer patients. Currently, to our knowledge, only our 2 previous reports on patients with high-grade gliomas18 (prospective) and resected pancreatic cancer17 (retrospective) examine the relationship between treatment-associated lymphopenia and survival outcomes. In both studies, patients who developed grade III to IV lymphopenia (< 500 cells/mm3) 2 months after beginning chemoradiation had worse survival compared with patients with grade 0 to II lymphopenia at the same time point.17,18 Multivariate analyses identified treatment-related lymphopenia as a significant independent predictor for survival in both studies (HRs of 1.7 and 2.2), whereas baseline lymphocyte status was not significantly associated with survival.17,18 The results of our study corroborate these findings in a new patient population, supporting the hypothesis that the association between treatment-related lymphopenia and survival may hold for malignancies of diverse stages and anatomic sites.
Aside from treatment-related lymphopenia, our multivariate analysis also identified 4 other independent prognostic factors for survival among patients with LAPC treated with definitive chemoradiation. Baseline nutritional status, as measured by serum albumin level, was the strongest independent predictor of survival (HR = 3.6, P = 0.0002) among all prognostic factors identified. This finding lends credence to the validity of our multivariate analysis, as baseline albumin level has been significantly associated with survival in several studies of patients with pancreatic cancer.24–29,48 Likewise, baseline BUN and baseline platelet count have been previously associated with outcomes in patients with pancreatic cancer.29 PTV was additionally identified as an independent prognostic factor for survival among our patient population (HR = 1.003, P = 0.0006). As PTV is generally highly correlated with gross tumor volume, it seems plausible that tumors with larger volumes and, thus, larger PTVs may predispose to inferior disease outcomes. Although the HR is small, the level of significance is high, suggesting that PTV merits further investigation as a prognostic factor in LAPC.
It is interesting that some commonly accepted prognostic factors for survival outcomes in pancreatic cancer, such as age, performance status, tumor grade, and baseline CA19-9, did not emerge as significant independent predictors of survival in our study. There are several possible explanations for these findings. Although age as a continuous variable did show a significant association with survival on univariate Cox regression analysis, it subsequently dropped out of the multivariate proportional hazards model, likely because its effects were reflected in other variables included in the model that happened to be more closely associated with survival in our cohort. Worse performance status was associated with inferior survival (HR = 1.210, P = 0.29), but did not reach significance, possibly due to our sample size being underpowered to detect a significant association. It is relatively unsurprising that tumor grade was not found to be prognostic for survival given that our cohort was composed of patients with unresectable pancreatic tumors requiring biopsy by fine needle aspiration. This biopsy method yields cytologic specimens that frequently are not amenable to histologic grading due to lack of architectural preservation. Consequently, 67% of tumors in our study were graded as adenocarcinoma not otherwise specified, almost certainly limiting the prognostic value that could be derived from this variable. CA19-9 was considered as a continuous variable in our study due to lack of a consensus, thoroughly validated threshold value for prognostic dichotomization in the literature to date; however, it is possible that treating CA19-9 as a continuous, rather than dichotomous, variable resulted in an artificially diminished association with prognosis.
Our study must be interpreted in the context of several limitations. The retrospective approach used is subject to inherent biases that require this study to be considered exploratory in nature. For instance, only patients who had baseline and follow-up lymphocyte counts collected at our institution were eligible for inclusion, possibly causing a selection bias. Furthermore, patients were drawn from a period of 15 years at a single institution during which chemoradiation planning, delivery, and treatment strategies evolved substantially, causing fractionation and dosing schemes to vary between patients. Patients also received heterogenous therapies after completing their initial course of chemoradiation. Finally, the retrospective design of this study necessitated that the 2-month time point used to dichotomize patients into high and low TLC groups be only an approximate, rather than an exact, time point. Consequently, some variability did exist in the timing of the 2-month TLC relative to both the start and completion of chemoradiation therapy. Our findings must be considered with these limitations in mind.
In summary, chemoradiation-related lymphopenia is common, sustained, and seems to be an independent predictor of survival in patients with LAPC. This finding spurs other important questions requiring investigation in both the clinical and laboratory settings, such as whether treatment-related lymphopenia is causally related to or merely associated with adverse outcomes. Prospective study of lymphocyte status and interventions to achieve immune system reconstitution in patients treated with chemoradiation for LAPC and other malignancies is warranted as a possible avenue for improving outcomes.
Acknowledgments
Supported by the AMA Foundation, the Claudio X. Gonzalez Family Foundation, the Simkins Family Foundation, the McKnight Family Foundation, the Flannery Family Foundation, the Alexander Family Foundation, the Keeling Family Foundation, and the DeSanti Family Foundation. A.T.W was supported by an AMA Foundation Research Seed Grant.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ehrlich P. On the current state of cancer research. Ned Tijdschr Geneeskd. 1909;5:273–290. [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Prestwich RJ, Errington F, Hatfield P, et al. The immune system— is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20:101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumagalli LA, Vinke J, Hoff W, et al. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26:394–402. doi: 10.1097/00002371-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Le Scodan R, Massard C, Jouanneau L, et al. Brain metastases from breast cancer: proposition of new prognostic score including molecular subtypes and treatment. J Neurooncol. 2012;106:169–176. doi: 10.1007/s11060-011-0654-x. [DOI] [PubMed] [Google Scholar]
- 8.Elnemr A, Ohta T, Yachia A, et al. Human pancreatic cancer cells disable function of fas receptors at several levels in fas signal transduction pathway. Int J Oncol. 2001;29:311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- 9.Elnemr A, Ohta T, Yachie A, et al. Human pancreatic cancer cells express non-functional fas receptors and counterattack lymphocytes by expressing fas ligand; a potential mechanism for immune escape. Int J Oncol. 2001;18:33–39. [PubMed] [Google Scholar]
- 10.Bonham CA, Lu L, Banas RA, et al. TGF-β 1 pretreatment impairs the allostimulatory function of human bone marrow-derived antigen-presenting cells for both naive and primed T cells. Transpl Immunol. 1996;4:186–191. doi: 10.1016/s0966-3274(96)80015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–932s. [PubMed] [Google Scholar]
- 12.Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 13.Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:456–460. doi: 10.1080/13651820701774891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga A, Miyamoto M, Cho Y, et al. CD8 + tumor-infiltrating lymphocytes together with CD4 + tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Mitry E, Hammel P, Deplanque G, et al. Safety and activity of masitinib in combination with gemcitabine in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:395–403. doi: 10.1007/s00280-010-1299-8. [DOI] [PubMed] [Google Scholar]
- 16.Faris JE, Arnott J, Zheng H, et al. A phase 2 study of oral MKC-1, an inhibitor of importin-b, tubulin, and the mTOR pathway in patients with unresectable or metastatic pancreatic cancer. Invest New Drugs. 2012;30:1614–1620. doi: 10.1007/s10637-011-9708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balmanoukian A, Ye X, Herman J, et al. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudra S, Narang AK, Pawlik TM, et al. Evaluation of predictive variables in locally advanced pancreatic adenocarcinoma patients receiving definitive chemoradiation. Pract Radiat Oncol. 2011;2:77–85. doi: 10.1016/j.prro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 21.Crane CH, Janjan NA, Evans DB, et al. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol. 2001;29:9–18. doi: 10.1385/IJGC:29:1:09. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Okada S, Tokuuye K, et al. Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer. 2001;91:490–495. doi: 10.1002/1097-0142(20010201)91:3<490::aid-cncr1027>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Huang PI, Chao Y, Li CP, et al. Efficacy and factors affecting outcome of gemcitabine concurrent chemoradiotherapy in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2009;73:159–165. doi: 10.1016/j.ijrobp.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Yi JH, Lee J, Park SH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011;80:175–180. doi: 10.1159/000328449. [DOI] [PubMed] [Google Scholar]
- 25.Chiang KC, Yeh CN, Lee WC, et al. Prognostic analysis of patients with pancreatic head adenocarcinoma less than 2 cm undergoing resection. World J Gastroenterol. 2009;15:4305–4310. doi: 10.3748/wjg.15.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 27.Marechal R, Demols A, Gay F, et al. Prognostic factors and prognostic index for chemonaive and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology. 2007;73:41–51. doi: 10.1159/000120627. [DOI] [PubMed] [Google Scholar]
- 28.Falconer JS, Fearon KC, Ross JA, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008;99:883–893. doi: 10.1038/sj.bjc.6604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 31.Cox D. Regression models and life tables. J R Stat Soc Ser. 1972;B34:187–220. [Google Scholar]
- 32.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The gastrointestinal tumor study group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Gastrointestinal tumor study group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 34.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 35.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plate J. Clinical trials of vaccines for immunotherapy in pancreatic cancer. Expert Rev Vaccines. 2011;10:825–836. doi: 10.1586/erv.11.77. [DOI] [PubMed] [Google Scholar]
- 37.Rotstein S, Blomgren H, Petrini B, et al. Long term effects on the immune system following local radiation therapy for breast cancer. I. cellular composition of the peripheral blood lymphocyte population. Int J Radiat Oncol Biol Phys. 1985;11:921–925. doi: 10.1016/0360-3016(85)90114-2. [DOI] [PubMed] [Google Scholar]
- 38.Tarpley JL, Potvin C, Chretien PB. Prolonged depression of cellular immunity in cured laryngopharyngeal cancer patients treated with radiation therapy. Cancer. 1975;35:638–644. doi: 10.1002/1097-0142(197503)35:3<638::aid-cncr2820350315>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Schrek R. Qualitative and quantitative reactions of lymphocytes to x rays. Ann NY Acad Sci. 1961;95:839–848. doi: 10.1111/j.1749-6632.1961.tb50080.x. [DOI] [PubMed] [Google Scholar]
- 40.Hendry JH, Roberst SA. Analysis of dose-incidence relationships for marrow failure in different species, in terms of radiosensitivity of tissue-rescuing units. Radiat Res. 1990;122:155–160. [PubMed] [Google Scholar]
- 41.Baird MS, Hendry JH, Testa NG. The radiosensitivity of human haemopoietic progenitor cells. Int J Radiat Biol. 1989;56:617–621. doi: 10.1080/09553008914551831. [DOI] [PubMed] [Google Scholar]
- 42.Nothdurft W, Steinbach KH, Fliedner TM. In vitro studies on the sensitivity of canine granulopoietic progenitor cells (GM-CFC) to ionizing radiation: differences between steady state GM-CFC from blood and bone marrow. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;43:133–140. doi: 10.1080/09553008314550141. [DOI] [PubMed] [Google Scholar]
- 43.Nothdurft W, Fliedner TM. The response of the granulocytic progenitor cells (CFU-C) of blood and bone marrow in dogs exposed to low doses of X irradiation. Radiat Res. 1982;89:38–52. [PubMed] [Google Scholar]
- 44.Week E. The development of lymphopenia in uremic patients undergoing extracorporeal irradiation of the blood with portable beta units. Radiat Res. 1973;56:554–559. [PubMed] [Google Scholar]
- 45.Matsubara S, Horiuchi J, Okuyama T, et al. Chromosome aberrations in the peripheral lymphocytes induced by brachytherapy and external cobalt teletherapy. Int J Radiat Oncol Biol Phys. 1985;11:1085–1094. doi: 10.1016/0360-3016(85)90054-9. [DOI] [PubMed] [Google Scholar]
- 46.Ford WL. The mechanism of lymphopenia produced by chronic irradiation of the rat spleen. Br J Exp Pathol. 1968;49:502–510. [PMC free article] [PubMed] [Google Scholar]
- 47.Hollingsworth JW, Carr J, Ford WL. Lymphopenia produced by polyethylene-32P strips applied to the rabbit appendix. Cell Immunol. 1972;4:407–415. doi: 10.1016/0008-8749(72)90042-1. [DOI] [PubMed] [Google Scholar]
- 48.Ueda M, Endo I, Nakashima M, et al. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104–110. doi: 10.1007/s00268-008-9807-2. [DOI] [PubMed] [Google Scholar]