Abstract
Conventional methods for studying paracrine signaling in vitro may not be sensitive to short-range effects resulting from signal dilution or decay. We employ a microfabricated culture substrate to maintain two cell populations in microscale proximity. Individual populations can be quickly retrieved for cell-specific readouts by standard high-throughput assays. We show that this platform is sensitive to short-range interactions that are not detectable by common methods such as conditioned media transfer or porous cell culture inserts, as revealed by gene expression changes in a tumor-stromal crosstalk model. In addition, we are able to detect population-specific gene expression changes that would have been masked in mixed co-culture. We thus demonstrate a tool for investigating an important class of intercellular communication that may be overlooked in conventional biological studies.
Introduction
Paracrine cell-cell signaling can be acutely range-dependent due to mechanisms such as ultrasensitivity in the response to a concentration gradient1 or rapid signal decay, for example by reactive oxygen species.2 In vitro studies of cell-cell signaling often employ compartmentalized culture models instead of mixed co-cultures in order to avoid confounding the readouts from two different cell populations. The most common approaches are conditioned media transfer between populations in separate wells, and the use of porous cell culture inserts that separate two populations by a semi-porous membrane and a distance of about 1 mm. However, these conventional approaches may not be sensitive to short-range paracrine effects.
Previously, we described the use of microfabricated comb substrates for the study of heterotypic cell-cell signaling in liver cultures.3–5 In this system, cells are grown on interdigitating sliding plates that can be positioned such that two populations are either in direct contact or separated by an 80-µm gap (Fig. 1a). While these previous studies focused on the importance of contact-dependent signaling, intriguingly, the data also suggested that cells co-cultured in close proximity displayed enhanced viability compared to cells co-cultured at a greater distance from each other.3,4 It has also been reported that Hedgehog signaling between prostate tumor cells and myofibroblasts was observed only when the populations were cultured in close proximity at a separation of 500 µm by using a microfluidic culture platform.6
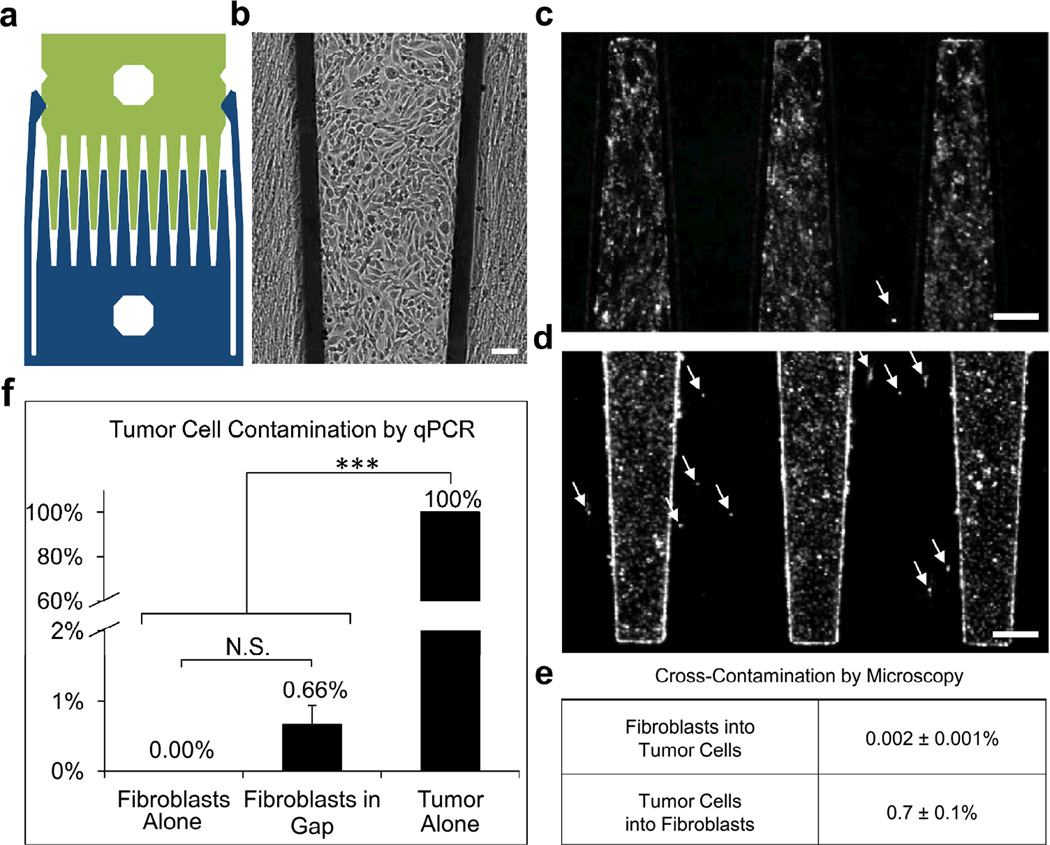
Figure 1.
Comb substrates allow cells to be cultured in close proximity with minimal cross-contamination. (a) Diagram of device with paired combs locked into the gap configuration. (b) Brightfield reflected light image of HT1080 tumor cells (cobblestone cells) and human lung fibroblasts (elongated cells) in gap co-culture after 48 h, scale bar 100 µm. (c–d) Fluorescent images with arrows highlighting (c) a DiI-labeled fibroblast contaminating the tumor population, and (d) DiI-labeled tumor cells contaminating the fibroblast population in gap co-culture, scale bar 250 µm. (e) Quantification of fluorescent images shows minimal cross-contamination. (f) Minimal tumor contamination of the fibroblast population in gap co-culture, as determined by qRT-PCR for the tumor cell marker, TERT, relative to HPRT. Samples displaying statistically significant changes (Student’s t-test) are indicated (*** = p < 0.005) and relevant changes that are not significant are denoted (N.S.). Error bars are SEM.
While these previous studies point to the importance of paracrine signaling range, there has not existed a high-throughput technique to screen for distance-dependent effects. In this report, we combine comb culture substrates with a quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) array to identify gene expression changes resulting from tumor-stromal crosstalk. We compare gene induction on our platform with conventional conditioned media transfer and porous membrane inserts in an effort to discover distance-dependent gene expression patterns.
Results and Discussion
To test our screening approach, we chose to study cellular crosstalk in a model system consisting of HT1080 human fibrosarcoma cells co-cultivated with human lung fibroblasts. This particular pairing was selected because the lung is a common site for fibrosarcoma metastasis.7 Combs were individually plated with pure populations, and then pairs were snapped together to form co-cultures with HT1080s and fibroblasts in close proximity but separated by a gap of 80 µm (Fig. 1b). While the gap prevents cell migration between adjacent fingers, it was still possible that cells could detach and float across the gap. In order to verify that the tumor and fibroblast populations remained pure during gap co-culture, we used fluorescent labeling to track cross-contamination between the two populations (Fig. 1c,d). After 48 h, we measured 0.7% contamination of HT1080s in the fibroblast population, and 0.002% contamination of fibroblasts in the HT1080 population. Contamination of the fibroblast population by HT1080s was additionally verified by qRT-PCR quantification, using telomerase reverse transcriptase (TERT) as a tumor cell marker. TERT levels in lysate collected from the fibroblast combs after 48 h of gap co-culture corresponded to 0.66% contamination by HT1080 cells (Fig. 1f), which correlated well with the fluorescent tracking data.
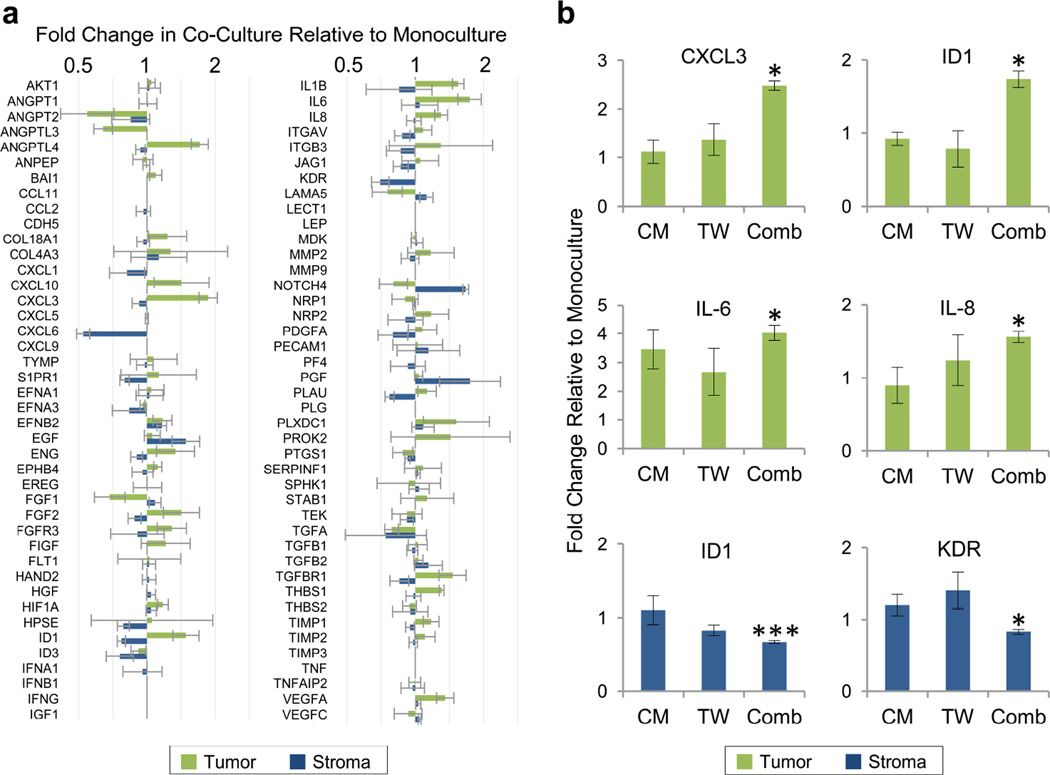
Given the ability to retrieve highly pure populations from gap co-cultures, we next assayed the gene expression changes induced in each population as a result of paracrine crosstalk during co-cultivation. Gap co-cultures were prepared alongside monoculture controls in an identical gap configuration but with a single cell type on both combs. After 48 h, the comb pairs were separated to retrieve pure tumor and fibroblast populations and the cells were immediately lysed off the combs. Following RNA isolation and cDNA generation, the samples were analyzed with a qRT-PCR array to measure the expression of 84 genes associated with tumor progression (Fig. 2a). Genes of interest were identified based on the magnitude and statistical significance of the differences in expression level in co-culture compared to monoculture. The expression of these genes was then quantified by conventional qRT-PCR in order to validate the results of the array. Specifically, we examined the expression of ANGPTL3, ANGPTL4, CXCL3, ID1, IL-1β, IL-6, IL-8, THBS1, and VEGFA in the tumor cells, and the expression of HPSE, ID1, and KDR in the fibroblasts. Although some of these genes of interest proved to be false positives, others were successfully validated by conventional qRT-PCR. In the HT1080s, the expression levels of CXCL3, ID1, IL-6, and IL-8 were significantly increased during gap co-culture relative to monoculture controls, by 2.54-fold, 1.72-fold, 4.38-fold, and 1.56-fold, respectively (Fig. 2b). In the fibroblasts, the expression levels of ID1 and KDR were significantly decreased during gap co-culture relative to monoculture controls, by 0.67-fold and 0.83-fold respectively (Fig. 2b).
Figure 2.
(a) Cell-specific gene expression changes on comb substrates, assayed by qRT-PCR array. Fold change in co- culture relative to monoculture is displayed for both HT1080 tumor cells (green) and lung fibroblasts (blue). (b) Gene induction as a function of cell proximity. Quantification by conventional qRT-PCR of gene expression in conditioned medium (CM), Transwell (TW), and comb gap (Comb) co-cultures. In all conditions, all assayed cells were cultured on comb substrates. Samples displaying statistically significant changes (Student’s t-test) versus monoculture controls are indicated (* = p < 0.05, *** = p < 0.005). Error bars are SEM.
In order to determine whether the close proximity of gap co-culture plays a role in driving the observed changes in gene expression, we repeated the experiment by using conditioned media transfer (CM) and Transwell porous membrane inserts (TW) to achieve paracrine signaling. Here, we focused on the six validated genes from above and used conventional qRT-PCR for quantification. Gene expression in the presence of paracrine crosstalk was normalized to monoculture controls. In order to control for substrate effects, all cells that were assayed for gene expression were cultured on combs. In terms of proximity, cells were most distant in conditioned media transfer, at an intermediate separation in Transwell culture, and closest in gap culture on comb substrates.
A striking proximity effect was observed for CXCL3 and ID1 in HT1080s, with significant induction observed in gap co-culture with lung fibroblasts, but no change in conditioned media or Transwell cultures (Fig. 2b). Likewise, IL-8 expression in HT1080s exhibited greater induction in gap co-culture, but IL-6 expression appeared to be less sensitive to proximity. In the fibroblasts, a weaker proximity effect was observed, with ID1 and KDR moderately inhibited relative to monoculture only in gap co-culture. The conditioned media and Transwell experiments were also repeated on conventional substrates, with similar trends generally observed (Fig. S1, ESI†).
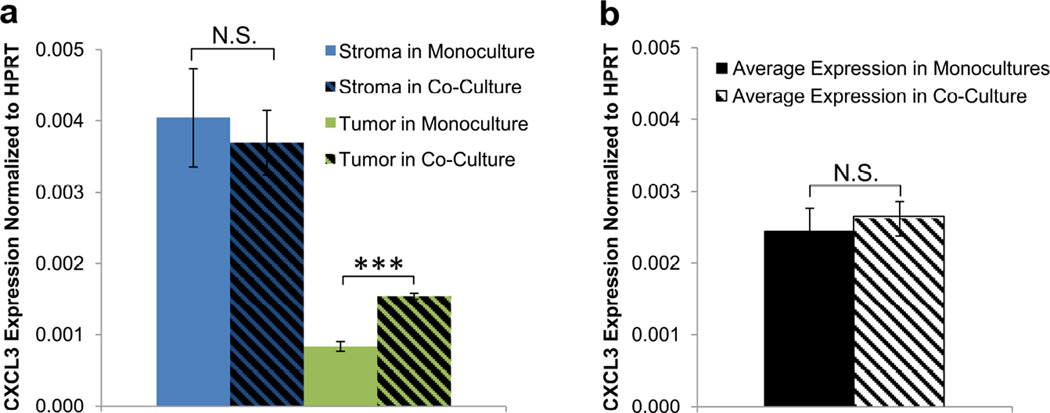
In our model system, we observed nearly 5-fold greater baseline expression of CXCL3 in the stromal cells versus the tumor cells (Fig. 3a). In short-range co-culture, tumor expression of CXCL3 increased by 2.5-fold, but without a cell-specific assay, this strong change would be largely masked by the stromal signal (Fig. 3b). Thus, the ability to retrieve pure populations out of co-culture is critical for enabling certain crosstalk responses to be observed. CXCL3 has been associated with an aggressive metastatic phenotype8,9 and also has been implicated as an autocrine growth factor.10 Therefore, our observations are consistent with stromal induction of tumor progression through both direct and indirect mechanisms: directly by paracrine secretion of CXCL3, and indirectly by stimulation of tumor cells to secrete autocrine CXCL3.
Figure 3.
Masking of cell-specific gene expression changes in mixed co-culture. (a) Baseline CXCL3 expression in fibroblasts (blue) is almost 5 times higher than in HT1080 cells (green). Upon co-culture, fibroblast expression of CXCL3 is unchanged (hatched blue), while HT1080 expression is significantly increased (hatched green). Here, upregulation of CXCL3 in HT1080s is clearly observed due to the cell-specific readout. (b) In a mixed co-culture without cell-specific readouts, the average CXCL3 expression (hatched) would not be significantly different from the average of CXCL3 expression in the two monoculture populations (black), and thus the upregulation in HT1080s would not be detected. Samples displaying statistically significant changes (Student’s t-test) are indicated (*** = p < 0.005) and relevant changes that are not significant are denoted (N.S.). Error bars are SEM.
The array data also indicated strong induction of Notch4 in the fibroblasts, and this result was validated with conventional qRT-PCR (10.5-fold). However, Notch4 expression was 555-fold higher in the HT1080s than in the fibroblasts. Since we measured approximately 1% contamination of HT1080s into fibroblasts, it was possible that the observed increase in Notch4 mRNA in the fibroblast lysate was partially due to tumor contamination. Hence, Notch4 was discarded as a gene of interest. With any cell pairing, it will be important to quantify cross-contamination to guard against false positives.
Unfortunately, we were not able to explore all of the potential genes of interest (such as CXCL6 and PLAU in fibroblasts) due to the limited volume of RNA isolate that could be extracted. Due to the limited surface area of the comb device,5 cells needed to be pooled from up to 18 combs per condition in order to obtain sufficient RNA quantity. Sample volume was a particular issue for the fibroblasts, which yielded less total RNA than the tumor cells. By employing new molecular analysis techniques such as barcoded hybridization (Nanostring nCounter) and RNA sequencing, it will be possible to profile larger numbers of genes from smaller sample volumes.
Conclusions
The comb device enables separation of co-cultures into pure populations, which can then be analyzed by a wide variety of standard approaches. Other methods for obtaining cell-specific readouts have included xenotypic co-cultures with species-specific primers,11 mass spectrometry of proteins from cells labeled with heavy isotopes,12,13 or genetic modification of cells to produce labeled mRNA,14 but these approaches offer less flexibility in terms of cell types and assays. Fluorescence-activated cell sorting can also produce purified populations from a mixed culture, but dissociation into single-cell suspensions is not always possible and signals can degrade during the lengthy process. Due to these disadvantages, along with considerations of cost and difficulty, compartmentalized co-culture techniques such as conditioned media transfer and porous membrane inserts remain heavily utilized in the current literature. As we have demonstrated, however, significant interactions can go undetected by these conventional methods. It is possible that a substantial subset of cell-cell interactions have been previously overlooked due to the limitations of conventional tools in detecting short-range paracrine signaling. Our method provides a useful technique for exploring this class of intercellular communication.
Materials and Methods
Cell Culture
HT1080 fibrosarcoma cells were obtained as a gift from Dr. Eric Stanbridge and were cultured in DMEM (Genesee, San Diego, CA) supplemented with 10% fetal bovine serum (FBS) (Fisher, Pittsburg, PA) and 1% Penicillin-Streptomycin (Genesee) and used between passages 80 and 90. Normal human lung fibroblasts (Lonza, Basel, Switzerland) were cultured in M199 (Genesee) with the same supplements and used between passages 13 and 14.
Comb Preparation and Co-culture
Comb substrates were microfabricated and prepared as previously described3 in the Integrated Nanosystems Research Facility at the University of California, Irvine. Briefly, combs were etched out of a silicon wafer, coated with polystyrene (Polysciences, Inc., Warrington, PA), and plasma-treated. Immediately before use, combs were sterilized in 70% EtOH (Fisher), washed in sterile phosphate buffered saline (PBS), and coated with 1% gelatin (Sigma Aldrich, St. Luis, MO) for 30 minutes. Tumor cells (4 × 105 in 1 mL culture media) and fibroblasts (1 × 105 in 1 mL culture media) were seeded separately onto comb pairs locked together in contact in 12-well plates. After 6 h, the combs were moved to a new well with fresh culture media and separated to the gap configuration. After an additional 6 h, the cells from the back portion of the comb were removed by a cell scraper (Fisher) to leave only the cells adhered to the comb teeth. The comb pairs were gently dipped twice in PBS, incubated in a new well with fresh media for 3 h, and then again dipped twice in fresh PBS in order to eliminate loose or detached cells and minimize contamination between paired combs. Combs were then paired and interlocked with either a comb plated with the same cell type (comb monoculture) or a comb with the opposing cell type (comb co-culture) in 2 mL of EGM-2 (Lonza), with a separation of 80 µm between the combs. As previously described, the comb device includes a self-alignment mechanism that enables a gap of 79±1 µm to be repeatedly achieved by simple manual manipulation.5 After 48 h, comb pairs were decoupled, quickly washed in PBS, and grouped with other combs from the same experimental condition. Cells were then lysed off the combs and collected. Material from 18 combs (for fibroblasts) or 12 combs (for tumor cells) was used for each condition of each replicate experiment. To prepare combs for reuse, they were cleaned in 10% bleach, then toluene (Fisher), and then Nanostrip (Cyantek, Fremont, CA). The stripped combs were then recoated with polystyrene and plasma-treated.
Cross-Contamination Tests
Combs were prepared as described for comb co-culture. After the cell-scraping step, half of the combs were incubated in DiI (Invitrogen, Grand Island, NY) for 3 h for cell labeling. Labeled combs were paired with unlabeled combs containing the opposing cell type for 48 h. Fluorescence and brightfield reflective light imaging was performed every 24 h using an upright Olympus U-TVO.63XC microscope to measure cross-contamination of the stained cells. Stained cells appearing on the unstained comb were counted, and this number was divided by the total number of cells on the unstained comb to calculate percent cross-contamination.
Conventional qRT-PCR Assays
RNA was isolated using a Qiagen RNeasy kit (Valencia, CA) and cDNA was generated using the Biorad Iscript cDNA Synthesis system (Hercules, CA). Primers were synthesized by IDT (San Diego, CA). Primer sequences are reported in Table S2, ESI†. Gene expression levels were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT).
qRT-PCR Array
RNA was isolated and cDNA was prepared as described above. Samples were added to the Human Angiogenesis RT2 Profiler PCR array (Qiagen) and qRT-PCR was performed. Gene expression levels were normalized to the geometric mean of the 6 housekeeper genes on the array.
Conditioned Medium Co-culture
For experiments on comb substrates, conditioned medium was collected from fibroblasts (1.5 × 105 cells per comb pair) or tumor cells (8 × 105 cells per comb pair) plated onto paired combs. For experiments performed under standard conditions, conditioned medium was collected from fibroblasts (1.5 × 105 cells per well) or tumor cells (3 × 105 cells per well) cultured for 48 h directly in a well of a 12-well plate in 2 mL of EGM-2. The target cells to be cultured in this conditioned media were plated on comb substrates as described above for comb co-cultures, or directly in 12-well plates with 7.5 × 104 cells per well for fibroblasts and 1 × 105 cells per well for tumor cells. After 48 h of culture in conditioned medium (2 mL of per well), the cells were washed and cell lysate was collected. Monoculture controls were set up identically but received fresh medium in place of conditioned medium. Cell material from 18 combs (for fibroblasts) or 12 combs (for tumor cells) was used for each condition of each replicate experiment. For conventional cultures without combs, cell material was pooled from 2 fibroblast wells or 1 tumor well for each condition of each replicate experiment.
Transwell Co-culture
5 × 104 fibroblasts or 5 × 104 tumor cells were seeded in each 8 µm Transwell insert (Millipore, Billerica, MA) in 12-well plates with 2 mL of medium per well. Target cells were plated on comb substrates as described above, or directly in 12-well plates at 7.5 × 104 cells per well for fibroblasts and 1 × 105 cells per well for tumor cells. The Transwell inserts were transferred to wells containing appropriate target cells and fresh EGM-2 medium (2 mL). Monoculture controls were set up identically but did not receive Transwell inserts. After 48 h of culture, the Transwell inserts were removed, the target cells were washed, and cell lysate was collected. Cell material from 18 combs (for fibroblasts) or 12 combs (for tumor cells) was used for each condition of each replicate experiment. For conventional cultures without combs, cell material was pooled from 2 fibroblast wells or 1 tumor well for each condition of each replicate experiment.
Supplementary Material
Insight, innovation, integration.
Short-range paracrine signaling may be important in vivo but overlooked in cell culture studies because of the limitations of conventional tools. Through the use of a microfabricated device, we compare paracrine signaling at length scales of 100 µm to 10 mm and find evidence of sharply range-dependent effects. Importantly, some responses require microscale proximity between the interacting cells and can be detected by our platform but not by conventional methods.
Acknowledgements
This work was supported in part by the Chao Family Comprehensive Cancer Center through an NCI Center Grant (P30A062203). E. E. H. was supported by the American Cancer Society (ACS/IRG 98-279-07). K. H. S. received fellowship support from the NSF (GRFP) and the NIH (T32-HD060555). C. C. W. H. is supported by NIH grant RO1HL60067.
Footnotes
Electronic Supplementary Information (ESI) available: (S1) Cell-cell proximity experiments performed with CM and TW on conventional substrates; (S2) Primer sequences for qRT-PCR. See DOI: 10.1039/b000000x/
Notes and references
- 1.Melen GJ, Levy S, Barkai N, Shilo BZ. Mol. Syst. Biol. 2005;1:1–11. doi: 10.1038/msb4100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winterbourn CC. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 3.Hui EE, Bhatia SN. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui EE, Li C, Agrawal A, Bhatia SN. J. Microelectromech. Syst. 2013 doi: 10.1109/JMEMS.2013.2278813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domenech M, Bjerregaard R, Bushman W, Beebe DJ. Integr. Bio. 2012;4:142–152. doi: 10.1039/c1ib00104c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto N, Yang M, Jiang P, Tsuchiya H, Tomita K, Moossa AR, Hoffman RM. Clin. Exp. Metastasis. 2003;20:181–185. doi: 10.1023/a:1022662927574. [DOI] [PubMed] [Google Scholar]
- 8.Egnl T, Relja B, Blumenberg C, Muller I, Ringel EM, Beecken W-D, Jonas D, Blaheta RA. Life Sci. 2006;78:1784–1793. doi: 10.1016/j.lfs.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Corre LL, Guinebretière J-M, Burlinchon S, Lidereau R, Lazennec G. Endocr. Relat. Cancer. 2007;14:1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 10.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Int. J. Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier NP, Soufi B, Walkowicz WE, Pedicord VA, Mavrakis KJ, Macek B, Gin DY, Sander C, Miller ML. Nat. Methods. 2013;10:768–773. doi: 10.1038/nmeth.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MR, Robinson KJ, Cleary MD, Doe CQ. Nat. Methods. 2009;6:439–44. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.