Abstract
Synthetic modification of a recombinant protein cage called a vault with stimuli-responsive smart polymers provides access to a new class of biohybrid materials; the polymer nanocapsules retain the structure of the protein cage and exhibit the responsive nature of the polymer. Vaults are naturally-occurring ubiquitous ribonucleoprotein particles 41 × 41 × 72.5 nm composed of a protein shell enclosing multiple copies of two proteins and multiple copies of one or more small untranslated RNAs. Recombinant vaults are structurally identical but lack the vault content proteins. Poly(N-isopropylacrylamide) (pNIPAAm), a polymer responsive to heat, was conjugated to recombinant vaults that were composed of ~78 copies of the major vault protein (MVP) modified to contain a cysteine rich region at the N-terminus (CP-MVP). The polymer was synthesized using reversible addition-fragmentation chain transfer (RAFT) polymerization to have a dansyl group at the alpha end and modified to have a thiol-reactive pyridyl disulfide at the omega end, which readily coupled to CP-MVP vaults. The resulting vault nanocapsules underwent reversible aggregation upon heating above the lower critical solution temperature (LCST) of the polymer as determined by electron microscopy (EM), dynamic light scattering experiments, and UV-Vis turbidity analysis. The vault structure remained entirely intact throughout the phase transition; suggesting its use in a myriad of biomedical and biotechnology applications.
Keywords: Vault, protein cage, thermo-responsive, protein-polymer conjugate, poly(N-isopropylacrylamide)
Naturally derived protein cages, such as virus capsids and ferritin, serve as excellent templates for functional biohybrid materials with precise architectures, unattainable by purely synthetic processes.1–5 Such materials have been developed for applications in the fields of nanotechnology,4,6 biotechnology,5,7 electronic materials,8,9 catalysis,10–12 and drug delivery.13–16 The regular arrangement of protein sub-units within protein cage structures allows for synthetic modification of specific regions and surfaces of the protein cage, such as the exterior shell or interior cavity. The conjugation of synthetic polymers to viruses, such as adenovirus, cowpea mosaic virus, tobacco mosaic virus, and others has been investigated.17–20 For example, poly(ethylene glycol) has been attached to adenovirus and cowpea mosaic virus and has been shown to decrease immunogenicity of the particles.17,19 Recently, Douglas and coworkers have polymerized 2-aminoethyl methacrylate from the interior of P22 capsids creating a particle capable of encapsulating magnetic resonance imaging (MRI) contrast agents.21 Although there are several examples of polymer conjugation to protein cages, to our knowledge there are no examples of stimuli-responsive smart polymers conjugated to protein cages. Yet such materials can reversibly alter the physical characteristics of the nanobiomaterial by an externally controlled trigger.
Smart polymers are responsive to external stimuli such as heat, pH, magnetic field, etc.,22 and the analysis of the micro- and nano-assembly transitions has been undertaken.23 Poly(N-isopropylacrylamide) (pNIPAAm) is a well known temperature responsive smart polymer that undergoes a reversible phase transition, where it becomes insoluble in water above the lower critical solution temperature (LCST).22 We and others have previously reported the preparation of protein-pNIPAAm conjugates, which exhibit the pNIPAAm phase behavior, while retaining the bioactivity of the protein.24–30 Polymer conjugation results in altered properties upon exposure to heat. For example, ligand binding sites can be closed, proteins can be reversibly aggregated, and the polymer can be used to purify biomolecules by mild precipitation.24,25,30 In this paper, we report that thermally-responsive polymers can be added to vault nanocapsules without disrupting the structure of the particle.
First reported in 1986 by Rome and Kedersha, vaults are highly conserved ribonucleoprotein complexes found in high concentrations in almost all eukaryotic cells.32–34 These massive particles weigh approximately 13 mDa and have a unique structure composed of a barrel waist and protruding caps at both ends (Scheme 1).35 In recent years, high-resolution crystal structures have confirmed low resolution cryo-EM structures.36 Naturally occurring vaults are comprised of several highly conserved proteins, with one protein, the 104 kDa major vault protein (MVP) accounting for over 70% of the vault mass. The MVP provides the basis for the vault structure, with ~78 MVPs aligned from C- to N-terminus from the cap of the vault to the waist, with all of the N-termini facing towards the interior. Vaults are dynamic, the MVPs are non-covalently associated and although they bind to each other very tightly, they do have a kinetic off rate which means that dynamically they can open and close to accommodate small molecules which slip in or large proteins or other macromolecules which bind to their interior.31 Vaults are also highly stable in vivo and in vitro to a variety of biological and physical stresses.37
Scheme 1.
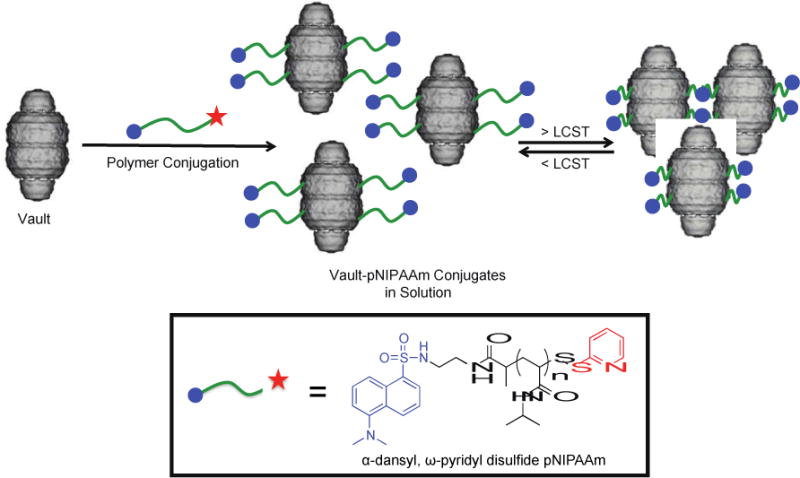
Preparation of thermo-responsive vault-pNIPAAm conjugates. The vault image was adapted from cryoEM reconstructions of recombinant vaults (P. Stewart, Vanderbilt University Medical Center, Nashville, TN).31
Expression of MVP in insect cells results in the spontaneous assembly of non-native vault structures measuring 45 × 45 × 75 nm.38 Although the natural biological function of vaults is unknown, recombinant vaults can be engineered for a variety of applications.39 For example, the N-terminus of MVP can be modified to contain a cysteine-rich (4 cysteine residues) 12 amino acid peptide sequence to form CP-MVP. Vaults assembled from this fusion protein have increased particle stability.39 Cell-specific targeting vaults have also been engineered by incorporating IgG binding peptides into recombinant MVPs for attachment of targeting antibodies.40 While in nature vaults contain multiple copies of two large proteins (VPARP and TEP1) and one or more small RNAs (vRNAs), recombinant vaults have a hollow interior with an approximate volume of 5 × 107 Å3, large enough to accommodate hundreds of proteins and many more small molecules.31,35,41–43 For example, we have packaged gold nanoparticles and incorporated conducting polymers non-covalently into the vault interior.42,43 In addition, a number of proteins have been packaged into the vault taking advantage of a binding site on MVP which faces the internal vault lumen. This site recognizes the vault protein VPARP through a protein domain at the VPARP C-terminus, which is referred to as INT for MVP interaction domain.39 INT fusion proteins bind with high affinity to the vault lumen, but they are slowly released. This strategy has been used to package antigens into vaults so that the vault can be used as an adjuvant and to package a chemokine, which activates the immune system.44–46 Herein we report the feasibility of preparing smart vault nanoparticle conjugates by the synthesis a vault-poly(N-isopropylacrylamide) conjugate. The synthesis and characterization of responsive properties are described.
Results and Discussion
Our approach to developing thermo-responsive vault nanoparticles is based upon the conjugation of thiol-reactive pNIPAAm to cysteine-rich CP-MVP vaults. We and others have prepared cysteine-reactive polymers by reversible addition-fragmentation chain transfer (RAFT) polymerization.47,48 RAFT is suitable for this application because it results in polymers with narrow molecular weight distributions.49,50 Well-defined polymers are particularly important for this study because they exhibit sharp LCSTs. We chose to use a pyridyl-disulfide pNIPAAm in order to achieve reversible conjugation to the vault. We also introduced a dansyl group, a fluorophore, at the other end group for ease of visualization.
The polymer was prepared as shown in Scheme 2. First a dansyl-modified CTA was synthesized by coupling N-(2-aminoethyl)-5-(dimethylamino)naphthalene-1-sulfonamide with 2-(((ethylthio)carbonothioyl)thio)propanoic acid (Scheme S1 and ESI for synthesis details and characterization). The product was obtained in 64% yield. This CTA was then utilized in the RAFT polymerization of NIPAAm with AIBN in DMF at 80 ºC. The polymerization was stopped after 1 h at 81% monomer conversion by 1H NMR spectroscopy. Polymer 1 was obtained after extensive dialysis against MeOH. The number-average molecular weight (Mn) was determined by 1H NMR (ESI, Figure S3) to be 12.4 kDa and the polydispersity index (PDI) by GPC (ESI, Figure S4) was 1.02. The trithiocarbonate end-group of polymer 1 was transformed into a cysteine reactive pyridyl-disulfide via aminolysis of the trithiocarbonate to the free thiol with ethanolamine, followed by an in situ disulfide exchange with Aldrithiol®. After dialysis of the reaction mixture against MeOH, the α-dansyl, ω-pyridyl disulfide pNIPAAm, polymer 2, was obtained. Molecular weight analysis indicated that the polymer had not coupled during the end group transformation because the Mn and PDI (12.4 kDa and 1.03, respectively) were the same as before the reaction (ESI, Scheme S6). By comparing the integration of the peaks corresponding to the pyridyl disulfide to the dansyl group in the 1H NMR spectrum (ESI, Figure S5), the conversion of the disulfide exchange was determined to be 81%.
Scheme 2.
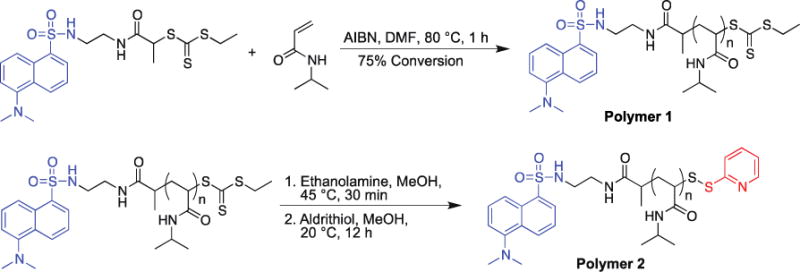
Synthesis of thiol-reactive pNIPAAm polymer
Polymers 2 was conjugated to CP-MVP vault by incubating the CP-MVP vault at a ratio of 100 : 1 polymer : CP-MVP vault (Scheme 3). The resulting conjugate was analyzed by SDS-PAGE and compared to unmodified CP-MVP vault. Under non-reducing conditions there was a clear shift to higher molecular weight compared to the ~100 kDa CP-MVP vault (Scheme 1a, lanes 5 versus 4), and under reducing conditions, SDS-PAGE stained with coomassie blue showed no difference in molecular weight as expected because of the reversibility of the disulfide bond of the polymer connection (Scheme 1a, lanes 2 and 3). Visualization of the gel with ultra-violet light confirmed conjugation; the dansyl fluorophore of the polymer overlaying with coomassie staining of the protein was clearly visible (Scheme 1b, lane 3). The results demonstrate that the conjugate with the CP-MVP vault was prepared and that the conjugation was reversible.
Scheme 3.
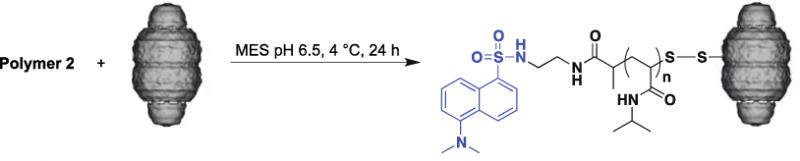
Synthesis of CP-MVP vault-pNIPAAm conjugates.
The thermo-responsive properties of the conjugate were studied by UV-Vis turbidity measurements. By UV-Vis turbidity studies (Figure 2) polymer 2 had an LCST of 30.5 ºC. The phase transition was sharp, as expected for a narrow molecular weight distribution polymer. The hydrophobic dansyl group lowered the LCST from the typical value of 32 ºC. Once the polymer was conjugated to the hydrophilic CP-MVP vault, the LCST was increased by the proximity of the hydrophilic protein to 35.9 ºC. The phase transition was broad, potentially due to a distribution of number of polymers and attachment sites. The vault itself had no LCST as expected (ESI Figure S7); no visible aggregation of the vault structure was observed up to at least 50 ºC, indicating that the phase transition for the conjugate was due to the polymer. The results together confirmed that the polymer is covalently attached to the protein and that the polymer confers thermal responsivity to the vault.
Figure 2.
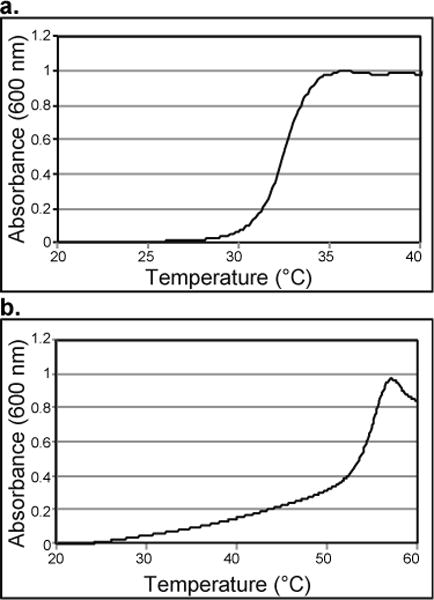
UV-Vis turbidity study of a) polymer 2 and b) conjugate. LCST is 10% of the maximum absorbance.
Dynamic light scattering (DLS) studies at different temperatures were performed to investigate the size of the conjugate (Figure 3). At 25 ºC, the conjugate closely resembled the unmodified CP-MVP vault (49.7 nm ± 2.2 nm, versus 48.69 nm ± 0.44 nm, respectively). However upon heating to 40 ºC, above the LCST of the polymer, the conjugate aggregated into micron-sized particles, while the size of the unmodified CP-MVP vault did not change (53.98 nm ± 5.0). Interestingly, upon cooling the observed aggregates disappeared and the conjugate returned to approximately its original size (58.76 nm ± 1.5 nm) suggesting that the aggregation was reversible.
Figure 3.
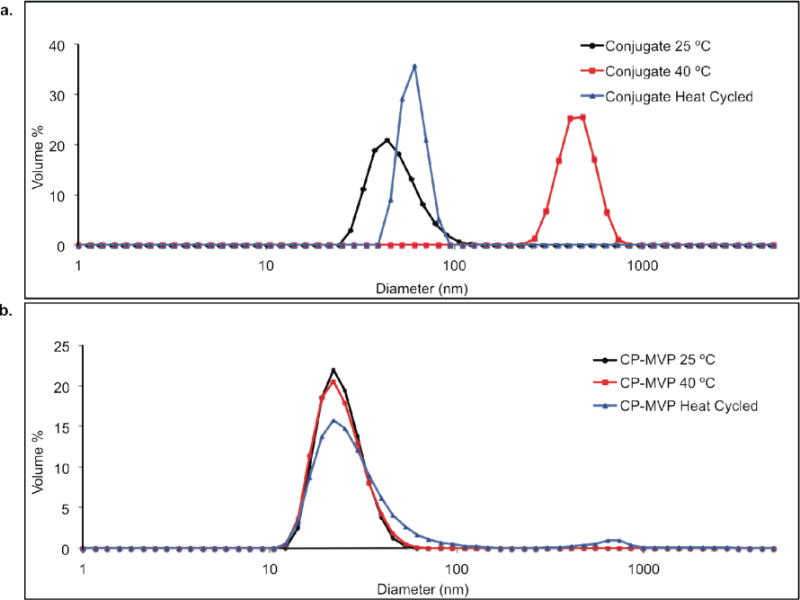
a. DLS analysis of conjugate at 25 ºC, 40 ºC, and heat cycled (25 ºC to 40 ºC to 25 ºC) conjugate cooled to 25 ºC. b. DLS analysis of unmodified CP-MVP vaults at 25 ºC, 40 ºC, and heat cycled (25 ºC to 40 ºC to 25 ºC).
This result was further investigated by electron microscopy. EM images of the conjugate and unmodified CP-MVP vault at 35 ºC showed intact vault structures (Figure 4a, c, respectively). This indicated that modification of CP-MVP vaults with polymer 2 did not alter the vault structure. However, when the samples were heated above the LCST to 45 ºC, the conjugate appeared aggregated, yet with intact vault structures still recognizable within the aggregates (Figure 4d), while the unmodified CP-MVP vault structures were unchanged (Figure 4b). Interestingly, when the conjugate was cooled back down to 4 ºC, the vault structures were no longer aggregated and looked the same as before heating (Figure 4e).
Figure 4.
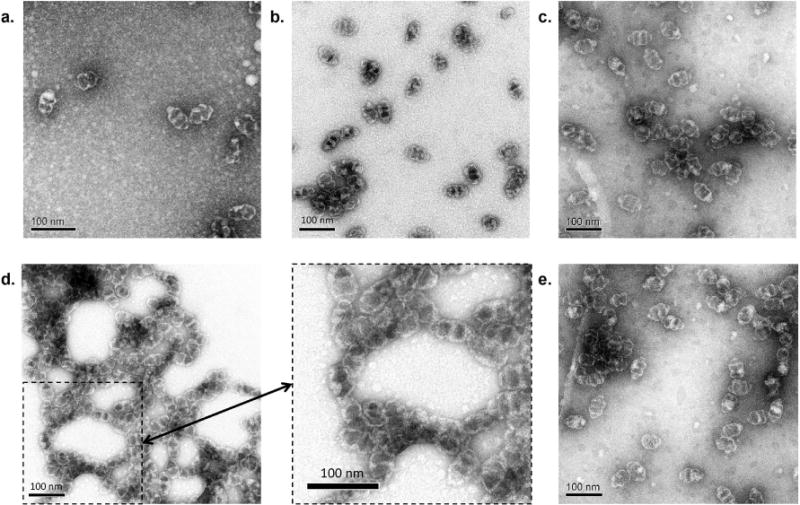
Negative stain TEM of unmodified CP-MVP vaults at a) 35 ºC and b) 45 ºC; Polymer 2-CP-MVP vault conjugates at c) 35 ºC, d) 45 ºC (inset image at the bottom middle indicated by the arrow is an expanded view of the dashed box in d), e) after cooling back down to 4 ºC.
The data together demonstrate that conjugation of the pNIPAAm does not interfere with or disrupt vault structure. Yet, the polymer once attached allows for reversible aggregation of vaults without destroying their structure. This result is interesting because it implies that cargo could be potentially loaded within the vault, while the polymer could be used to precipitate the vaults at a site of interest. It is well known that pNIPAAm can form aggregates at tumors, while remaining soluble in other tissue due to the differential in temperatures between the cancerous and normal tissues.51,52 An aggregating vault conjugate would potentially undergo similar selectivity, yet at the same time allow for protein or drug delivery from the vault nanocapsules. Drug loading and delivery within the vault is possible and has been demonstrated with vaults engineered to contain lipids.39
It is not known if the pNIPAAm is attached to the cysteine-rich region at the N-terminus in the interior or to free cysteines at the exterior. Upon examination of the crystal structure of the rat MVP,33 there are six cysteines on the exterior of the vault; however all but one appear to be not surface accessible. Cysteine 356 may be exposed or at least partially exposed. CP-MVP is engineered to contain an additional four cysteines near the N-terminus of the protein, which is in the interior of the vault. Due to the dynamic nature of the CP-MVP vaults, polymer should be able to enter the structure to react. Thus, likely the polymer is attached a both the exterior and interior. It also may be possible that polymer in the interior could thread out and participate in aggregation of the vaults. Our initial attempts to investigate the location(s) of the chains were to associate gold nanoparticle-modified streptavidin with polymers that had biotin groups instead of a dansyls (data now shown). However, the location of the polymer was unclear. Cysteine mutagenesis experiments may provide the desired information. Yet, it is clear from the results that attachment of the polymer inside the vault does not disrupt the structure, as full vault structures were observed. The results also demonstrate that conjugates with pNIPAAm that contain a fluorophore can be synthesized. This implies that other end groups could be prepared, and multifunctional vaults could be made with this strategy. Vaults are stable, non-immunogenic, readily engineered and expressed; making them a versatile platform for the development of sophisticated hybrid nanomaterials. Thus, we envision that the thermally-responsive vaults described herein are an interesting and versatile nanoplatform for drug delivery and other applications.
Conclusion
Novel biohybrid nanoparticles have been synthesized through covalent conjugation of pNIPAAm to recombinant CP-MVP vaults. Pyridyl disulfide pNIPAAm with a dansyl group at the other end group was conjugated to CP-MVP vaults via reaction with free cysteines. The resulting conjugates were thermally-responsive. UV-Vis turbidity measurements showed that the conjugates had an LCST. DLS studies demonstrated that upon heating, aggregates formed, which were reversible with cooling. This result was confirmed by EM studies, whereby reversible aggregation of polymer-modified vaults was observed, while the vault nanocapsules remained intact regardless of the temperature. We envision that these smart vault conjugates may be used for drug delivery applications in conjunction with previously reported vault-based hydrophobic drug delivery methods and emerging in vivo localized heating techniques,41,53,54 to build up depots of therapeutic drugs in tumors for a sustained release of drugs to tumor cells, and other interesting medical and biotechnology applications.
Methods
Materials
Chemicals were purchased from Sigma-Aldrich, Fisher Scientific, and Acros. AIBN was recrystallized twice from acetone. NIPAAm was recrystallized twice from hexanes. Rat CP-MVP was expressed from a baculovirus system in Sf9 insect cells, which do not contain endogenous vaults according to our literature procedure.39
Instrumental
NMR spectra were obtained on a Bruker 600 MHZ DRX spectrometer. Proton NMR spectra were acquired with a relaxation delay of 30 sec for all polymers. UV-Vis spectra were obtained on a Biomate 5 Thermo Spectronic UV-Vis spectrometer and a Hewlett-Packard HP8453 diode-array UV-Vis spectrophotometer with Peltier temperature control spectrometer with quartz cells. GPC was conducted on a Shimadzu HPLC system equipped with a refractive index detector RID-10A, one Polymer Laboratories PLgel guard column, and two Polymer Laboratories PLgel 5 μm mixed D columns. LiBr (0.1 M) in DMF at 40 °C was used as an eluent (flow rate: 0.80 mL/min). Calibration was performed using near-monodisperse PMMA standards from Polymer Laboratories. DLS was performed on a Malvern Zetasizer Nano-S. SDS-PAGE was performed using Bio-Rad Any kD Mini-PROTEAN-TGX gels.
Polymerization of NIPAAm in the presence of dansyl CTA to afford α-dansyl pNIPAAm
The dansyl CTA (46 mg, 0.095 mmol), NIPAAm (1.4 g, 12.0 mmol), and AIBN (1.6 mg, 0.113 mmol), were added to a Schlenk tube containing a stirring bar, and DMF (1.9 mL) was added to dissolve the solids. Freeze-pump-thaw cycles were repeated five times and the reaction was performed at 70 ºC in an oil bath. The reaction was stopped after 1 h at 81% monomer conversion by cooling with liquid nitrogen and exposing the reaction to atmosphere. Polymer 1 was purified by dialyzing against MeOH (MWCO 3,500 g/mol) giving the α-dansyl pNIPAAm. 1H NMR (600 MHz, MeOD) δ: 8.55 (d), 8.36–8.28 (m), 8.24–8.15 (m), 8.12–7.35 (NH, pNIPAAM), 7.26 (d), 4.60 (s), 4.13–3.77 (bs), 3.43–3.23 (m), 2.86 (s), 2.25–1.80 (CH, pNIPAAm), 1.78–1.45 (CH2, pNIPAAm), 1.26–0.93 (NCH3, pNIPAAm). Mn by 1H NMR was 12,400 g/mol (targeted 12,000 g/mol). Mn(GPC) was 14,200 g/mol and the PDI was 1.02.
In situ aminolysis and disulfide exchange of dansyl pNIPAAm with Aldrithiol® to form polymer 2
Polymer 1 (70 mg, 0.005 mmol) was added to a Schlenk tube equipped with a stirring bar and dissolved in MeOH (0.7 mL). In a second Schlenk tube equipped with a stirring bar, ethanolamine (14 μg, 0.230 μmol) was added. In a third Schlenk tube equipped with a stirring bar, Aldrithiol® (75 mg, 0.340 mmol) was dissolved in MeOH (0.7 mL). All three Schlenk tubes were subjected to five freeze-pump-thaw cycles. The contents of the first Schlenk tube, containing α-dansyl pNIPAAm and MeOH, were then transferred into the second Schlenk tube, containing ethanolamine, using a syringe. The second Schlenk tube was then immersed in an oil bath at 45 ºC and the solution was allowed to stir. After 30 min, the second Schlenk tube was removed from the oil bath and the contents, dansyl pNIPAAm, MeOH, and ethanolamine, were transferred to the third Schlenk tube, containing Aldrithiol and MeOH, via syringe. The third Schlenk tube containing dansyl pNIPAAm, ethanolamine, Aldrithiol® and MeOH was stirred at 20 °C for 12 h. The product was purified by dialyzing against MeOH (MWCO 3,500 g/mol) giving polymer 2. End-group conversion of the trithiocarbonate to a pyridyl disulfide was 81% by 1H-NMR. 1H NMR (600 MHz, MeOD) δ: 8.55 (d, Ar CH), 8.40–8.27 (m, Ar CH), 8.24–8.14 (NH, pNIPAAm), 7.26 (Ar CH), 7.22–7.15 (Ar CH), 3.95 (CH3, pNIPAAm), 3.45–3.20 (CH2CH2), 2.87 (NCH3), 2.34–1.76 (CH, pNIPAAm), 1.76–1.46 (CH2, pNIPAAM), 1.26–1.20 (NCH3, pNIPAAm). Mn (1H NMR) = 12,400 g/mol; Mn (GPC) = 14,200 g/mol; PDI = 1.03.
pNIPAAm conjugation to CP-MVP
A pH 6.5 20 mM 2-(N-morpholino) ethanesulfonic acid) (MES) buffer was degassed with argon gas for 30 min. CP-MVP (0.50 mg, 0.05μmol) in 250 μL buffer was treated with triscarboxylethylphosphine (TCEP) resin for 2 h at 4 °C, then filtered through a 0.22 μm syringe filter. Polymer 2 (6.20 mg, 5.00 μmol) was dissolved in 250 μL of buffer. The filtered CP-MVP solution was added to the solution containing polymer 2. The reaction was allowed to stand at 4 °C for 24 h before purification by ultrafiltration (MWCO 100,000 g/mol) to give the conjugate.
LCST determination
A solution of the polymer, conjugate, or unmodified CP-Vault-pNIPAAm conjugate at a concentration of 1 mg mL−1 in pH 6.5 20 mM MES was placed in a 100 μL quartz cuvette. The cuvette was placed in a Hewlett-Packard HP8453 diode-array UV-Vis spectrophotometer with Peltier temperature control. The following temperature program was used: temperature was elevated at 0.5 °C min−1 and held for 30 s prior to measuring the absorbance at 600 nm. The LCST was determined at 10% of the maximum absorbance.
DLS size determination
A solution of CP-Vault-pNIPAAm conjugate at a concentration of 1 mg mL−1 in pH 6.5, 20 mM MES was placed in a 40 μL disposable cuvette. The cuvette was placed in a Malvern Zetasizer Nano-S. The temperature was allowed to equilibrate for three minutes before beginning the DLS measurement. This process was repeated three times, sizes are reported as an average of three measurements.
Preparation of the TEM sample
A 40 μL aliquot of a 0.2 μg μL−1 solution of CP-Vault-pNIPAAm conjugate was warmed to a set temperature (35 °C or 45 °C or heated to 45 °C then cooled to 4 °C) and incubated for 7 min. 20 μL of each solution was then pipetted onto the surface of a heating block at the set temperature. A carbon-coated EM grid was floated up-side down on the sample to allow the conjugate to adsorb to the grid surface. After 7 min, the grid was removed from the sample and blotted on filter paper to remove excess liquid. The grid was then floated up-side down in 500 μL of a 1% uranyl acetate solution at the set temperatures. After 7 min, the grid was removed from the uranyl acetate solution and blotted on filter paper. TEM was conducted on a JEOL JEM1200-EX transmission electron microscope. Images were taken using a Gatan BioScan 600W 1×1K digital camera and Digital Micrograph acquisition software.
Supplementary Material
Figure 1.
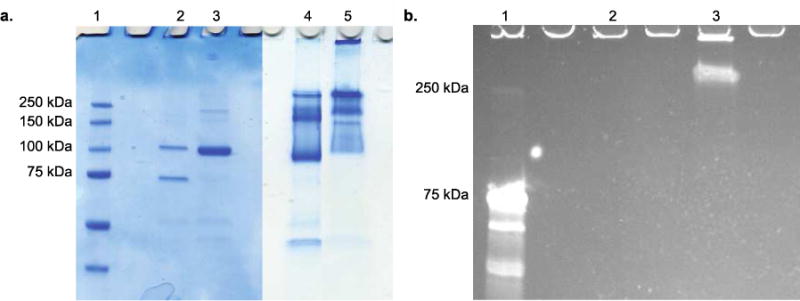
SDS-PAGE of CP-MVP vault and conjugate visualized by a. coomassie blue staining (Lane 1: protein marker; Lane 2: CP-MVP vault reducing conditions; Lane 3: Conjugate reducing conditions; Lane 4: CP-MVP vault non-reducing conditions; Lane 5: Conjugate non-reducing conditions) and b. UV-Vis excitation (Lane 1: protein marker; Lane 2: CP-MVP vault non-reducing conditions; Lane 3: Conjugate non-reducing conditions).
Acknowledgments
This work was funded by the National Institutes of Health (R21 CA 137506-01). N. Matsumoto thanks the National Science Foundation for a Graduate Research Fellowship (DGE-0707424). V. Kickhoefer is thanked for many helpful discussions. H. Roseboro is thanked for expression and purification of the CP-MVP vaults. J. Mrazek is thanked for assistance with EM preparation and imaging.
Footnotes
Full citations of references 39, 44, 45, and 49, synthesis and NMR spectra of the dansyl CTA, polymer NMR spectra and GPC traces, UV-Vis turbidity study of CP-MVP vaults. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Douglas T, Young M. Virus Particles as Templates for Materials Synthesis. Adv Mater. 1999;11:679–681. [Google Scholar]
- 2.Witus LS, Francis MB. Using Synthetically Modified Proteins to Make New Materials. Acc Chem Res. 2011;44:774–783. doi: 10.1021/ar2001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LA, Niu ZW, Wang Q. Viruses and Virus-Like Protein Assemblies-Chemically Programmable Nanoscale Building Blocks. Nano Res. 2009;2:349–364. [Google Scholar]
- 4.Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, Varpness Z, Liepold LO, Young M, Douglas T. Biological Containers: Protein Cages as Multifunctional Nanoplatforms. Adv Mater. 2007;19:1025–1042. [Google Scholar]
- 5.Soto CM, Ratna BR. Virus Hybrids as Nanomaterials for Biotechnology. Curr Opin Biotechnol. 2010;21:426–438. doi: 10.1016/j.copbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Jutz G, Boker A. Bionanoparticles as Functional Macromolecular Building Blocks – a New Class of Nanomaterials. Polymer. 2011;52:211–232. [Google Scholar]
- 7.Steinmetz NF, Evans DJ. Utilisation of Plant Viruses in Bionanotechnology. Org Biomol Chem. 2007;5:2891–2902. doi: 10.1039/b708175h. [DOI] [PubMed] [Google Scholar]
- 8.Endo M, Fujitsuka M, Majima T. Porphyrin Light-Harvesting Arrays Constructed in the Recombinant Tobacco Mosaic Virus Scaffold. Chem-Eur J. 2007;13:8660–8666. doi: 10.1002/chem.200700895. [DOI] [PubMed] [Google Scholar]
- 9.Nam KT, Kim D-W, Yoo PJ, Chiang C-Y, Meethong N, Hammond PT, Chiang Y-M, Belcher AM. Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science. 2006;312:885–888. doi: 10.1126/science.1122716. [DOI] [PubMed] [Google Scholar]
- 10.Comellas-Aragones M, Engelkamp H, Claessen VI, Sommerdijk N, Rowan AE, Christianen PCM, Maan JC, Verduin BJM, Cornelissen JJLM, Nolte RJM. A Virus-Based Single-Enzyme Nanoreactor. Nat Nanotechnol. 2007;2:635–639. doi: 10.1038/nnano.2007.299. [DOI] [PubMed] [Google Scholar]
- 11.Minten IJ, Claessen VI, Blank K, Rowan AE, Nolte RJM, Cornelissen JJLM. Catalytic Capsids: The Art of Confinement. Chem Sci. 2011;2:358–362. [Google Scholar]
- 12.Patterson DP, Prevelige PE, Douglas T. Nanoreactors by Programmed Enzyme Encapsulation inside the Capsid of the Bacteriophage P22. ACS Nano. 2012;6:5000–5009. doi: 10.1021/nn300545z. [DOI] [PubMed] [Google Scholar]
- 13.MaHam A, Tang ZW, Wu H, Wang J, Lin YH. Protein-Based Nanomedicine Platforms for Drug Delivery. Small. 2009;5:1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 14.Manchester M, Singh P. Virus-Based Nanoparticles (Vnps): Platform Technologies for Diagnostic Imaging. Adv Drug Deliv Rev. 2006;58:1505–1522. doi: 10.1016/j.addr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Ma YJ, Nolte RJM, Cornelissen JJLM. Virus-Based Nanocarriers for Drug Delivery. Adv Drug Deliv Rev. 2012;64:811–825. doi: 10.1016/j.addr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Garcea RL, Gissmann L. Virus-Like Particles as Vaccines and Vessels for the Delivery of Small Molecules. Curr Opin Biotechnol. 2004;15:513–517. doi: 10.1016/j.copbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Hybrid Virus-Polymer Materials. 1. Synthesis and Properties of PEG-Decorated Cowpea Mosaic Virus. Biomacromolecules. 2003;4:472–476. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 18.Schlick TL, Ding Z, Kovacs EW, Francis MB. Dual-Surface Modification of the Tobacco Mosaic Virus. J Am Chem Soc. 2005;127:3718–3723. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- 19.O’Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. Pegylation of Adenovirus with Retention of Infectivity and Protection from Neutralizing Antibody in Vitro and in Vivo. Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 20.Sikkema FD, Comellas-Aragones M, Fokkink RG, Verduin BJM, Cornelissen JJLM, Nolte RJM. Monodisperse Polymer-Virus Hybrid Nanoparticles. Org Biomol Chem. 2007;5:54–57. doi: 10.1039/b613890j. [DOI] [PubMed] [Google Scholar]
- 21.Lucon J, Qazi S, Uchida M, Bedwell GJ, LaFrance B, Prevelige PE, Douglas T. Use of the Interior Cavity of the P22 Capsid for Site-Specific Initiation of Atom-Transfer Radical Polymerization with High-Density Cargo Loading. Nat Chem. 2012;4:781–788. doi: 10.1038/nchem.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alarcon CdlH, Pennadam S, Alexander C. Stimuli Responsive Polymers for Biomedical Applications. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 23.Antipina MN, Sukhorukov GB. Remote Control over Guidance and Release Properties of Composite Polyelectrolyte Based Capsules. Adv Drug Deliv Rev. 2011;63:716–729. doi: 10.1016/j.addr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Chen GH, Hoffman AS. Preparation and Properties of Thermoreversible, Phase-Separating Enzyme-Oligo(N-Isopropylacrylamide) Conjugates. Bioconjugate Chem. 1993;4:509–514. doi: 10.1021/bc00024a013. [DOI] [PubMed] [Google Scholar]
- 25.Stayton PS, Shimoboji T, Long C, Chilkoti A, Chen GH, Harris JM, Hoffman AS. Control of Protein-Ligand Recognition Using a Stimuli-Responsive Polymer. Nature. 1995;378:472–474. doi: 10.1038/378472a0. [DOI] [PubMed] [Google Scholar]
- 26.Heredia KL, Bontempo D, Ly T, Byers JT, Halstenberg S, Maynard HD. In Situ Preparation of Protein – “Smart” Polymer Conjugates with Retention of Bioactivity. J Am Chem Soc. 2005;127:16955–16960. doi: 10.1021/ja054482w. [DOI] [PubMed] [Google Scholar]
- 27.Heredia KL, Grover GN, Tao L, Maynard HD. Synthesis of Heterotelechelic Polymers for Conjugation of Two Different Proteins. Macromolecules. 2009;42:2360–2367. doi: 10.1021/ma8022712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Kaddis CS, Loo RRO, Grover GN, Loo JA, Maynard HD. Synthesis of Maleimide-End-Functionalized Star Polymers and Multimeric Protein-Polymer Conjugates. Macromolecules. 2009;42:8028–8033. doi: 10.1021/ma901540p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vazquez-Dorbatt V, Tolstyka ZP, Maynard HD. Synthesis of Aminooxy End-Functionalized pNIPAAm by RAFT Polymerization for Protein and Polysaccharide Conjugation. Macromolecules. 2009;42:7650–7656. doi: 10.1021/ma9013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C-W, Nguyen TH, Maynard HD. Thermoprecipitation of Glutathione S-Transferase by Glutathione-Poly(N-Isopropylacrylamide) Prepared by RAFT Polymerization. Macromol Rapid Commun. 2010;31:1691–1695. doi: 10.1002/marc.201000333. [DOI] [PubMed] [Google Scholar]
- 31.Poderycki MJ, Kickhoefer VA, Kaddis CS, Raval-Fernandes S, Johansson E, Zink JI, Loo JA, Rome LH. The Vault Exterior Shell Is a Dynamic Structure That Allows Incorporation of Vault-Associated Proteins into Its Interior. Biochemistry. 2006;45:12184–12193. doi: 10.1021/bi0610552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedersha NL, Rome LH. Isolation and Characterization of a Novel Ribonucleoprotein Particle – Large Structures Contain a Single Species of Small Rna. J Cell Biol. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedersha NL, Miquel MC, Bittner D, Rome LH. Vaults. 2. Ribonucleoprotein Structures Are Highly Conserved among Higher and Lower Eukaryotes. J Cell Biol. 1990;110:895–901. doi: 10.1083/jcb.110.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suprenant KA. Vault Ribonucleoprotein Particles: Sarcophagi, Gondolas, or Safety Deposit Boxes? Biochemistry. 2002;41:14447–14454. doi: 10.1021/bi026747e. [DOI] [PubMed] [Google Scholar]
- 35.Kong LB, Siva AC, Rome LH, Stewart PL. Structure of the Vault, a Ubiquitous Cellular Component. Struct Fold Des. 1999;7:371–379. doi: 10.1016/s0969-2126(99)80050-1. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Kato K, Yamashita E, Sumizawa T, Zhou Y, Yao M, Iwasaki K, Yoshimura M, Tsukihara T. The Structure of Rat Liver Vault at 3.5 Angstrom Resolution. Science. 2009;323:384–388. doi: 10.1126/science.1164975. [DOI] [PubMed] [Google Scholar]
- 37.Esfandiary R, Kickhoefer VA, Rome LH, Joshi SB, Middaugh CR. Structural Stability of Vault Particles. J Pharm Sci. 2009;98:1376–1386. doi: 10.1002/jps.21508. [DOI] [PubMed] [Google Scholar]
- 38.Stephen AG, Raval-Fernandes S, Huynh T, Torres M, Kickhoefer VA, Rome LH. Assembly of Vault-Like Particles in Insect Cells Expressing Only the Major Vault Protein. J Biol Chem. 2001;276:23217–23220. doi: 10.1074/jbc.C100226200. [DOI] [PubMed] [Google Scholar]
- 39.Kickhoefer VA, Garcia Y, Mikyas Y, Johansson E, Zhou JC, Raval-Fernandes S, Minoofar P, Zink JI, Dunn B, Stewart PL, et al. Engineering of Vault Nanocapsules with Enzymatic and Fluorescent Properties. P Natl Acad Sci USA. 2005;102:4348–4352. doi: 10.1073/pnas.0500929102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kickhoefer VA, Han M, Raval-Fernandes S, Poderycki MJ, Moniz RJ, Vaccari D, Silvestry M, Stewart PL, Kelly KA, Rome LH. Targeting Vault Nanoparticles to Specific Cell Surface Receptors. ACS Nano. 2009;3:27–36. doi: 10.1021/nn800638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buehler DC, Toso DB, Kickhoefer VA, Zhou ZH, Rome LH. Vaults Engineered for Hydrophobic Drug Delivery. Small. 2011;7:1432–1439. doi: 10.1002/smll.201002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldsmith LE, Pupols M, Kickhoefer VA, Rome LH, Monbouquette HG. Utilization of a Protein “Shuttle” to Load Vault Nanocapsules with Gold Probes and Proteins. ACS Nano. 2009;3:3175–3183. doi: 10.1021/nn900555d. [DOI] [PubMed] [Google Scholar]
- 43.Ng BC, Yu M, Gopal A, Rome LH, Monbouquette HG, Tolbert SH. Encapsulation of Semiconducting Polymers in Vault Protein Cages. Nano Lett. 2008;8:3503–3509. doi: 10.1021/nl080537r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champion CI, Kickhoefer VA, Liu GC, Moniz RJ, Freed AS, Bergmann LL, Vaccari D, Raval-Fernandes S, Chan AM, Rome LH, et al. A Vault Nanoparticle Vaccine Induces Protective Mucosal Immunity. Plos One. 2009;4:e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kar UK, Jiang JN, Champion CI, Salehi S, Srivastava M, Sharma S, Rabizadeh S, Niazi K, Kickhoefer V, Rome LH, et al. Vault Nanocapsules as Adjuvants Favor Cell-Mediated over Antibody-Mediated Immune Responses Following Immunization of Mice. Plos One. 2012;7:e38553. doi: 10.1371/journal.pone.0038553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kar UK, Srivastava MK, Andersson A, Baratelli F, Huang M, Kickhoefer VA, Dubinett SM, Rome LH, Sharma S. Novel Ccl21-Vault Nanocapsule Intratumoral Delivery Inhibits Lung Cancer Growth. Plos One. 2011;6:e18758. doi: 10.1371/journal.pone.0018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT Polymerization. Chem Rev. 2009;109:5402–5436. doi: 10.1021/cr9001403. [DOI] [PubMed] [Google Scholar]
- 48.Grover GN, Maynard HD. Protein-Polymer Conjugates: Synthetic Approaches by Controlled Radical Polymerizations and Interesting Applications. Curr Opin Chem Biol. 2010;14:818–827. doi: 10.1016/j.cbpa.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules. 1998;31:5559–5562. [Google Scholar]
- 50.Moad G, Rizzardo E, Thang SH. Living Radical Polymerization by the RAFT Process – a Third Update. Aust J Chem. 2012;65:985–1076. [Google Scholar]
- 51.Bawa P, Pillay V, Choonara YE, Toit LCd. Stimuli-Responsive Polymers and Their Applications in Drug Delivery. Biomed Mater. 2009;4:022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Liu SY. Polymeric Nanocarriers Possessing Thermoresponsive Coronas. Soft Matter. 2008;4:1745–1749. [Google Scholar]
- 53.Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug Targeting Using Thermally Responsive Polymers and Local Hyperthermia. J Control Release. 2001;74:213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 54.Skirtach AG, Javier AM, Kreft O, Kohler K, Alberola AP, Mohwald H, Parak WJ, Sukhorukov GB. Laser-Induced Release of Encapsulated Materials inside Living Cells. Angew Chem-Int Edit. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


