Abstract
Background
Perinatally HIV-infected adolescents may be susceptible to aggregate atherosclerotic cardiovascular disease (CVD) risk, as measured by the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) coronary arteries (CA) and abdominal aorta (AA) risk scores, due to prolonged exposure to HIV and antiretroviral therapy.
Methods and Results
CA and AA PDAY scores were calculated for 165 perinatally HIV-infected adolescents, using a weighted combination of modifiable risk factors: dyslipidemia, cigarette smoking, hypertension, obesity, and hyperglycemia. Demographic and HIV-specific predictors of scores ≥ 1 were identified and trends in scores over time were assessed. 48% and 24% of the perinatally HIV-infected adolescents had CA and AA scores ≥ 1, representing increased CVD risk factor burden. Significant predictors of CA scores ≥ 1 included male sex, history of an AIDS-defining condition, longer duration of use of a ritonavir-boosted protease inhibitor, and no prior use of tenofovir. Significant predictors of AA scores ≥ 1 included suppressed viral load, history of an AIDS-defining condition, and longer duration of boosted protease inhibitor use. No significant changes in CA and AA risk scores were observed over the 4-year study period.
Conclusions
A substantial proportion of perinatally HIV-infected youth have high PDAY scores reflecting increased aggregate atherosclerotic CVD risk factor burden. High scores were predicted by HIV disease severity and boosted protease inhibitor use. PDAY scores may be useful in identifying high-risk youth who may benefit from early lifestyle or clinical interventions.
Keywords: Perinatally HIV-infected adolescents, cardiovascular disease, boosted protease inhibitor use
Introduction
Advances in diagnosis and treatment of perinatal HIV infection in the United States have significantly improved life expectancy.1,2 However, prolonged exposure to HIV and highly active antiretroviral therapy (HAART) has been associated with long-term complications. Atherosclerotic cardiovascular disease (CVD) risk factors, including hyperlipidemia, lipodystrophy, diabetes, and hypertension have increased in prevalence and severity with the advent of HAART.3–15 These CVD risk factors have been studied in adults,3–6 with expanding research in children.7–15 Potential contributors to CVD risk and clinical CVD events in adult HIV-infected populations include traditional risk factors such as male sex and life style habits (e.g., exercise, diet, and smoking), as well as HIV-specific risk factors, particularly exposure to HAART and specific antiretroviral drugs including protease inhibitors, such as indinavir and lopinavir/ritonavir, and nucleoside reverse transcriptase inhibitors, such as stavudine, didanosine, and abacavir.3–6 In children, protease inhibitors have been primarily associated with CVD risk factors, although some studies note hypercholesterolemia and lipodystrophy with nucleoside reverse transcriptase inhibitors, particularly stavudine.7–15
The atherosclerotic disease process begins at an early age with severity and progression into adulthood associated with established CVD risk factors.16–19 Therefore, CVD risk should be tracked from childhood on. To identify and motivate high-risk children who may benefit from early interventions, it is clinically useful to obtain a measure of aggregate CVD risk by utilizing a scoring system that sums the risks associated with each individual risk factor.20 The Framingham Risk Calculator is among the most widely accepted instruments in predicting the 10-year overall risk of coronary heart disease based on a simple, validated algorithm that includes demographics, lifestyle behaviors, blood pressure, and lipid testing.21 However, this risk score has not been validated for an adolescent population. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) scoring system may be useful for measuring long-term CVD risk in youth and young adults by identifying those with current advanced atherosclerosis.18
Developed using autopsy data from over 1100 15–34 year-olds who died of external causes, the PDAY scoring system estimates the risk of currently having an advanced atherosclerotic lesion in the coronary arteries (CA) or the abdominal aorta (AA) relative to an individual of the same age and sex without any CVD risk factors.18 The CA risk score predicts coronary artery calcium and carotid artery intima-media thickness measures of atherosclerosis in a number of population-based cohorts.22–24 The risk factors included in the CA and AA scores are also associated with non-invasive measures of atherosclerosis among HIV-infected children.25,26 Thus, as a validated measure of the atherosclerotic disease process, we utilized PDAY scores to describe CVD risk in perinatally HIV-infected adolescents enrolled in the Pediatric HIV/AIDS Cohort Study. We additionally identified demographic and HIV-specific predictors of high scores and evaluated trends in scores over the 4-year study follow-up period.
Methods
The Adolescent Master Protocol of the Pediatric HIV/AIDS Cohort Study was designed to evaluate the impact of HIV-infection and antiretroviral therapy on youth with perinatal HIV-infection. Between March 2007 and December 2009, 451 infected and 227 uninfected youth from 15 study sites in the United States were enrolled if they were born to HIV-infected mothers, were 7 up to 16 years of age, and had complete medical history of antiretroviral therapy use, plasma HIV RNA (viral load) concentrations, and lymphocyte subset measurements since birth.
The Adolescent Master Protocol was approved by the institutional review board at the Harvard School of Public Health and at each participating site. Written informed consent was obtained from each participant’s parent or legal guardian. Assent was obtained from child participants according to local institutional review board guidelines. Of the 451 infected participants, 225 were in the validated age range for the PDAY score (≥ 15 years) as of April 1, 2012, and 165 had the clinical measures needed to calculate the PDAY score for at least one time point (Supplemental Table 1).
CA and AA risk scores were calculated at each annual visit after 15 years of age with available clinical data, by summing the scores associated with modifiable measures of atherosclerotic risk including lipids, glucose, smoking, blood pressure, and obesity defined by body mass index (BMI, kg/m2).18 In the PDAY study, CA and AA risk scores ≥1 were shown to indicate at least an 18% (95% confidence interval (CI): 14–22%) or 29% (95% CI: 23%–35%) increased odds of currently having an advanced atherosclerotic lesion in the CA or AA respectively, relative to a score of 0.18
Fasting serum and plasma were drawn annually to measure levels of total and high-density lipoprotein (HDL) cholesterol, and glucose, respectively. Hyperglycemia was defined as a fasting plasma glucose concentration ≥126 mg/dL, a diagnosis of diabetes, or reporting medication used to treat diabetes. The serum thiocyanate measure of smoking used in the development of the PDAY scores closely correlated with smoking an average of one pack per day (i.e. heavy smoking).27 Smoking an average of at least one pack per day in the past 3 months was collected using an Audio Computer Assisted Self-Interview. Hypertension was defined as an average systolic and/or diastolic blood pressure that was ≥95th percentile for age, sex, and height at three or more clinic visits.28
Demographic characteristics considered as potential predictors of CA and AA scores included age, sex, and race/ethnicity. HIV-specific clinical characteristics included measures of disease severity: CD4 count, HIV viral load, and Centers for Disease Control and Prevention (CDC) clinical classification for HIV disease: N/A: not/mildly symptomatic, B: moderately symptomatic, C: severely symptomatic (i.e. meet the case definition for acquired immunodeficiency syndrome (AIDS));29 and antiretroviral therapy previously associated with CVD risk: HAART, any ritonavir-boosted protease inhibitor, lopinavir/ritonavir, indinavir, didanosine, abacavir, stavudine, zidovudine, lamivudine, and tenofovir disoproxil fumarate.3–15 All characteristics were collected either through self-report (e.g., race/ethnicity) or medical chart abstraction (e.g., immunological, virological, and antiretroviral characteristics). Current measures were those closest to the most recent visit at which a PDAY score could be calculated within 90 days before to 7 days after the visit. Nadir CD4 count and peak viral load were defined as the lowest CD4 count and the highest viral load documented prior to or at the most recent visit with a PDAY score. HAART was defined as concomitant use of at least three drugs from at least two classes of antiretrovirals. Antiretroviral use was classified as use at the most recent visit (i.e., current use), prior or ever use, and cumulative duration of use up to and including the most recent visit with a PDAY score.
Statistical analyses were conducted separately for CA and AA scores. Demographic and HIV-specific characteristics were compared among children with low scores (≤ 0) and high scores (≥ 1) at their most recent visit using the Wilcoxon Rank Sum test or the Fisher’s Exact test as appropriate. The cut-off for low versus high scores was based on the distribution of scores in the study population and was consistent with definitions of low risk utilized in previous studies.22–24 To identify significant predictors of a high risk score, univariable predictors of a high score at the p<0.10 level were included in a multivariable logistic regression model. Among highly correlated univariable predictors, the predictor with the highest univariable c-statistic (c) was included in the final multivariable model. Sensitivity analyses were conducted restricting the study population to virologically-suppressed (HIV viral load ≤ 400 copies/mL) participants. Mixed-effects models with time as the independent variable and an assumed compound symmetry correlation structure were used to describe changes in CA and AA scores over the study follow-up period, starting with the baseline visit and including all study visits at which a PDAY score could be calculated. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary NC).
Results
The 165 perinatally HIV-infected adolescents in the study population were 15–19 years old (median age: 16.7 years) at their most recent visit with a PDAY score (Table 1). Forty-nine percent were male. Sixty-eight percent self-identified as black and 24% as Hispanic. At their most recent visit with a PDAY score, the majority (57%) of the adolescents had CD4 counts >500 cells/mm3, but a large proportion (41%) had a history of immunosuppression as evidenced by a low nadir CD4 count (<200 cells/mm3). A similar pattern of disease severity over time was observed with HIV viral load where 59% of the study population was virologically suppressed (≤400 copies/mL) at their most recent visit, but 68% had a history of viral load >100,000 copies/mL. Almost all of the adolescents had prior HAART exposure (97%). Seventy-four percent had been exposed to a boosted protease inhibitor regimen, with 58% currently on a boosted protease inhibitor as of their most recent visit.
Table 1.
Demographic and clinical characteristics of the 165 perinatally HIV-infected children in the study population.
| Characteristic | Total (n=165) |
|---|---|
| Current† age, years, median (q1, q3) | 16.7 (15.9, 17.8) |
| Female, n (%) | 84 (51%) |
| Race/Ethnicity, n (%) | |
| White/Other | 12 (7%) |
| Black | 113 (68%) |
| Hispanic | 39 (24%) |
| Missing | 1 (1%) |
| Current† CD4 count, cells/mm3, n (%) | |
| <200 | 19 (12%) |
| 200–500 | 46 (28%) |
| >500 | 94 (57%) |
| Missing | 6 (4%) |
| Nadir CD4 count, cells/mm3, n (%) | |
| <200 | 68 (41%) |
| 200–500 | 81 (49%) |
| >500 | 16 (10%) |
| Current† HIV viral load, copies/mL, n (%) | |
| ≤ 400 | 98 (59%) |
| 401–5,000 | 25 (15%) |
| >5,000 | 35 (21%) |
| Missing | 7 (4%) |
| Peak HIV viral load, copies/mL, n (%) | |
| <10,000 | 7 (4%) |
| 10,000–100,000 | 45 (27%) |
| >100,000 | 113 (68%) |
| Current† CDC category, n (%) | |
| N/A | 59 (36%) |
| B | 55 (33%) |
| C | 51 (31%) |
| HAART | |
| Current† use, n (%) | 143 (87%) |
| Ever use, n (%) | 160 (97%) |
| Cumulative duration of use, years, median (q1, q3) | 11.0 (7.7, 12.5) |
| Boosted protease inhibitor | |
| Current† use, n (%) | 95 (58%) |
| Ever use, n (%) | 122 (74%) |
| Cumulative duration of use, years, median (q1, q3) | 4.0 (0.0, 7.4) |
| Lopinavir/ritonavir | |
| Current† use, n (%) | 48 (29%) |
| Ever use, n (%) | 95 (58%) |
| Cumulative duration of use, years, median (q1, q3) | 1.4 (0.0, 4.8) |
| Indinavir | |
| Current† use, n (%) | 0 (0%) |
| Ever use, n (%) | 18 (11%) |
| Cumulative duration of use, years, median (q1, q3) | 0.0 (0.0, 0.0) |
| Didanosine | |
| Current† use, n (%) | 17 (10%) |
| Ever use, n (%) | 138 (84%) |
| Cumulative duration of use, years, median (q1, q3) | 3.3 (1.0, 7.1) |
| Abacavir | |
| Current† use, n (%) | 46 (28%) |
| Ever use, n (%) | 79 (48%) |
| Cumulative duration of use, years, median (q1, q3) | 0.0 (0.0, 3.3) |
| Stavudine | |
| Current† use, n (%) | 11 (7%) |
| Ever use, n (%) | 136 (82%) |
| Cumulative duration of use, years, median (q1, q3) | 6.1 (1.8, 8.5) |
| Zidovudine | |
| Current† use, n (%) | 18 (11%) |
| Ever use, n (%) | 145 (88%) |
| Cumulative duration of use, years, median (q1, q3) | 3.7 (1.6, 7.4) |
| Lamivudine | |
| Current† use, n (%) | 53 (32%) |
| Ever use, n (%) | 149 (90%) |
| Cumulative duration of use, years, median (q1, q3) | 5.7 (2.8, 9.4) |
| Tenofovir disoproxil fumarate | |
| Current† use, n (%) | 80 (48%) |
| Ever use, n (%) | 100 (61%) |
| Cumulative duration of use, years, median (q1, q3) | 0.9 (0.0, 3.2) |
Current defined as measurement at most recent visit.
CDC: Centers for Disease Control and Prevention (N/A: not/mildly symptomatic, B: moderately symptomatic, C: severely symptomatic (AIDS definition)), HAART: highly active antiretroviral therapy.
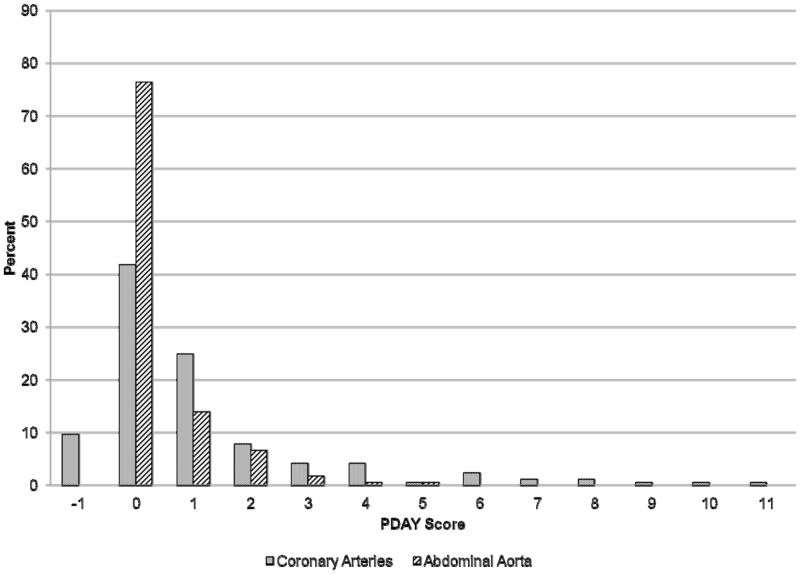
Figure 1 shows the distribution of most recent CA and AA scores in the study population. Forty-eight percent had CA scores ≥ 1 with 24%, 16%, and 12% having CA scores ≥ 2, ≥ 3, and ≥ 4, respectively. High CA scores were primarily attributed to increased levels of non-HDL cholesterol, with additional risk from low levels of HDL cholesterol and high BMI (Table 2, Supplemental Table 2). Few adolescents were defined as hypertensive (1%) or hyperglycemic (1%), and none were defined as heavy smokers. Two adolescents with CA scores ≥ 10 had elevated levels of non-HDL cholesterol in the range of 160–189 mg/dL and were obese (BMI >30 kg/m2). Twenty-four percent of the study population had AA scores ≥ 1. Ten percent, 3%, and 1% had AA scores ≥ 2, ≥ 3, and ≥ 4, respectively. High AA scores were also primarily attributable to dyslipidemia.
Figure 1.
Distribution of Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores.
Table 2.
Distribution of modifiable atherosclerotic risk factors included in the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) scoring system among the 165 perinatally HIV-infected children in the study population at their most recent visit with a PDAY score.
| Risk Factor | Coronary Arteries Points* | Abdominal Aorta Points* | n (%) |
|---|---|---|---|
| Modifiable risk factors | |||
| Non-HDL cholesterol, mg/dL | |||
| <130 | 0 | 0 | 128 (78) |
| 130–159 | 2 | 1 | 24 (14) |
| 160–189 | 4 | 2 | 12 (7) |
| 190–219 | 6 | 3 | 1 (1) |
| ≥220 | 8 | 4 | 0 (0) |
| HDL cholesterol, mg/dL | |||
| <40 | 1 | 0 | 48 (29) |
| 40–59 | 0 | 0 | 96 (58) |
| ≥ 60 | −1 | 0 | 21 (13) |
| Heavy smoking† | |||
| No | 0 | 0 | 165 (100) |
| Yes | 1 | 4 | 0 (0) |
| Blood pressure‡ | |||
| Not hypertensive | 0 | 0 | 163 (99) |
| Hypertensive | 4 | 3 | 2 (1) |
| Obesity (BMI), kg/m2 | |||
| Male | |||
| ≤30 | 0 | 0 | 74 (45) |
| >30 | 6 | 0 | 7 (4) |
| Female | |||
| ≤30 | 0 | 0 | 70 (42) |
| >30 | 0 | 0 | 14 (9) |
| Glycemia§ | |||
| Not hyperglycemic | 0 | 0 | 163 (99) |
| Hyperglycemic | 5 | 3 | 2 (1) |
Adapted from McMahan et al. 2005.18 Copyright © 2005, American Medical Association. All rights reserved.
Heavy smoking was defined as smoking an average of at least one pack/day in the past 3 months.
Hypertensive was defined as an average systolic and/or diastolic blood pressure ≥ 95th percentile for age, sex, and height at three or more visits.
Hyperglycemic was defined as a fasting plasma glucose concentration ≥126 mg/dL, a diagnosis of diabetes, or using diabetes medication.
HDL: high-density lipoprotein, BMI: body mass index.
Univariable predictors of CA scores ≥ 1 versus ≤ 0 at the p<0.10 level included male sex, CDC category C disease (AIDS-defining conditions), current use of a boosted protease inhibitor, prior use of a boosted protease inhibitor, longer cumulative duration of boosted protease inhibitor use, current use of lopinavir/ritonavir, prior use of lopinavir/ritonavir, and no prior use of tenofovir (Table 3, Supplemental Table 3). Current, ever, and duration of boosted protease inhibitor use and current and ever use of lopinavir/ritonavir are highly correlated. Thus, the one with the highest c-statistic was entered into the final multivariable model. Male sex, CDC category C disease (relative to category B), longer cumulative duration of boosted protease inhibitor use, and no prior use of tenofovir were all significant predictors of CA scores ≥ 1 at p<0.05 with a final model c-statistic of 0.74 (Table 4). Results were consistent among the virologically-suppressed subset of the population (Supplemental Table 4).
Table 3.
Univariable predictors of Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores among the 165 perinatally HIV-infected children in the study population at their most recent visit.
| Characteristic | Coronary Arteries PDAY Score | Abdominal Aorta PDAY Score | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 0 (n=85) | ≥ 1 (n=80) | P* | C- statistic | 0 (n=126) | ≥1 (n=39) | P* | C-statistic | |
| Female, n (%) | 50 (59%) | 34 (43%) | 0.04 | 0.58 | NS | |||
| Current† HIV viral load, copies/mL, n (%) | 0.01 | 0.64 | ||||||
| ≤400 | NS | 68 (54%) | 30 (77%) | |||||
| 401–5,000 | 24 (19%) | 1 (3%) | ||||||
| >5,000 | 29 (23%) | 6 (15%) | ||||||
| Missing | 5 (4%) | 2 (5%) | ||||||
| Current† CDC category, n (%) | 0.05 | 0.60 | 0.06 | 0.61 | ||||
| N/A | 33 (39%) | 26 (33%) | 47 (37%) | 12 (31%) | ||||
| B | 33 (39%) | 22 (28%) | 46 (37%) | 9 (23%) | ||||
| C | 19 (22%) | 32 (40%) | 33 (26%) | 18 (46%) | ||||
| HAART | ||||||||
| Current† use, n (%) | NS | 105 (83%) | 38 (97%) | 0.03 | 0.57 | |||
| Boosted protease inhibitor | ||||||||
| Current† use, n (%) | 41 (48%) | 54 (68%) | 0.02 | 0.60 | 63 (50%) | 32 (82%) | <.01 | 0.66 |
| Ever use, n (%) | 56 (66%) | 66 (83%) | 0.02 | 0.58 | 88 (70%) | 34 (87%) | 0.04 | 0.59 |
| Cumulative duration of use, years, median (q1, q3) | 2.8 (0.0, 7.5) | 4.8 (1.3, 7.3) | 0.09 | 0.63 | 3.2 (0.0, 7.3) | 06.0 (4.5, 8.2) | <.01 | 0.71 |
| Lopinavir/ritonavir | ||||||||
| Current† use, n (%) | 18 (21%) | 30 (38%) | 0.03 | 0.58 | 26 (21%) | 22 (56%) | <.01 | 0.68 |
| Ever use, n (%) | 42 (49%) | 53 (66%) | 0.04 | 0.58 | 66 (52%) | 29 (74%) | 0.02 | 0.61 |
| Cumulative duration of use, years, median (q1, q3) | NS | 0.4 (0.0, 4.1) | 4.5 (0.0, 6.8) | <.01 | 0.66 | |||
| Abacavir | ||||||||
| Current† use, n (%) | NS | 29 (23%) | 17 (44%) | 0.02 | 0.60 | |||
| Lamivudine | ||||||||
| Current† use, n (%) | NS | 35 (28%) | 18 (46%) | 0.05 | 0.59 | |||
| Tenofovir disoproxil fumarate | ||||||||
| Ever use, n (%) | 58 (68%) | 42 (53%) | 0.06 | 0.58 | 81 (64%) | 19 (49%) | 0.09 | 0.58 |
Wilcoxon Test p-value for continuous variables, and Fisher’s Exact Test p-value for categorical variables.
Current defined as measurement at most recent visit.
CDC: Centers for Disease Control and Prevention (N/A: not/mildly symptomatic, B: moderately symptomatic, C: severely symptomatic (AIDS definition)), HAART: highly active antiretroviral therapy, NS: Not significant at p<0.10.
Table 4.
Predictors of coronary arteries Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores ≥1 versus ≤ 0 among the 165 perinatally HIV-infected children in the study population at their most recent visit.
| Characteristic | Multivariable Odds Ratio (95% CI) | P |
|---|---|---|
| Sex | 0.01 | |
| Male | 2.47 (1.24, 4.92) | |
| Female | Reference | |
| Current*,† CDC category | 0.05 | |
| N/A | Reference | |
| B | 0.67 (0.30, 1.51) | |
| C | 1.97 (0.84, 4.60) | |
| Cumulative duration of boosted protease inhibitor use, years | 0.04 | |
| 0 | Reference | |
| 1–4 | 1.80 (0.70, 4.64) | |
| 5–8 | 3.97 (1.54, 10.24) | |
| >8 | 1.75 (0.65, 4.71) | |
| Ever tenofovir disoproxil fumarate use | 0.02 | |
| Yes | 0.43 (0.22, 0.87) | |
| No | Reference |
Current defined as measurement at most recent visit.
A significant association was seen for current CDC category C relative to category B -multivariable odds ratio (95% CI): 2.94 (1.23, 7.05).
CDC: Centers for Disease Control and Prevention (N/A: not/mildly symptomatic, B: moderately symptomatic, C: severely symptomatic (AIDS definition)).
Univariable predictors of AA scores ≥ 1 versus 0 at the p<0.10 level included current HIV viral load ≤ 400 copies/mL, CDC category C disease, current HAART use, current and prior use of a boosted protease inhibitor, a longer cumulative duration of boosted protease inhibitor use, current and prior use of lopinavir/ritonavir, a longer cumulative duration of lopinavir/ritonavir use, current abacavir use, current lamivudine use, and no prior tenofovir use (Table 3). In the final model, current HIV virologic suppression (≤ 400 copies/mL versus 401–5,000 copies/mL), CDC clinical category C disease (relative to category B), and longer cumulative duration of boosted protease inhibitor use significantly predicted AA scores ≥ 1 at p<0.05 with a total model c-statistic of 0.84 (Table 5). Results of sensitivity analyses were consistent with those observed in the overall population (Supplemental Table 5).
Table 5.
Predictors of abdominal aorta Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores ≥ 1 versus 0 among the 165 perinatally HIV-infected children in the study population at their most recent visit.
| Characteristic | Multivariable Odds Ratio (95% CI) | P |
|---|---|---|
| Current* HIV viral load, copies/mL | 0.04 | |
| ≤400 | Reference | |
| 401–5,000 | 0.08 (0.01, 0.67) | |
| >5,000 | 0.45 (0.14, 1.40) | |
| Current*,† CDC category | 0.04 | |
| N/A | Reference | |
| B | 0.30 (0.09, 1.02) | |
| C | 1.37 (0.49, 3.82) | |
| Cumulative duration of boosted protease inhibitor use, years | <0.01 | |
| 0 | Reference | |
| 1–4 | 0.70 (0.15, 3.28) | |
| 5–8 | 5.85 (1.62, 21.17) | |
| >8 | 3.82 (0.92, 15.78) | |
| Current* abacavir use | 0.30 | |
| Yes | 1.77 (0.60, 5.27) | |
| No | Reference | |
| Current* lamivudine use | 0.21 | |
| Yes | 2.19 (0.65, 7.43) | |
| No | Reference | |
| Ever tenofovir disoproxil fumarate use | 0.57 | |
| Yes | 0.75 (0.28, 2.03) | |
| No | Reference |
Current defined as measurement at most recent visit.
A significant association was seen for current CDC category C relative to category B -multivariable odds ratio (95% CI): 4.59 (1.42, 14.78).
CDC: Centers for Disease Control and Prevention (N/A: not/mildly symptomatic, B: moderately symptomatic, C: severely symptomatic (AIDS definition)).
Figure 2 shows the mean PDAY scores during the 4 year study follow-up period (mean follow-up of study population: 2.5 years). The slope of the CA scores was 0.10 per year and did not significantly change over time (95% CI: −0.05, 0.24, p=0.19). AA scores were also stable over time, with a slope of 0.03 per year (95% CI: −0.03, 0.08, p=0.34).
Figure 2.
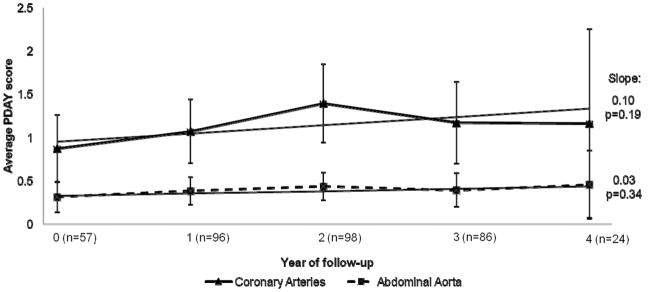
Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk scores over study follow-up.
Discussion
Previous studies of perinatally HIV-infected children raised concern regarding increased CVD risk due to distributions of individual risk factors in this population.7–15 Our study uniquely sought to measure and describe aggregate CVD risk due to the combination of risk factors in perinatally HIV-infected youth by utilizing the validated PDAY scoring system which integrates individual risk factors into a single score for the CA and AA. Forty-eight percent of perinatally HIV-infected youth had high scores; with 12% having scores associated with combinations of dyslipidemia plus high individual score risk factors such as obesity, high blood pressure, and hyperglycemia.
Twenty-two percent of our study population had non-HDL cholesterol levels ≥ 130 mg/dL and 29% had HDL cholesterol <40 mg/dL. These findings are consistent with the high prevalence of dyslipidemia observed in perinatally HIV-infected cohorts compared to national youth estimates.7–10,13, 30 The prevalence of obesity in our study population was 13% which is lower than the 18% prevalence of obesity recently reported among adolescents in the United States.31 This is consistent with previous studies.9,10 However, a recent study showed that HIV-infected children have similar trends in obesity relative to the general population.32 The combination of dyslipidemia and obesity in the perinatally HIV-infected population would further the concern for premature atherosclerotic CVD.
Males in our study population were more likely to have high CA scores compared to females. Among studies of CVD risk factors that evaluated sex differences among perinatally HIV-infected adolescents, one found significantly higher cholesterol levels in males relative to females.7 Three found no sex differences in lipodystrophy or insulin resistance,12–14 while three others found females to have a body fat distribution associated with increased CVD risk.10,11 Independent of their CVD risk due to traditional risk factors, males had a higher risk of having a lesion in the CA compared to females in the PDAY study.18
A history of an AIDS-defining event also predicted higher PDAY scores in our study. Low levels of HDL cholesterol, often associated with inflammation and endothelial activation, are reported in HIV-infected adults with AIDS or untreated HIV infection.33,34 Also, independent of traditional CVD risk factors, HIV disease severity is correlated with higher levels of T-cell activation that is further associated with the presence of subclinical carotid artery lesions.35 In our study, suppressed HIV viral loads also predicted high PDAY scores for the AA relative to HIV viral loads in the 401–5000 copies/mL range. These effects may reflect toxicities associated with exposure to antiretroviral medications, as children currently on effective therapy are likely to have suppressed viral loads. Our observation is also consistent with studies among HIV-infected adults that show the risk for vascular disease with ongoing inflammation is still increased in the presence of viral suppression.35
As expected from prior studies in HIV-infected youth,7–15 boosted protease inhibitor use was a strong predictor of high CA and AA scores in our study population. Protease inhibitors promote hyperlipidemia by upregulating fatty acid and cholesterol biosynthesis and secretion, and by increasing lipoprotein production in the liver.36 Protease inhibitors can also suppress leptin expression and glucose transporter type 4 activity in adipocytes, thereby promoting lipodystrophy, insulin resistance and diabetes.36 While 5–8 years of exposure to boosted protease inhibitors predicted high CA and AA scores, greater than 8 years of exposure did not. This may be due to a lack of power to observe a significant effect due to few observed events among those with greater than 8 years of exposure. Alternatively our results may reflect clinician practice to switch children with preliminary indications of hyperlipidemia off of boosted protease inhibitors to more lipid friendly regimens.
A recent study of HIV-infected adults found tenofovir was significantly associated with progression of subclinical atherosclerosis, mediated perhaps through the lack of reduction in plasma monocyte chemotactic protein 1 levels relative to therapy with abacavir and lamivudine.37 Among our population of perinatally HIV-infected adolescents, however, tenofovir use predicted a lower likelihood of having high CA scores, consistent with other studies among HIV-infected adults showing improvement of dyslipidemia and arterial stiffness with nucleoside reverse transcriptase inhibitor switches to tenofovir.38 With these contrasting results, the association between tenofovir and CVD requires further investigation.
Over our 4-year study follow-up, there were no significant changes in CA or AA scores. We may be observing the consistent tracking of CVD risk factors over time or the duration of follow-up of our cohort may not have been sufficiently long to observe the acquisition of more adult lifestyle risk factors, such as smoking and physical inactivity. Continued follow-up of adolescents in our study as they age into adulthood will be necessary to better evaluate longitudinal trends in CVD risk and identify associated factors.
Our study had some limitations. We were unable to validate the PDAY scoring system against non-invasive measures of atherosclerosis or CVD events. The Adolescent Master Protocol did not utilize these measures and there is a low probability of observing CVD events in our relatively young study population. The CA and AA risk scores utilized in this study were developed from autopsy data, so the definitions of some of the risk factors (e.g., hypertension) were adapted using measures available in clinical practice. The difference in definitions may, therefore, not directly correlate with the odds ratio interpretations of the PDAY risk scores as stated by McMahan et al.18 We utilized the PDAY score as an estimate of the aggregate atherosclerotic CVD risk in our population. The PDAY score, however, may underestimate the risk of atherosclerotic lesions in this population because of the independent effects of HIV and antiretroviral therapy on the development and progression of atherosclerosis.35 Studies among HIV-infected adults suggest that risk scores incorporating both traditional and HIV-specific parameters may better predict CVD risk in HIV-infected populations.39,40 Although our study has a relatively small sample size compared to other studies that have utilized PDAY scores,22–24 it represents an understudied population of perinatally HIV-infected youth aging into young adulthood.
In conclusion, a substantial proportion of perinatally HIV-infected adolescents have PDAY scores reflecting increased aggregate atherosclerotic risk factor burden. PDAY scores may be useful in identifying and tracking such high risk adolescents. Our findings suggest lifestyle modifications (e.g., diet and exercise) and switching to new antiretroviral regimens less likely to cause metabolic abnormalities should be considered.41 Finally, our study contributes to growing literature showing increased global risk for premature CVD disease morbidity and mortality in the perinatally HIV-infected population, including risk of cardiomyopathy and cardiometabolic disease.7–15,42,43 A comprehensive assessment and treatment plan based on validated screening and interventions may be useful for those at highest risk in the perinatally HIV-infected population.
Supplementary Material
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01 HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). The following institutions, clinical site investigators, and staff participated in conducting PHACS AMP in 2012, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton, Deyana Leon; Children’s Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; St. Christopher’s Hospital for Children: Janet Chen, Latreca Ivey, Maria Garcia Bulkley, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
Sources of Funding: The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1). Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or United States Department of Health and Human Services.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Laufer M, Scott GB. Medical management of HIV disease in children. Pediatr Clin North Am. 2000;47:127–153. doi: 10.1016/S0031-3955(05)70198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K, Hernán MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, Seage GR, 3rd Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents infected with HIV-1: A ten-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte Ad, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, Monforte AD, Friis-Møller N, Kirk O, Fontas E, Weller I, Phillips A, Lundgren J. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 6.Aragonès G, Pardo-Reche P, Fernández-Sender L, Rull A, Beltrán-Debón R, Rodríguez-Gallego E, Camps J, Joven J, Alonso-Villaverde C. The deleterious influence of tenofovir-based therapies on the progression of atherosclerosis in HIV-Infected patients. Mediators Inflamm. 2012;2012:372305. doi: 10.1155/2012/372305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter RJ, Wiener J, Abrams EJ, Farley J, Nesheim S, Palumbo P, Bulterys M Perinatal AIDS Collaborative Transmission Study-HIV Follow-up after Perinatal Exposure (PACTS-HOPE) Group. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 8.Tassiopoulos K, Williams PL, Seage GR, 3rd, Crain M, Oleske J, Farley J International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldrovandi GM, Lindsey JC, Jacobson DL, Zadzilka A, Sheeran E, Moye J, Borum P, Meyer WA, 3rd, Hardin DS, Mulligan K Pediatric AIDS Clinical Trials Group P1045 team. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller TL, Orav EJ, Lipshultz SE, Arheart KL, Duggan C, Weinberg GA, Bechard L, Furuta L, Nicchitta J, Gorbach SL, Shevitz A. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr. 2008;153:491–497. doi: 10.1016/j.jpeds.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, Chen JS, McFarland EJ, Borkowsky W, Silio M, Fielding RA, Siminski S, Miller TL Pediatric HIV/AIDS Cohort Study. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: Outcomes from the Pediatric HIV/AIDS Cohort Study (PHACS) Am J Clin Nutr. 2011;94:1485–1495. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpadi SM, Cuff PA, Horlick M, Wang J, Kotler DP. Lipodystrophy in HIV-infected children is associated with high viral load and low CD4+-lymphocyte count and CD4+-lymphocyte percentage at baseline and use of protease inhibitors and stavudine. J Acquir Immune Defic Syndr. 2001;27:30–34. doi: 10.1097/00126334-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Beregszaszi M, Dollfus C, Levine M, Faye A, Deghmoun S, Bellal N, Houang M, Chevenne D, Hankard R, Bresson JL, Blanche S, Levy-Marchal C. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 14.Geffner ME, Patel K, Miller TL, Hazra R, Silio M, Van Dyke RB, Borkowsky W, Worrell C, DiMeglio LA, Jacobson DL Pediatric HIV/AIDS Cohort Study. Factors associated with insulin resistance among children and adolescents perinatally-infected with HIV-1 in the Pediatric HIV/AIDS Cohort Study (PHACS) Horm Res Paediatr. 2011;76:386–391. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bitnun A, Sochett E, Dick PT, To T, Jefferies C, Babyn P, Forbes J, Read S, King SM. Insulin sensitivity and beta-cell function in protease inhibitor-treated and -naive human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2005;90:168–174. doi: 10.1210/jc.2004-0125. [DOI] [PubMed] [Google Scholar]
- 16.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 18.McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill HC., Jr Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165:883–890. doi: 10.1001/archinte.165.8.883. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 (Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Gidding SS, McMahan CA, McGill HC, Colangelo LA, Schreiner PJ, Williams OD, Liu K. Prediction of coronary artery calcium in young adults using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score: the CARDIA study. Arch Intern Med. 2006;166:2341–2347. doi: 10.1001/archinte.166.21.2341. [DOI] [PubMed] [Google Scholar]
- 23.McMahan CA, Gidding SS, Viikari JS, Juonala M, Kähönen M, Hutri-Kähönen N, Jokinen E, Taittonen L, Pietikäinen M, McGill HC, Jr, Raitakari OT. Association of Pathobiologic Determinants of Atherosclerosis in Youth risk score and 15-year change in risk score with carotid artery intima-media thickness in young adults (from the Cardiovascular Risk in Young Finns Study) Am J Cardiol. 2007;100:1124–1129. doi: 10.1016/j.amjcard.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AS, Dolan LM, Gao Z, Kimball TR, Urbina EM. Clustering of risk factors: a simple method of detecting cardiovascular disease in youth. Pediatrics. 2011;127:e312–e318. doi: 10.1542/peds.2010-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charakida M, Loukogeorgakis SP, Okorie MI, Masi S, Halcox JP, Deanfield JE, Klein NJ. Increased arterial stiffness in HIV-infected children: risk factors and antiretroviral therapy. Antivir Ther. 2009;14:1075–1079. doi: 10.3851/IMP1437. [DOI] [PubMed] [Google Scholar]
- 26.Mikhail IJ, Purdy JB, Dimock DS, Thomas VM, Muldoon NA, Clauss SB, Cross RR, Pettigrew RI, Hazra R, Hadigan C, Gharib AM. High rate of coronary artery abnormalities in adolescents and young adults infected with human immunodeficiency virus early in life. Pediatr Infect Dis J. 2011;30:710–712. doi: 10.1097/INF.0b013e31820f6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foss OP, Lund-Larsen PG. Serum thiocyanate and smoking: interpretation of serum thiocyanate levels observed in a large health study. Scand J Clin Lab Invest. 1986;46:245–251. doi: 10.3109/00365518609083666. [DOI] [PubMed] [Google Scholar]
- 28.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 29.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 30.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 32.Arbeitman L, Somarriba G, O’Brien R, Ludwig D, Miller T, Messiah S, Neri D, Scott G. Prevalence of obesity in HIV-infected children in a Miami cohort [Abstract]. Pediatric Academic Societies Annual Meeting; 2012 Apr 38– May 1; Boston, MA. Abstract number 1518.310. [Google Scholar]
- 33.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 34.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, Duprez D, Neaton JD. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201:285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triant VA, Grinspoon SK. Immune dysregulation and vascular risk in HIV-infected patients: implications for clinical care. J Infect Dis. 2011;203:439–441. doi: 10.1093/infdis/jiq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui DY. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42:81–92. doi: 10.1016/s0163-7827(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 37.Aragonès G, Pardo-Reche P, Fernández-Sender L, Rull A, Beltrán-Debón R, Rodríguez-Gallego E, Camps J, Joven J, Alonso-Villaverde C. The deleterious influence of tenofovir-based therapies on the progression of atherosclerosis in HIV-infected patients. Mediators Inflamm. 2012;2012:372305. doi: 10.1155/2012/372305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinn K, Richardson R, Carr A. Lower arterial stiffness and Framingham score after switching abacavir to tenofovir in men at high cardiovascular risk. AIDS. 2010;24:2403–2405. doi: 10.1097/QAD.0b013e32833d568f. [DOI] [PubMed] [Google Scholar]
- 39.Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, Phillips A, Sabin CA, Lundgren JD, Law MG DAD study group. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 40.Parra S, Coll B, Aragonés G, Marsillach J, Beltrán R, Rull A, Joven J, Alonso-Villaverde C, Camps J. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 41.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed September 10, 2012];Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2011 Aug 11;:1–268. Available at http://aidsinfo.nih.gov/ContentFiles/lvguidelines/PediatricGuidelines.pdf.
- 42.Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR, 3rd International Maternal Pediatric Adolescent AIDS Clinical Trials 219219C Study Team. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS. 2012;26:2027–2037. doi: 10.1097/QAD.0b013e3283578bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipshultz SE, Williams PL, Wilkinson JD, Leister E, Van Dyke R, Shearer WT, Rich KC, Hazra R, Kaltman J, Jacobson D, Dooley LB, Scott GW, Rabideau N, Colan SD for the Pediatric HIV/AIDS Cohort Study. Cardiac status of HIV-infected children treated with long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the NIH multicentre Pediatric HIV/AIDS Cohort Study. JAMA Pediatrics. 2013;167:520–527. doi: 10.1001/jamapediatrics.2013.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.