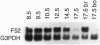Abstract
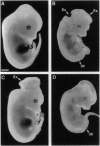
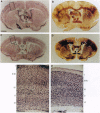
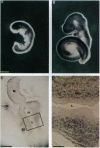
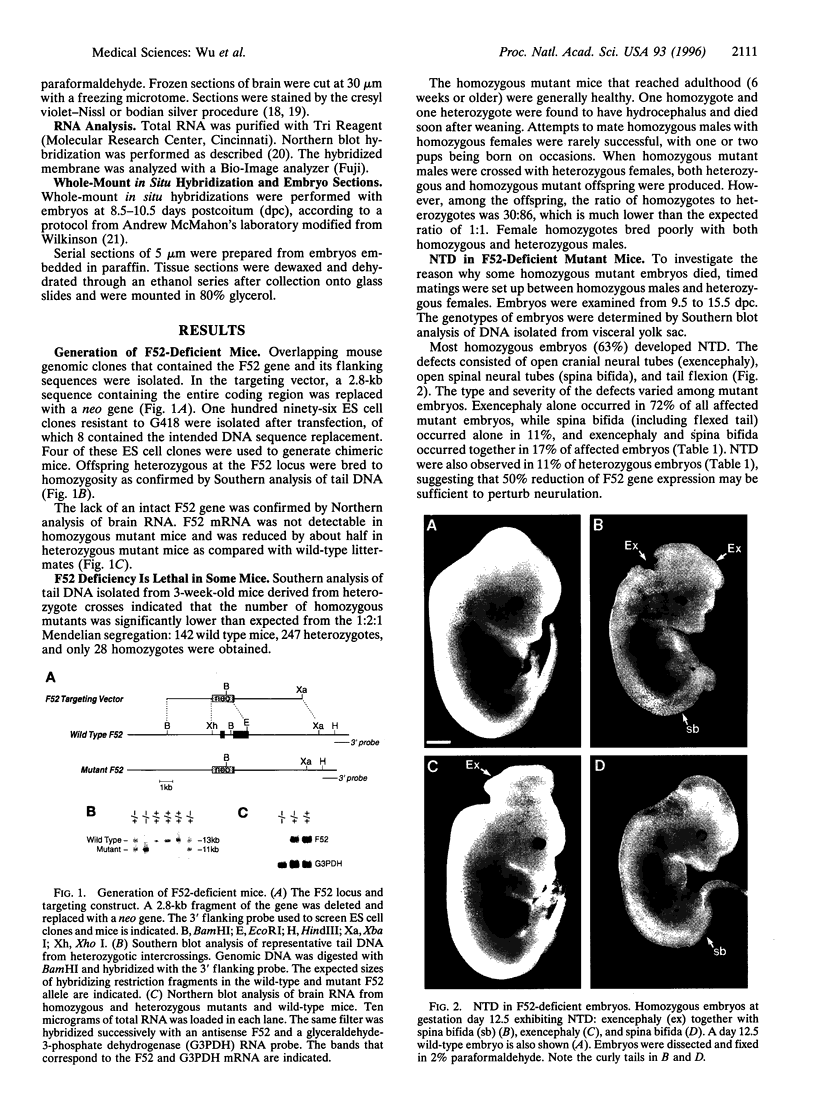
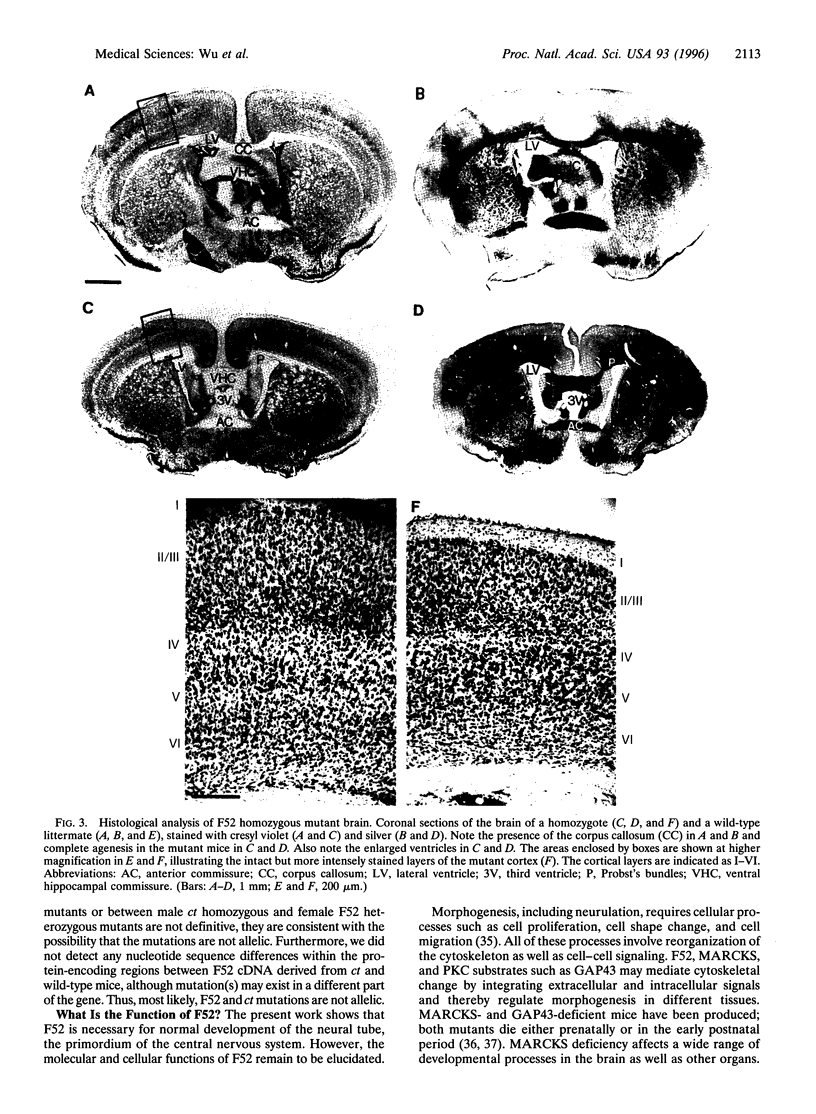
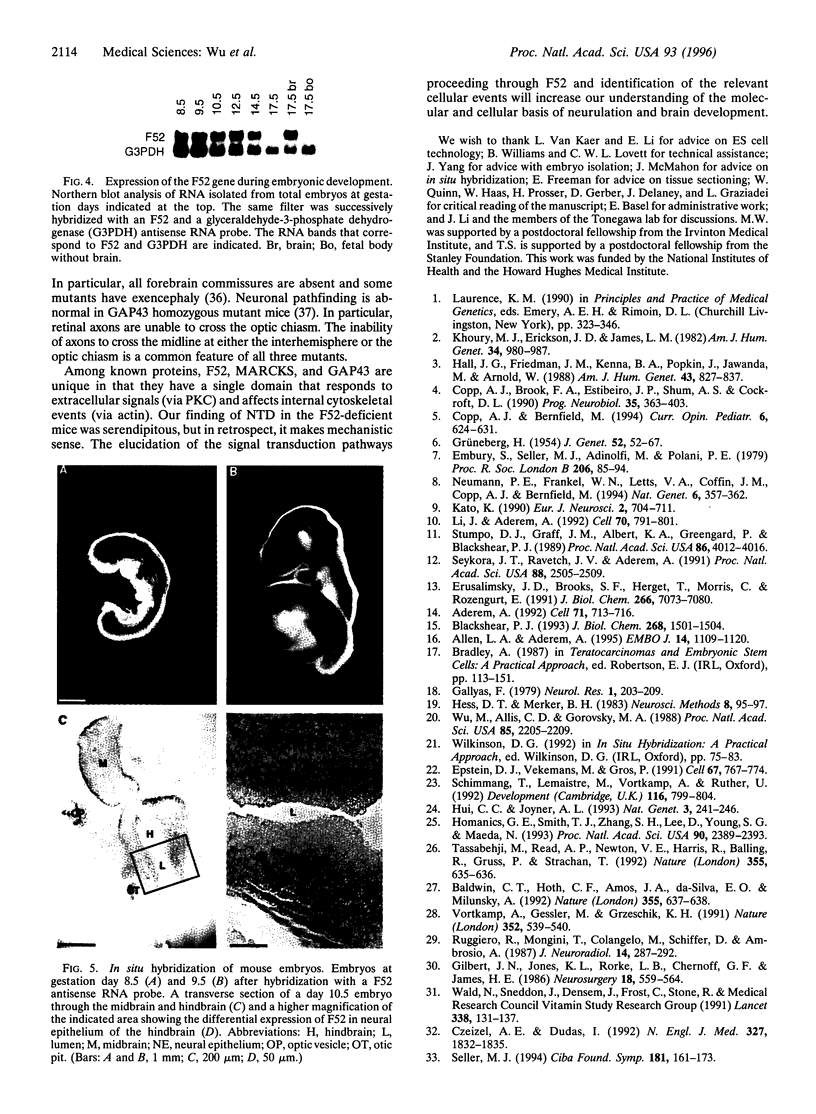
F52 is a myristoylated, alanine-rich substrate for protein kinase C. We have generated F52-deficient mice by the gene targeting technique. These mutant mice manifest severe neural tube defects that are not associated with other complex malformations, a phenotype reminiscent of common human neural tube defects. The neural tube defects observed include both exencephaly and spina bifida, and the phenotype exhibits partial penetrance with about 60% of homozygous embryos developing neural tube defects. Exencephaly is the prominent type of defect and leads to high prenatal lethality. Neural tube defects are observed in a smaller percentage of heterozygous embryos (about 10%). Abnormal brain development and tail formation occur in homozygous mutants and are likely to be secondary to the neural tube defects. Disruption of F52 in mice therefore identifies a gene whose mutation results in isolated neural tube defects and may provide an animal model for common human neural tube defects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Allen L. A., Aderem A. Protein kinase C regulates MARCKS cycling between the plasma membrane and lysosomes in fibroblasts. EMBO J. 1995 Mar 15;14(6):1109–1121. doi: 10.1002/j.1460-2075.1995.tb07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. T., Hoth C. F., Amos J. A., da-Silva E. O., Milunsky A. An exonic mutation in the HuP2 paired domain gene causes Waardenburg's syndrome. Nature. 1992 Feb 13;355(6361):637–638. doi: 10.1038/355637a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–1504. [PubMed] [Google Scholar]
- Copp A. J., Bernfield M. Etiology and pathogenesis of human neural tube defects: insights from mouse models. Curr Opin Pediatr. 1994 Dec;6(6):624–631. doi: 10.1097/00008480-199412000-00002. [DOI] [PubMed] [Google Scholar]
- Copp A. J., Brook F. A., Estibeiro J. P., Shum A. S., Cockroft D. L. The embryonic development of mammalian neural tube defects. Prog Neurobiol. 1990;35(5):363–403. doi: 10.1016/0301-0082(90)90037-h. [DOI] [PubMed] [Google Scholar]
- Czeizel A. E., Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992 Dec 24;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Embury S., Seller M. J., Adinolfi M., Polani P. E. Neural tube defects in curly-tail mice. I. Incidence, expression and similarity to the human condition. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):85–94. doi: 10.1098/rspb.1979.0092. [DOI] [PubMed] [Google Scholar]
- Epstein D. J., Vekemans M., Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991 Nov 15;67(4):767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Erusalimsky J. D., Brooks S. F., Herget T., Morris C., Rozengurt E. Molecular cloning and characterization of the acidic 80-kDa protein kinase C substrate from rat brain. Identification as a glycoprotein. J Biol Chem. 1991 Apr 15;266(11):7073–7080. [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1(2):203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gilbert J. N., Jones K. L., Rorke L. B., Chernoff G. F., James H. E. Central nervous system anomalies associated with meningomyelocele, hydrocephalus, and the Arnold-Chiari malformation: reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery. 1986 May;18(5):559–564. doi: 10.1227/00006123-198605000-00008. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Friedman J. M., Kenna B. A., Popkin J., Jawanda M., Arnold W. Clinical, genetic, and epidemiological factors in neural tube defects. Am J Hum Genet. 1988 Dec;43(6):827–837. [PMC free article] [PubMed] [Google Scholar]
- Hess D. T., Merker B. H. Technical modifications of Gallyas' silver stain for myelin. J Neurosci Methods. 1983 May;8(1):95–97. doi: 10.1016/0165-0270(83)90055-9. [DOI] [PubMed] [Google Scholar]
- Homanics G. E., Smith T. J., Zhang S. H., Lee D., Young S. G., Maeda N. Targeted modification of the apolipoprotein B gene results in hypobetalipoproteinemia and developmental abnormalities in mice. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2389–2393. doi: 10.1073/pnas.90.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. C., Joyner A. L. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993 Mar;3(3):241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Kato Kikuya. A Collection of cDNA Clones with Specific Expression Patterns in Mouse Brain. Eur J Neurosci. 1990;2(8):704–711. doi: 10.1111/j.1460-9568.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Khoury M. J., Erickson J. D., James L. M. Etiologic heterogeneity of neural tube defects. II. Clues from family studies. Am J Hum Genet. 1982 Nov;34(6):980–987. [PMC free article] [PubMed] [Google Scholar]
- Li J., Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell. 1992 Sep 4;70(5):791–801. doi: 10.1016/0092-8674(92)90312-z. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Rochelle J. M., Watson M. L., Seldin M. F., Blackshear P. J. Nucleotide sequence, expression, and chromosomal mapping of Mrp and mapping of five related sequences. Genomics. 1993 Jul;17(1):194–204. doi: 10.1006/geno.1993.1301. [DOI] [PubMed] [Google Scholar]
- Neumann P. E., Frankel W. N., Letts V. A., Coffin J. M., Copp A. J., Bernfield M. Multifactorial inheritance of neural tube defects: localization of the major gene and recognition of modifiers in ct mutant mice. Nat Genet. 1994 Apr;6(4):357–362. doi: 10.1038/ng0494-357. [DOI] [PubMed] [Google Scholar]
- Ruggiero R., Mongini T., Colangelo M., Schiffer D., Ambrosio A. Brain malformation associated with neural tube defects. Report of three cases of partial agenesis of the corpus callosum and myelomeningocele. J Neuroradiol. 1987;14(3):287–292. [PubMed] [Google Scholar]
- Schimmang T., Lemaistre M., Vortkamp A., Rüther U. Expression of the zinc finger gene Gli3 is affected in the morphogenetic mouse mutant extra-toes (Xt). Development. 1992 Nov;116(3):799–804. doi: 10.1242/dev.116.3.799. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Smith J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990 Jun;109(2):243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Seller M. J. Vitamins, folic acid and the cause and prevention of neural tube defects. Ciba Found Symp. 1994;181:161–179. doi: 10.1002/9780470514559.ch10. [DOI] [PubMed] [Google Scholar]
- Seykora J. T., Ravetch J. V., Aderem A. Cloning and molecular characterization of the murine macrophage "68-kDa" protein kinase C substrate and its regulation by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2505–2509. doi: 10.1073/pnas.88.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Fankhauser C., Huang P. L., Mashimo H., Fishman M. C. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995 Feb 10;80(3):445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Bock C. B., Tuttle J. S., Blackshear P. J. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M., Read A. P., Newton V. E., Harris R., Balling R., Gruss P., Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992 Feb 13;355(6361):635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Gessler M., Grzeschik K. H. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991 Aug 8;352(6335):539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Gorovsky M. A. Cell-cycle regulation as a mechanism for targeting proteins to specific DNA sequences in Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2205–2209. doi: 10.1073/pnas.85.7.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]