Abstract
Every hip fracture begins with a microscopic crack, which enlarges explosively over microseconds. Most hip fractures in the elderly occur on falling from standing height, usually sideways or backwards. The typically moderate level of trauma very rarely causes fracture in younger people. Here, this paradox is traced to the decline of multiple protective mechanisms at many length scales from nanometres to that of the whole femur. With normal ageing, the femoral neck asymmetrically and progressively loses bone tissue precisely where the cortex is already thinnest and is also compressed in a sideways fall. At the microscopic scale of the basic remodelling unit (BMU) that renews bone tissue, increased numbers of actively remodelling BMUs associated with the reduced mechanical loading in a typically inactive old age augments the numbers of mechanical flaws in the structure potentially capable of initiating cracking. Menopause and over-deep osteoclastic resorption are associated with incomplete BMU refilling leading to excessive porosity, cortical thinning and disconnection of trabeculae. In the femoral cortex, replacement of damaged bone or bone containing dead osteocytes is inefficient, impeding the homeostatic mechanisms that match strength to habitual mechanical usage. In consequence the participation of healthy osteocytes in crack-impeding mechanisms is impaired. Observational studies demonstrate that protective crack deflection in the elderly is reduced. At the most microscopic levels attention now centres on the role of tissue ageing, which may alter the relationship between mineral and matrix that optimises the inhibition of crack progression and on the role of osteocyte ageing and death that impedes tissue maintenance and repair. This review examines recent developments in the understanding of why the elderly hip becomes fragile. This growing understanding is suggesting novel testable approaches for reducing risk of hip fracture that might translate into control of the growing worldwide impact of hip fractures on our ageing populations.
Keywords: Hip fracture, Ageing, Osteoporosis, Cortical thinning, Osteocyte, Toughness
Highlights
-
•
Multiple mechanisms at many length scales protect against hip fracture but are degraded with ageing.
-
•
Compression failure in the supero-lateral cortex maybe the immediate precipitating cause of intra-capsular hip fracture.
-
•
This cortex becomes progressively thinner throughout life so it can be overloaded in a fall through crushing or buckling.
-
•
The bone tissue of the proximal femur becomes progressively more brittle as mechanisms that inhibit crack propagation deteriorate.
-
•
The roles of osteocyte ageing and associated loss of mineralized tissue maintenance are discussed alongside possible countermeasures.
Introduction
Hip fracture risk increases exponentially with age but unlike vertebral fracture its incidence varies widely between populations [1]. Patterns of exponentially increasing disease incidence are typically associated with multiple causes [2]. Conventionally, hip fractures are classified into two main types: cervical and trochanteric. The former have offered more possibilities of clinical investigation because it is common practice to insert a prosthesis making the fracture site recoverable; but investigators have had modest success in differentiating hip fracture types by clinical risk factors and geometry [3]. Normal younger adult bone has excellent mechanical properties resulting from its nature as a composite material formed of mineral and matrix that limits the occurrence or spread of damage. Damage control mechanisms are found at every length scale, from that of the individual collagen molecule to the grand scale of society itself, which aims to minimize trauma. Here we review our understanding of how the skeleton progressively loses its remarkable mechanical properties. Age-related hip fragility is considered from the patient's perspective: what led her, or increasingly his, femur to fracture when it would not previously have done so [4]? A secondary theme is the underappreciated relevance of modern research on structural failure in complex systems both biological and non-biological.
The femoral neck functions as a lever. In levers, resistance to fracture depends on their mass and geometry as well as on their material properties. To date, local bone mass is the only related factor accepted as a clinical measure of hip fracture risk [5,6], commonly referred to as “bone density”: this is a ratio of mass to femoral neck width over a standard length of 15 mm (here referred to as aBMD with units of g cm− 2). The other risk factor routinely considered clinically is a patient's age as a surrogate for various other unquantified factors. Yet, after accounting for age, mortality and aBMD, as well as other factors used in several guidelines, Kanis et al. were unable to account for the more than 10-fold geographical differences in risk of hip fracture seen worldwide [1] making it necessary to examine hip fracture at a more fundamental level.
Ageing and the hip
Scientific interest in the proximal femur has been partly driven by the attractive hypotheses its cantilevered shape encouraged regarding the effects of mechanical forces on bone [7]. A detailed study of the anatomy of the cadaveric femur led Backman to suggest that the femur was weaker in a fall onto the greater trochanter than in stance [8]. Hirsch and Brodetti removed the proximal femur's internal trabeculae, finding that this reduced its strength [9]. Throughout the 19th and 20th centuries, the relative importance of nutritional or sunlight deficiencies, lifestyle, generalised osteoporosis and age-related changes in the properties of the collagenous matrix of bone in the developing fragility of the elderly hip were debated [7,10,11]. Disquietingly, 50% of elderly hip fracture cases are not classified as being osteoporotic by densitometry [1]; and while elderly UK hip fracture cases had femoral neck cortices thinned locally by up to 30% compared to controls [12,13], they also had normal-for-age femoral neck trabecular bone density [14,15]. Meanwhile, non-First World populations such as elderly Mediterranean and West African women farmers have relative freedom from hip fractures [16–18] despite falling and frequently being osteoporotic by First World standards [18]. The role of the non-mineral collagenous matrix in fracture resistance has been underestimated until recently. Changes to inter- and intrafibrillar collagen cross-linking, as well as collagen content, can reduce the energy required to cause bone failure (through reducing the material property called toughness), and increase fracture risk, particularly with accumulation of advanced glycosylation end products (AGEs) as occurs especially in diabetes mellitus [19]. So, while collagen may have less effect on bone's strength and stiffness [20] than does mineral, it may have a much greater effect on bone fragility [10].
Up to now, bone quality as the sum of characteristics other than its mass that influence a bone's resistance to fracture has been difficult to assess [21]. Yet studies of normal ageing have provided clues. Younger people have much thicker supero-lateral femoral neck cortices than older people [14]. This contrast was attributed to reduced variety of physical activity so that walking, the principal physical activity of our later years, might be insufficient to maintain the strength of a cortex that is mechanically sensitive and adapts structure over many length scales to function [22]. Even in historical times, the geometry of the European proximal femur appears to have become more fragile as physical activity patterns have become more sedentary [23].
If an unaccustomed heavy load is experienced by a young person's femur, (as occurs in contact sports) there is high resistance to crack initiation, extension and consequent fracture [24]. The energy of a sideways fall must be dissipated and absorbed in the whole femur and surrounding soft tissue. Fracture requires deformation of a bone beyond the point where its integrity can be maintained. If it is deformed without fracture, its shape may recover completely or nearly so. If deformation occurs under a sudden load that increases beyond the so-called yield point of the bone tissue, deformation is incompletely recoverable and is defined as “plastic” rather than “elastic”. Once plastic deformation begins, bone tissue starts to harden and further deformation will eventually lead to complete rupture. Plastic strain reflects the absorption of the energy generated in a fall. The plastic strain that develops as a bone is loaded beyond its yield point may eventually lead to complete rupture; the amount of plastic strain a bone can tolerate before rupture may also decline as it ages [25]. A bone is characterised as being tough when it can tolerate a large amount of plastic strain, as the proximal femur is exposed to in a fall; or as brittle when it can only tolerate a small amount of plastic strain.
This intriguing preservation of bending resistance by the elderly hip, calculated from DXA-based hip strength analysis [26], alongside the failure of declining aBMD to explain much of the age-related risk of hip fracture [27] has triggered a re-evaluation of fracture mechanisms in old people.
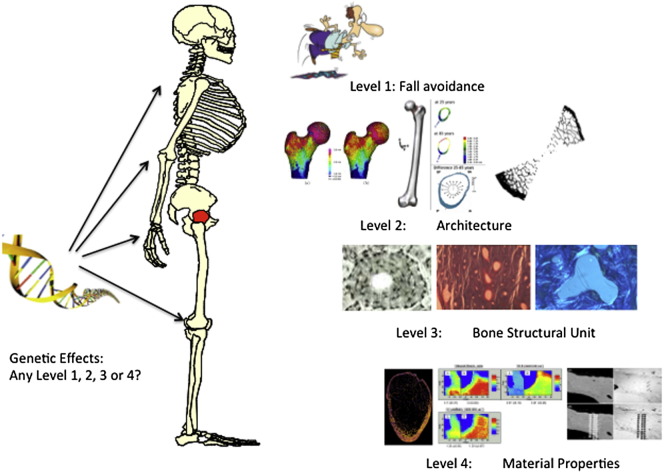
Fracture resistance at multiple levels of organisation (Fig. 1)
Fig. 1.
Caption: Levels of bio-mechanical organisation active in lowering risk of hip fracture in decreasing order of length scale: Level 1 cognitive and societal mechanisms reducing the risk of trauma; Level 2 architectural disposition of bone tissue to optimise strength while preserving gravitational lightness; Level 3 remodelling and renewal of bone tissue- the key role of the osteocyte; Levels 4 and higher-material properties of bone tissue and their resistance to crack propagation.
All bio-mineralized structures are organised hierarchically over a wide range of length scales. In consequence, an effect observable at a microscopic level is clinically significant only if it results in reduced mechanical performance at a more macroscopic scale – immediately or after some delay [28]. The highest level of defence against fracture, here called Level 11, is personal and societal and depends on modifying or adapting to the environment. Age-specific fall rates are quite variable with a tenfold range in the reported fall rates between the highest (Oslo) and lowest (Rotterdam) centres of the European Prospective Osteoporosis Study [29]. Environmental differences are not necessarily the cause of the higher fracture rates in “high fall rate” societies: the true cause might be differences in neurological, locomotor or muscular function between elderly populations, leading to impairment of the fall avoidance reflexes learned in childhood. Vitamin D may however play a role at several levels. At Level 1 it may contribute to defence against hip fracture since repletion may reduce fall rates in institutionalized fallers [30].
The second level of fracture resistance relates to the architectural structure of the femur as visible by the naked eye. The third level is provided by the femur's microstructure as visualized in conventional bone histomorphometry. Microscopic sculpting at this length scale is accomplished by teams of osteoblasts and osteoclasts organised into the multi-cellular Basic Metabolic Units (BMUs) which create structural units (BSUs) “glued” together by a collagen-poor osteopontin-containing cement [31] that appears as “cement” lines under the microscope. Fourth and higher levels of organisation (up to seven have been proposed) are common to the whole skeleton and extend from the micro- through the nano-scales.
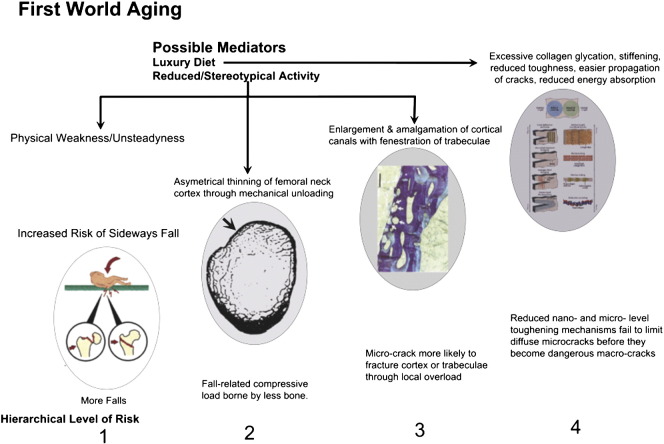
Fig. 2 is a cartoon showing risk mechanisms that might contribute to risk of hip fracture or its prevention at different hierarchical levels. We now examine organizational levels 2–4 for their involvement in fracture prevention in further detail.
Fig. 2.
Risk mechanisms arising from ageing-related failures in biological protection against hip fracture. These are classified by length scale aligned with the protective mechanisms seen in younger bone. (Scale) Level 1: failure to avoid sideways falls, involving personal physical decline and societal acceptance of fall risk to preserve personal freedom. Repeated falls might in principle weaken the femoral cortex through the sort of “delamination” mechanisms that can occur in man-made composites and making a fracture more likely at the next fall (see text). Level 2: Thinning of the supero-lateral half of femoral neck cortex leads to risk of its local buckling or crushing under compression in a sideways fall, progressing inevitably to complete intracapsular hip fracture. Level 3: Increased remodelling with simultaneous resorption of several adjacent canals (or trabecular surfaces) can destroy the integrity of bone's microstructure if thin bone structures are fenestrated. Here 4 osteons are arrowed: two are undergoing resorption and are about to amalgamate with two others in the resting phase near the periosteal surface of the femoral neck. The walls between osteons are hard to re-create subsequently and the resulting composite osteons have been postulated to constitute the first phase in the gradual “trabecularization” of the femoral neck cortex with ageing [86]. Level 4: The multiple toughening mechanisms operating at nano- through micro-scale are shown in this cartoon from Launey et al. to which the reader is referred for a detailed explanation [98]. The known or postulated effects of age-related disease and disabilities to limit the effectiveness of these mechanisms include: exposure to products that glycate collagen, so stiffening it (especially in diabetes mellitus) and e.g. fracturing crack bridges; loss of heterogeneity of mineralization that might otherwise deflect growing cracks with energy absorption; increased crystallinity that might make some abnormally large apatite crystals vulnerable to fracture; loss with cell death or dysfunction of defined low density structures such as osteocyte canaliculi that deflect cracks; (see text).
Organisation level 2: Architecture
Alongside the material properties of bone tissue, the contribution of the femur's architecture to optimising the mechanical work needed to induce fracture has long been appreciated. Galileo argued that as mammals grow heavier, they need proportionately wider femurs to avoid fracture [32]. Darwin observed that the heavier wing and weaker leg bones of the wild compared to the domestic duck reflected different patterns of mechanical usage [33]. Wolff also proposed that bones adapt their internal structure efficiently so as to minimise the bending effects of the mechanical loads placed upon them [34]. Koch [35] then controversially [36] suggested that the architecture of cortical and trabecular bone in the femoral neck was ideally adapted to resist bending.
But compression can cause fracture in other ways. Bone (like marble, concrete and other familiar materials) can disintegrate by crumbling under high compression, while thin or unsupported bony structures may sometimes buckle at rather lower strains. Buckling explains the collapse of osteoporotic cancellous bone in the spine, which is made more likely as the disappearance of horizontal trabeculae results in fewer connections within the load-bearing vertical trabeculae [37]. In a fall onto the greater trochanter, the supero-lateral cortex, normally loaded weakly in tension, becomes heavily loaded in compression. Because of its thinness in the elderly [38], this cortex is a target for research into fracture prevention for its potential to buckle in a sideways fall.
Ageing effects
DXA-based in vivo 2D measurements were used to estimate the fall forces needed for fracture in bending by Yoshikawa et al. [39]. Engineers estimate resistance to bending by a structure's moment of inertia or by the related but size-adjusted property section modulus. Each of these depends on the bone's mass and width and the distance of each measured voxel of bone tissue from the bone cross-section's centre of mass. Yoshikawa et al. estimated moment of inertia assuming bending in a fall was in the plane of DXA measurement. It was found by using comparable methods that the section modulus of the hip remains nearly constant during normal ageing [40]. This contrasts with the fact that, from age 40, there is a 10-fold increase in hip fracture risk every 20 years [2]. Rapid loss of hip bending resistance does occur eventually, with the onset of frailty [26]. DXA aBMD typically declines somewhat faster than bending resistance. This is explained by the gradual widening of the femoral neck diameter with age [41], which for algebraic reasons reduces aBMD while it increases section modulus. Widening of the femoral neck is driven by periosteal bone formation associated with compensatory endosteal resorption [42] and is associated with markers of sex hormone deficiency [43].
These findings stimulated 3D studies, in and ex vivo. As we age, the contrast between the thickness of the infero-medial and the thinness of the supero-lateral femoral neck cortex increased because the infero-medial remained largely unchanged as the supero-lateral grew thinner [14,44,45]. This change had a small effect on bending resistance. But in a sideways fall, a thinned supero-lateral cortex taking the full impact load might fracture through being over-compressed and either crumble or buckle. Risk of buckling is also increased [15] because internally supportive trabeculae become less dense in the femoral neck [44,46] and inter-trochanteric regions [47]. Such trabeculae are potentially important because the force required for a structure to buckle is inversely proportional to its unsupported length.
Architectural changes associated with hip fracture: Fracture prediction
While unfractured controls showed small changes with age in DXA-assessed bending resistance, in female cases of hip fracture bending resistance was substantially reduced compared to controls, equivalent to 2 decades of age-related changes [26,48]. There was, however, substantial between-group overlap. Cases also had thinner [12,46] and more porous [49] bone cortices. But differences in 2D imaging were somewhat [50] or little better than aBMD in predicting hip fracture [51,52]. Some aspect of age-related hip fragility remained uncaptured by any form of DXA-based assessment, due either to the geometric limitations of 2D DXA, its exclusive focus on mineralisation or its coarse resolution.
This stimulated the application of 3D approaches, made possible by the increasing resolution of computed tomograms of the hip region [53]. Finite Element Simulation or Analysis (FEA) is an approach to modelling the properties of complex 3D shapes that relies on visualizing the proximal femur as being formed of a large number of contiguous “elements” each with its own material properties. The three steps in FEA are firstly to construct a model in which the geometry is divided into a usually substantial number of elements connected at discrete points called nodes. Some of these nodes are fixed in space while others have prescribed mechanical loads. Typically, special purpose software is used to overlay a mesh onto a 3D scan. This data-set is used as input to solve a series of algebraic linear or non-linear equations relating the externally applied forces and the displacements at each node. Non-linear equations are required if mechanical loading with the potential to induce plastic strain is being investigated because the stress–strain curve in each element affected by plastic deformation is highly likely to be non-linear. Recent studied on the spinal vertebrae have shown considerable promise that FEA modelling surpasses in accuracy DXA-based methods for assessing vertebral fracture resistance [54]. In the AGES Rejkyavik population-based cohort study, ageing of the individual was associated with loss of Finite Element Analysis (FEA)-assessed strength [55] of the hip in both stance and falling configurations. This was more rapid in women than men, while contrastingly, hip fracture prediction was improved by FEA more in men than women compared to BMD. In a subsequent analysis of data from the same study, simultaneous FEA assessment of 3D CT scan data both at hip and spine appeared to significantly enhance 2D DXA-based (hip) or 3D trabecular density (spine) predictions of future fracture [56].
Cortical thinning and fracture mechanics
In a third AGES-based analysis, fracture was also associated with thinning of the superior femoral neck cortex [45]. This finding was supported by two case–control studies [46,57], one suggesting that patchy rather than uniform thinning of the supero-lateral cortex could underlie hip fragility [57]. Fast cine investigation of ex vivo hip fracture has shown that the initiating crack frequently begins in the supero-lateral cortex [58]. It can be shown mathematically that section modulus of the femoral neck collapses suddenly after the crack becomes macroscopic; the infero-medial cortex, no longer braced by the supero-lateral cortex, then fractures in bending as a secondary event [14]. If the initial mode of cortical failure is by buckling not crushing, supporting trabeculae may be critical because they have a surprisingly large effect to prevent buckling while having little capacity to prevent crushing [9,15,59].
It is now understood [60] that local critical stress, in which both the stress experienced by a section of femoral neck cortex and its curvature normal to that stress are important determinants, is a measure of resistance to buckling [14,15,61]. While in vivo CT-based 3D studies modelled with FEA can assess the potential for crushing [62] of the thinned supero-lateral cortex, realistic predictions of cortical buckling, which has an absolute requirement for non-linear modelling and other refinements to avoid unrealistic stress calculations at the individual voxel level, remain to be incorporated successfully into FEA modelling of hip fracture.
Organisation level 3
With much of the age-related increased risk of hip fracture unlikely to be explained by whole hip architecture, we must examine age-related changes in properties of femoral neck bone at more microscopic levels. The young person's Bone Structural Unit BSU, termed an osteon in cortical bone, contains osteocytes at varying densities ranging from 40 to 90*103 mm− 3 [63]; lining cells interposed between the matrix and the osteocytes' source of nutrition; and if it is actively undergoing renewal, osteoblasts and osteoclasts increasing or reducing its mineralized mass. The remodelling BSU is called a Basic Multicellular Unit (BMU) and is supervised by endocrine and possibly sympathetic [64] pathways. Osteocytes play crucial roles in sensing and initiating responses to mechanical loading by regulating the composition of the mineralized matrix [65,66] and deploying a range of local and systemic signals, including nitric oxide [67], prostaglandins, RANK ligand [68] and the osteoblast inhibitor sclerostin [69]. They also regulate plasma phosphate levels, which must be normal to avoid osteomalacia, implicated in a minority of hip fracture cases [70,71]. Osteocytic dendrites interconnect with other osteocytes, surface lining cells and the cement line through microscopic canaliculi that track mostly radially across the thickness of most osteons. These canaliculi may play an important permissive role in absorbing the energy associated with microcracks that develop under load [72], which may occur as a result of neighbouring osteocyte lacunae acting as stress-enhancing “flaws” in the structure of bone tissue. This aspect of function is discussed later.
Increased remodelling and fracture risk
Remodelling is increased after menopause in association with reduced oestrogen levels. A typical remodelling cycle, lasting 3 months or more, results in transient fragility since the affected structure is temporarily thinned or made porous. A second general effect of menopause is to alter the balance between formation and resorption towards bone resorption in part at least through apoptosis of osteocytes [73] which induces osteoclastic activity [74]. Other effects of estrogen decline include dysinhibition of sclerostin and RANKL signalling [75]. Together, these observations might explain why a variety of different drugs that reduce markers of bone resorption to young adult levels reduce hip and other non-vertebral fractures [76] and why the size of this effect may be larger than explained by increased bone density. Another cause of increased remodelling is the secondary hyperparathyroidism associated with reduced vitamin D levels, commonly seen in the elderly. Before it develops into frank osteomalacia with demineralization of bone tissue, this commonly results in accelerated net bone loss, cortical thinning and trabecular fenestration as reviewed by Chavassieux et al. [77].
In the femoral neck of hip fracture cases, bone remodelling was higher in the main compression cortex in cases than controls [78] and increased cortical porosity was due to a moderate number of abnormally large canals, formed apparently by the merging of 2 or more osteons. Ageing in men is associated both with increased porosity of the femoral cortex and with increasing stiffness as assessed by acoustic microscopy [20]. Remodelling osteons in both healthy subjects [79] and in hip fracture are clustered anatomically [80], suggesting that cortical remodelling is simultaneously initiated within several capillary branches of a terminal intra-osseous arteriole. New imaging techniques such as synchrotron radiation CT now make it possible to investigate the detailed 3D structure of individual osteons in the femoral cortex and the anatomical relationships between them [81,82]. These offer promising new avenues for understanding how conversion of the cortex to trabecular bone, leading to cortical thinning [83,84], develops and how osteonal structures might help preserve the toughness of bone once a crack threatens to expand beyond the osteon's own territory (see [85] and below).
The location of this increased remodelling, in the thick anterior and inferior cortices [78,86,87], suggested it was not secondary to fatigue damage. The elderly femoral neck cortex also contains regions of highly mineralized and therefore probably brittle bone or calcified cartilage [88]. The location of this calcified cartilage as well as its persistence in the face of increased remodelling is puzzling, because of its frequent distance from tendon insertions where hypermineralization is usually found.
Cellular regulation of increased remodelling: BMU and osteon-level effects on strength
To cause structural weakness, cortical osteoclasts may not need to resorb to an excessive depth. Close clustering of resorbing BMUs allows the breakthrough of osteoclastic resorption from one simultaneously remodelling osteon into another [86] and may explain the statistical association between clustering of remodelling osteons and the giant canals seen to excess in hip fracture cases. This “breakthrough” resorption is topologically analogous to loss of whole trabeculae after they are resorbed on both surfaces, which is considered to reduce connectivity in cancellous bone and is seen in vertebral osteoporosis [89]. Tsangari et al. found no evidence for disordered remodelling in inter-trochanteric trabecular bone from hip fracture patients [90]; but even if a resorbing trabecula remains unperforated, the effect of a resorption pit on its strength can be substantial and unpredictable [91]. Large magnification finite element studies of the femoral cortex ex vivo are just becoming practical [92] and van Rietbergen et al. found evidence that the trabecular bone in the osteoporotic proximal femur was subject to higher and more uneven stresses than in control bone, increasing the risk of local mechanical failure [93].
In trabecular bone, remodelling may occur following strenuous activity, where calculation suggests that in vertebrae microscopic damage might occur [94]. Tough lamellar bone [95] replacing the damaged tissue, usually following a preliminary repair by weaker woven bone, can only be formed at a maximum rate of about 10− 3 mm/day [96]. So full repair of a 200 mm*10− 3 thick trabecula following a microfracture typically takes over 8 months.
Organisation levels 4 and higher
Understanding of mechanical breakage was revolutionised nearly a century ago by the celebrated work of Griffiths on crack initiation in materials that can like glass be ruptured by stresses 100-fold lower than those necessary to rupture the atomic bonds they contained [97]. Yet we have so far followed clinical tradition and considered hip fracture as a macroscopic event, so far hardly considering its origins as a crack at the sub-osteon level, initiating in a microscopic flaw, that enlarges explosively to result in organ failure. Unlike glass, there are multiple microscopic levels of defence against crack enlargement in bone tissue that provide an abundance of mechanisms for absorbing the energy delivered by trauma [98]. Do these mechanisms deteriorate with age?
Safe engineering practice depends on applying fracture mechanics to the prevention of disastrous crack enlargement [99]; and this knowledge has inspired several recent reviews considering the basic science of bone quality at sub-BSU and sub-osteon levels of organization [98,100,101]. The possible effects of widespread osteocyte death [102] on hip fragility are considered later. Here we focus on mechanisms limiting crack growth relevant to human hip fracture.
Microscopic cracks, their development, capture and persistence
Stresses concentrate in a loaded bone at microscopic cavities and notches, risking crack initiation [103]. Normal mature human femoral bone subjected to cyclic loading develops microcracks that first grow then rapidly decelerate [104]. This reflects multiple toughening mechanisms that absorb the energy associated with growing cracks. Moreover, each acts without excessive sacrifice of strength [85,95]. Their length scales range from the molecular (e.g. reformable bonding of collagen [105] and cross-links [106]) through that of the potentially crack-deflecting osteocyte canaliculus (~ 250 nm diameter) or smaller [72] to the whole osteon, with its diameter some 1000-fold larger.
Left unchecked, cracks in brittle materials tend to accelerate in a single direction. Deflecting its direction, through as wide an angle as possible, absorbs a crack's energy. When the matrix of bone is not homogeneous, a developing crack will experience multiple deflections that sap its energy and its potential to grow into a complete clinical fracture [107]. So heterogeneity of its material properties is desirable as well as being intrinsic to lamellar bone [101,108].
On a larger scale, a high density of osteons might reduce overall tissue density while increasing toughness, simply because osteons absorb or deflect cracks [109]. Repair of bone sufficiently damaged by the development of an enlarging crack may necessitate remodelling.
Since the supero-lateral cortex of the femoral neck can become as thin as 0.5–1.0 mm in old age [15,110], only a few intact cortical osteonal systems capable of crack capture can remain within it. This makes thin cortices, like trabeculae, dependent on the more microscopic crack-capturing mechanisms. Because femoral cortical bone is more liable to crack longitudinally, cracks that start transversely are usually diverted longitudinally (except intriguingly in patients with the sub-trochanteric fractures seen following long-term treatment with anti-resorptive agents [111,112]) so that a tendency to cortical splitting, akin to the delamination of plywood, might be the first adverse consequence of a heavy fall onto the greater trochanter.
Unremodelled cracks accumulate with ageing in the femur, notably in women [113]. Zioupos and Currey found differences between the cracks seen in older and younger cortical bone, with older subjects showing less evidence of crack deflection [114]. Diab et al. [115] induced cracking in fatigue tests and showed that older bone formed linear microcracks in preference to diffuse damage with an exponential decrease in fatigue life with age. By analogy with man-made composites, longitudinal splitting, in which cracking occurs parallel to the periosteal surface [58], must increase the tendency of the supero-lateral cortex to buckle under load [116]. At the nano-scale level, an additional effect of ageing is that collagen molecules undergo more glycation, which is likely to make bone harder and reduce toughness [117]. The degree of glycation is in part a function of tissue age, which is relatively high in the femoral cortex at approximately 20 years [118], and also of circulating levels of glycating sugars. Variations in the molecular nature of collagen cross links may also be influential [119]. Recent work has contrasted the beneficial effects of enzymatic glycation on bone's toughness with the deleterious effects of non-enzymic glycation, which is associated with linear cracking [120] and has been implicated as a contributing cause of atypical femoral fractures [121].
Diabetics provide up to 10% of hip fracture cases and have a 1.6 to 1.7-fold increased risk; they suffer osteoporotic fractures of the spine and hip at higher aBMD levels [122–125]. The relationship of undercarboxylated osteocalcin on the one hand to carbonate substitution and mineral crystallinity and on the other to adiponectin and the control of blood sugar might reflect a causal relationship between diabetes and reduced material properties. However, advanced glycation end products (AGEs), increased in diabetics and potentially affecting the toughness of bone collagen, are quite strongly implicated in their increased susceptibility to vertebral fractures, which are poorly predicted by aBMD measurements [126].
Osteocytes and the regulation of bone strength
Osteocytes may function collectively as a syncytium [127], are sensitive to mechanical loading, promote [68] bone breakdown and regulate its growth [69], may locally regulate matrix mineralization [128] and also mobilise calcium [66,129] in response to PTH signalling [130]. Their functions in vertebrates may have evolved with terrestrial living [131]. The concept that osteocytes function collectively has given rise to the idea that they might form part of a so-called “small world network” [132], which is a way of efficiently delivering a complex service at low economic cost in systems as diverse as parts of the brain [133] and large airline networks. Osteocytes, in a small world network, might deliver diverse and complex interactions with other cells and systems such as osteoblasts, osteoclasts, phosphate and calcium homeostasis in both plasma and local bone matrix, etc. Any small world network carries with it a potential risk of systemic breakdown in the event that key elements (e.g. certain osteocytes or groups of osteocytes) became disabled.
Osteocyte ageing and death
Osteocyte death is widespread in the elderly proximal femur, where the average newly entombed osteocyte must wait two or more decades before it is liberated from its bone matrix “prison” by osteoclastic remodelling [102,134]. While osteocytes throughout the skeleton are located frequently 0.1 mm or further from the circulation and hence their source of nutrients, osteocyte death is much less prevalent in more actively remodelling bone such as the iliac crest. In the femoral shaft, individual osteocyte lacunae shrink in volume as the subject ages, but the numbers of lacunae in the matrix per unit volume of bone tissue does not change [135]. Experimentally, Noble et al. showed that bone experiencing reduced loading contained more osteocytes undergoing apoptosis [136]. When loading exceeded normal limits, rapidly developing micro-damage was also associated [137] with greatly increased osteocyte apoptosis. This preceded the appearance of florid osteoclastic resorption and remodelling followed by infilling [136]. Parathyroid hormone (PTH) and oestrogen can also suppress sclerostin, promote osteocyte survival [73,138] and encourage a positive remodelling balance through increasing bone formation. Sclerostin is normally secreted by osteocytes and chondrocytes but not by osteoblasts or lining cells [69,139,140] and is likely to modify the local architecture of remodelling bone – a potential effect that would be lost in bone lacking osteocytes.
Osteocyte apoptosis may not be the most important osteocytic death mechanism in the proximal femur. Long-lived cells depend for their survival on the efficiency of their autophagy mechanisms. Targeted experimental suppression of autophagy, which leads to the premature senescence of osteocytes, results in a premature skeletal ageing phenotype in mice [141]. It is currently believed that premenopausal rates of remodelling result in healthier, stronger bones. But if remodelling is over-suppressed, eg by prolonged anti-resorptive treatment, the demands this might place on the long-term efficiency of osteocyte autophagy could become unachievable in the proximal femur.
Birth of osteocytes
Recent 3D studies of osteocyte densities [63,142] are at variance with previous suggestions [96] that excess pre-osteocytes are formed from osteoblasts in the birth of new BMUs; based on observed osteoblast densities [143], most if not all have a predestined future as osteocytes based on these new data. The matrix density of osteocytes also becomes reduced as infilling progresses [63]. Compared to controls, in hip fracture cases full osteonal closure appears prolonged, explaining in part the observed increases in cortical porosity. As the cortical osteon is filled in, osteocytes were confirmed to be embedded at ever declining rates relative to the formation of mineralized matrix in both cases and controls, more so in the former [63,144]. In the fracture cases, the proportion of osteocytes expressing sclerostin increased more rapidly with maturation of the osteon [145] while also expressing nitric oxide synthases (NOS) less frequently [146,147]; NOS are involved in osteocytic endocrine signalling [148]. If ongoing mineralization was observed, it was associated with a higher density of recently embedded osteocytes adjacent to the osteonal canal, yet osteocyte densities adequate by this criterion were rarely achieved in the hip fracture cases [144].
Other ageing effects affecting bone's material properties
Matrix hardening
Tjhia et al. [149] compared iliac biopsies from normal and osteoporotic subjects and found group differences between mineral content and nano-indentation-derived measures of hardness and resistance to plastic deformation. For a given mineralization level, hardness and deformation resistance increased with ageing, an effect that was increased in vertebral fracture cases. These increases suggested increased brittleness. In naïve-to-treatment case–control studies of hip fracture there was a small but statistically significant decrease in matrix mineralisation in fracture cases [150,151]. However, cases and controls had similar indentation modulus and hardness. This also suggests that the organic phase is stiffer in hip fracture and potentially less tough [150,151], consistent with the finding of Norman et al. that reduced mineralization is positively associated with diffuse damage and microcrack density [152].
Tissue-level and within-osteon heterogeneity
Ciarelli et al. suggested that the variation in mineralization between light and dark lamellae needs to be maintained for crack deflection to be optimal, while the lower levels seen in osteoporotic patients specifically with vertebral fracture may fail to deflect microcracks sufficiently [153]. In hip fracture cases naïve to anti-osteoporosis treatment, heterogeneity of both the mineral:matrix ratio and carbonate:phosphate ratios were reduced while crystallinity was increased [154], compared to controls.
Cortical osteons from young people have a steeper gradient of mineralization from the canal to the cement line than in older subjects, with the highest mineralization levels being adjacent to the canal [155], where the volumetric density of osteocytic nuclei is lowest [63,144]. This mineralization gradient may have been the first demonstration of an important source of heterogeneity, but it has yet to be related to the toughness of cortical bone.
Regulation of the size and orientation of mineral crystals: a role for osteocytes?
Kerschnitski et al. applied synchrotron small-angle X-ray scattering in tandem with confocal laser scanning microscopy to the study of bone mineral particles in relation to osteocytic dendrites in their canaliculi. They demonstrated that most ovine bone crystals are less than 1*10− 6 mm from the nearest osteocyte canaliculus and that the degree of regularity in the size and orientation of the crystals is statistically associated with proximity to an osteocytic structure [142]. Closer proximity was also associated with crystals of better mechanical characteristics.
The concept that the mineral phase provides strength in compression and the organic matrix strength in tension has also been re-examined. The protein matrix contributes to bone's integrity especially in tension or shear [10] and to the measured hardness of bone as assessed by nano-indentation. Gao et al. modelled the mineral platelets aligned in the direction of tensile stress, linked by the protein matrix that experiencing alternating zones of shear and tension [156]. When the crystals were restricted in length to about 30 nm, crystal imperfections (e.g. arising from inclusions of proteinaceous material within their substance) were of little importance in determining tensile strength. Larger crystals had an increased requirement for perfection. Crystal size grows with tissue ageing [157] sometimes spectacularly in hip fracture cases [158]. Gupta et al. showed that the mineral particles observed in bone can survive up to twice the fracture strain seen for bulk apatite [159]. This might allow healthy bone to avoid crack nucleation, if the mineral particles do not exceed a critical size. The load sharing mechanism between mineral and collagen may result in damage shielding, preventing the fibrils from being exposed to excessive strains. However, osteoporotic human bone showed raised levels of crystallinity and maturity in its mineral phase [160], which was thought to reduce its resistance to cracking.
Crack reorientation and capture within and between osteons and BSUs
The cement lines between BSUs and between osteons normally appear densely mineralized [161] and may provide routes for crack deflection. Lamellae near the cement line and tissue in the immediate vicinity of viable osteocytes are associated with reduced local tissue hardness, but may also be tougher [162]. The interesting observation by Boyde and Jones that aberrant non-mineralized cement lines may form in elderly bone [163] needs to be explored further in light of the cement line's likely toughening role. Guo et al. have found that crack “capture” by osteons was enhanced by lower levels of mineralisation [164]. Conceivably, increased remodelling, as is seen in the nearly hip fracture-free Gambians with their low calcium diet and high indices of bone remodelling [165] might result in tougher but less dense bone tissue due to higher osteocyte survival, less hard matrix and less advanced secondary mineralisation.
Treatment with bisphosphonates reduces the heterogeneity of both mineralization and the carbonate:phosphate ratio; interestingly even without such treatments hip fracture cases have reductions in these ratios [154]. There is some evidence that these specific types of heterogeneity might reduce proliferation of micro-cracks [166–168], crack initiation and fatigue failure [169,170]. Renders et al. [167] who used Finite Element Analysis to simulate trabecular bone under mechanical load, found that increased heterogeneity tended to remove higher strain levels into the interior of the trabeculae, a potentially valuable means for reducing the initiation of dangerous cracks.
Synthesis
Each hip fracture begins as a microscopic crack. The young adult human femur has multiple defences against crack enlargement at varying length scales that finally fail during a hip fracture some decades later. Each line of defence absorbs some of the potential energy delivered to any new or old microscopic crack by moderate trauma, for example a sideways fall to the ground. In doing so these defences collectively reduce the kinetic energy available to the crack, energy that is essential to drive its directional expansion into a clinical fracture. The multiple effects of ageing on the proximal femur progressively increase the likelihood of mechanical failure either by increasing the local strain experienced during the fall or else by reducing the effectiveness of local mechanisms that impede or divert crack growth. To the interactions between these multiply declining safety mechanisms can be attributed the exponential rise in the risk of hip fracture with ageing.
At the macroscopic level, attention has become focused on the regions of the proximal femur that lose cortical thickness or trabecular volumetric density most rapidly [45]. Analysis of normal first world physical activity patterns suggest that the supero-lateral femoral neck cortex is one of the zones least loaded mechanically. Local cortical bone losses lead to corresponding increases in strain on direct compression in a fall, increasing the likelihood of plastic deformation to the point of catastrophic failure, which might occur with or without buckling of the cortex. Co-existing microscopic age-related changes collectively reduce the toughness of the remaining bone tissue with its capacity to absorb energy by plastic deformation; and they leave tell-tale signatures such as the greater linearity of non-catastrophic cracks that can be observed microscopically [85,114].
Recent studies of their morphology and function suggest that embedded live osteocytes have one or more key roles that are relevant to the multiple toughening processes operating at sub-osteon level. They are the only cellular presence in close proximity to the mineral–matrix interface, the mechanical performance of which is now known to be sensitive to variations in matrix and crystal characteristics (such as crystal size and thickness) as well as the interactions between mineral and matrix. Half of all osteocytes die in situ in the normal elderly proximal femoral cortex [102,134]; but if osteocytes form a potentially energy efficient and damage-resistant small or single world network [142], this level of mortality might leave some toughening functions largely intact, provided the minority of osteocytes with the most profuse connections, i.e. those potentially functioning in communications parlance as “hubs”, are protected. To investigate further the osteocyte's role in avoiding hip fracture requires that functional studies of the osteocyte network be put in the correct 3-dimensional anatomical context. An age-dependent decline in osteocyte lacunar size over the human lifespan [135] is also suggestive of generally changed function of surviving osteocytes, since progressive maturation is associated with shrinkage in volume and changes in the profile of the bioactive molecules osteocytes express [65].
To study the propagation of fragility effects in older persons across hierarchical levels requires both experimental and correlative clinical studies. Alongside the study of the most microscopic (Level 4) effects of ageing on bone fragility and toughness, we must also develop better ways to determine modes of hip fracture, e.g. to differentiate crushing from buckling failure of the femoral neck cortex in compression. High-resolution imaging is used to study failure in metal alloys and non-biological composites. If it becomes feasible to image clinically a specific region of weakness e.g. with advanced imaging [57], it is a matter of elementary anatomy that the proximal femur is very accessible via the percutaneous route if an effective local strengthening treatment, deliverable prior to hip fracture, e.g. under CT control via needle, could be developed.
There is urgency because of the limited impact on public health of current anti-osteoporosis treatments, despite their effectiveness at the individual level. Johannesdottir et al. found that in the ninth decade normal physical activity is insufficient to prevent the supero-lateral proximal femur experiencing fast ongoing cortical thinning [45]. Simply projecting this thinning trend towards the average woman's 100th birthday leads one to expect, if she survives, that the supero-lateral femoral neck cortex could effectively disappear leaving the hip structurally unable to bear her weight in motion. And a century of survival is expected to become tenfold more likely for women born in 1950 compared to those born in 1910 [171].
Cracking parallel to the periosteal surface in elderly femoral bone to create apparent delaminations is a possibility suggested by the study of failure in man-made composites. These would naturally enlarge further under forces otherwise insufficient to buckle the structure [116] and might explain why repeated fallers can sometimes suffer hip fractures after falling less violently than on previous occasions when the hip did not break. The long-term consequence of delamination is a potential reduction in the critical buckling stress that might be progressive in a hip subjected to repeated falls. Such a hip might fracture more easily than the amount of cortical and trabecular bone suggests – a possibility that requires ex vivo study. Indeed, clinicians will soon expect to calculate from clinical CT scans the now measurable [57] minimum safe thickness of the supero-lateral cortex that will not enlarge the pre-existing cracks known to populate the elderly female femoral cortex [172]. This may require an understanding of the resistance to buckling provided by the trabeculae to which the cortex is attached [15]. They will also wish to use novel technology [56] in trials of hip strengthening interventions, which are providing some encouragement even using older DXA monitoring [173].
While the risk for hip fracture is partly heritable [174], the fact that many common genetic variants have modest effects on fracture risk [175] and that these are pleiotropic [176] is consistent with the data presented here. The possibility that some reversible aspects of western lifestyles have contributed to the large 20th century increases in age-specific hip fracture risk encourages study of gene–environment interactions.
Conflict of interest
Both authors state that they have no conflicts of interest.
Acknowledgments
This is based in part on the Charles Dent lecture given to the Bone Research Society Cambridge meeting 2011. The authors acknowledge support from grants from the UK MRC (G9321536 & G0501550), Arthritis Research UK (grant no 17173), ASBMR (Bridge Funding Research Grants Program), BBSRC BB/D004624, NIHR Biomedical Research Centre (Cambridge) and EU FP7 (F2-2008–201099 & 201865).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Length scales are ordered from largest to smallest because of the potential need to add further scales at the more microscopic levels.
Contributor Information
Jonathan Reeve, Email: jonathan.reeve@ndorms.ox.ac.uk.
Nigel Loveridge, Email: nl10003@cam.ac.uk.
References
- 1.Kanis J.A., Odén A., McCloskey E.V., Johansson H., Wahl D.A., Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melton L.J., III A Gompertzian view of osteoporosis. Calcif Tissue Int. 1990;46:285–286. doi: 10.1007/BF02563815. [DOI] [PubMed] [Google Scholar]
- 3.Pulkkinen P., Glüer C.C., Jämsä T. Investigation of differences between hip fracture types: a worthy strategy for improved risk assessment and fracture prevention. Bone. 2011;49:600–604. doi: 10.1016/j.bone.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Bell G.H. Bone as a skeletal structure. Proc Nutr Soc. 1952;6:405–409. doi: 10.1079/bjn19520045. [DOI] [PubMed] [Google Scholar]
- 5.Cummings S.R., Bates D., Black D.M. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [erratum 2825] [DOI] [PubMed] [Google Scholar]
- 6.NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:85–95. [Google Scholar]
- 7.Cooke A.M. Osteoporosis. Lancet. 1955:877–882. doi: 10.1016/s0140-6736(55)90374-3. 929-937. [DOI] [PubMed] [Google Scholar]
- 8.Backman S. Karolinska Sjukhuset; Stockholm, Sweden: 1957. The proximal end of the femur: investigations with special reference to the etiology of femoral neck fractures. Diagnostic Radiology, pp. 170. [PubMed] [Google Scholar]
- 9.Hirsch C., Brodetti A. The weight-bearing capacity of structural elements in femoral necks. Acta Orthop Scand. 1956;26:15–24. [PubMed] [Google Scholar]
- 10.Burr D.B. The contribution of the organic matrix to bone's material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 11.Johnell O., Borgstrom F., Jonsson B., Kanis J.A. Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int. 2007;18:333–337. doi: 10.1007/s00198-006-0245-4. [DOI] [PubMed] [Google Scholar]
- 12.Bell K., Loveridge N., Power J., Garrahan N., Stanton M., Lunt M. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res. 1999;14:112–120. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree N., Loveridge N., Parker M., Rushton N., Power J., Bell K.L. Intracapsular hip fracture and the region-specific loss of cortical bone: analysis by Quantitative Computed Tomography (pQCT) J Bone Miner Res. 2001;16:1318–1328. doi: 10.1359/jbmr.2001.16.7.1318. [DOI] [PubMed] [Google Scholar]
- 14.Mayhew P.M., Thomas C.D.L., Clement J.G., Loveridge N., Beck T.J., Bonfield W. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 15.Thomas C.D.L., Mayhew P.M., Power J., Poole K.E.S., Loveridge N., Clement J.G. Femoral neck trabecular bone: loss with ageing and role in preventing fracture. J Bone Miner Res. 2009;24:1808–1818. doi: 10.1359/jbmr.090504. [DOI] [PubMed] [Google Scholar]
- 16.Adebajo A.O., Cooper C., Evans J.G. Fractures of the hip and distal forearm in West Africa and the United Kingdom. Age Ageing. 1991;20:435–438. doi: 10.1093/ageing/20.6.435. [DOI] [PubMed] [Google Scholar]
- 17.Elffors I., Allander E., Kanis J.A., Gullberg B., Johnell O., Dequeker J. The variable incidence of hip fracture in Southern Europe: the MEDOS study. Osteoporos Int. 1994;4:253–263. doi: 10.1007/BF01623349. [DOI] [PubMed] [Google Scholar]
- 18.Aspray T.J., Prentice A., Cole T., Sawo Y., Reeve J., Francis R.M. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11:1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 19.Yan W., Li X. Impact of diabetes and its treatments on skeletal diseases. Front Med. 2013;7:81–90. doi: 10.1007/s11684-013-0243-9. [DOI] [PubMed] [Google Scholar]
- 20.Malo M.K., Rohrbach D., Isaksson H., Töyräs J., Jurvelin J.S., Tamminen I.S. Longitudinal elastic properties and porosity of cortical bone tissue vary with age in human proximal femur. Bone. 2013;53:451–458. doi: 10.1016/j.bone.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Sievanen H., Kannus P., Jarvinen L.N. Bone quality: an empty term. PLoS Med. 2007;4:e27. doi: 10.1371/journal.pmed.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner C.H. Bone strength: current concepts. Ann N Y Acad Sci. 2006;1068:429–446. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 23.Sievanen H., Josza L., Pap I., Jarvinen M., Jarvinen T.A., Kannus P. Fragile external phenotype of modern human proximal femur in comparison with medieval bone. J Bone Miner Res. 2007;22:537–543. doi: 10.1359/jbmr.070102. [DOI] [PubMed] [Google Scholar]
- 24.Currey J.D. Hierarchies in biomineral structures. Science. 2005;309:253–254. doi: 10.1126/science.1113954. [DOI] [PubMed] [Google Scholar]
- 25.Currey J., Brear K., Zioupos P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J Biomech. 1995;29:257–260. doi: 10.1016/0021-9290(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 26.Beck T.J., Oreskovic T.L., Stone K.L., Ruff C.B., Ensrud K., Nevitt M.C. Structural adaptation to changing skeletal load in the progression towards hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 27.de Laet C.E.D.H., van Hout B.A., Burger H., Hofman A., Pols H.A.P. Bone density and the risk of hip fracture in men and women: cross sectional analysis. BMJ. 1997;315:221–225. doi: 10.1136/bmj.315.7102.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez C.J., Keaveny T.M. A biomechanical perspective on bone quality. Bone. 2006;39:1173–1181. doi: 10.1016/j.bone.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy D.K., Pye S.R., Lunt M., O'Neill T.W., Todd C.J., Raspe H.H. and the EPOS Study Group. Falls explain between centre differences in the incidence of limb fracture across Europe. Bone. 2002;31:712–717. doi: 10.1016/s8756-3282(02)00909-2. [DOI] [PubMed] [Google Scholar]
- 30.Cameron I.D., Gillespie L.D., Robertson M.C., Hill K.D., Murray G.R., Cumming R.G. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev. Jan 20 2012;(1):CD005465. doi: 10.1002/14651858.CD005465.pub2. [see also update in: Cochrane Database Syst Rev. 2012;12:CD005465. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005465.pub3/full 2013-12-20] [DOI] [PubMed] [Google Scholar]
- 31.McKee M.D., Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Galilei Linceo Galileo. Elsevier; Leiden: 1638. Discorsi e dimostrazioni matematiche intorno a due nuove scienze attenti alla macanica e i movimenti locali. [Google Scholar]
- 33.Darwin C. John Murray; London: 1859. On the origin of species by means of natural selection. [Google Scholar]
- 34.Wolff J. A Hirschwald; Berlin: 1892. Das Gesetz der Transformation der Knochen. [Google Scholar]
- 35.Koch J. The laws of bone architecture. Am J Anat. 1917;21:177–298. [Google Scholar]
- 36.Skedros J.G., Baucom S.L. Mathematical analysis of trabecular ‘trajectories’ in apparent trajectorial structures: the unfortunate historical emphasis on the human proximal femur. J Theor Biol. 2007;244:15–45. doi: 10.1016/j.jtbi.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Bell G.H., Dunbar O., Beck J.S., Gibb A. Variations in strength of vertebrae with age and their relation to osteoporosis. Calcif Tissue Res. 1967;1:75–86. doi: 10.1007/BF02008077. [DOI] [PubMed] [Google Scholar]
- 38.Crabtree N., Lunt M., Holt G., Kröger H., Burger H., Grazio S. Hip geometry, bone mineral distribution and bone strength in European men and women: the EPOS study. Bone. 2000;27:151–160. doi: 10.1016/s8756-3282(00)00300-8. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa T., Turner C.H., Peacock M., Slemenda C.W., Weaver C.M., Teegarden D. Geometric structure of the femoral neck measured using dual-energy X-ray absorptiometry. J Bone Miner Res. 1994;9:1053–1064. doi: 10.1002/jbmr.5650090713. [DOI] [PubMed] [Google Scholar]
- 40.Kaptoge S., Dalzell N., Jakes R., Wareham N., Khaw K.T., Beck T.J. Hip section modulus, a measure of bending resistance. Is more strongly related to physical activity than BMD. Osteoporos Int. 2003;14:941–949. doi: 10.1007/s00198-003-1484-2. [DOI] [PubMed] [Google Scholar]
- 41.Kaptoge S., Dalzell N., Loveridge N., Beck T.J., Khaw K.T., Reeve J. Effects of gender, anthropometric variables and aging on the evolution of hip strength in men and women aged over 65. Bone. 2003;32:561–570. doi: 10.1016/s8756-3282(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 42.Power J., Loveridge N., Rushton N., Parker M., Reeve J. Intracapsular bone formation on the external ‘periosteal’ surface of the femoral neck: an investigation in cases of hip fracture and controls. Osteoporos Int. 2003;14:146–151. doi: 10.1007/s00198-002-1333-8. [DOI] [PubMed] [Google Scholar]
- 43.Kaptoge S., Dalzell N., Folkerd E., Doody D., Khaw K.T., Beck T.J. Sex hormone status may modulate rate of expansion of proximal femur diameter in older women alongside other skeletal regulators. J Clin Endocrinol Metab. 2007;92:304–313. doi: 10.1210/jc.2006-0893. [DOI] [PubMed] [Google Scholar]
- 44.Poole K.E.S., Mayhew P.M., Rose C.M., Brown J.K., Bearcroft P.J., Loveridge N. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res. 2010;25:482–491. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 45.Johannesdottir F., Aspelund T., Reeve J., Poole K.E.S., Sigurdsson S., Harris T.B. Age-related regional losses of cortical and trabecular bone in femoral neck in elderly women and men: the AGES-Reykjavik longitudinal study. J Bone Miner Res. 2013;28:2165–2176. doi: 10.1002/jbmr.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L., Udall W.J., McCloskey E.V., Eastell R. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int. 2014;25:251–263. doi: 10.1007/s00198-013-2401-y. [DOI] [PubMed] [Google Scholar]
- 47.Tsangari H., Findlay D.M., Fazzalari N.L. Structural and remodeling indices in the cancellous bone of the proximal femur across adulthood. Bone. 2007;40:211–217. doi: 10.1016/j.bone.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Crabtree N.J., Kroger H., Martin A., Pols H.A.P., Lorenc R., Nijs J. Improving risk assessment: hip geometry, bone mineral distribution and bone strength in hip fracture cases and controls. The EPOS study. Osteoporos Int. 2002;13:48–54. doi: 10.1007/s198-002-8337-y. [DOI] [PubMed] [Google Scholar]
- 49.Bell K.L., Loveridge N., Power J., Stanton M., Meggitt B.F., Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 50.Naylor K.E., McCloskey E.V., Eastell R., Yang L. Use of DXA-based finite element analysis of the proximal femur in a longitudinal study of hip fracture. J Bone Miner Res. 2013;28:1014–1021. doi: 10.1002/jbmr.1856. [DOI] [PubMed] [Google Scholar]
- 51.Kaptoge S., Beck T.J., Reeve J., Stone K.L., Hillier T.A., Cauley J.A. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaCroix A.Z., Beck T.J., Cauley J.A., Lewis C.E., Bassford T., Jackson R. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelke K. Assessment of bone quality and strength with new technologies. Curr Opin Endocrinol Diabetes Obes. 2012;19:474–482. doi: 10.1097/MED.0b013e32835a2609. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Sanyal A., Cawthon P.M., Palermo L., Jekir M., Christensen J. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27:808–816. doi: 10.1002/jbmr.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang T.F., Sigurdsson S., Karlsdottir G., Oskarsdottir D., Sigmarsdottir A., Chengshi J. Age-related loss of proximal femoral strength in elderly men and women: the age gene/environment susceptibility study—Reykjavik. Bone. 2012;50:743–748. doi: 10.1016/j.bone.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopperdahl D.L., Aspelund T., Hoffmann P.F., Sigurdsson S., Siggeirsdottir K., Harris T.B. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. Aug 16 2013 doi: 10.1002/jbmr.2069. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole K.E.S., Treece G.M., Mayhew P.M., Vaculík J., Dungl P., Horák M. Cortical thickness mapping to identify focal osteoporosis in patients with hip fracture. PLoS One. 2012;7:e38466. doi: 10.1371/journal.pone.0038466. [Epub 2012 Jun 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Bakker P.M., Manske S.L., Ebacher V., Oxland T.R., Cripton P.A., Guy P. During sideways falls proximal femur fractures initiate in the superolateral cortex: evidence from high-speed video of ex vivo fractures. J Biomech. 2009;42:1917–1925. doi: 10.1016/j.jbiomech.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Manske S.L., Liu-Ambrose T., Cooper D.M.L., Kontulainen S., Guy P., Forster B.B. Cortical and trabecular bone in the femoral neck both contribute to proximal femur failure load prediction. Osteoporos Int. 2009;20:445–453. doi: 10.1007/s00198-008-0675-2. [DOI] [PubMed] [Google Scholar]
- 60.Timoshenko S.P., Gere J.M. McGraw Hill; New York: 1979. Theory of elastic stability. [Google Scholar]
- 61.Hetenyi M. University of Michigan Press; Detroit: 1946. Beams on elastic foundation: theory with applications in the fields of civil and mechanical engineering. [Google Scholar]
- 62.Carpenter R.D., Beaupré G.S., Lang T.F., Orwoll E.S., Carter D.R., Osteoporotic fractures in men (MrOS) study group New QCT analysis approach shows the importance of fall orientation on femoral neck strength. J Bone Miner Res. 2005;20:1533–1542. doi: 10.1359/JBMR.050510. [DOI] [PubMed] [Google Scholar]
- 63.Hannah K.M., Thomas C.D.L., Clement J.G., De Carlo F., Peele A.G. Bimodal distribution of osteocyte lacunar size in the human femoral cortex as revealed by micro-CT. Bone. 2010;47:866–871. doi: 10.1016/j.bone.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 64.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 65.Dallas S.L., Prideaux M., Bonewald L.F. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wysolmerski J.J. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone. 2013;54:230–236. doi: 10.1016/j.bone.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaman G., Pitsillides A.A., Rawlinson S.C.F., Suswillo R.L.F., Mosley J.R., Cheng M.Z. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14:1123–1131. doi: 10.1359/jbmr.1999.14.7.1123. [DOI] [PubMed] [Google Scholar]
- 68.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 69.Poole K.E., van Bezooijen R.L., Loveridge N., Hamersma H., Papapoulos S.E., Lowik C. Sclerostin is a delayed secretion product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 70.Hordon L.D., Peacock M. Osteomalacia and osteoporosis in femoral neck fracture. Bone Miner. 1990;11:247–259. doi: 10.1016/0169-6009(90)90063-l. [DOI] [PubMed] [Google Scholar]
- 71.Compston J.E., Vedi S., Croucher P.I. Low prevalence of osteomalacia in elderly patients with hip fracture. Age Ageing. 1991;20:132–134. doi: 10.1093/ageing/20.2.132. [DOI] [PubMed] [Google Scholar]
- 72.Ebacher V., Guy P., Oxland T.R., Wang R.Z. Sub-lamellar microcracking and roles of canaliculi in human cortical bone. Acta Biomater. 2012;8:1093–1100. doi: 10.1016/j.actbio.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Tomkinson A., Reeve J., Shaw R.W., Noble B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metabol. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 74.Kogianni G., Mann V., Noble B.S. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23:915–927. doi: 10.1359/jbmr.080207. [DOI] [PubMed] [Google Scholar]
- 75.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1226–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mackey D.C., Black D.M., Bauer D.C., McCloskey E.V., Eastell R., Mesenbrink P. Effects of antiresorptive treatment on nonvertebral fracture outcomes. J Bone Miner Res. 2011;26:2411–2418. doi: 10.1002/jbmr.446. [DOI] [PubMed] [Google Scholar]
- 77.Chavassieux P., Seeman E., Delmas P.D. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 78.Bell K., Loveridge N., Power J., Rushton N., Reeve J. Intracapsular hip fracture: increased cortical remodelling in the thinned and porous anterior region of the femoral neck. Osteoporos Int. 1999;10:248–257. doi: 10.1007/s001980050223. [DOI] [PubMed] [Google Scholar]
- 79.Bell K.L., Loveridge N., Reeve J., Thomas C.D.L., Feik S.A., Clement J.G. Super-osteons (remodelling clusters) in the cortex of the femoral shaft: influence of age and gender. Anat Rec. 2001;264:378–386. doi: 10.1002/ar.10014. [DOI] [PubMed] [Google Scholar]
- 80.Jordan G., Loveridge N., Bell K.L., Power J., Rushton N., Reeve J. Spatial clustering of osteonal remodelling: a cause of focal weakness in the femoral neck cortex in hip fracture. Bone. 2000;26:305–313. doi: 10.1016/s8756-3282(99)00272-0. [DOI] [PubMed] [Google Scholar]
- 81.Arhatari B.D., Cooper D.M.L., Thomas C.D.L., Clement J.G., Peele A.G. Imaging the 3D structure of secondary osteons in human cortical bone using phase-retrieval tomography. Phys Med Biol. 2011;56:5265–5274. doi: 10.1088/0031-9155/56/16/012. [DOI] [PubMed] [Google Scholar]
- 82.Cooper D.M.L., Erickson B., Peele A.G., Hannah K., Thomas C.D., Clement J.G. Visualization of 3D osteon morphology by synchrotron radiation micro-CT. J Anat. 2011;219:481–489. doi: 10.1111/j.1469-7580.2011.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zebaze R.M., Ghasem-Zadeh A., Bohte A., Iuliano-Burns S., Mirams M., Price R.I. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 84.Kersh M.E., Pandy M.G., Bui Q.M., Jones A.C., Arns C.H., Knackstedt M.A. The heterogeneity in femoral neck structure and strength. J Bone Miner Res. 2013;28:1022–1028. doi: 10.1002/jbmr.1827. [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann E.A., Schaible E., Bale H., Barth H.D., Tang S.Y., Reichert P. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A. 2011;108:14416–14421. doi: 10.1073/pnas.1107966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bell K.L., Loveridge N., Jordan G.R., Power J., Constant C.R., Reeve J. A novel mechanism for the induction of the increased cortical porosity in cases of intra-capsular hip fracture. Bone. 2000;27:297–304. doi: 10.1016/s8756-3282(00)00318-5. [DOI] [PubMed] [Google Scholar]
- 87.Power J., Loveridge N., Lyon A., Rushton N., Parker M., Reeve J. Osteoclastic cortical erosion as a determinant of sub-periosteal osteoblastic bone formation in the femoral neck's response to BMU imbalance. Effects of stance-related loading and hip fracture. Osteoporos Int. 2005;6:1049–1056. doi: 10.1007/s00198-004-1803-2. [DOI] [PubMed] [Google Scholar]
- 88.Vajda E.G., Bloebaum R.D. Age-related hyper-mineralization in the female proximal human femur. Anat Rec. 1999;255:202–211. doi: 10.1002/(SICI)1097-0185(19990601)255:2<202::AID-AR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 89.Reeve J. A stochastic analysis of iliac trabecular bone dynamics. Clin Orthop Relat Res. 1986;213:264–278. [PubMed] [Google Scholar]
- 90.Tsangari H., Kuliwaba J.S., Fazzalari N. Trabecular bone remodelling and subcapital femoral fracture. J Musculoskelet Neuronal Interact. 2007;7:69–73. [PubMed] [Google Scholar]
- 91.Hernandez C.J., Gupta A., Keaveny T.M. A biomechanical analysis of the effects of resorption cavities on cancellous bone strength. J Bone Miner Res. 2006;21:1248–1255. doi: 10.1359/jbmr.060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verhulp E., van Rietbergen B., Huiskes R. Load distribution in the healthy and osteoporotic human proximal femur during a fall to the side. Bone. 2008;42:30–35. doi: 10.1016/j.bone.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 93.van Rietbergen B., Huiskes R., Eckstein F., Ruegsegger P. Trabecular bone tissue strains in the healthy and osteoporotic human femur. J Bone Miner Res. 2003;18:1781–1788. doi: 10.1359/jbmr.2003.18.10.1781. [DOI] [PubMed] [Google Scholar]
- 94.Kopperdahl D.L., Keaveny T.M. Yield strain behaviour of trabecular bone. J Biomech. 1998;31:601–608. doi: 10.1016/s0021-9290(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 95.Peterlik H., Roschger P., Klaushofer K., Fratzl P. From brittle to ductile fracture of bone. Nat Mater. 2006;5:52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- 96.Parfitt A.M. The physiological and clinical significance of bone histomorphometric data. In: Recker R., editor. Bone histomorphometry, techniques and interpretation. CRC Press; Boca Raton FL: 1983. pp. 143–224. [Google Scholar]
- 97.Griffiths A.A. The phenomena of rupture and flow in solids. Philos Trans R Soc Lond A. 1921;221:163–198. [Google Scholar]
- 98.Launey M.E., Buehler M.J., Ritchie R.O. On the mechanistic origins of toughness in bone. Annu Rev Mater Res. 2010;40:25–53. [Google Scholar]
- 99.Broek D. Martinus Nijhoff; The Hague: 1982. Elementary engineering fracture mechanics. [Google Scholar]
- 100.Ruppel M.E., Miller L.M., Burr D.B. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int. 2008;19:1251–1265. doi: 10.1007/s00198-008-0579-1. [DOI] [PubMed] [Google Scholar]
- 101.Fratzl P. When the cracks begin to show. Nat Mater. 2008;7:610–612. doi: 10.1038/nmat2240. [DOI] [PubMed] [Google Scholar]
- 102.Dunstan C.R., Evans R.A., Hills E., Wong S.Y., Higgs R.J. Bone death in hip fracture in the elderly. Calcif Tissue Int. 1990;47:270–275. doi: 10.1007/BF02555908. [DOI] [PubMed] [Google Scholar]
- 103.Currey J.D. Stress concentrations in bone. Q J Microsc Sci. 1962;103:111–133. [Google Scholar]
- 104.Akkus O., Rimnac C.M. Cortical bone tissue resists fatigue fracture by deceleration and arrest of microcrack growth. J Biomech. 2001;34:757–764. doi: 10.1016/s0021-9290(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 105.Thompson J.B., Kindt J.H., Drake B., Hansma H.G., Morse D.E., Hansma P.K. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414:773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 106.Paschalis E.P., Tatakis D.N., Robins S., Fratzl P., Manjubala I., Zoehrer R. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone. 2011;49:1232–1241. doi: 10.1016/j.bone.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nalla R.K., Stolken J.S., Kinney J.H., Ritchie R.O. Fracture in human cortical bone: local fracture criteria and toughening mechanisms. J Biomech. 2005;38:1517–1525. doi: 10.1016/j.jbiomech.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 108.Fratzl P., Gupta H.S., Fischer F.D., Kolednik O. Hindered crack propagation in materials with periodically varying young's modulus—lessons from biological materials. Adv Mater. 2007;19:2657–2661. [Google Scholar]
- 109.Yeni Y.N., Norman T.L. Fracture toughness of human femoral neck: effect of microstructure, composition, and age. Bone. 2000;26:499–504. doi: 10.1016/S8756-3282(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 110.Johannesdottir F., Poole K.E.S., Reeve J., Siggeirsdottir K., Aspelund T., Mogensen B. Distribution of cortical bone in the femoral neck and hip fracture: a prospective case–control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone. 2011;48:1268–1276. doi: 10.1016/j.bone.2011.03.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schilcher J., Koeppen V., Ranstam J., Skripitz R., Michaëlsson K., Aspenberg P. Atypical femoral fractures are a separate entity, characterized by highly specific radiographic features. A comparison of 59 cases and 218 controls. Bone. 2013;52:389–392. doi: 10.1016/j.bone.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 112.van der Meulen M.C., Boskey A.L. Atypical subtrochanteric femoral shaft fractures: role for mechanics and bone quality. Arthritis Res Ther. 2012;14:220. doi: 10.1186/ar4013. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schaffler M.B., Boyce T.M., Lundin-Cannon K.D., Milgrom C., Fyhrie D.P. Age-related architectural changes and microdamage accumulation in the human femoral neck cortex. Trans Orthop Res Soc. 1995;20:549. [Google Scholar]
- 114.Zioupos P., Currey J.D. Changes in the stiffness, strength and toughness of human cortical bone with age. Bone. 1998;22:57–66. doi: 10.1016/s8756-3282(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 115.Diab T., Sit S., Rho J., Vashishth D. Age-dependent fatigue behaviour of human cortical bone. Eur J Morphol. 2005;42:53–59. doi: 10.1080/09243860500095539. [DOI] [PubMed] [Google Scholar]
- 116.Nilsson K.-F., Asp L.E., Alpman J.E., Nystedt L. Delamination buckling and growth for delaminations at different depths in a slender composite panel. Int J Solids Struct. 2001;38:3039–3071. [Google Scholar]
- 117.Wang X., Shen X., Li X., Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 118.Wand J.S., Smith T., Green J.R., Hesp R., Bradbeer J.N., Reeve J. Whole body and site-specific bone remodelling in patients with previous femoral fractures. Relationships between reduced physical activity, reduced bone mass and increased bone resorption. Clin Sci. 1992;83:665–675. doi: 10.1042/cs0830665. [DOI] [PubMed] [Google Scholar]
- 119.Banse X., Sims T.J., Bailey A.J. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002;17:1621–1628. doi: 10.1359/jbmr.2002.17.9.1621. [DOI] [PubMed] [Google Scholar]
- 120.Karim L., Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ettinger B., Burr D.B., Ritchie R.O. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013;55:495–500. doi: 10.1016/j.bone.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 122.Schwartz A., Sellmeyer D., Ensrud K., Cauley J., Tabor H., Schreiner P. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endrocrinol Metab. 2001;86:7. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 123.Forsén L., Meyer H.E., Midthjell K., Edna T.H. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 124.Ottenbacher K.J., Ostir G.V., Peek M.K., Goodwin J.S., Markides K.S. Diabetes mellitus as a risk factor for hip fracture in Mexican American older adults. J Gerontol A Biol Sci Med Sci. 2002;57:M648–M653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- 125.Norris R., Parker M. Diabetes mellitus and hip fracture: a study of 5966 cases. Injury. 2011;42:1313–1316. doi: 10.1016/j.injury.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 126.Yamaguchi T., Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus [Review] Endocr J. 2011;58:613–624. doi: 10.1507/endocrj.ej11-0063. [DOI] [PubMed] [Google Scholar]
- 127.Palumbo C., Ferretti M., Marotti G. Osteocyte dendrogenesis in static and dynamic bone formation: an ultrastructural study. Anat Rec. 2004;278A:474–480. doi: 10.1002/ar.a.20032. [DOI] [PubMed] [Google Scholar]
- 128.Atkins G.J., Findlay D.M. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int. 2012;23:2067–2079. doi: 10.1007/s00198-012-1915-z. [DOI] [PubMed] [Google Scholar]
- 129.Talmage D.W., Talmage R.V. Calcium homeostasis: how bone solubility relates to all aspects of bone physiology. J Musculoskelet Neuronal Interact. 2007;7:108–112. [PubMed] [Google Scholar]
- 130.Saini V., Marengi D.A., Barry K.J., Fulzele K.S., Heiden E., Liu X. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Witten P.E., Huysseune A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev. 2009;84:315–346. doi: 10.1111/j.1469-185X.2009.00077.x. [DOI] [PubMed] [Google Scholar]
- 132.Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 133.Bullmore E., Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 134.Power J., Noble B.S., Loveridge N., Bell K.L., Rushton N., Reeve J. Osteocyte viability in the femoral neck cortex: an association with cortical remodelling in cases of hip fracture and controls. Calcif Tissue Int. 2001;69:13–19. doi: 10.1007/s00223-001-0013-6. [DOI] [PubMed] [Google Scholar]
- 135.Carter Y., Thomas C.D., Clement J.G., Cooper D.M.L. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. J Struct Biol. 2013;183:519–526. doi: 10.1016/j.jsb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 136.Noble B., Peet N., Stevens H., Brabbs A., Mosley J., Reilly G. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:934c–944c. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 137.Verborgt O., Gibson G.J., Schaffler M.B. Loss of osteocyte integrity in association with microdamage and bone remodelling after fatigue in vivo. J Bone Miner Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 138.Jilka R.L., Weinstein R.S., Bellido T., Robertson P., Parfitt A.M., Manolagas S.C. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Bezooijen R.L., Roelen B.A., Visser A., van der Wee-Pals L., de Wilt E., Karperien M. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atkins G.J., Rowe P.S., Lim H.P., Welldon K.J., Ormsby R., Wijenayaka A.R. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011;26:1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Onal M., Piemontese M., Xiong J., Wang Y., Han L., Ye S. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288:17432–17440. doi: 10.1074/jbc.M112.444190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kerschnitzki M., Kollmannsberger P., Burghammer M., Duda G.N., Weinkamer R., Wagermaier W. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res. 2013;28:1837–1845. doi: 10.1002/jbmr.1927. [DOI] [PubMed] [Google Scholar]
- 143.Schen S., Villanueva A.R., Frost H.M. Number of osteoblasts per unit area of osteoid seam in cortical human bone. Can J Physiol Pharmacol. 1965;43:319–325. doi: 10.1139/y65-031. [DOI] [PubMed] [Google Scholar]
- 144.Power J., Doube M., van Bezooijen R., Loveridge N., Reeve J. Osteocyte recruitment declines as the osteon fills in: interacting effects of osteocytic sclerostin and previous hip fracture on the size of cortical canals in the femoral neck. Bone. 2012;50:1107–1114. doi: 10.1016/j.bone.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 145.Power J., Poole K.E., van Bezooijen R., Doube M., Caballero-Alías A.M., Lowik C. Sclerostin and the regulation of bone formation: effects in hip osteoarthritis and femoral neck fracture. J Bone Miner Res. 2010;25:1867–1876. doi: 10.1002/jbmr.70. [DOI] [PubMed] [Google Scholar]
- 146.Caballero A., Loveridge N., Pitsillides A., Parker M., Kaptoge S., Lyon A. Osteocytic expression of constitutive NO synthase isoforms in the femoral neck cortex: a case–control study of intra-capsular hip fracture. J Bone Miner Res. 2005;20:268–273. doi: 10.1359/JBMR.041103. [DOI] [PubMed] [Google Scholar]
- 147.Loveridge N., Fletcher S., Power J., Caballero A.M., Das-Gupta V., Rushton N. Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone. 2002;30:866–871. doi: 10.1016/s8756-3282(02)00732-9. [DOI] [PubMed] [Google Scholar]
- 148.Sunters A., Armstrong V.J., Zaman G., Kypta R.M., Kawano Y., Lanyon L.E. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) receptor sensitivity to anbient IGF, leading to phosphoinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem. 2010;285:8743–8758. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tjhia C.K., Stover S.M., Rao D.S., Odvina C.V., Fyhrie D.P. Relating micromechanical properties and mineral densities in severely suppressed bone turnover patients, osteoporotic patients and normal subjects. Bone. 2012;51:114–122. doi: 10.1016/j.bone.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loveridge N., Power J., Reeve J., Boyde A. Bone mineralization density and femoral neck fragility. Bone. 2004;35:929–941. doi: 10.1016/j.bone.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 151.Fratzl-Zelman N., Roschger P., Gourrier A., Weber M., Misof B.M., Loveridge N. Combination of nanoindentation and quantitative backscattered electron imaging revealed altered bone material properties associated with femoral neck fragility. Calcif Tissue Int. 2009;85:335–343. doi: 10.1007/s00223-009-9289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Norman T.L., Little T.M., Yeni Y.N. Age-related changes in porosity and mineralization and in-service damage accumulation. J Biomech. 2008;41:2868–2873. doi: 10.1016/j.jbiomech.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 153.Ciarelli T.E., Tjhia C.K., Rao D.S., Qiu S., Parfitt A.M., Fyhrie D.P. Trabecular packet-level lamellar density patterns differ by fracture status and bone formation rate in white females. Bone. 2009;45:903–908. doi: 10.1016/j.bone.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 154.Gourion-Arsiquaud S., Lukashova L., Power J., Loveridge N., Reeve J., Boskey A.L. Fourier transformed infra-red imaging of femoral neck bone: reduced heterogeneity of mineral:matrix and carbonate:phosphate and more variable crystallinity in treatment-naïve fracture cases compared to fracture-free controls. J Bone Miner Res. 2013;2013:150–161. doi: 10.1002/jbmr.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ortner D.J. Aging effects on osteon remodeling. Calcif Tissue Res. 1975;18:27–36. doi: 10.1007/BF02546224. [DOI] [PubMed] [Google Scholar]
- 156.Gao H., Ji B., Jager I.L., Arzt E., Fratzl P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci U S A. 2003;100:5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chatterji S.K., Wall J.C., Jeffery J.W. Age-related changes in the orientation and particle size of the mineral phase in human femoral cortical bone. Calcif Tissue Int. 1981;33:567–574. doi: 10.1007/BF02409493. [DOI] [PubMed] [Google Scholar]
- 158.Kent G.N., Dodds R.A., Klenerman L., Watts R.W., Bitensky L., Chayen J. Changes in crystal size and orientation of acidic glycosaminoglycans at the fracture site in fractured necks of femur. J Bone Joint Surg Br. 1983;65:189–194. doi: 10.1302/0301-620X.65B2.6826629. [DOI] [PubMed] [Google Scholar]
- 159.Gupta H.S., Seto J., Wagermaier W., Zaslansky P., Boesecke P., Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci U S A. 2006;103:17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paschalis E.P., Betts F., DiCarlo E., Mendelsohn R., Boskey A.L. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 161.Skedros J.G., Holmes J.L., Vajda E.G., Bloebaum R.D. Cement lines of secondary osteons in human bone are not mineral-deficient: new data in a historical perspective. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:781–803. doi: 10.1002/ar.a.20214. [DOI] [PubMed] [Google Scholar]
- 162.Follon E.L., Loveridge N., Stokes D., Bonfield W. Osteonal material properties in the fractured femoral neck. J Bone Miner Res. 2002;17:12. [abstract] [Google Scholar]
- 163.Boyde A., Jones S.J. Scanning electron microscopy of bone: instrument, specimen, and issues. Microsc Res Tech. 1996;33:92–120. doi: 10.1002/(SICI)1097-0029(19960201)33:2<92::AID-JEMT2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 164.Guo X.E., Liang L.C., Goldstein S.A. Micromechanics of osteonal cortical bone fracture. J Biomech Eng. 1998;120:112–117. doi: 10.1115/1.2834290. [DOI] [PubMed] [Google Scholar]
- 165.Prentice A., Jarjou L.M., Stirling D.M., Buffenstein R., Fairweather-Tait S. Biochemical markers of calcium and bone metabolism during 18 months of lactation in Gambian women accustomed to a low calcium intake and in those consuming a calcium supplement. J Clin Endocrinol Metab. 1998;83:1056–1066. doi: 10.1210/jcem.83.4.4737. [DOI] [PubMed] [Google Scholar]
- 166.Keaveny T.M., Hayes W.C. A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng. 1993;115:534–542. doi: 10.1115/1.2895536. [DOI] [PubMed] [Google Scholar]
- 167.Renders G.A., Mulder L., van Ruijven L.J., Langenbach G.E., van Eijden T.M. Mineral heterogeneity affects predictions of intratrabecular stress and strain. J Biomech. 2011;44:402–407. doi: 10.1016/j.jbiomech.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 168.Donnelly E., Meredith D.S., Nguyen J.T., Gladnick B.P., Rebolledo B.J., Shaffer A.D. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res. 2012;27:672–678. doi: 10.1002/jbmr.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kosmopoulos V., Schizas C., Keller T.S. Modeling the onset and propagation of trabecular bone microdamage during low-cycle fatigue. J Biomech. 2008;41:515–522. doi: 10.1016/j.jbiomech.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 170.Schaffler M.B. Role of bone turnover in microdamage. Osteoporos Int. 2003;14:s73–s77. doi: 10.1007/s00198-003-1477-1. [and s77-s80] [DOI] [PubMed] [Google Scholar]
- 171.United Nations Population Fund December 20 2013. http://www.unfpa.org/webdav/site/global/shared/documents/publications/2012/UNFPA-Report-Chapter1.pdf
- 172.Shaffler M., Choi K., Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 173.Allison S.J., Folland J.P., Rennie W.J., Summers G.D., Brooke-Wavell K. High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone. 2013;53:321–328. doi: 10.1016/j.bone.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 174.Kanis J.A., Johansson H., Oden A., Johnell O., De Laet C., Eisman J.A. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 175.Estrada K., Styrkarsdottir U., Evangelou E., Hsu Y.H., Duncan E.L., Ntzani E.E. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moayyeri A., Hsu Y.-H, Karasik D., Estrada K., Xiao S.-M, Nielson C. Genetic determinants of heel bone properties: Genome-wide association meta-analysis and replication in the GEFOS/GENOMOS consortium. Human Molecular Genetics. 2014, Jan 14 doi: 10.1093/hmg/ddt675. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]