Abstract
Injuries to the superficial digital flexor tendon (SDFT) are an important cause of morbidity and mortality in equine athletes, but the healing response is poorly understood. One important drive for the healing of connective tissues is the inflammatory cascade, but the role of inflammation in tendinopathy has been contentious in the literature. This article reviews the processes involved in the healing of tendon injuries in natural disease and experimental models. The importance of inflammatory processes known to be active in tendon disease is discussed with particular focus on recent findings related specifically to the horse.
Whilst inflammation is necessary for debridement after injury, persistent inflammation is thought to drive fibrosis, a perceived adverse consequence of tendon healing. Therefore the ability to resolve inflammation by the resident cell populations in tendons at an appropriate time would be crucial for successful outcome. This review summarises new evidence for the importance of resolution of inflammation after tendon injury. Given that many anti-inflammatory drugs suppress both inflammatory and resolving components of the inflammatory response, prolonged use of these drugs may be contraindicated as a therapeutic approach. We propose that these findings have profound implications not only for current treatment strategies but also for the possibility of developing novel therapeutic approaches involving modulation of the inflammatory process.
Keywords: Tendon, Injury, Tendinopathy, Pathogenesis, Inflammation, Resolution
Abbreviations: COX, cyclooxygenase; ECM, extracellular matrix; FPR2/ALX, lipoxin A4 receptor; IFN-γ, interferon gamma; IL-1β, interleukin-1beta; LXA4, lipoxin A4; Mφ, macrophage; mPGES-1, microsomal prostaglandin E synthase-1; MMP, matrix metalloproteinase; NSAIDs, non-steroidal anti-inflammatory drugs; PGE2, prostaglandin E2; SDFT, superficial digital flexor tendon; TNF-α, tumour necrosis factor alpha
1. Introduction
Pathologic changes in tendons due to repetitive use are a significant cause of morbidity in athletic humans and horses (Avella et al., 2009; Kujala et al., 2005; Thorpe et al., 2010). The importance of inflammation in both the pathogenesis of tendon injury and the healing process has been contentious in recent years and is poorly understood. Equine clinicians are familiar with the clinical signs of inflammation immediately after a tendon injury occurs, but these signs are not so evident during the chronic phase of injury. Overstrain injury in humans is generally considered to result from a primarily degenerative condition where clinical signs of inflammation and invading inflammatory cells are rarely observed (Alfredson and Lorentzon, 2002; Astrom and Rausing, 1995; Jarvinen et al., 1997; Jozsa et al., 1990; Kannus and Jozsa, 1991; Webbon, 1978). However, this lack of perceived clinical inflammation may be attributable to factors such as the later presentation of human patients, frequently with recurrent injury and the availability of tissues for analysis at different times after injury. Furthermore, the absence of clinically evident inflammation does not preclude an integral role for inflammatory mediators during the pathogenesis and healing of tendon injuries at a cellular level. Current controversy regarding the role of inflammation in tendon injury and healing is similar to that previously debated in joint disease. Use of the term ‘arthritis’ with its emphasis on inflammation, has generally replaced the term ‘arthrosis’ when referring to a wide variety of joint disease conditions (Attur et al., 2002). This change in terminology reflects the fact that inflammatory cytokines have recently been shown to play a pivotal role in the development of joint disease even when clinical signs of inflammation may not be detected. Terminology commonly used to refer to tendon pathology is further described in Box 1.
Box 1. Terminology.
The terms below are used commonly in a variety of clinical scenarios but have not been well defined. For the purposes of this review, we have attempted to clarify what would be considered the most appropriate use of these terms.
‘Tendinopathy’ is used to describe disorders affecting tendons, including tendon rupture and chronic pain. This term does not assume any knowledge about the underlying pathology.
‘Tendonitis’ is used to describe a painful tendon and implies that tendon injury is accompanied by an inflammatory response.
‘Tendinosis’ is used to describe a painful tendon and implies that tendon injury develops as a consequence of a degenerative process and implies absence of inflammation.
‘Tendon disease’ implies that injury develops as a consequence of repetitive cyclic loading and the effects of ageing resulting in cumulative micro-damage. Often exacerbated by overstrain injury, the most common manifestation in the horse is a central core lesion.
‘Tendon injury’ encompassing tendon disease but also occurs as a consequence of traumatic injury when the tendon is lacerated after cutaneous injury.
Several laboratory animal models have been used to investigate tendon injury; however, these induced injury models do not accurately reproduce the naturally occurring conditions that are detected in human and equine patients (Lui et al., 2011). Horses, on the other hand suffer a high frequency of clinical overstrain injury involving a wide spectrum of tendons and ligaments. Therefore the horse presents an attractive large animal model not only for equine related studies but also as a relevant model of human injury due to the shared characteristics of aging phenotypes (Dudhia et al., 2007; Strocchi et al., 1991), elastic energy storing function common to the weight-bearing tendons of both species (Ker et al., 2000; Wilson et al., 2001) and injury induced by natural athletic activity. Equine tendon healing processes are traditionally classified into three distinct but overlapping phases in naturally occurring injury; the acute inflammatory phase occurs immediately after the initial trauma lasting only a few days, followed by the sub-acute reparative phase (peaking at 3–6 weeks but lasting several months and chronic remodelling phase (>3 months after injury) (Dowling et al., 2000). Whilst this temporally coordinated but overlapping sequence of events describes very well the clinical progression of SDFT lesions and other injuries such as some suspensory branch desmopathies in the horse, injuries to the human Achilles tendon manifest differently with less well defined phases characterised by persistent pain and ‘failed healing’ (Longo et al., 2009). These clinical descriptions of the healing phases suggest that inflammation drives acute and chronic phases of injury. However recent insights into the underlying molecular events suggest that some components of the inflammatory cascade are necessary for resolution of injury. This review will discuss the inflammatory mediators relevant to tendon disease and illustrate that in addition to pro-inflammatory roles, inflammation triggers important resolution processes, which can potentially be harnessed for beneficial therapeutic effect.
2. Inflammation related mediators in tendon health and disease
2.1. Cytokines in tendinopathy
Cytokines are small proteins with the ability to influence the biological activities of cells and operate in an autocrine/paracrine manner. They are highly potent and exert their effects at picomolar concentrations. The interaction between a cytokine and its receptor triggers a series of intracellular signalling events culminating in a physiological response (Evans, 1999). Cytokines are reported to have important roles in tendon and ligament homeostasis by regulating cellular differentiation and activity (Evans, 1999; Lin et al., 2006; Molloy et al., 2003) and the synthesis of tendon matrix (Millar et al., 2009; Riley, 2005; Sun et al., 2008). However, samples of injured equine SDFT stain positively for pro-inflammatory cytokines IL-1α, IL-1β, TNF-α and IFN-γ which were not found in normal tendons (Hosaka et al., 2002). Hence in conjunction with their proposed homeostatic roles, cytokines are also potential contributors to the development of tendinopathy.
In addition to the study of natural tendon disease, rodent models of induced tendon injury and in vitro cell/tissue culture studies have been utilised to investigate the roles of cytokines in tendinopathy. Treadmill running of rats to simulate tendon overuse showed up-regulation of inflammation-related genes IL-11, IL-15, IL-6, TNF-α (Millar et al., 2009). The fatigue loading of rat patellar tendons produced structural changes accompanied by increased expression of both MMP-13 and IL-1 β (Sun et al., 2008). Furthermore, both repetitive cyclical stretching of tendon fibroblasts in vitro and stimulation with IL-1β induced MMP production and expression of COX-2 and PGE2 (Archambault et al., 2002; Tsuzaki et al., 2003; Yang et al., 2005). Not only are cytokines associated with the onset of injury, the macrophages releasing these cytokines are potentially important for effective debridement and subsequent healing of the injured tendon. For example, healing was shown to be inferior in a surgical model of patellar tendon injury in IL-6 knockout mice, even though IL-6 has both pro and anti-inflammatory effects (Lin et al., 2006).
2.2. Prostaglandin lipid mediators in tendinopathy
Prostaglandins also have potentially important roles in tendon health and disease. Prostaglandins are synthesised from arachadonic acid by COX-1 and COX-2. Arachadonic acid is in turn derived from the cell membrane phospholipid bilayer by the activity of phospholipase enzymes in response to trauma, cytokines and growth factors (Funk, 2001). Prostaglandins act in an autocrine or paracrine fashion at nanomolar concentrations (Stables and Gilroy, 2011). There are 3 series of prostaglandins; the 1 series prostaglandins give rise to PGE1 and PGF1α, 2 series prostaglandins include PGD2, PGE2 and PGF2α and the 3 series prostaglandins PGE3 and PGF3α (Abayasekara and Wathes, 1999). Prostaglandins of the 1 and 3 series are considered to be less biologically active than those of the 2 series (Irvine, 1982; Lands, 1992). These lipid mediators exert their biological effects by binding to a series of receptors present on the surface of cell membranes. Multiple receptor subtypes have been identified in mice and humans, including the prostaglandin D receptors DP1, DP2 and the prostaglandin F receptor FP. A series of four Prostaglandin E (EP) receptor subtypes are responsible for mediating the downstream effects of PGE2, which include EP1, EP2, EP3 and EP4.
Constitutive prostaglandin production is a prerequisite for normal physiologic processes in many tissues and cells of the body including bone remodelling, modification of blood flow (both vasoconstriction or vasodilation), vascular permeability, smooth muscle tone and platelet aggregation (Dunn, 1987; Graham et al., 2009; Petrucci et al., 2011; Saito et al., 1988; Williams, 1979). Prostaglandin production appears to be a normal physiologic response of tendon cells to repetitive motion. For example, PGE2 levels increased in the peri-tendinous Achilles region of healthy exercising humans (Langberg et al., 1999) and in murine patellar and Achilles tendons following treadmill exercise (Zhang and Wang, 2010). These observations are supported by in vitro experiments whereby tendon fibroblasts in culture release PGE2 in response to repetitive cyclic strain (Almekinders et al., 1993, 1995; Wang et al., 2004).
In addition to constitutive production, prostaglandins are produced in response to injury (Tilley et al., 2001). PGE2 is released by tendon fibroblasts in vitro in response to stimulation with IL-1β in a COX-2 dependent manner (Tsuzaki et al., 2003; Yang et al., 2005) and the inducible terminal synthase of PGE2 (microsomal prostaglandin E synthase-1, mPGES-1) is also up-regulated by IL-1β (Jakobsson et al., 1999; Kojima et al., 2002). The role of prostaglandins in tendinopathy is complex and diverse with evidence supporting both deleterious and beneficial effects. Deleterious effects were observed after peri-tendinous injection of PGE1 into the rat Achilles tendon leading to increased tendon cross sectional area and degenerative changes similar to those observed in human tendinopathy after 5 weeks (Sullo et al., 2001). Similarly, repeated intratendinous injection of 500 ng PGE2 into rabbit patellar tendons culminated in degenerative changes such as focal hyper cellularity, disorganised tissue architecture and reduced collagen fibril diameter compared with control tendons (Khan et al., 2005). This may be effectuated via the prostaglandin EP4 receptor that appears to mediate the degradative effects of IL-1 induced prostaglandins (Thampatty et al., 2007). In addition to IL-1, PGE2 potentiates ECM catabolism in cartilage, periodontal ligament (Attur et al., 2008; Ruwanpura et al., 2004) and tendon via increased expression of MMP-1 and MMP-3 mRNA (Thampatty et al., 2007; Tsuzaki et al., 2003; Yang et al., 2005) and proteins (Tsuzaki et al., 2003).
However, evidence also exists to support a positive role of PGE2 in tendon healing. Peri-tendinous injections of 800 ng PGE2 into the rat patellar tendon improved tendon mechanical properties compared to non-treated controls or saline-injected tendons (Ferry et al., 2012). The role of PGE2 in equine tendons has not been extensively studied, although we have shown that higher doses of PGE2 countered the catabolic effects of IL-1β in an in vitro model of equine tendon inflammation (Dakin et al., 2012a). NSAIDs inhibit prostaglandin production and are widely used pharmacological agents for the treatment of inflammatory diseases and to alleviate the pain associated with tendinopathy. Despite evidence suggesting inflammation also having detrimental effects on the healing process of connective tissues, non-specific blockade of the inflammatory response may not necessarily be beneficial to the healing process (Magra and Maffulli, 2006; Marsolais et al., 2003). Inhibition of prostaglandin synthesis in tendon fibroblasts undergoing cyclical stretch in vitro results in a compensatory elevation in levels of the pro-inflammatory leukotriene B4, potentially exacerbating inflammation (Li et al., 2004). Studies have revealed the potential for COX inhibitors to impair healing of tendons (Cohen et al., 2006; Ferry et al., 2007) and reduce exercise-induced increases in collagen synthesis in the human patellar tendon (Christensen et al., 2011). Moreover PGE2 generated during inflammation, activates specialised pro-resolving lipid mediators such as lipoxins and resolvins, which are essential in restoring tissue homeostasis after inflammation or injury (Levy et al., 2001; Serhan et al., 2000a, 2004). Hence ‘switching off’ prostaglandins is likely to have a negative impact upon the body's endogenous mechanisms to regulate inflammation (Gilroy et al., 1999; Serhan, 2005).
Prostaglandins also exhibit immuno-modulatory properties, for example PGE2 down-regulates the inflammatory response via inhibiting production of pro-inflammatory IL-12 whilst stimulating production of the anti-inflammatory cytokine IL-10 (van der Pouw Kraan et al., 1995). Hence prostaglandins such as PGE2 exhibit double-edged sword properties in terms of their perceived beneficial and detrimental effects on tendons. This may be influenced by the dose of PGE2 and experimental model used, however the concept of maintaining a balance to ensure regulation of prostaglandin production in tendons is likely to be of physiological importance.
2.3. Inflammation triggers resolution
An intriguing dimension to the physiology of inflammation is the identification of specialised pro-resolving mediators (SPM) encompassing lipoxins such as lipoxin A4 (LXA4) (Serhan et al., 1984) acting upon the resolving receptor FPR2/ALX (Ye et al., 2009) and more recently, the resolvins and maresins (Serhan et al., 2002, 2009). FPR2/ALX is expressed by monocytes and macrophages (Yang et al., 2001) and is central in controlling the duration and magnitude of the inflammatory response, providing endogenous stop signals for inflammation (Serhan et al., 2000b). Resolution pathways are programmed responses activated during inflammation, resulting in a switch in lipid mediators from the dominance of prostaglandins to the lipoxins to promote resolution of inflammation and the return of tissues to their normal homeostatic state (Levy et al., 2001; Serhan et al., 2008, 1984, 2000b). Importantly, resolution has been shown to be an active and highly regulated physiological process (Serhan et al., 2007; Serhan and Chiang, 2008) rather than simple passive cessation of inflammation as previously thought. Key events in resolution of inflammation include phagocytosis of apoptotic cells, modification of inflammatory cell infiltration to the inflamed site and modulation of vascular permeability (Serhan et al., 2007). However, whilst resolution is well studied in experimental murine models of inflammation, there is limited information documenting this process in naturally diseased connective tissues and resolution has only recently been identified in injured equine tendons (Dakin et al., 2012a,b).
3. Recent developments on the role of inflammation in equine tendinopathy: implications for current clinical practice
There is little published information that has addressed the role of inflammation in equine tendon disease. A persistent inflammatory stimulus with sustained production of growth factors, proteolytic enzymes and cytokines is a common feature of fibrotic diseases, stimulating the deposition of connective tissue elements that are detrimental to normal tissue architecture (Wynn, 2004). Fibrosis is the final common pathogenic process for many forms of chronic inflammatory disease (Leask et al., 2002). Although the deposition of scar tissue is an integral component of the healing response, the altered composition of the tendon ECM compromises the structural and functional properties of tendons and potentially contributes towards the high risk of re-injury (Crevier-Denoix et al., 1997; Dakin et al., 2011). Through the study of natural tendon injury in the horse, we have demonstrated that aberrations in the enzymes regulating PGE2 metabolism occur soon after injury (Dakin et al., 2012a). In addition, components of inflammation have also been shown to be highly active at this stage, demonstrated by the presence of M1 polarised macrophages (Mφ) (Dakin et al., 2012b), which release pro-inflammatory mediators such as IL-1β and PGE2. During the later phase of tendon healing, the M2Mφ phenotype predominates (releasing anti-inflammatory cytokines) and we have proposed that these cells are actively surveying the healing tendon for ‘stress signals’ to protect this susceptible region from re-injury.
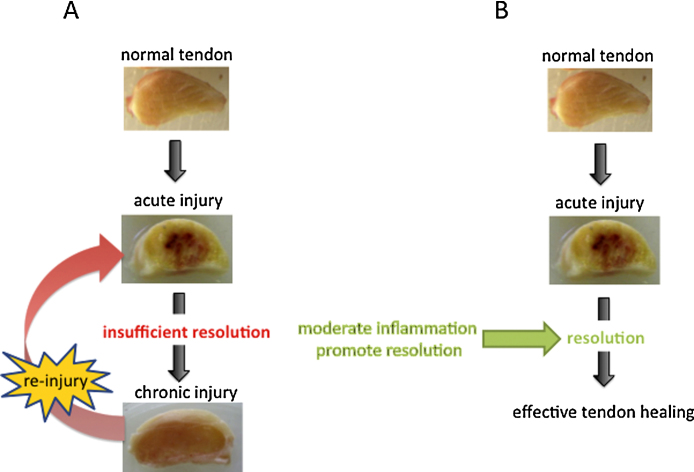
The practice of using steroids and non-steroidal anti-inflammatory drugs (NSAIDs) to relieve pain associated with tendon injury is controversial. Whilst appropriate short term systemic use of these drugs is unlikely to be associated with deleterious patient side effects, prolonged use of NSAIDs or intratendinous injection of steroid preparations are associated with adverse effects on tendon mechanical properties (Virchenko et al., 2004) and propensity for rupture (Shrier et al., 1996), respectively. Furthermore, adverse systemic effects of NSAIDs also include gastrointestinal ulceration (Videla and Andrews, 2009), which occurs due to disruption of normal homeostatic mechanisms involving prostaglandin synthesis. Whilst the inflammatory component of tendinopathy has received much attention, the processes concerned with resolution of tendon inflammation have been neglected from a therapeutic perspective. Improved understanding of inflammation is crucial to the development of novel anti-inflammatory agents that preserve the beneficial, yet remove the detrimental components of the cascade. Although the field of resolving inflammation is relatively new, understanding of pro-resolution processes is rapidly evolving using murine models of experimentally induced inflammation (Navarro-Xavier et al., 2010; Rajakariar et al., 2008). Inhibiting inflammation has been shown to ‘switch off’ the activation of key inflammation-resolving mechanisms (Gilroy et al., 1999). Harnessing the potential of pro-resolving mediators represents a new approach to managing inflammatory diseases in the future. Pro-resolving pathways have not been widely studied in injured tendons, although we have demonstrated increased expression of the pro-resolving receptor FPR2/ALX (Dakin et al., 2012b) and the switch from pro-inflammatory prostaglandins to the production of pro-resolving lipids such as LXA4 during the early stage of tendon injury (Dakin et al., 2012a). However, it is hypothesised that this resolving response is somehow dysregulated or of insufficient duration or magnitude, as tendon inflammation is not adequately ‘switched off’ (Dakin et al., 2012b). This favours the development of chronic inflammation and the formation of a fibrogenic repair scar (Fig. 1). In support of this concept, expression of the pro-resolving receptor FPR2/ALX (a critical component in mediating pro-resolving mechanisms) has been shown to reduce with time after tendon injury and with ageing (Dakin et al., 2012a). This is likely to have implications for sustaining chronic tendon injury if acute inflammation is insufficiently resolved, and in the development of re-injury with age because the ability to resolve tendon inflammation diminishes with ageing.
Fig. 1.
Schematic to propose the relationship between inflammation and resolution in the development of tendon injury. (A) In early stage injury, inflammation triggers a tendon resolution response, which appears to be transient and reduces with age and time after injury. During the later stages of healing (chronic injury) we propose that insufficient or dysregulated resolution allows low-level inflammation to persist, increasing the propensity for fibrotic healing and re-injury. (B) To improve the healing response of tendon, a potential therapeutic strategy is to moderate inflammation whilst simultaneously enhancing the tendons resolution response.
4. Conclusions
Selectively managing tendon injuries according to their disease stage is likely to be an important aspect determining the success of therapy. Future therapeutic management of early stage tendon injury may require addressing both sides of the inflammation equation, whereby inflammatory processes can be modulated to prevent inappropriate or prolonged ECM degradation, whilst simultaneously enhancing pro-resolving processes. In the chronic stage of tendon disease it may be more appropriate to avoid the use of NSAIDs due to their perceived adverse effects on collagen synthesis (Christensen et al., 2011). Instead, treatment of chronic tendon injury could focus on promoting pro-resolution processes by guiding chronically inflamed tissues into a resolving pathway. This may be accomplished by addressing novel therapeutic targets such as pro-resolving lipids or proteins (Gilroy, 2010; Gilroy et al., 2004; Morris et al., 2010), which may be beneficial in reducing the propensities for fibrotic tendon healing and re-injury.
The capacity to prevent tendon injury remains the ultimate goal; however this is currently precluded by our inability to accurately identify sub-clinical injuries in vivo. Sub-clinical injuries are attributable to the effects of exercise and have been identified during post mortem examination of equine SDFTs (Birch et al., 1998; Smith et al., 1999). Future research should aim to identify parameters that determine the inflammatory status of injured tendons in vivo. This encompasses the development of biomarkers of tendon injury (Dakin et al., 2014; Jackson et al., 2003; Smith and Heinegard, 2000), which may facilitate identification of sub-clinical injuries prior to the onset of overt clinical disease. This review highlights the importance of a finely balanced inflammatory response in tendon healing. The recent identification of pro-resolving mediators in healing equine tendons provides novel therapeutic opportunities in the quest for effective prevention and treatment.
Conflict of interest
The authors have no conflicts of interests to declare.
Acknowledgements
This study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) UK and Ceva (France) grant number BB/F018258/1.
References
- Abayasekara D.R., Wathes D.C. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot. Essent. Fatty Acids. 1999;61:275–287. doi: 10.1054/plef.1999.0101. [DOI] [PubMed] [Google Scholar]
- Alfredson H., Lorentzon R. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr. Drug Targets. 2002;3:43–54. doi: 10.2174/1389450023348028. [DOI] [PubMed] [Google Scholar]
- Almekinders L.C., Banes A.J., Ballenger C.A. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. [PubMed] [Google Scholar]
- Almekinders L.C., Baynes A.J., Bracey L.W. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. Am. J. Sports Med. 1995;23:119–123. doi: 10.1177/036354659502300120. [DOI] [PubMed] [Google Scholar]
- Archambault J., Tsuzaki M., Herzog W., Banes A.J. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J. Orthop. Res. 2002;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Astrom M., Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin. Orthop. Relat. Res. 1995:151–164. [PubMed] [Google Scholar]
- Attur M., Al-Mussawir H.E., Patel J., Kitay A., Dave M., Palmer G., Pillinger M.H., Abramson S.B. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J. Immunol. 2008;181:5082–5088. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- Attur M.G., Dave M., Akamatsu M., Katoh M., Amin A.R. Osteoarthritis or osteoarthrosis: the definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthritis Cartilage. 2002;10:1–4. doi: 10.1053/joca.2001.0488. [DOI] [PubMed] [Google Scholar]
- Avella C.S., Ely E.R., Verheyen K.L., Price J.S., Wood J.L., Smith R.K. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons. Equine Vet. J. 2009;41:449–454. doi: 10.2746/042516409x391042. [DOI] [PubMed] [Google Scholar]
- Birch H.L., Bailey A.J., Goodship A.E. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Christensen B., Dandanell S., Kjaer M., Langberg H. Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J. Appl. Physiol. 2011;110:137–141. doi: 10.1152/japplphysiol.00942.2010. [DOI] [PubMed] [Google Scholar]
- Cohen D.B., Kawamura S., Ehteshami J.R., Rodeo S.A. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am. J. Sports Med. 2006;34:362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- Crevier-Denoix N., Collobert C., Pourcelot P., Denoix J.M., Sanaa M., Geiger D., Bernard N., Ribot X., Bortolussi C., Bousseau B. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet. J. Suppl. 1997:23–26. doi: 10.1111/j.2042-3306.1997.tb05046.x. [DOI] [PubMed] [Google Scholar]
- Dakin S.G., Dudhia J., Werling N.J., Werling D., Abayasekara D.R., Smith R.K. Inflamm-aging and arachadonic acid metabolite differences with stage of tendon disease. PLoS One. 2012;7:e48978. doi: 10.1371/journal.pone.0048978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S.G., Jespers K., Warner S., O’Hara L.K., Dudhia J., Goodship A.E., Wilson A.M., Smith R.K. The relationship between in vivo limb and in vitro tendon mechanics after injury: a potential novel clinical tool for monitoring tendon repair. Equine Vet. J. 2011;43:418–423. doi: 10.1111/j.2042-3306.2010.00303.x. [DOI] [PubMed] [Google Scholar]
- Dakin, S.G., Smith, R.K.W., Heinegard, D., Onnerfjord, P., Khabut, A., Dudhia, J., 2014. Proteomic analysis of tendon extracellular matrix reveals disease stage-specific fragmentation and differential cleavage of COMP. J. Biol. Chem. doi: 10.1074/jbc.M113.511972. [DOI] [PMC free article] [PubMed]
- Dakin S.G., Werling D., Hibbert A., Abayasekara D.R., Young N.J., Smith R.K., Dudhia J. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7:e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling B.A., Dart A.J., Hodgson D.R., Smith R.K. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000;32:369–378. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- Dudhia J., Scott C.M., Draper E.R., Heinegard D., Pitsillides A.A., Smith R.K. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Dunn M. The role of arachidonic acid metabolites in renal homeostasis. Non-steroidal anti-inflammatory drugs renal function and biochemical, histological and clinical effects and drug interactions. Drugs. 1987;33(Suppl. 1):56–66. doi: 10.2165/00003495-198700331-00009. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999;28:71–76. doi: 10.2165/00007256-199928020-00001. [DOI] [PubMed] [Google Scholar]
- Ferry, S.T., Afshari, H.M., Lee, J.A., Dahners, L.E., Weinhold, P.S., 2012. Effect of prostaglandin E2 injection on the structural properties of the rat patellar tendon. Sports Med., Arthrosc., Rehabilitat., Ther. Technol.: SMARTT 4, 2. [DOI] [PMC free article] [PubMed]
- Ferry S.T., Dahners L.E., Afshari H.M., Weinhold P.S. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am. J. Sports Med. 2007;35:1326–1333. doi: 10.1177/0363546507301584. [DOI] [PubMed] [Google Scholar]
- Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gilroy D.W. Eicosanoids and the endogenous control of acute inflammatory resolution. Int. J. Biochem. Cell Biol. 2010;42:524–528. doi: 10.1016/j.biocel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Gilroy D.W., Colville-Nash P.R., Willis D., Chivers J., Paul-Clark M.J., Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Graham S., Gamie Z., Polyzois I., Narvani A.A., Tzafetta K., Tsiridis E., Helioti M., Mantalaris A. Prostaglandin EP2 and EP4 receptor agonists in bone formation and bone healing: In vivo and in vitro evidence. Expert Opin. Investig. Drugs. 2009;18:746–766. doi: 10.1517/13543780902893051. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Kirisawa R., Yamamoto E., Ueda H., Iwai H., Takehana K. Localization of cytokines in tendinocytes of the superficial digital flexor tendon in the horse. J. Vet. Med. Sci. 2002;64:945–947. doi: 10.1292/jvms.64.945. [DOI] [PubMed] [Google Scholar]
- Irvine R.F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem. J. 1982;204:3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B.E., Smith R.K., Price J.S. A molecular marker of type I collagen metabolism reflects changes in connective tissue remodelling associated with injury to the equine superficial digital flexor tendon. Equine Vet. J. 2003;35:211–213. doi: 10.2746/042516403776114135. [DOI] [PubMed] [Google Scholar]
- Jakobsson P.J., Thoren S., Morgenstern R., Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen M., Jozsa L., Kannus P., Jarvinen T.L., Kvist M., Leadbetter W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Jozsa L., Reffy A., Kannus P., Demel S., Elek E. Pathological alterations in human tendons. Arch. Orthop. Trauma Surg. 1990;110:15–21. doi: 10.1007/BF00431359. [DOI] [PubMed] [Google Scholar]
- Kannus P., Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Ker R.F., Wang X.T., Pike A.V. Fatigue quality of mammalian tendons. J. Exp. Biol. 2000;203:1317–1327. doi: 10.1242/jeb.203.8.1317. [DOI] [PubMed] [Google Scholar]
- Khan M.H., Li Z., Wang J.H. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin. J. Sport Med. 2005;15:27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Kojima F., Naraba H., Sasaki Y., Okamoto R., Koshino T., Kawai S. Coexpression of microsomal prostaglandin E synthase with cyclooxygenase-2 in human rheumatoid synovial cells. J. Rheumatol. 2002;29:1836–1842. [PubMed] [Google Scholar]
- Kujala U.M., Sarna S., Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin. J. Sport Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- Lands W.E. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992;6:2530–2536. doi: 10.1096/fasebj.6.8.1592205. [DOI] [PubMed] [Google Scholar]
- Langberg H., Skovgaard D., Karamouzis M., Bulow J., Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J. Physiol. 1999;515(Pt 3):919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Holmes A., Abraham D.J. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr. Rheumatol. Rep. 2002;4:136–142. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Li Z., Yang G., Khan M., Stone D., Woo S.L., Wang J.H. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am. J. Sports Med. 2004;32:435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- Lin T.W., Cardenas L., Glaser D.L., Soslowsky L.J. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Longo U.G., Ronga M., Maffulli N. Achilles tendinopathy. Sports Med. Arthrosc. 2009;17:112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- Lui P.P., Maffulli N., Rolf C., Smith R.K. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sports. 2011;21:3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Magra M., Maffulli N. Nonsteroidal antiinflammatory drugs in tendinopathy: friend or foe. Clin. J. Sport Med. 2006;16:1–3. doi: 10.1097/01.jsm.0000194764.27819.5d. [DOI] [PubMed] [Google Scholar]
- Marsolais D., Cote C.H., Frenette J. Nonsteroidal anti-inflammatory drug reduces neutrophil and macrophage accumulation but does not improve tendon regeneration. Lab. Invest. 2003;83:991–999. doi: 10.1097/01.lab.0000078688.07696.ac. [DOI] [PubMed] [Google Scholar]
- Millar N.L., Wei A.Q., Molloy T.J., Bonar F., Murrell G.A. Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Joint Surg. Br. 2009;91:417–424. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- Molloy T., Wang Y., Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- Morris T., Stables M., Colville-Nash P., Newson J., Bellingan G., de Souza P.M., Gilroy D.W. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Xavier R.A., Newson J., Silveira V.L., Farrow S.N., Gilroy D.W., Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J. Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- Petrucci G., De Cristofaro R., Rutella S., Ranelletti F.O., Pocaterra D., Lancellotti S., Habib A., Patrono C., Rocca B. Prostaglandin E2 differentially modulates human platelet function through the prostanoid EP2 and EP3 receptors. J. Pharmacol. Exp. Ther. 2011;336:391–402. doi: 10.1124/jpet.110.174821. [DOI] [PubMed] [Google Scholar]
- Rajakariar R., Lawrence T., Bystrom J., Hilliard M., Colville-Nash P., Bellingan G., Fitzgerald D., Yaqoob M.M., Gilroy D.W. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G.P. Gene expression and matrix turnover in overused and damaged tendons. Scand. J. Med. Sci. Sports. 2005;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Ruwanpura S.M., Noguchi K., Ishikawa I. Prostaglandin E2 regulates interleukin-1beta-induced matrix metalloproteinase-3 production in human gingival fibroblasts. J. Dent. Res. 2004;83:260–265. doi: 10.1177/154405910408300315. [DOI] [PubMed] [Google Scholar]
- Saito H., Nakamaru M., Ogihara T., Rakugi H., Kumahara Y., Inagami T., Shimamoto K. Effect of vasodilator prostaglandins on the vascular renin-angiotensin system. Life Sci. 1988;43:1557–1563. doi: 10.1016/0024-3205(88)90405-5. [DOI] [PubMed] [Google Scholar]
- Serhan C.N. Novel omega—3-derived local mediators in anti-inflammation and resolution. Pharmacol. Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Serhan C.N., Brain S.D., Buckley C.D., Gilroy D.W., Haslett C., O’Neill L.A., Perretti M., Rossi A.G., Wallace J.L. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 2008;153(Suppl. 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Gotlinger K., Hong S., Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serhan C.N., Hamberg M., Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U. S. A. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Takano T., Chiang N., Gronert K., Clish C.B. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis. Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am. J. Respir. Crit. Care Med. 2000;161:S95–S101. doi: 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- Serhan C.N., Yang R., Martinod K., Kasuga K., Pillai P.S., Porter T.F., Oh S.F., Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier I., Matheson G.O., Kohl H.W., 3rd Achilles tendonitis: are corticosteroid injections useful or harmful? Clin. J. Sport Med. 1996;6:245–250. [PubMed] [Google Scholar]
- Smith R.K., Birch H., Patterson-Kane J., Firth E.C., Williams L., Cherdchutham W., van Weeren W.R., Goodship A.E. Should equine athletes commence training during skeletal development?.: changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet. J. 1999;(Suppl. 30):201–209. doi: 10.1111/j.2042-3306.1999.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Smith R.K., Heinegard D. Cartilage oligomeric matrix protein (COMP) levels in digital sheath synovial fluid and serum with tendon injury. Equine Vet. J. 2000;32:52–58. doi: 10.2746/042516400777612053. [DOI] [PubMed] [Google Scholar]
- Stables M.J., Gilroy D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Strocchi R., De Pasquale V., Guizzardi S., Govoni P., Facchini A., Raspanti M., Girolami M., Giannini S. Human Achilles tendon: morphological and morphometric variations as a function of age. Foot Ankle. 1991;12:100–104. doi: 10.1177/107110079101200207. [DOI] [PubMed] [Google Scholar]
- Sullo A., Maffulli N., Capasso G., Testa V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: a possible animal model of chronic Achilles tendinopathy. J. Orthop. Sci. 2001;6:349–357. doi: 10.1007/s007760100031. [DOI] [PubMed] [Google Scholar]
- Sun H.B., Li Y., Fung D.T., Majeska R.J., Schaffler M.B., Flatow E.L. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin. Orthop. Relat. Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thampatty B.P., Li H., Im H.J., Wang J.H. EP4 receptor regulates collagen type-I MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene. 2007;386:154–161. doi: 10.1016/j.gene.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Clegg P.D., Birch H.L. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010;42:174–180. doi: 10.2746/042516409X480395. [DOI] [PubMed] [Google Scholar]
- Tilley S.L., Coffman T.M., Koller B.H. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki M., Guyton G., Garrett W., Archambault J.M., Herzog W., Almekinders L., Bynum D., Yang X., Banes A.J. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4 IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan T.C., Boeije L.C., Smeenk R.J., Wijdenes J., Aarden L.A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla R., Andrews F.M. New perspectives in equine gastric ulcer syndrome. Vet. Clin. North Am. Equine Pract. 2009;25:283–301. doi: 10.1016/j.cveq.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Virchenko O., Skoglund B., Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am. J. Sports Med. 2004;32:1743–1747. doi: 10.1177/0363546504263403. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Li Z., Yang G., Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clin. Orthop. Relat. Res. 2004:243–250. doi: 10.1097/01.blo.0000126337.65685.e4. [DOI] [PubMed] [Google Scholar]
- Webbon P.M. A histological study of macroscopically normal equine digital flexor tendons. Equine Vet. J. 1978;10:253–259. doi: 10.1111/j.2042-3306.1978.tb02275.x. [DOI] [PubMed] [Google Scholar]
- Williams T.J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br. J. Pharmacol. 1979;65:517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.M., McGuigan M.P., Su A., van Den Bogert A.J. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- Wynn T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Chen Q., Le Y., Wang J.M., Oppenheim J.J. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J. Immunol. 2001;166:4092–4098. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- Yang G., Im H.J., Wang J.H. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R.D., Boulay F., Wang J.M., Dahlgren C., Gerard C., Parmentier M., Serhan C.N., Murphy P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang J.H. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J. Orthop. Res. 2010;28:198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]