Abstract
The aim of this study was to determine whether skin temperature measurement by digital thermography on hands and feet is useful for diagnosis of Raynaud's phenomenon (RP). Fifty-seven patients with RP (primary RP, n = 33; secondary RP, n = 24) and 146 healthy volunteers were recruited. After acclimation to room temperature for 30 min, thermal imaging of palmar aspect of hands and dorsal aspect of feet were taken. Temperature differences between palm (center) and the coolest finger and temperature differences between foot dorsum (center) and first toe significantly differed between patients and controls. The area under curve analysis showed that temperature difference of the coolest finger (cutoff value: 2.2℃) differentiated RP patients from controls (sensitivity/specificity: 67/60%, respectively). Temperature differences of first toe (cutoff value: 3.11℃) also discriminated RP patients (sensitivity/specificity: about 73/66%, respectively). A combination of thermographic assessment of the coolest finger and first toe was highly effective in men (sensitivity/specificity : about 88/60%, respectively) while thermographic assessment of first toe was solely sufficient for women (sensitivity/specificity: about 74/68%, respectively). Thermographic assessment of the coolest finger and first toe is useful for diagnosing RP. In women, thermography of first toe is highly recommended.
Graphical Abstract
Keywords: Raynaud Disease, Thermography, Digital Thermography, Temperature
INTRODUCTION
Raynaud's phenomenon (RP) is characterized by a three-phase color change of blanching, cyanosis, and rubor in distal extremities after exposure to cold and subsequent rewarming (1). RP is not easily diagnosed in the clinic. It is difficult to observe the triphasic color change even in winter because most patients wait in a warm waiting room long enough for any color change to disappear. Thermography on hands has been used to assess RP for a long time. Typically, an infrared camera was used to determine skin temperature distributions by measuring thermal radiation (2). Whether RP is primary or secondary, the symptoms and signs of RP are manifested via vasospasms of the small muscular arteries and arterioles of the digits (3). Blood carries heat to the skin of the fingers, but vasospasms reduce blood flow, resulting in low temperature of the fingers. However, concerns about the reproducibility of thermography have limited its use (4). As RP affects both hands and feet (5, 6) we used infrared thermography to measure skin temperature differences between the palm of the hand and the fingers and between the dorsum of the feet and the toes in patients with RP and normal healthy controls.
MATERIALS AND METHODS
Subjects
We recruited 57 patients with RP (primary RP, n = 33; secondary RP, n = 24). All patients with primary RP (21 women, 12 men) satisfied the diagnostic criteria of LeRoy and Medsger (7) and had no clinical or immunological evidence of connective tissue disease, including normal nailfold capillary finding. Patients with secondary RP (20 women, 4 men) had the following underlying diseases: systemic sclerosis (n = 10), mixed connective tissue disease (n = 2), rheumatoid arthritis (n = 3), Sjogren's syndrome (n = 6), systemic lupus erythematosus (n = 1), Buerger's disease (n = 1), or atherosclerosis (n = 1). In addition, 146 healthy people (86 women, 60 men) who has no underlying disease such as diabetes mellitus or hypertension volunteered for the study. Infrared digital thermography was performed at a single rheumatism center in Incheon (Korea).
Thermography protocol
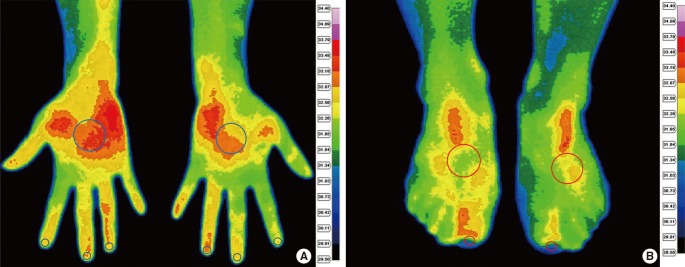
Thermography images were acquired using an infrared thermography camera (IRIS-XP®, Medicore, Seoul). A technician who has acquired official qualification of infrared thermography and has experience of 5 yr in Inha University Hospital performed thermography in this study. After subjects were acclimated to room temperature for 30 min in a temperature-controlled room at 24℃ and images of palmar aspects of both hands and dorsal aspects of both feet were taken. As there were technical difficulties to take images of the palmar aspect of both feet, the dorsal aspect was alternatively chosen to represent skin temperature of the feet. For each subject, hand images were analyzed as shown in Fig. 1A. The temperature within a circle at the palm center (diameter 1 cm) and at the distal phalanx (diameter 2-3 mm) were measured. Thumbs are known to be spared in both primary and secondary RP (8) and 5th finger tip is small and has a low temperature in both normal controls and RP patients which would result in less discriminatory power. Thus, second, third and fourth fingers were assessed and the finger with lowest temperature (the coolest finger) was selected, reflecting RP involvement most likely. The temperature difference between the palm and the coolest finger was calculated. In the case of feet, first toe was chosen to represent RP involvement of the toes because of too small a size of the third, fourth and fifth toes to assess the temperature distribution of the toes. In the pilot study, the receiver operating curve was drawn for both the first and second toes to select for effective thermographic parameters of the toes and the first toe showed best discriminatory power with the largest area under the curve and a confidence interval (CI) (data not shown). The temperature difference between the center of the foot dorsum and the first toe was calculated in the same manner as the hand and is shown in Fig. 1B. To evaluate test reproducibility, 57 controls (32 women, 25 men) were assessed two times in one day. No physical exercise was permitted and controls were required to rest for 30 min intervals between tests.
Fig. 1.
Thermographic images of palmar aspect of hands (A) and dorsal aspect of feet (B). The region of interest of the distal finger/toe, palm of the hands and dorsum of the feet are drawn in circles. The color chart provides a temperature scale. First, the coolest finger was chosen among the second, third and fourth fingers and then temperature difference was determined by subtracting the temperature at the coolest finger from the temperature at the palm. Temperature difference of first toe was determined in the same manner as in the hand.
Statistical methods
Results were analyzed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Skin temperature differences were evaluated by independent samples t-tests. Receiver operating characteristic (ROC) analysis was carried out and the optimal cutoff value was determined for the maximum sum of sensitivity and specificity. Intraclass correlation coefficients (ICCs) in the second, third and fourth fingers and the first toe were calculated to evaluate the reproducibility of the test in 57 normal controls. The interpretation of the ICC was in accordance to Fleiss (<0.40, poor reliability; 0.40-0.75, good reliability; >0.75 excellent reliability) (9). The P value<0.05 was considered significant.
Ethics statement
Approval by the institutional review board of Inha University Hospital (IUH-IRB 11-1295) was obtained for this study and all subjects provided written informed consent before participating in the study.
RESULTS
Reproducibility
ICCs of temperature differences between the palm and the second, third and fourth fingers of dominant hand and between the foot dorsum and the first toe showed excellent reproducibility (>0.75) except, of fourth finger of the non-dominant hand (0.73).
Skin temperature distributions in patients and controls
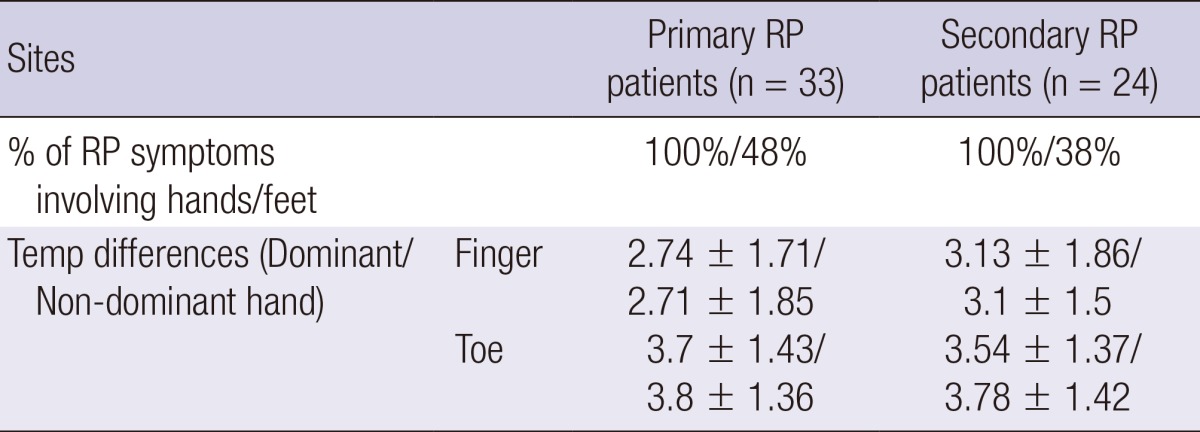
All patients in the study reported symptoms of RP involving hands whereas twenty-five patients out of total 57 patients (44%) with RP reported symptoms involving feet. The distribution of the coolest finger in the normal control/RP patients is shown in Fig. 2. The temperature differences between the palm and ipsilateral coolest finger and between the dorsum and ipsilateral first toes were significantly larger in RP patients than in the normal controls (P≤0.001, at all times). When study participants were classified by sex, temperature differences of the coolest fingers and of first toes were mostly significantly bigger in RP patients than in controls both in men and in women, except temperature differences of the coolest finger of the non-dominant hand which did not contrast between RP patients and normal controls in women (Table 1). When restricted to normal controls, temperature differences between the palm and the coolest fingers were significantly bigger in normal female controls than in normal male controls (P<0.001) whereas temperature differences between the foot dorsum and the first toe did not contrast between normal female controls and normal male controls. Among the RP patients, there was no gender gap in temperature differences of the coolest fingers and the first toes. There were 20 smokers in the normal male controls and 7 smokers amongst the male RP patients. When temperature differences of the coolest fingers and the first toes were compared between smokers and non-smokers both in male controls and male RP patients, there was no temperature differences between smokers and non smokers in both groups. There was only one smoker each in the normal female controls and in the female RP patient group and their temperature differences of the coolest fingers and the first toes did not vary from the others. Lastly, there was no significant contrast in temperature difference of the coolest fingers and the first toes between patients with primary RP or secondary RP (Table 2).
Fig. 2.
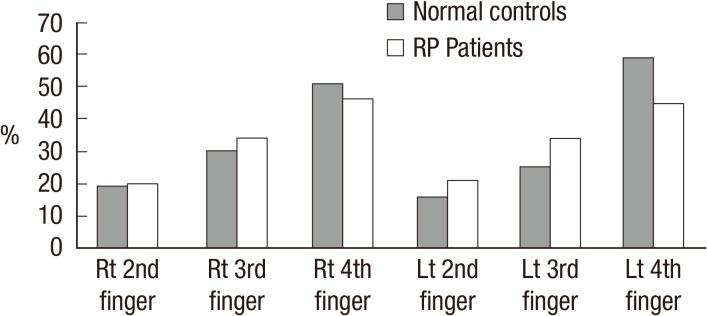
The distribution of coolest finger in normal controls and RP patients. Rt/Lt denotes right/left, respectively.
Table 1.
Temperature (Temp) differences of coolest finger and first toe in total population, men and women. Values were expressed as dominant hands/non-dominant hands
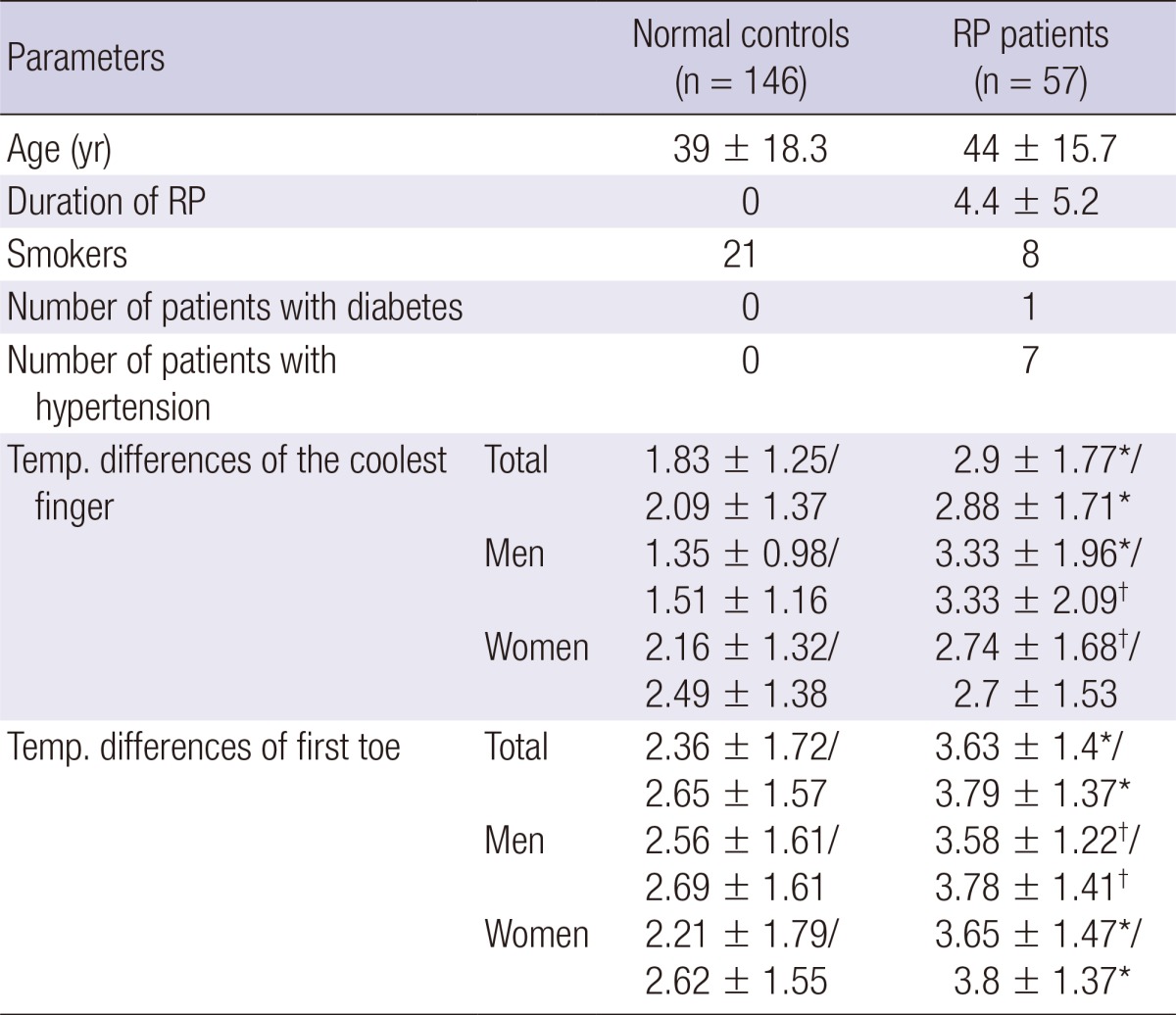
*Indicates P≤0.001 and †indicates P<0.05 between normal controls and RP patients. Temp., temperature.
Table 2.
Temperature (Temp) differences of coolest finger and first toe in primary and secondary RP patients
ROC analysis
ROC curves were drawn for temperature differences of the coolest fingers and first toes. The optimal cut off values of the coolest finger and the first toe were shown to be 2.2℃ and 3.11℃, respectively. Sensitivities, and specificities for the coolest finger and the first toe in the total population, men and women were calculated and are shown in Table 3.
Table 3.
Sensitivities and specificities for temperature differences of coolest finger and first toe. Sensitivities and specificities were determined at a cut off value of 2.2℃ for temperature differences of the coolest finger and at a cut off value of 3.11℃ for temperature differences of the first toe in total population, men and women
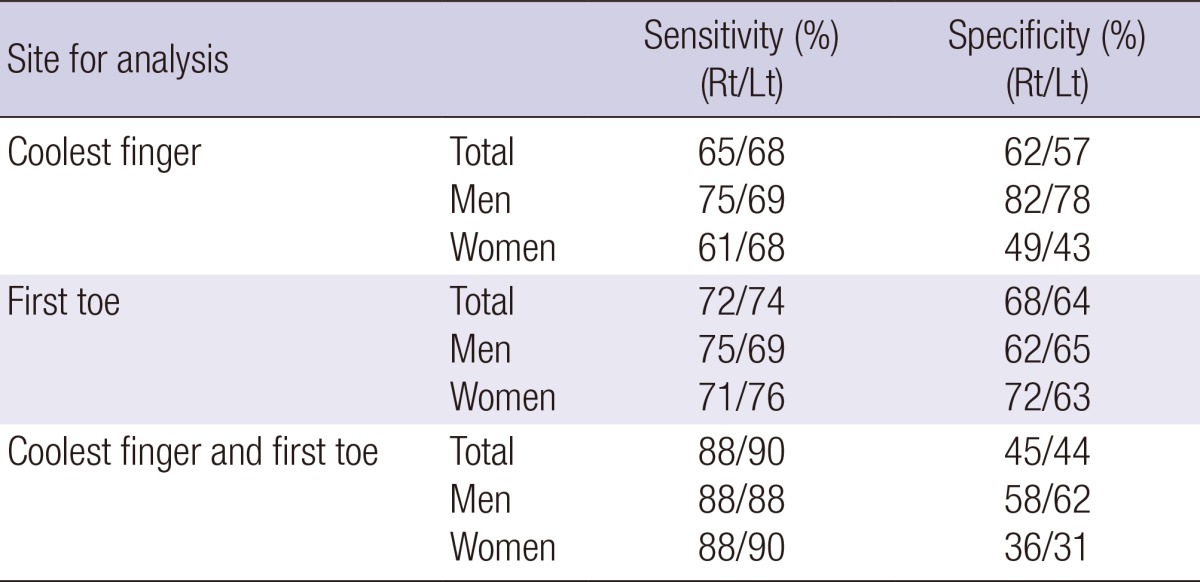
Rt, right; Lt, left.
DISCUSSION
This is the first study to perform infrared thermography on both hands and feet. Skin temperature differences between the center of palm and the coolest finger and between the center of the foot dorsum and the first toe was significantly bigger in RP patients than in controls, irrespective of RP symptom involving either hands or feet. RP is characterized by recurrent episodes of discoloration affecting the fingers and toes (10). According to a study of systemic sclerosis by La Montagna et al. about 90% of patients were presented with features of RP in the feet (11) in contrast to 44% of the patients in our study. However, most studies using thermography to assess RP have focused solely on the fingers (12, 13, 14). Presumably, this is because patients complained about RP symptoms more frequently in their hands than in their feet and both patients and examiners disliked removing patients' shoes in the clinic, thus toes were more difficult to be examined than fingers. Irrespective of RP symptom either involving hands or feet, temperature differences of first toe (cutoff value: 3.11℃) successfully discriminated RP patients from normal controls. Heslop et al. reported long ago that the thumb and little finger were least affected by RP (15) and the second, third and fourth fingers were included for the assessment of RP. As the dominant hands of RP patients were reported to be cooler than those of controls in summer (16), the finger which is easily affected by RP would have lower temperature. By simply checking temperature distribution in finger tips, the coolest finger could be easily chosen in a thermographic image of hands. Thus, the coolest finger was selected among the second, third and fourth fingers in our study to choose the best thermographic parameter among the fingers. A thermographic assessment of the coolest finger was highly effective in men with a high sensitivity and specificity. However, it did not work well for women probably because of low temperatures of the fingers in normal female controls where temperature differences of the left coolest finger did not even differ between normal female controls and female RP patients. In case of the feet, the first toe was found to best reflect RP involvement of the foot. Advantage of thermography on the feet was that there was no gender gap in temperature differences of the toes among normal controls, unlike differences found in the hands. Thus, temperature difference between the foot dorsum and the first toe successfully distinguished RP patients from normal controls in both men and women. Among women, temperature differences of the first toe were more sensitive and specific than temperature difference of the coolest finger. In particular, the first toe provided the best thermographic parameter on the foot than any of the other toes. It has the additional benefit of being larger than other toes with more obvious boundaries, which allows confident location for regions of interest to be evaluated. Therefore, it is highly recommended to perform thermographic assessments on the feet, even if patients are not complaining about color changes of their feet.
A limitation of this study requires consideration. Our study failed to differentiate between primary and secondary RP. Secondary RP is associated with underlying disorders (e.g., systemic sclerosis) and may be associated with irreversible vessel and tissue damage. The results of previous studies with cold challenges were in controversy as some studies succeeded to differentiate disease states of RP (12, 13) while other study failed (17). It was previously studied that abnormal nailfold capillaroscopic findings reflect increased plasma endothelin-1 level in patients with RP, helping early diagnosis of connective tissue disease (18). Therefore, we believe that infrared thermography on hands and feet is not solely adequate and a combined approach with thermography, nailfold capillary examination and autoantibody tests would be able to detect secondary RP.
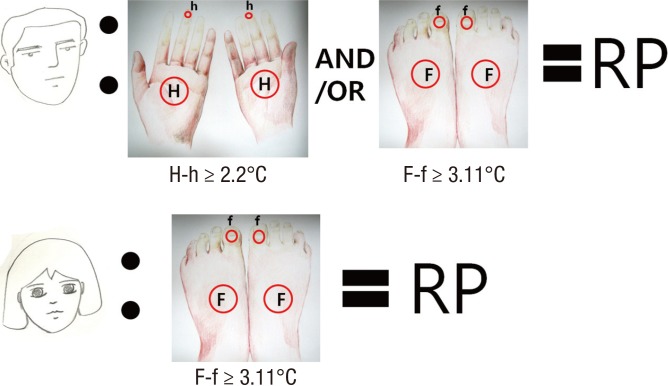
In conclusion, our study indicates that thermographic assessment on the hands and feet can aid in the diagnosis of RP in both women and men (Fig. 3). In particular, thermographic assessment on the feet, especially of the first toe, can be more helpful for women and highly recommended.
Fig. 3.
The schematic summary of infrared thermography in Raynaud's phenomenon (RP). H, palm of the hand; h, coolest finger; F, dorsum of the foot; f, first toe.
Footnotes
Authors declare no conflicts of interest.
References
- 1.Coffman JD. Raynaud's phenomenon. New York: Oxford University Press; 1989. [Google Scholar]
- 2.Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging. 1998;17:1019–1027. doi: 10.1109/42.746635. [DOI] [PubMed] [Google Scholar]
- 3.Block JA, Sequeira W. Raynaud's phenomenon. Lancet. 2001;357:2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- 4.Herrick AL, Clark S. Quantifying digital vascular disease in patients with primary Raynaud's phenomenon and systemic sclerosis. Ann Rheum Dis. 1998;57:70–78. doi: 10.1136/ard.57.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambova S, Hermann W, Müller-Ladner U. Capillaroscopic pattern at the toes of systemic sclerosis patients: does it "tell" more than those of fingers? J Clin Rheumatol. 2011;17:311–314. doi: 10.1097/RHU.0b013e31822be4e8. [DOI] [PubMed] [Google Scholar]
- 6.Sari-Kouzel H, Hutchinson CE, Middleton A, Webb F, Moore T, Griffin K, Herrick AL. Foot problems in patients with systemic sclerosis. Rheumatology (Oxford) 2001;40:410–413. doi: 10.1093/rheumatology/40.4.410. [DOI] [PubMed] [Google Scholar]
- 7.LeRoy EC, Medsger TA., Jr Raynaud's phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10:485–488. [PubMed] [Google Scholar]
- 8.Chikura B, Moore TL, Manning JB, Vail A, Herrick AL. Sparing of the thumb in Raynaud's phenomenon. Rheumatology (Oxford) 2008;47:219–221. doi: 10.1093/rheumatology/kem353. [DOI] [PubMed] [Google Scholar]
- 9.Fleiss JL. Reliability of measurement. In: Fleiss JL, editor. The design and analysis of clinical experiments. New York: John Wiley & Sons; 1986. pp. 1–32. [Google Scholar]
- 10.Coffman JD. Raynaud's phenomenon: an update. Hypertension. 1991;17:593–602. doi: 10.1161/01.hyp.17.5.593. [DOI] [PubMed] [Google Scholar]
- 11.La Montagna G, Baruffo A, Tirri R, Buono G, Valentini G. Foot involvement in systemic sclerosis: a longitudinal study of 100 patients. Semin Arthritis Rheum. 2002;31:248–255. doi: 10.1053/sarh.2002.29493. [DOI] [PubMed] [Google Scholar]
- 12.Anderson ME, Moore TL, Lunt M, Herrick AL. The 'distal-dorsal difference': a thermographic parameter by which to differentiate between primary and secondary Raynauds phenomenon. Rheumatology (Oxford) 2007;46:533–538. doi: 10.1093/rheumatology/kel330. [DOI] [PubMed] [Google Scholar]
- 13.Clark S, Hollis S, Campbell F, Moore T, Jayson M, Herrick A. The "distal-dorsal difference" as a possible predictor of secondary Raynaud's phenomenon. J Rheumatol. 1999;26:1125–1128. [PubMed] [Google Scholar]
- 14.Schlager O, Gschwandtner ME, Herberg K, Frohner T, Schillinger M, Koppensteiner R, Mlekusch W. Correlation of infrared thermography and skin perfusion in Raynaud patients and in healthy controls. Microvasc Res. 2010;80:54–57. doi: 10.1016/j.mvr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Heslop J, Coggon D, Acheson ED. The prevalence of intermittent digital ischaemia (Raynaud's phenomenon) in a general practice. J R Coll Gen Pract. 1983;33:85–89. [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner-Medwin JM, Macdonald IA, Taylor JY, Riley PH, Powell RJ. Seasonal differences in finger skin temperature and microvascular blood flow in healthy men and women are exaggerated in women with primary Raynaud's phenomenon. Br J Clin Pharmacol. 2001;52:17–23. doi: 10.1046/j.0306-5251.2001.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauling JD, Flower V, Shipley JA, Harris ND, McHugh NJ. Influence of the cold challenge on the discriminatory capacity of the digital distal-dorsal difference in the thermographic assessment of Raynauds phenomenon. Microvasc Res. 2011;82:364–368. doi: 10.1016/j.mvr.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Do JH, Kim HY. Increased plasma endothelin-1 and abnormal nailfold capillaroscopic findings in patients with connective tissue diseases. Korean J Med. 2004;66:275–283. [Google Scholar]