Abstract
The aim of this study was to assess the prognostic value of combined use of white blood cell (WBC), hemoglobin (Hb), and platelet distribution width (PDW) in patients with acute myocardial infarction (AMI). This study included 1,332 consecutive patients with AMI. Patients were categorized into complete blood cell (CBC) group 0 (n=346, 26.0%), 1 (n=622, 46.7%), 2 (n=324, 24.3%), and 3 (n=40, 3.0%) according to the sum of the value defined by the cut-off levels of WBC (1, ≥14.5×103/µL; 0, <14.5×103/µL), Hb (1, <12.7 g/dL; 0, ≥12.7 g/dL), and PDW (1, ≥51.2%; 0: <51.2%). In-hospital death occurred in 59 (4.4%) patients. Patients who died during index hospitalization had higher WBC and PDW and lower Hb. The patients could be stratified for in-hospital mortality according to CBC group; 1.2%, 2.7%, 9.0%, and 22.5% in CBC groups 0, 1, 2, and 3 (P<0.001), respectively. In multivariate logistic regression analysis, CBC group≥2 (odds ratio, 3.604; 95% confidence interval, 1.040-14.484, P=0.043) was an independent predictor for in-hospital death. The prognostic impact of the combined use of CBC markers remained significant over 12 months. In conclusions, combination of WBC, Hb, and PDW, a cheap and simple hematologic marker, is useful in early risk stratification of patients with AMI.
Keywords: Hemoglobins, Leukocytes, Platelet Distribution Width, Myocardial Infarction
INTRODUCTION
Effective risk stratification is important for management of patients with acute myocardial infarction (AMI) (1, 2). Complete blood cell (CBC) is a simple, easily and widely performed laboratory test. Among several CBC markers, the predictive value of white blood cell (WBC) and hemoglobin (Hb) for short- and long-term survival in patients with AMI has been relatively well established (3, 4, 5, 6, 7, 8), although some studies have refuted this association (9, 10, 11).
Although thromboembolic events are among the major causes of mortality in patients with AMI (12, 13), limited evaluation of platelet distribution width (PDW), a progressive platelet activation marker, has been conducted in patients with coronary artery disease (CAD), with conflicting results (14, 15, 16, 17).
Simple combination of these CBC markers, all connected in some way with AMI prognosis, has never been tested in risk assessment for AMI. We attempted to determine whether the combined use of WBC, Hb, and PDW had prognostic value in patients with AMI.
MATERIALS AND METHODS
This observational study included 1,365 consecutive patients with AMI who were enrolled in the Korea Acute Myocardial Infarction Registry (KAMIR) from the authors' single center between November 2005 and February 2009. KAMIR is a Korean, prospective, open, observational, multicenter on-line registry of AMI supported by the Korean Society of Cardiology since November 2005. Details of KAMIR have been published (18). PDW levels were collected retrospectively, because they had not been entered into the KAMIR database. AMI was diagnosed by characteristic clinical presentation, serial changes on ECG suggesting infarction, and an increase in cardiac enzymes (19). ST-segment elevation myocardial infarction (STEMI) was defined by new ST elevation in ≥2 contiguous leads, measuring >0.2 mV in leads V1-3, or 0.1 mV in all other leads. Peripheral blood samples for laboratory tests, except for lipid profile, were collected at admission. Peripheral venous blood specimens were sampled in Vacutainer™ tubes containing K-ethylenediaminetetraacetic acid and applied immediately to an automated hematologic analyzer, K-4500 (TOA Medical Electronics, Kobe, Japan) for WBC count, Hb concentration, and PDW. Other biochemical variables were measured using standard laboratory techniques. The estimated glomerular filtration rate (eGFR) was calculated according to the Cockcroft-Gault formula. In addition to laboratory findings, baseline demographic characteristics, initial vital signs, and cardiovascular risk factors were evaluated. Hypertension was defined as a previous medical diagnosis of hypertension, use of antihypertensive medications or blood pressure≥140/90 mmHg. History of hyperlipidemia was defined as the diagnosis previously made by a physician or treatment with lipid-lowering medications. Analysis of ECG in all patients was performed by the attending cardiologists.
We assessed in-hospital and 12-month mortalities. At 12 months after the index AMI, follow-up data were obtained by review of the medical records and/or telephone interview with the patient or family members.
Data are presented as mean±standard deviation for continuous variables and percentages for categorical variables. N-terminal Pro-B-type natriuretic peptide (NT-ProBNP) was log-transformed to reduce the effect of skewed distribution of the data. Patients were categorized as with and without in-hospital mortality. Receiver-operating characteristics (ROC) curve analysis was used for determination of the cut-off values for prediction of in-hospital mortality. Comparisons were made using the Student's t-test for continuous variables and the chi-square test for categorical variables. Multivariate logistic regression analysis and Cox proportional hazard model were used for determination of independent parameters for in-hospital and 12-month mortalities, respectively. Sequential models were developed in order to examine the incremental prognostic value of the parameters. Incremental factors added to the model at each step were considered significant when the difference in the log-likelihood associated with each model corresponded to P<0.05. Twelve-month survival was analyzed by the Kaplan-Meier method. The P values were 2-sided, and P<0.05 was considered significant. SPSS version 15.0 for Windows (SPSS Inc, Chicago, IL, USA) was used in performance of statistical analysis.
Ethics statement
KAMIR was approved by the institutional review board of Kyungpook National University Hospital (No. KNUH-07-0093). Informed consent was waived by the board.
RESULTS
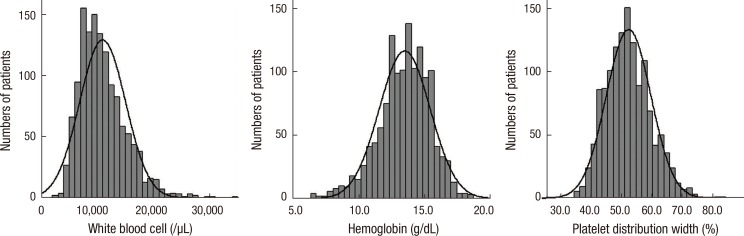
Of the consecutive 1,365 patients, 15 patients were on hemodialysis due to end stage renal disease and 18 patients who had underlying malignancy were excluded, leaving 1,332 patients in the final analysis. Baseline characteristics of the patients are shown in Table 1. The mean age of patients was 64±12 yr and 901 (67.6%) were men. Mean levels of WBC, Hb, and PDW were 10.9±4.1×103/µL, 13.5±1.9 g/dL, and 52.2±7.4%, respectively (Fig. 1). WBC count showed positive correlation with the Killip class (r=0.148), levels of creatinine (r=0.081), peak cardiac troponin I (cTnI) (r=0.189), and Hb (r=0.174), but showed negative correlation with age (r=-0.130) and left ventricular ejection fraction (LVEF) (r=-0.091) (Table 2). Hb level showed negative correlation with age (r=-0.442), Killip class (r=-0.242), levels of creatinine (r=-0.289), and log NT-ProBNP (r=-0.427), but showed positive correlation with LVEF (r=0.137) and WBC count (r=0.174). PDW showed weak positive correlation with Killip class (r=0.091) and levels of creatinine (r=0.075) and log NT-ProBNP (r=0.096).
Table 1.
Baseline clinical characteristics of the patients with and without in-hospital death
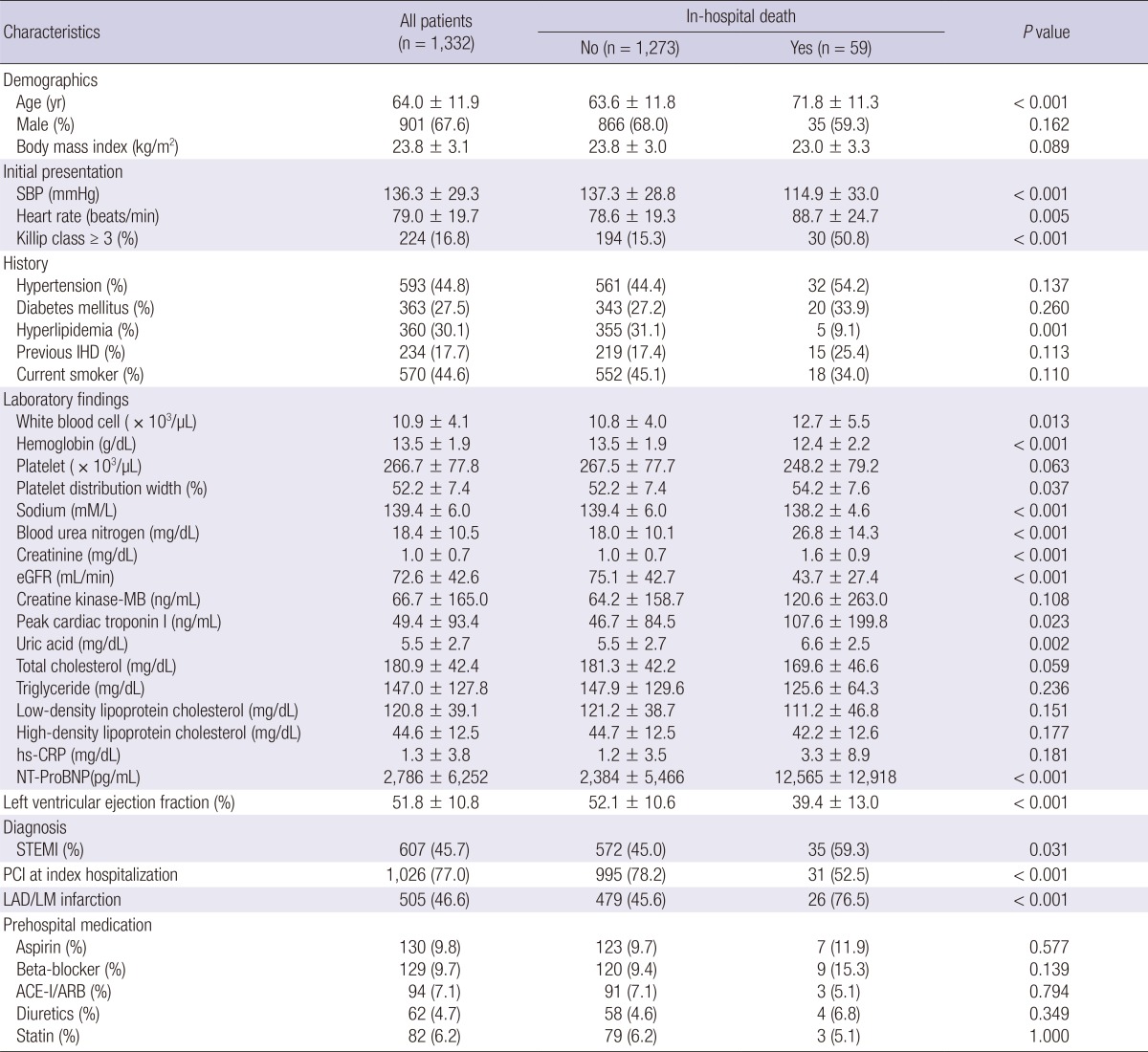
SBP, systolic blood pressure; IHD, ischemic heart disease; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; NT-ProBNP, N-terminal Pro-B-type natriuretic peptide; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; LAD/LM, left anterior descending/Left main; ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker.
Fig. 1.
Histograms of the distribution of baseline white blood cell (10.9±4.1×103/µL, range 2.7-34.8±103/µL), hemoglobin (13.5±1.9 g/dL, range 6.4-18.6 g/dL), and platelet distribution width (52.2±7.4%, range 35.3-82.9%).
Table 2.
Correlation among baseline characteristics including white blood cell, hemoglobin, and PDW
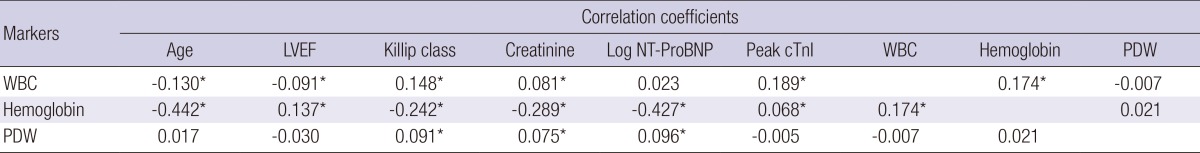
*Means P value<0.05. LVEF, left ventricular ejection fraction; Log NT-ProBNP, log N-terminal Pro-B-type natriuretic peptide; Peak cTnI, peak cardiac troponin I; WBC, white blood cell; PDW, platelet distribution width.
During the index admission, there were 59 (4.4%) in-hospital deaths. In the univariate analysis, age, heart rate, Killip class≥3, levels of WBC, PDW, blood urea nitrogen, peak cTnI, uric acid, and NT-ProBNP were significantly higher; and systolic blood pressure (SBP), previous history of hyperlipidemia, levels of Hb, sodium, and eGFR were significantly lower in patients with in-hospital death. Echocardiographic data were not available in 95 patients, including 34 (58%) who died before undergoing echocardiographic examination. Therefore, it was not included in the multivariate analysis. STEMI was more common in patients with in-hospital death. Percutaneous coronary intervention (PCI) was performed less frequently at index hospitalization among patients with in-hospital death and left anterior descending artery location of infarct-related artery was significantly higher in patients with in-hospital death. However, regarding gender, body mass index, current smoking status, pre-hospital medication, history of hypertension, diabetes mellitus, and ischemic heart disease, levels of creatine kinase-MB, cholesterol, and high-sensitivity C-reactive protein, no significant differences were observed between the groups.
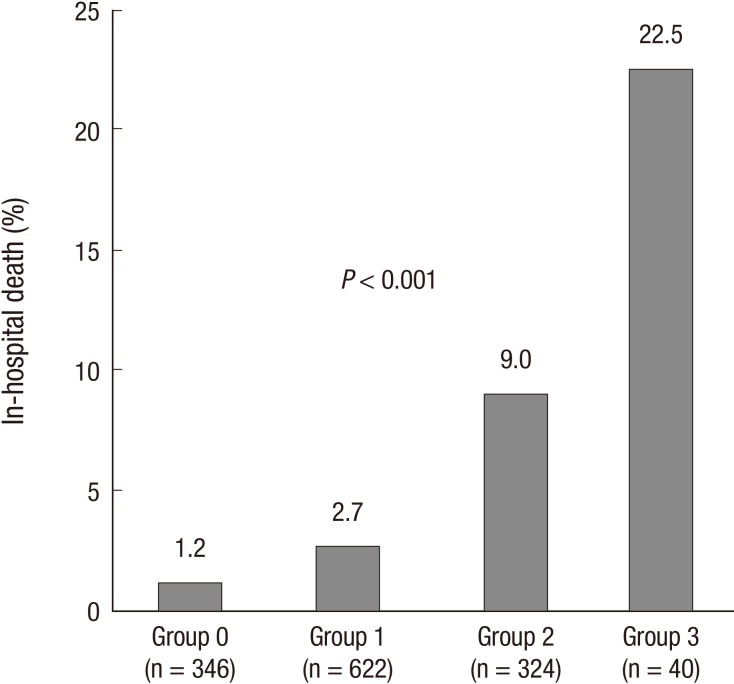
WBC count (12.7±5.5×103/µL vs 10.8±4.0×103/µL, P=0.013) and PDW (54.2±7.6% vs 52.2±7.4%, P=0.037) were significantly higher in the group with in-hospital death than in the group without. However, Hb level was significantly lower in patients with in-hospital death (12.4±2.2 g/dL vs 13.5±1.9 g/dL, P<0.001). Area under the ROC curve for prediction of in-hospital death was 0.602 (95% CI, 0.517-0.687) for WBC count, 0.669 (95% CI, 0.595-0.742) for Hb, and 0.581 (95% CI, 0.507-0.655) for PDW. The best cut-off levels of WBC, Hb, and PDW by ROC curve analysis were 14.5×103/µL, 12.7 g/dL, and 51.2%, respectively. Patients with high Killip class and high serum levels of creatinine and NT-ProBNP had significantly lower hemoglobin level and higher WBC and PDW (Table 3). Patients were categorized into four CBC groups; group 0 (n=346, 26.0%), 1 (n=622, 46.7%), 2 (n=324, 24.3%), and 3 (n=40, 3.0%) according to the sum of values defined by the cut-off levels of WBC (1, ≥14.5×103/µL; 0, <14.5×103/µL), Hb (1, <12.7 g/dL; 0, ≥12.7 g/dL), and PDW (1, ≥51.2%; 0, <51.2%). CBC group showed positive correlation with age (r=0.189, P<0.001), Killip class (r=0.243, P<0.001), levels of creatinine (r=0.238, P<0.001), and log NT-ProBNP (r=0.278, P<0.001), but showed negative correlation with LVEF (r=-0.124, P<0.001). Significant differences in in-hospital death were observed among CBC group 0 (1.2%), 1 (2.7%), 2 (9.0%), and 3 (22.5%) (P<0.001) (Fig. 2).
Table 3.
Clinical characteristics of patients with acute myocardial infarction according to the levels of white blood cell, hemoglobin, and platelet distribution width
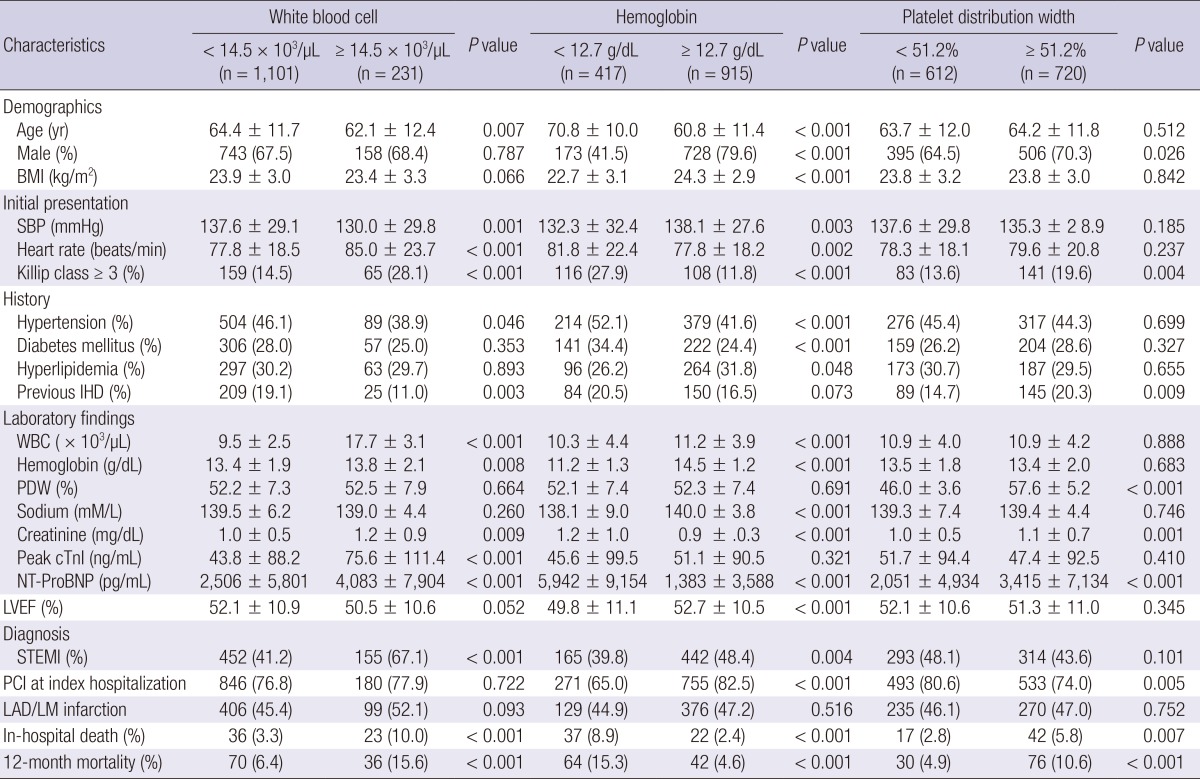
BMI, body mass index; SBP, systolic blood pressure; IHD, ischemic heart disease; WBC, white blood cell; PDW, platelet distribution width; Peak cTnI, peak cardiac troponin I; NT-ProBNP, N-terminal Pro-B-type natriuretic peptide; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; LAD/LM, left anterior descending/Left main.
Fig. 2.
In-hospital death categorized according to the cut-off levels of white blood cell, hemoglobin, and platelet distribution width. Significant differences in in-hospital death were observed among CBC groups 0 (1.2%), 1 (2.7%), 2 (9.0%), and 3 (22.5%) (P<0.001).
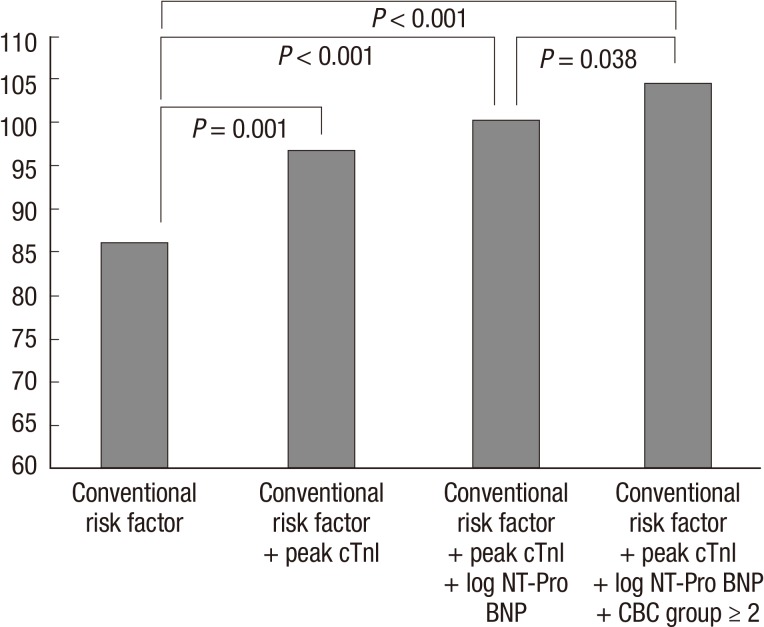
In multivariate logistic regression analysis, after adjusting for multiple clinical prognostic factors, WBC≥14.5×103/µL (odds ratio [OR], 2.077; 95% confidence interval [CI], 0.674-6.398, P=0.203), Hb<12.7 g/dL (OR, 1.285; 95% CI, 0.374-4.415, P=0.691), and PDW≥51.2% (OR, 1.697; 95% CI, 0.495-5.819, P=0.401) were not independent predictors for in-hospital death when they were entered separately into the model (Table 4). However, when the sum of the three CBC markers was entered into the model, CBC group≥2 (OR, 3.604; 95% CI, 1.040-12.484, P=0.043) was an independent predictor for in-hospital death in addition to SBP (OR, 0.962; 95% CI, 0.943-0.981, P<0.001), Killip class≥3 (OR, 3.432; 95% CI, 1.053-11.181, P=0.041), left anterior descending artery infarction (OR, 4.194; 95% CI, 1.046-16.812, P=0.043), log NT-ProBNP (OR, 3.853; 95% CI, 1.461-10.162, P=0.006), and peak cTnI (OR, 1.004; 95% CI, 1.001-1.006, P=0.012). In addition, CBC group≥2 had incremental prognostic value (chi-square=4.3, P=0.038) (Fig. 3).
Table 4.
Multivariate logistic regression analysis for the in-hospital death (n = 1,332)
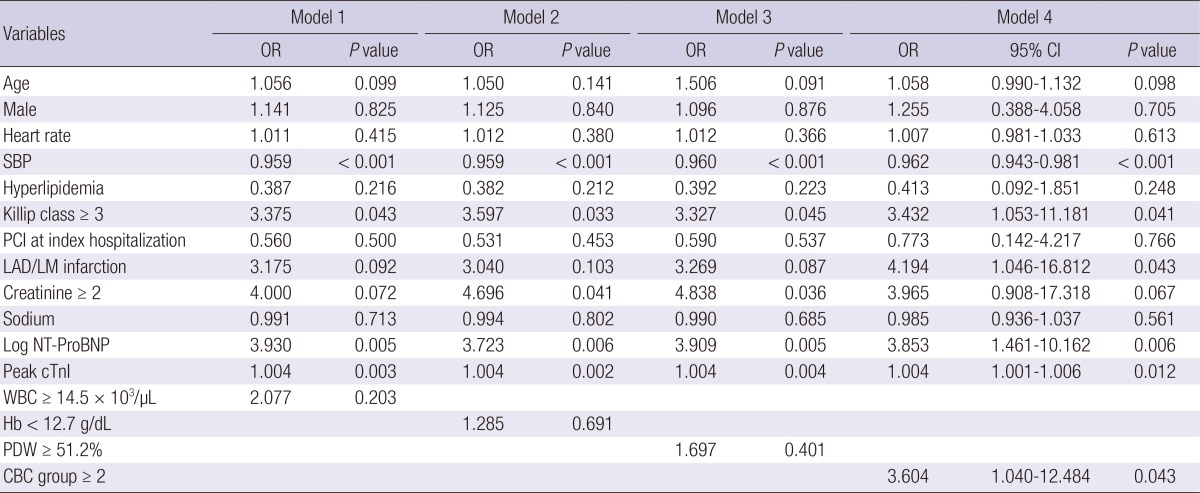
OR, odds ratio; BMI, body mass index; CI, confidence interval; SBP, systolic blood pressure; PCI, percutaneous coronary intervention; LAD/LM, left anterior descending/Left main; Log NT-ProBNP, log N-terminal Pro-B-type natriuretic peptide; Peak cTnI, peak cardiac troponin I; WBC, white blood cell; PDW, platelet distribution width; CBC, complete blood cell.
Fig. 3.
Incremental prognostic value of combined use of white blood cell, hemoglobin, and platelet distribution width in the Cox proportional-hazard model. CBC Group ≥2 has incremental prognostic value. Conventional risk factors included age, gender, heart rate, systolic blood pressure, hyperlipidemia, Killip class ≥3, percutaneous coronary intervention at index hospitalization, left anterior descending artery infarction, creatinine ≥2 mg/dL, and sodium.
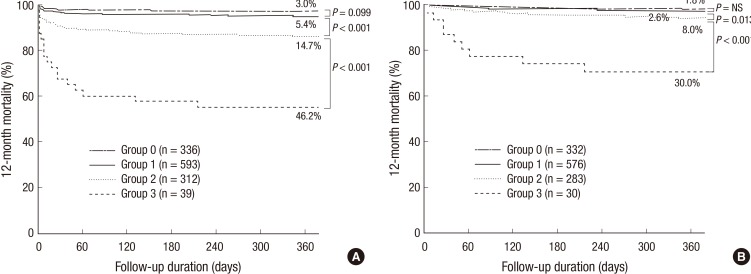
During the 12-month follow-up period after the index admission, 52 patients were lost, leaving 1280 patients in the analysis for 12-month mortality, and there were 106 (8.3%) all cause deaths. Kaplan-Meier curves showed a significant gradual increase in the risk of 12-month mortality according to the sum of the CBC markers (3.0%, 5.4%, 14.7%, and 46.2% of groups 0, 1, 2, and 3, P<0.001) (Fig. 4A). When patients with in-hospital death were excluded, 12-month mortality was still significantly different among the CBC groups (1.8%, 2.6%, 6.0%, and 30.0% of group 0, 1, 2, and 3, P<0.001) (Fig. 4B). In the Cox-proportional hazard model, CBC group≥2 (hazard ratio, 2.067; 95% CI, 1.017-4.204, P=0.045) was an independent predictor for 12-month mortality (Table 5).
Fig. 4.
Kaplan-Meier curves for 12-month mortality according to the sum of values defined by the cut-off levels of CBC markers in all patients (A) and in-hospital survivors (B).
Table 5.
Cox-Proportional Hazard Model for 12-month death (n = 1,280)
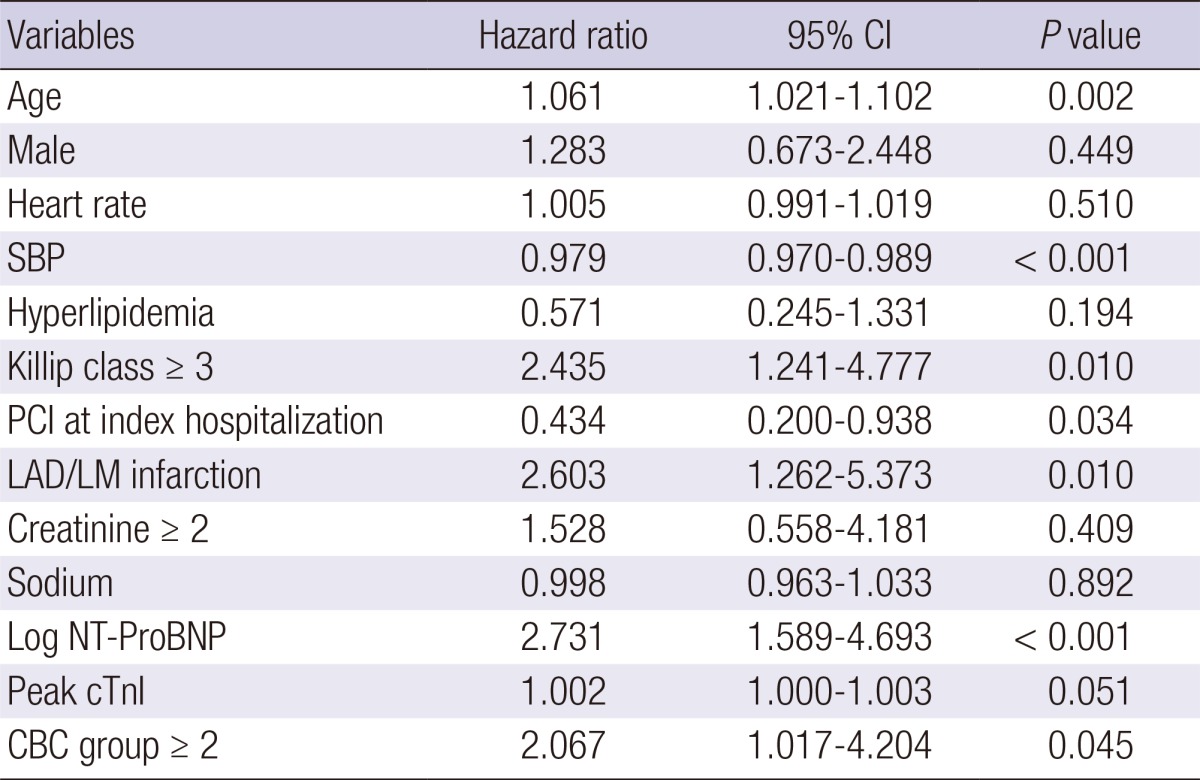
CI, confidence interval; SBP, systolic blood pressure; PCI, percutaneous coronary intervention; LAD/LM, left anterior descending/Left main; Log NT-ProBNP, log N-terminal Pro-B-type natriuretic peptide; Peak cTnI, peak cardiac troponin I; CBC, complete blood cell.
DISCUSSION
In the current study, the combination of WBC, Hb, and PDW was useful in prediction of in-hospital death in patients with AMI, although they did not have independent value, individually. In addition, it had an important incremental prognostic value to the combination of conventional risk factors, peak cTnI and NT-ProBNP, which are strong prognostic markers in AMI, both STEMI and non-STEMI (1, 20). The prognostic impact of the combined use of CBC markers remained significant over 12 months after index admission.
CBC, a cheap and simple hematologic marker, has been used for risk stratification of patients with AMI (3, 4, 5, 6, 7, 8). Among several CBC markers, the predictive values of WBC and hemoglobin in relation to short- and long-term survival in patients with AMI has been relatively well established (3, 4, 5, 6, 7, 8). Inflammation has been shown to be an important risk factor for development of cardiovascular events and several studies have also reported an association of elevated WBC count with increased risk of short- and long-term mortality in patients with AMI (3, 4, 5, 6, 7). Although the mechanism responsible for these associations is unknown, several hypotheses have been postulated, including a leukocyte-mediated diminished microcirculatory perfusion (21) and greater thrombus formation at the site of the atherosclerotic plaque (22), leukocyte-mediated no reflow (23), indirect cardiotoxicity mediated through proinflammatory cytokines (24), and negative inotropic effects on the myocardium via the nitric oxide synthesis pathway (25). Anemia, also a common comorbidity in patients with AMI, is associated with increased mortality (6, 7, 8). In a recent study with STEMI patients, lower hemoglobin level ≤12.5 g/dL, similar to the cut-off level of hemoglobin (12.65 g/dL) in our study, was an independent predictor for in-hospital mortality (8). The relationship between low hemoglobin and increased mortality could basically be explained by lower oxygen delivery to myocardial tissue (26, 27). However, negative studies for the prognostic value of CBC markers in AMI patients have also been reported (9, 10, 11). In STEMI patients who underwent primary PCI, WBC count did not show statistical association with an increased incidence of one-month and/or 12-month death (9, 10). Hemoglobin was not an independent predictor for 12-month death in patients with STEMI who underwent PCI (11). In our previous study with AMI patients, WBC and hemoglobin were not associated with six-month major adverse cardiac events (28). In the current study, after adjusting for multiple confounding variables, an elevated WBC count and lower hemoglobin were also not independent predictors for in-hospital and 12-month death. Patients with high WBC count and/or low hemoglobin had concomitantly low LVEF, high Killip class, high creatinine, and high NT-ProBNP.
Unlike WBC and hemoglobin, limited evaluation of PDW has been conducted in patients with CAD, with conflicting results (14, 15, 16, 17). In a small study reported by Ihara et al. (16), lower PDW was observed in patients with angiographic coronary artery stenosis. However, in a small study of 194 patients reported by Khandekar et al. (17), significantly higher PDW was observed in patients with AMI and unstable angina, compared to those with stable CAD and the control group. Thromboembolic events are a major cause of mortality in patients with AMI (12, 13). Reliable markers of platelet activation, such as thrombin-antithrombin complex, β-thromboglobulin, and soluble platelet P-selectin have been investigated. However, these indices cannot be included in routine laboratory tests. Activation of platelets causes morphologic changes, including both spherical transformation and pseudopodia formation (29). Progressively activated platelets with pseudopodia formation could have heterogeneous size, giving larger PDW. Therefore, PDW could be an important, simple, effortless, and cost effective tool that can be used more extensively for prediction of impending acute events. In the current study, PDW of patients with in-hospital death was significantly higher than that of those without in-hospital death.
The combination of WBC and Hb was studied with regard to the combined use of CBC markers for risk stratification (6, 7). In the ANIN Myocardial Infarction Registry, a U-shaped relationship of Hb levels and mortality was observed for patients with higher leukocytosis, whereas, in patients with lower WBC count, higher mortality was related solely to the lowest Hb quintile (6). Another study from the Korean Acute Myocardial infarction Registry reported that patients with anemia and high neutrophil/lymphocyte (N/L) had higher mortality at six months after STEMI, compared to patients with no anemia and low N/L (7). However, to the best of our knowledge, a triple combination of CBC markers, all connected in some way with AMI prognosis, had never been used in risk assessment for AMI. In the current study, combined use of WBC, hemoglobin, and PDW, which are readily available CBC data in the early in-hospital period, showed good correlation with age, Killip class, NT-ProBNP, and LVEF which are well known as prognostic markers in patients with AMI. Furthermore, a triple combination of CBC markers was useful for prediction of in-hospital and 12-month mortality in patients with AMI.
There are several limitations to this study. First a single cut-off level for hemoglobin (12.7 g/dL) was used for prediction of in-hospital death. In general, differences in hemoglobin levels and definition of anemia were observed between male and female. However, because best cut-off level of hemoglobin by ROC curve analysis did not differ between genders, we used a single cut-off value for hemoglobin. Second, information on the WBC differential count was not available. However, Gurm et al. reported that total WBC count was a better prognostic marker for long-term prognosis than the WBC components (30).
In conclusion, combination of WBC, Hb, and PDW, a cheap and simple hematologic marker, is useful in early risk stratification of patients with AMI.
Footnotes
This research was supported by Kyungpook National University Research Fund, 2012
The authors have no conflicts of interest to disclose.
References
- 1.Haaf P, Balmelli C, Reichlin T, Twerenbold R, Reiter M, Meissner J, Schaub N, Stelzig C, Freese M, Paniz P, et al. N-terminal pro B-type natriuretic peptide in the early evaluation of suspected acute myocardial infarction. Am J Med. 2011;124:731–739. doi: 10.1016/j.amjmed.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Jeong MH, Rhee JA, Choi JS, Lee KH, Lee MG, Sim DS, Park KH, Yoon NS, Yoon HJ, et al. Predictors of long-term survival in acute coronary syndrome patients with left ventricular dysfunction after percutaneous coronary intervention. Korean Circ J. 2012;42:692–697. doi: 10.4070/kcj.2012.42.10.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron HV, Cannon CP, Murphy SA, Braunwald E, Gibson CM. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. 2000;102:2329–2334. doi: 10.1161/01.cir.102.19.2329. [DOI] [PubMed] [Google Scholar]
- 4.Han YC, Yang TH, Kim DI, Jin HY, Chung SR, Seo JS, Jang JS, Kim DK, Kim DK, Kim KH, et al. Neutrophil to lymphocyte ratio predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013;43:93–99. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmerini T, Mehran R, Dangas G, Nikolsky E, Witzenbichler B, Guagliumi G, Dudek D, Genereux P, Caixeta A, Rabbani L, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation. 2011;123:2829–2837. doi: 10.1161/CIRCULATIONAHA.110.985564. [DOI] [PubMed] [Google Scholar]
- 6.Kruk M, Przyłuski J, Kalińczuk L, Pregowski J, Kadziela J, Kaczmarska E, Petryka J, Kepka C, Klopotowski M, Chmielak Z, et al. Hemoglobin, leukocytosis and clinical outcomes of ST-elevation myocardial infarction treated with primary angioplasty: ANIN Myocardial Infarction Registry. Circ J. 2009;73:323–329. doi: 10.1253/circj.cj-08-0370. [DOI] [PubMed] [Google Scholar]
- 7.Cho KH, Jeong MH, Ahmed K, Hachinohe D, Choi HS, Chang SY, Kim MC, Hwang SH, Park KH, Lee MG, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107:849–856. doi: 10.1016/j.amjcard.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 8.Dündar C, Oduncu V, Erkol A, Tanalp AC, Sırma D, Karagöz A, Karabay CY, Kılıçgedik A, Pala S, Tigen K, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol. 2012;101:37–44. doi: 10.1007/s00392-011-0361-9. [DOI] [PubMed] [Google Scholar]
- 9.Smit JJ, Ottervanger JP, Slingerland RJ, Kolkman JJ, Suryapranata H, Hoorntje JC, Dambrink JH, Gosselink AT, de Boer MJ, Zijlstra F, et al. Comparison of usefulness of C-reactive protein versus white blood cell count to predict outcome after primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol. 2008;101:446–451. doi: 10.1016/j.amjcard.2007.09.088. [DOI] [PubMed] [Google Scholar]
- 10.Pellizzon GG, Dixon SR, Stone GW, Cox DA, Mattos L, Boura JA, Grines LL, Addala S, O'Neill WW, Grines CL. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (the Stent PAMI Trial) Am J Cardiol. 2003;91:729–731. doi: 10.1016/s0002-9149(02)03416-1. [DOI] [PubMed] [Google Scholar]
- 11.Vis MM, Engström AE, Sjauw KD, Tjong FV, Baan J, Jr, Koch KT, DeVries JH, Tijssen JG, de Winter RJ, Piek JJ, et al. Plasma glucose and not hemoglobin or renal function predicts mortality in patients with STEMI complicated with cardiogenic shock. J Cardiovasc Med (Hagerstown) 2010;11:827–831. doi: 10.2459/JCM.0b013e32833cdc6d. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–132. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Heestermans AA, van Werkum JW, Zwart B, van der Heyden JA, Kelder JC, Breet NJ, van't Hof AW, Dambrink JH, Koolen JJ, Brueren BR, et al. Acute and subacute stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: incidence, predictors and clinical outcome. J Thromb Haemost. 2010;8:2385–2393. doi: 10.1111/j.1538-7836.2010.04046.x. [DOI] [PubMed] [Google Scholar]
- 14.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Ihara A, Matsumoto K, Kawamoto T, Shouno S, Kawamoto J, Katayama A, Yoshitatsu M, Izutani H. Relationship between hemostatic markers and platelet indices in patients with aortic aneurysm. Pathophysiol Haemost Thromb. 2006;35:451–456. doi: 10.1159/000102053. [DOI] [PubMed] [Google Scholar]
- 16.Ihara A, Kawamoto T, Matsumoto K, Shouno S, Hirahara C, Morimoto T, Noma Y. Relationship between platelet indexes and coronary angiographic findings in patients with ischemic heart disease. Pathophysiol Haemost Thromb. 2006;35:376–379. doi: 10.1159/000097692. [DOI] [PubMed] [Google Scholar]
- 17.Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006;59:146–149. doi: 10.1136/jcp.2004.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, Kim YJ, Kim KS, Hur SH, Seong IW, et al. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry) Am J Cardiol. 2009;104:182–189. doi: 10.1016/j.amjcard.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 21.Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997;78:310–314. [PubMed] [Google Scholar]
- 22.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001;21:2093–2098. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 23.Engler RL, Schmid-Schönbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983;111:98–111. [PMC free article] [PubMed] [Google Scholar]
- 24.Mann DL, Young JB. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest. 1994;105:897–904. doi: 10.1378/chest.105.3.897. [DOI] [PubMed] [Google Scholar]
- 25.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 26.Levy PS, Kim SJ, Eckel PK, Chavez R, Ismail EF, Gould SA, Ramez Salem M, Crystal GJ. Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol. 1993;265:H340–H349. doi: 10.1152/ajpheart.1993.265.1.H340. [DOI] [PubMed] [Google Scholar]
- 27.Wahr JA. Myocardial ischaemia in anaemic patients. Br J Anaesth. 1998;81:10–15. [PubMed] [Google Scholar]
- 28.Bae MH, Lee JH, Lee SH, Park SH, Yang DH, Park HS, Cho Y, Jun JE, Chae SC. Serum uric acid as an independent and incremental prognostic marker in addition to N-terminal pro-B-type natriuretic peptide in patients with acute myocardial infarction. Circ J. 2011;75:1440–1447. doi: 10.1253/circj.cj-10-0952. [DOI] [PubMed] [Google Scholar]
- 29.Jagroop IA, Clatworthy I, Lewin J, Mikhailidis DP. Shape change in human platelets: measurement with a channelyzer and visualisation by electron microscopy. Platelets. 2000;11:28–32. doi: 10.1080/09537100075760. [DOI] [PubMed] [Google Scholar]
- 30.Gurm HS, Bhatt DL, Lincoff AM, Tcheng JE, Kereiakes DJ, Kleiman NS, Jia G, Topol EJ. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89:1200–1204. doi: 10.1136/heart.89.10.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]